Summary
The extracellular matrix (ECM) is a diverse microenvironment that maintains bidirectional communication with surrounding cells to regulate cell and tissue homeostasis. The classical definition of the ECM has more recently been extended to include non-fibrillar proteins that either interact or are structurally affiliated with the ECM, termed the ‘matrisome.’ In addition to providing the structure and architectural support for cells and tissue, the matrisome serves as a reservoir for growth factors and cytokines, as well as a signaling hub via which cells can communicate with their environment and vice-versa. The matrisome is a master regulator of tissue homeostasis and organ function, which can dynamically and appropriately respond to any stress or injury. Failure to properly regulate these responses can lead to changes in the matrisome that are maladaptive. Hepatic fibrosis is a canonical example of ECM dyshomeostasis, leading to accumulation of predominantly collagenous ECM; indeed, hepatic fibrosis is considered almost synonymous with collagen accumulation. However, the qualitative and quantitative alterations of the hepatic matrisome during fibrosis are much more diverse than simple accumulation of collagens and occur long before fibrosis is histologically detected. A deeper understanding of the hepatic matrisome and its response to injury could yield new mechanistic insights into disease progression and regression, as well as potentially identify new biomarkers for both. In this review, we discuss the role of the ECM in liver diseases and look at new “omic” approaches to study this compartment.
Keywords: Extracellular matrix, ECM, Proteomics, Liver disease, Fibrosis, Regeneration
Abbreviations: AUROC, area under the receiver operating characteristic curve; CCl4, carbon tetrachloride; ECM, extracellular matrix; HCC, hepatocellular carcinoma; MMP, matrix metalloproteinase; NAFLD, non-alcoholic fatty liver disease; NPV, negative predictive value; POSTN, periostin; PPV, positive predictive values; TGFβ, transforming growth factor beta
Key points.
-
•
The extracellular matrix is a dynamic niche critical for liver homeostasis.
-
•
Proteomic approaches can document changes to the matrisome.
-
•
Changes to the hepatic matrisome occur well before fibrosis.
-
•
Fibrotic changes extend beyond collagens to the entire matrisome.
-
•
Proteomic analysis of the extracellular matrix may yield new surrogate/mechanistic biomarkers.
Introduction
The extracellular matrix (ECM) consists of a diverse range of components that work bidirectionally with surrounding cells to create a microenvironment that regulates cell and tissue homeostasis. The basic definition of the ECM comprises fibrillar proteins (e.g., collagens, glycoproteins, and proteoglycans). This definition has more recently been extended to include ECM-affiliated proteins (e.g., collagen-related proteins, transmembrane proteoglycans), ECM regulators and modifiers (e.g., lysyl oxidases, transglutaminases, matrix metalloproteinases or MMPs) and secreted factors that bind to the ECM (e.g., transforming growth factor-β [TGFβ], and other cytokines)1; this broader definition has been termed the ‘matrisome’.2 The ECM not only provides structure and support for the cells in a tissue, but also acts as a reservoir for growth factors and cytokines and as a signalling hub via which cells can communicate with their environment and vice-versa.3,4
Genetic mutations in ECM components can cause a myriad of connective tissue pathologies,[5], [6], [7] if not embryonic lethality.8,9 Alterations in the ECM composition and dysregulation of its functions have been associated with a plethora of diseases including cardiovascular diseases,10,11 skin diseases,12 fibrosis,13 and cancers,14,15 among others. The ECM can thus be viewed as a master regulator of tissue homeostasis and organ function. As such, it must be able to dynamically respond to any variations such as stress or injury. Subcutaneous wound healing is an excellent example of appropriate ECM changes in response to injury/stress; the orchestrated crosstalk between the coagulation cascade and the inflammatory response not only mediate wound closure, but also lay a provisional ECM for recovery and restitution16; these molecular mechanisms in the skin are also at play in other organs, including the liver.17
Failure to properly regulate ‘wound healing’ responses can lead to ECM changes that are maladaptive and eventually lead to fibrosis.18 For example, ‘aging’ of the ECM (i.e., increased crosslinking) is hypothesized to contribute to dysfunction in several organ systems, including the liver.[18], [19], [20], [21] Hepatic fibrosis is a canonical example of ECM dyshomeostasis, leading to accumulation of fibrillar ECM, such as collagens. Although originally viewed as “end-stage liver disease,” it is now understood that hepatic fibrosis is potentially reversible. This phenomenon was first identified in experimental models,22,23 and later in human cohorts.[24], [25], [26], [27] The resolution of hepatic fibrosis is mediated not only by a decrease in the production of ECM, but also by an increase in the degradation of existing ECM by MMPs.28 Several key events have been identified that drive fibrosis resolution, such as stellate cell deactivation/apoptosis and changes to the inflammatory/wound healing response29,30; however, degradation of the accumulated ECM is a key (and by histologic definition, the key) response for fibrosis resolution.
Hepatic fibrosis is considered almost synonymous with collagen accumulation.13,31 Given the robust collagen ECM deposition found in fibrosis and cirrhosis and the ease of visualizing this with histochemical stains (e.g. Masson's trichrome, picrosirius red; Fig. 1), this focus is not necessarily surprising. However, the qualitative and quantitative alterations of the hepatic ECM during fibrosis are much more diverse than simple accumulation of collagens.[32], [33], [34], [35] The roles of other ECM proteins in hepatic fibrosis progression are incompletely understood. Moreover, the expanded definition of the ECM to encompass non-fibrillar proteins found in that microenvironmental niche has not been fully explored in the context of liver diseases.36,37 Yet, a deeper understanding of the roles of the ECM in liver diseases could yield new mechanistic insights into disease progression and regression, as well as potentially identify new biomarkers for both. In this review, we discuss the role of the ECM in liver diseases and look at new “omic” approaches to study this compartment.
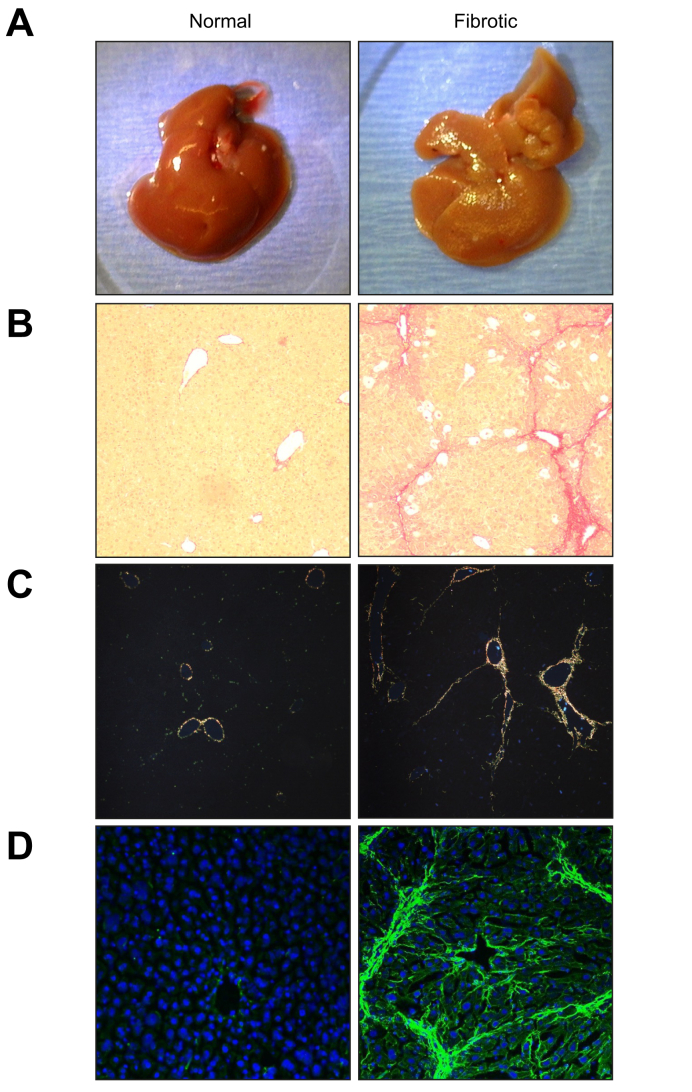
Fig. 1.
Macroscopic and microscopic depictions of normal and fibrotic mouse livers.
Representative images depicting hepatic changes in ECM in mouse liver caused by experimental fibrosis (“Fibrotic” right panels) compared to naïve control livers (“Normal” left panels); fibrosis was induced by administering CCl4 (1 ml/kg i.p.; 2x/wk) for 4 weeks. (A) Macroscopic changes to Glisson's capsule during fibrosis (see Section 1). (B) Collagen accumulation depicted by brightfield analysis of picrosirius red staining (10× magnification). (C) Collagen I (orange-red) and III (green) accumulation depicted by polarized light analysis of picrosirius red staining (10× magnification). (D) Collagen Iα1 accumulation depicted by immunofluorescent detection (20× magnification). CCl4, carbon tetrachloride; ECM, extracellular matrix.
Compartments of the hepatic ECM: the interstitium and the basement membrane
In a normal liver section, the ECM comprises a relatively small portion of the overall area.38 The best characterised function of the ECM is that of providing support and structure to tissues. The hepatic ECM is comprised of proteins from both hepatic and extrahepatic (e.g., the coagulation cascade) sources. Under basal conditions, almost all hepatocellular cells contribute to the ECM; hepatocytes, cholangiocytes and sinusoidal endothelial cells all synthesize components of the fibrillar ECM.39 Kupffer cells do not normally synthesize fibrillar ECM, but they do produce several secreted factors (e.g., cytokines) that are associated with the ECM or alter ECM metabolism. Although it is unclear if hepatic stellate cells (HSCs) generate significant ECM during normal tissue homeostasis, activated HSCs transdifferentiate into a myofibroblast-like phenotype and generate ECM.40 Furthermore, other myofibroblast-like cells have been identified in the liver, such as fibrocytes and periportal fibroblasts.[41], [42], [43], [44] The amount and content of ECM components produced by these cells change in response to injury or stress.45
In most tissues, there are 2 structurally distinct types of ECM: the interstitial ECM and the basement membrane.46 Interstitial ECM proteins (e.g., fibronectins, elastin, and fibrillar collagens) form networks that provide support to the overall superstructure that shapes and encapsulates the organ.5 The hepatic interstitial ECM comprises the Glisson's capsule (Fig. 1A), which extends into sheaths around hepatic ducts, arteries and portal venules.47 Although poorly studied, it has been long known that Glisson's capsule thickens and gets ‘rough’ with liver disease48,49 (Fig. 1A); indeed, more recent studies suggest that these changes may be tracked to determine overall hepatic fibrosis.50,51 The basement membrane is usually defined as a thin, electron-dense sheet of ECM that is the foundation for epithelial and endothelial cells. The basement membrane is an ancient and specialised form of ECM that is found at the interface between cell layers and connective tissues and around a variety of cell types, including adipocytes, Schwann cells and myotubes.52 In most tissues, this dense layer is a true barrier between the epithelial/endothelial cells and the adjacent cell layers. In the liver, it is present around larger blood vessels but is mostly absent from the fenestrated endothelium that forms sinusoidal capillaries, although some ECM components can be found in the perisinusoidal space, the space of Disse (Fig. 2). The basement layer found in the space of Disse is, in contrast, much less dense and contains fenestrations.40 Although it possesses similar ECM proteins (e.g., collagen type IV and laminin),53 this region acts more like a structural filter that facilitates bidirectional exchange of proteins and xenobiotics between the sinusoidal blood and hepatocytes. Although it is clear that the liver does not have a basal lamina, whether or not the ECM found in the space of Disse should be considered a basement membrane is the subject for a histological, rather than functional, debate.46
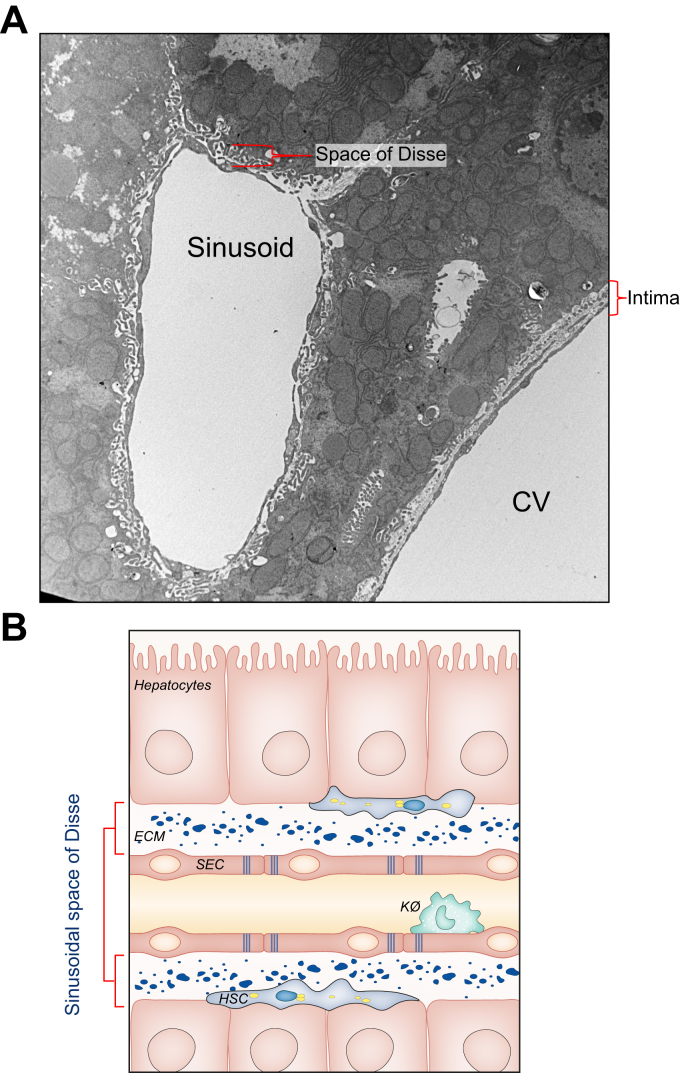
Fig. 2.
Hepatic ECM in the liver.
In normal livers, the ECM comprises only a small portion of the total tissue area. The ECM of the hepatic sinusoid in the space of Disse is discontinuous and fenestrated. This contrasts with normal basement membranes that are continuous and are true barriers between the luminal space and the parenchymal cells, such as the interstitial space surrounding central veins. (A) Transmission electron micrograph depicting the difference between discontinuous ECM in the space of Disse with continuous ECM in the interstitial space between CV lumens and hepatic tissue. Image courtesy of D. Stolz, University of Pittsburgh Center for Biologic Imaging. (B) Schematic representation of the structure of the space of Disse. Parenchymal cells (hepatocytes) are separated from the sinusoidal lumen by the space of Disse and SECs. Location of other cell types, such as HSCs and Kø are also depicted. CV, central venule; ECM, extracellular matrix; HSCs, hepatic stellate cells; Kø, Kuppfer cells; SECs, sinusoidal endothelial cells.
Remodelling of the hepatic ECM in regeneration and diseases
ECM and liver regeneration
The capacity of the liver to regenerate in response to injury is unique among encapsulated organs in mammalian species. As the main detoxifying organ in the body, the liver is at increased risk of toxic injury. Due to its regenerative properties, however, the liver can be restored to full size, ensuring survival. The liver can fully regenerate within 7–10 days in experimental models (e.g., mice).54 Although hepatocytes rarely proliferate in the healthy adult liver, virtually all surviving hepatocytes replicate at least once after 70% partial hepatectomy (PHx). Residual hepatocytes upregulate both proliferative and liver-specific gene expression in order to preserve tissue-specific function. In addition to hepatocyte proliferation, there is a tightly coordinated response to complement the regenerative process, so that the entire organ can be reconstituted within days. This complex and synchronized regenerative response in the liver can be perturbed and can thereby impact on normal tissue recovery from injury or damage. Indeed, it is now clear that impaired regeneration and/or restitution is critical to the chronicity of numerous hepatic diseases.55
Liver regeneration is often discussed in the context of 3 phases: priming, proliferation, and growth termination. The ECM plays key roles in all 3 phases and is critical for normal and adaptive liver regeneration. The priming phase is characterised by activation of several proteases found in the ECM (e.g., MMPs).56 These proteases not only break down the normal hepatic ultrastructure, which restricts proliferation, but also proteolytically release preformed growth initiation factors sequestered in the ECM (e.g., interleukin-6 and tumour necrosis factor-α).57,58 During the proliferation phase, hepatocytes divide in response to growth factors (e.g., hepatocyte growth factor), which are also initially released via proteolytic activation in the matrisome.56,59 This initial wave of proliferation forms avascular clusters of hepatocytes and non-parenchymal cells; continued proliferation is further supported by remodelling of the ECM to generate a new ultrastructure and to support angiogenesis.57 The robust regenerative response of the liver is matched by an equally robust termination of this response, once the organ has regained the original mass (i.e., the “hepatostat”)60; this process is again mediated in part by remodelling of the ECM to facilitate terminal differentiation of the new liver cells and to complete the building of the ultrastructure.57 Hepatic regeneration has been studied for several decades and the hepatic ECM is under extensive investigation for the purposes of regenerative medicine and liver tissue engineering.64 Our current understanding of the role of the ECM in liver regeneration is built upon both hypothesis-driven experiments, as well as “omic” approaches that have profiled changes in gene expression or global protein abundance.[61], [62], [63] Future analyses of this unique process using proteomic strategies specifically aimed at profiling ECM proteins (see below) would likely be highly informative.
Disruption of the architecture and composition of the liver ECM in diseases
As mentioned, in some areas of research (e.g., subcutaneous wound healing), the global changes to the ECM in response to acute injury are well-understood.16 In contrast, research on the hepatic ECM in the context of liver disease has been largely ‘collagenocentric’ and ‘fibrosocentric,’ or primarily focused on the role of the collagen ECM during fibrogenesis65,66 (Fig. 1). However, it is well-known that there are multiple ECMs that change qualitatively and quantitatively during hepatic fibrosis,32,33 and these changes are not solely relegated to fibrosis.
Although a critical foundation of scientific understanding, hypothesis-driven science has philosophical and technical limitations.67,68 First, reductionist approaches assume that the behaviour of individual components does not change when incorporated into the system as a whole. This assumption has already been criticised for studying the ECM, as many of these components are interlinked and function as a group.4 Moreover, hypothesis-driven research, by definition, precludes discovery-based experiments, as they rely on an a priori understanding of the expected results. In contrast, agnostic, data-intensive, approaches are often criticised as unfocused or ‘fishing’ exhibitions that may lead to false positive results. However, “omic” approaches, coupled with hypothesis-driven step-wise informatics analyses (i.e., iterative data-intensive studies) can overcome these concerns and yield new information and ideas.67
Proteomic profiling of the hepatic matrisome
Despite technical challenges linked to studying highly glycosylated and cross-linked biomolecules ,2 over the past decade mass spectrometry-based approaches have been developed to characterise the protein composition of the ECM of tissues (reviewed in36,[69], [70], [71]). In brief, these approaches exploit the fact that ECM proteins are relatively insoluble compared to most intracellular proteins, and thus can be enriched by incubating tissue samples in buffers that deplete non-ECM proteins (i.e., decellularisation). ECM proteins obtained from tissues can then be digested into peptides whose sequences can be inferred by mass spectrometry analysis.36,69,72,73 Of note, which ECM proteins and how many of them are identified in a proteomic screen depend on several variables, including the stringency of the decellularisation approach, the method used to digest proteins into peptides (e.g. enzymatic vs. non-enzymatic), the extent of protein or peptide fractionation, and the mass spectrometry acquisition parameters (e.g. length of the liquid chromatography gradient). For example, decellularisation buffers containing higher concentrations of detergents risk extracting some ECM-associated proteins. Although it is beyond the scope of this review to discuss these points here, we invite our readers to refer to a recent review for more information.69
The liver matrisome is composed of 150+ distinct ECM and ECM-associated proteins
In a previous study focused on primary colorectal cancers and their hepatic metastases, we reported the in-depth characterisation of the ECM of human liver samples from healthy individuals and showed that it is composed of over 150 distinct ECM proteins,74 a number higher than anyone could have predicted. In this section, we review this first draft of the healthy human liver matrisome and discuss how the proteins composing it can further be classified into different categories, which can shed light on their potential roles in liver physiology.2,75 These first drafts of the healthy human and murine liver matrisomes provide a reference for studies that aim to identify changes in the ECM composition accompanying the aetiology of liver diseases (see below).
Core matrisome proteins of the liver
Components of the basement membrane
The basement membrane “toolkit” is the ensemble of 40 proteins that can assemble to form a functional basement membrane. It comprises the ECM glycoproteins laminins, nidogens, agrin, hemicentin, nidogens, nephronectin, papilin and netrins, type IV and type VI collagens, and the proteoglycan perlecan.53,76 Proteomic analysis of the ECM of healthy human liver has identified 21 of these proteins (Table 1) including 7 laminin chains that can assemble to form heterotrimeric laminins α2β1γ1, α2β2γ1, α4β1γ1, α4β2γ1, and α5β1γ1,77 agrin, nephronectin, nidogen 1, papilin, and perlecan (Table 1). Mass spectrometry also identified 5 collagen IV chains, which are network-forming collagen chains that form heterotrimers comprising either the α1(IV), α2(IV), α3(IV) chains, the α3(IV), α4(IV), α5(IV) chains, or the α5(IV)2, α6 (IV) chains,78,79 although the α5(IV) chain was not detected. Last, mass spectrometry identified 4 collagen VI chains forming heterotrimers comprising either the α1(VI), α2(VI), α3(VI) chains or the α1(VI), α2(VI), α6(VI) chains.80
Table 1.
List of basement membrane proteins identified by mass spectrometry in healthy human liver samples.
| ECM glycoproteins | Collagens | Proteoglycan |
|---|---|---|
| Laminin α chains: α1, α2, α4 |
Collagen IV chains: 4α1, 4α2, 4α3, 4α4, 4α6 |
Perlecan (HSPG2) |
| Laminin β chains: β1, β2, β3 |
Collagen VI chains: 6α1, 6α2, 6α3, 6α6 |
|
| Laminin γ1 | ||
| Agrin (AGRN) | ||
| Nidogen-1 (NID1) | ||
| Nephronectin (NPNT) | ||
| Papilin (PAPLN) |
(Adapted from Naba et al., BMC Cancer, 201474). ECM, extracellular matrix.
Collagens
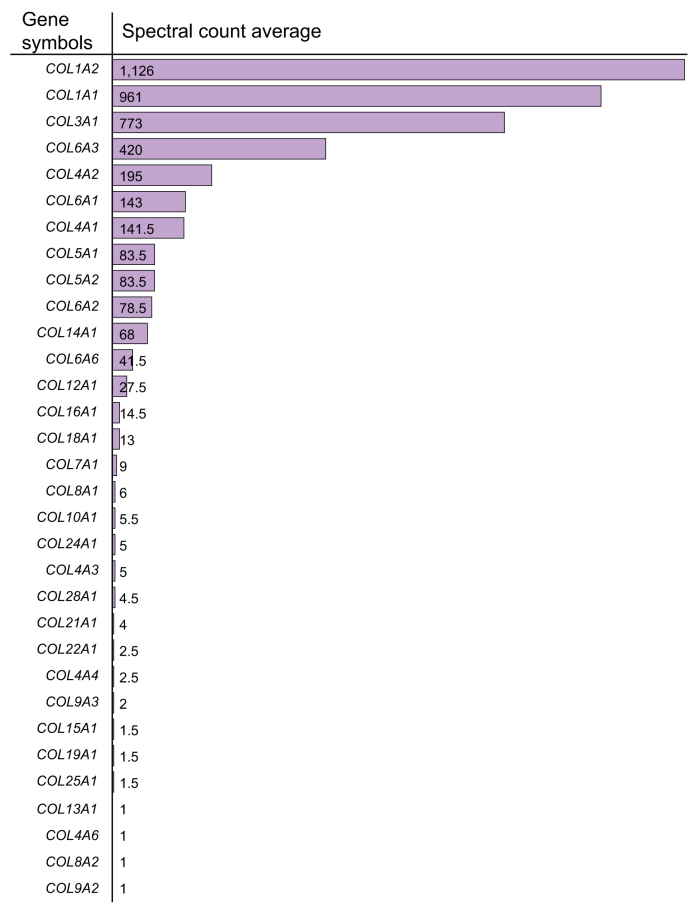
The human genome encodes 44 collagen genes that are further classified based on their structures and supramolecular assembly types.79 In addition to the 9 chains of network-forming basement membrane collagens, 23 other collagen chains were detected, including the fibril-forming collagens α1(I), α2(I), α1(III), α1(V), α2(V), α1(XXVII); the fibril-associated collagens with interrupted triple helices (FACIT) α2(IX), α3(IX), α1(XII), α1(XIV), α1(XVI), α1(XIX), α1(XXI), α1(XXII); the network-forming collagens α1(VIII), α2(VIII), α1(X); collagen α1(VII); the multiplexins collagens α1(XV) and α1(XVIII), and the transmembrane collagens α1(XIII) and α1(XXV) (Box 1). These proteins were found in vastly different quantities with fibrillar collagens being the most abundant and FACITs being detected in lower abundance74 (Fig. 3).
Box 1.
List of matrisome-associated proteins identified by mass spectrometry in healthy human liver samples. (Adapted from Naba et al., BMCCancer, 201474). ECM, extracellular matrix.
Fig. 3.
List of collagens identified by mass spectrometry in healthy human liver samples.
Spectral count is an indication of the relative protein abundance. Adapted from.74
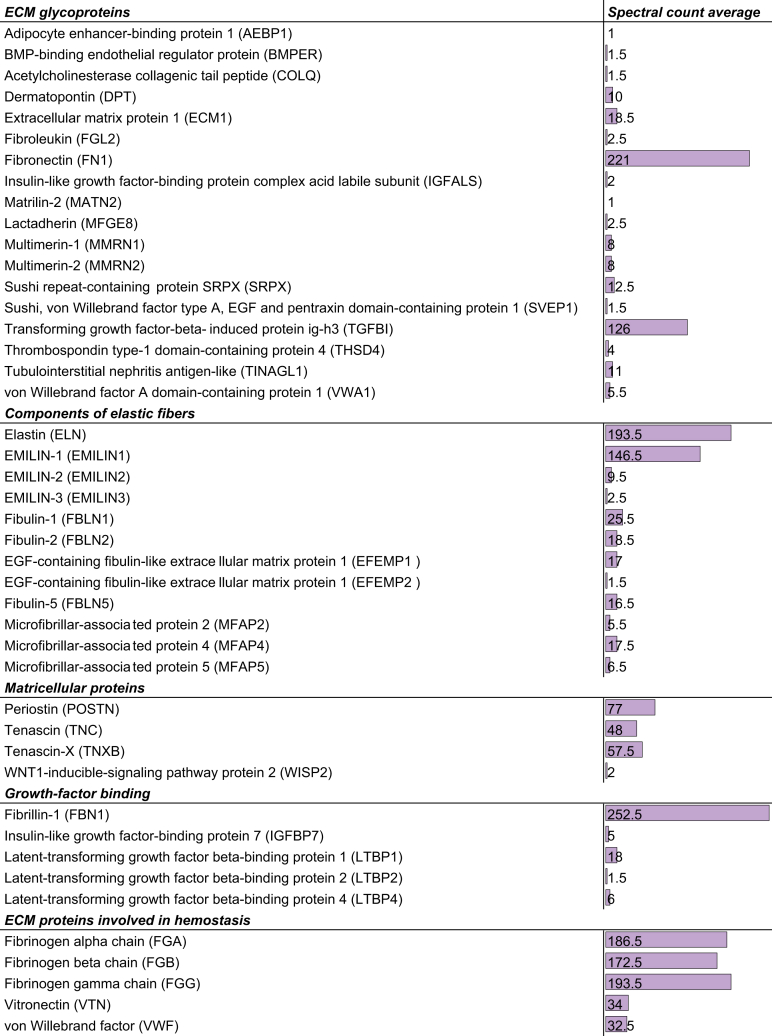
ECM glycoproteins
In Fig. 4, we present the list of 44 ECM glycoproteins that were identified in our study and that we further classified based on their structure or functions. Among the most abundant proteins detected (based on the spectral count, a semi-quantitative measure of protein abundance) are those synthesised in the liver, including fibrinogens (that play a role in haemostasis) and fibronectin. We also detected a large number of proteins associated with the formation of elastic fibres,81 the matricellular proteins82 periostin, the tenascins C and X and WISP2, and TGFβ-binding ECM proteins including fibrillin-1, and the latent TGFβ-binding proteins 1, 2 and 4.83 Interestingly, recent work by Fan et al.84 has shown that the protein glycoprotein ECM1 restricts activation of TGFβ during fibrogenesis in mice. Periostin has also been shown to participate in the aetiology of intrahepatic cholangiocarcinoma85 and hepatic fibrogenesis.86
Fig. 4.
List of ECM glycoproteins identified by mass spectrometry in healthy human liver samples.
Spectral count is an indication of the relative protein abundance. Adapted from.74 ECM, extracellular matrix.
Proteoglycans
As it is with collagens and glycoproteins, proteoglycans can be further categorised based on their structural properties.87 Six of the 11 proteoglycans detected by mass spectrometry (asporin, biglycan, decorin, lumican, mimecan and prolargin) belong to the family of small leucine-rich repeat proteoglycans (or SLRPs) that are characterised by the presence of leucine-rich repeats in the protein core of these components, 1, versican, belongs to the hyalectan family and 3 are understudied proteins: proteoglycan 2, proteoglycan 3, and proteoglycan 4 (or lubricin). Interestingly, it has recently been proposed that certain proteoglycans could serve as biomarkers and potential therapeutic targets in hepatocellular carcinoma (HCC).88
Matrisome-associated proteins of the liver
In addition to the core matrisome components, i.e. proteins forming the architecture of the liver ECM,1,2 we have previously proposed including other proteins that can be found within ECMs in the definition of the matrisome. Among these are proteins that are structurally related to ECM proteins (termed “ECM-affiliated proteins”), ECM-remodelling enzymes and their regulators (termed “ECM regulators”), as well as other secreted proteins that can bind to the ECM including growth factors (termed “secreted factors”).2,3,75 We have compiled in Box 1, the 15 ECM-affiliated proteins, 46 ECM regulators, including a large number of proteases (cathepsins and matrix metalloproteinases) and crosslinking enzymes (transglutaminase 2 and lysyl oxidase like 1), and 21 secreted factors found in our previous study.74
Since murine models are broadly used to study liver development, diseases and regeneration, recent proteomic studies have focused on the characterisation of the matrisome of healthy murine liver samples. Krasny and collaborators compared side-by-side different ECM decellularisation and solubilisation strategies and identified a total of 40 matrisome proteins in mouse liver samples,89 of which 30 were also discovered in healthy human liver samples. Goddard and collaborators reported the identification of 35 matrisome proteins using a global proteomic approach on the ECM of normal rat livers,90 apart from the ECM-affiliated protein PlexinA2, all of these proteins were also detected in healthy human liver samples. Furthermore, Goddard and collaborators also employed a technology known as QConCAT for Quantitative conCATamers synthetically made of the assembly of peptides of interest that are released upon enzymatic digestion,91 to specifically measure the abundance of 57 selected ECM proteins in rat liver samples.90 This study identified collagens I and VI as well as fibronectin, thrombospondin 1 and transglutaminase 2 as the most abundant proteins of the 57 quantified in the rat liver, mirroring the data obtained on human liver samples. Of note, differences in the number of matrisome proteins detected in different studies can be partly attributed to the depth of the analyses performed (e.g. extent of peptide fractionation, length of the liquid chromatography gradient, dynamic exclusion of highly abundant peptides during the mass spectrometry scan).
Changes in the composition of the ECM during liver fibrosis identified using proteomics
Similar to other organs, the acute phase response in the liver involves several of the ECM proteins found in subcutaneous wound healing.[92], [93], [94] Using proteomics on ECM-enriched/decellularised samples, we demonstrated that the hepatic matrisome changes robustly in response to acute injury (e.g., acute lipopolysaccharide), even under conditions in which the ECM appears histologically unchanged65 (Fig. 5A). These sub-histologic transitional changes to the matrisome appear to resolve after acute injury.65,95 With chronic injury (alcohol exposure), we showed that the transitional matrisome is replaced by collagenous scarring in the liver, which is again in line with subcutaneous wound healing.16 Improvements in referral practices and non-invasive tests have increased the rate of early detection of asymptomatic chronic liver diseases.96 This paradigm change has created the opportunity for mechanism-based therapies to halt disease progression during earlier (i.e., pre-fibrotic) phases of the disease progression.
Fig. 5.
Proteomic profiling of the matrisome in different models of liver fibrosis.
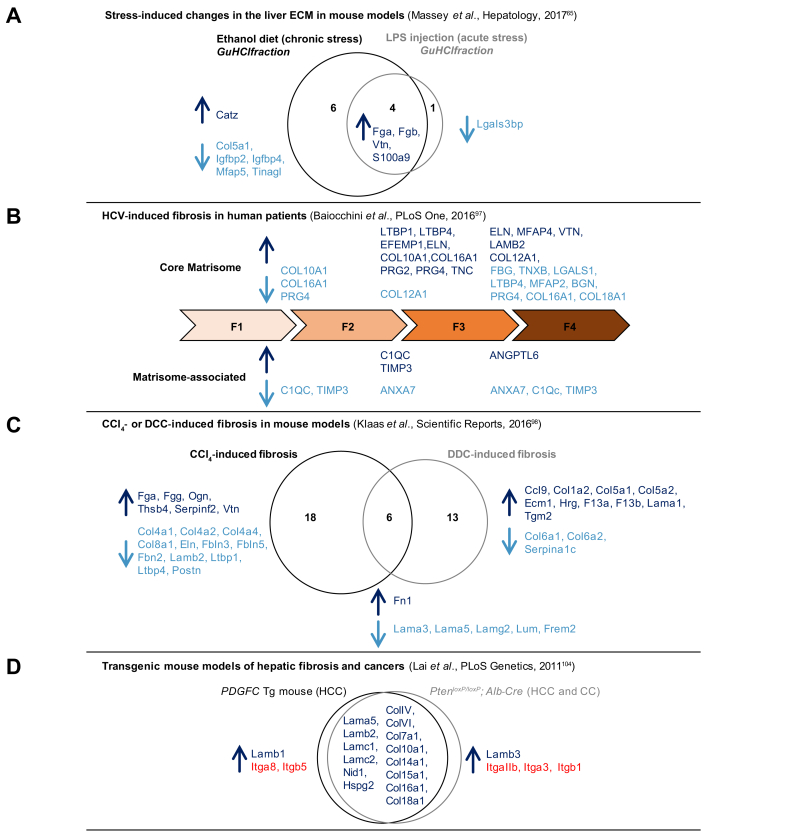
(A) Changes in the liver ECM in mouse models in response to acute (LPS injection) or chronic (ethanol exposure) stress.65 (B) HCV-induced fibrosis in human patients.97 (C) CCl4- or DDC-induced fibrosis in mouse models.98 (D) Transgenic mouse models of hepatic fibrosis and cancers.104 ECM proteins detected in higher abundance between conditions are indicated in dark blue, those detected in lower abundance between conditions are indicated in light blue. CCl4, carbon tetrachloride; DDC, diethoxycarbonyl dihydrocollidine; ECM, extracellular matrix; LPS, lipopolysaccharide.
Proteomics was also recently employed to study changes in the hepatic matrisome during fibrosis. In a 2016 study, Baiocchini and collaborators employed proteomics to characterise the ECM composition of fibrotic liver samples obtained from 57 HCV-infected patients.97 They profiled the ECM of decellularised liver samples at different stages of fibrosis: the F1 stage, characterised by portal fibrosis without septa (13 patients), the F2 stage by portal fibrosis with few septa (19 patients), the F3 by numerous septa without cirrhosis (14 patients), and the F4 stage characterised by cirrhosis (10 patients). The study revealed that the abundance of certain core matrisome and matrisome-associated proteins varies between fibrotic stages (Fig. 5B). Interestingly, they also showed that the abundance of the fibrillar collagens I and III increased between F1 and F2, and F2 and F3, but decreased in cirrhotic samples (stage F4). In another 2016 study, Klaas and collaborators aimed to determine the extent to which the ECM composition of the liver changes during fibrosis. To do so, they used ECM-enriched samples from 2 mouse models in which liver fibrosis was induced either by intraperitoneal injection of carbon tetrachloride (CCl4) or by feeding mice with a 0.1% diethoxycarbonyl dihydrocollidine (DDC)-supplemented diet for 2 weeks.98 Importantly, this study demonstrated that the nature of the insult triggering a fibrotic response (CCl4 or DDC) induced the production of a different set of ECM components (Fig. 5C). This is likely because different cell types are recruited and/or activated upon various insults and different signalling pathways are activated downstream of these insults. Interestingly, this study further showed that compositional changes were associated with changes in tissue microarchitecture and elasticity of the liver, 2 parameters that can be used as biomarkers of liver disease progression.[99], [100], [101]
Although fibrosis is characterised by an accumulation of ECM, it has been proposed that the fibrotic ECM is not properly assembled and that ECM components secreted in excess fail to incorporate into an insoluble ECM, and thereby may be subjected to increased turnover.102 In addition, in pathological contexts, ECM proteins may be subjected to degradation or crosslinking, which also impact protein solubility and subsequently ECM-mediated cell signalling. Changes in ECM protein solubility can be assessed by analysing protein fractions from tissues using quantitative detergent solubility profiling developed by the Mann lab.103 In the aforementioned study, we profiled 4 distinct biochemical fractions containing proteins soluble in sodium chloride buffer, in SDS-containing buffer, in guanidine hydrochloride, and the remaining insoluble proteins. Using this approach we showed that in addition of changes in the ECM composition, changes in ECM protein solubility occurred upon acute or chronic stress.65
Changes in the composition of the ECM accompany HCC progression
ECM deposition is also a hallmark of cancers.14 Lai and colleagues used global proteomics to compare changes in the core matrisome composition occurring over time in 2 transgenic mouse models of liver cancers104: a first model in which the human PDGF-C is expressed in a liver-specific manner by the albumin promoter and which results in the development of fibrosis, steatosis and HCC105; and a second model in which Pten is deleted specifically from hepatocytes (Ptenfl/fl; Alb-Cre), which results in the development of steatosis, followed by HCC and cholangiocarcioma.106 In their analysis, the authors exclusively focused on core matrisome components, specifically collagens and ECM glycoproteins of the basement membrane, and on those components detected in higher abundance between healthy and fibrotic tissues and between fibrotic tissues and tumour samples. Interestingly, they reported that the majority of changes occurred in both models, with only 1 differing basement-membrane component between the 2 systems: the laminin β1 chain being detected in higher abundance in HCC arising in the PDGF-C model and the laminin β3 chain detected in higher abundance in the HCC arising in the Pten model (Fig. 5D). This suggests that different laminin trimers may be assembled in different contexts, however, of note, none of the chains capable of assembling with the β3 chain (namely the α3A or α3B chains) were reported in the study. In addition to interrogating the ECM protein composition of HCCs, this study also reported the profiling of the complement of ECM receptors (CD44, integrins) detected in the different samples, which hints at the signalling pathways activated in different contexts (Fig. 5D). In line with these observations, Mazza et al.107 demonstrated in a recent study that the cirrhotic ECM may differentially favour key steps of hepatocarcinogenesis (e.g., epithelial to mesenchymal transitions), which may explain why HCC almost exclusively occurs on the background of cirrhosis.
Changes in the hepatic ECM upon metastatic seeding
Over 130 years ago, Paget proposed the “seed and soil” hypothesis of cancer metastasis.108 He proposed that the propensity of metastases to “home” to specific organs is regulated by interaction and cooperation between the metastatic cells (seed) and the target organ microenvironment (soil).108 The content and metabolism of the ECM is key in mediating both intravasation and escape of the metastasis from the primary tumour, as well as extravasation, growth, and invasion at the site of metastasis.
The liver is one of the preferred sites of metastasis of multiple cancer types.109,110 As mentioned, the impact of the ECM on liver diseases has historically focused on fibrotic changes. Although primary liver cancer (HCC) occurs almost exclusively on the background of fibrosis/cirrhosis, no such association has been shown in the context of metastasis to the liver.111 Steatosis/steatohepatitis is commonly found during resection surgery for metastases to the liver, but these changes are often attributed to chemotherapy-induced fatty liver injury.112,113 However, some studies have indicated that earlier stages of chronic liver diseases (e.g., NAFLD) are associated with a higher incidence of metastases to the liver, regardless of chemotherapeutic regimen.111,114 Several hypotheses have been proposed to explain why the liver is such a supportive environment for metastatic seeding and outgrowth, one of which is the presence of a hospitable and tuneable ECM.74 Recently, proteomics has been employed to determine the extent of the remodelling of the hepatic matrisome upon metastatic seeding of colorectal tumour cells74 or mammary tumour cells.115 Given the novel recognition that the hepatic matrisome responds much more dynamically to liver injury than previously appreciated, these changes may increase the fertility of the hepatic “soil” for metastases. Some studies have been performed to computationally predict these possibilities,116,117 but a deeper understanding of this potential interaction is needed.
Integrating proteomic data on the liver ECM into MatrisomeDB
The development of proteomic approaches to characterise the matrisome of tissues has made it possible to catalogue ECM components and changes in their abundance and solubility across various states. However, this is only the first step towards understanding the specific roles of each ECM component in driving disease progression. Thanks to the efforts of the scientific community, raw mass spectrometry data are usually publicly available via repositories such as ProteomeXchange118 or PRIDE.119 To assist with that effort, we have developed a searchable database, MatrisomeDB (http://www.pepchem.org/matrisomedb), that integrates proteomic data on the ECM of tissues.120 In its latest release, and of interest to our readers, we have included 4 datasets on the liver ECM for which mass spectrometry data were publicly available.65,74,97,98 Multiple parameters can be used to interrogate MatrisomeDB including one or multiple matrisome categories, species, or tissue types, or, more specifically, a particular gene or gene signature.120 MatrisomeDB query output include heatmaps indicative of relative protein abundance across tissues as well as detailed lists of detected proteins.
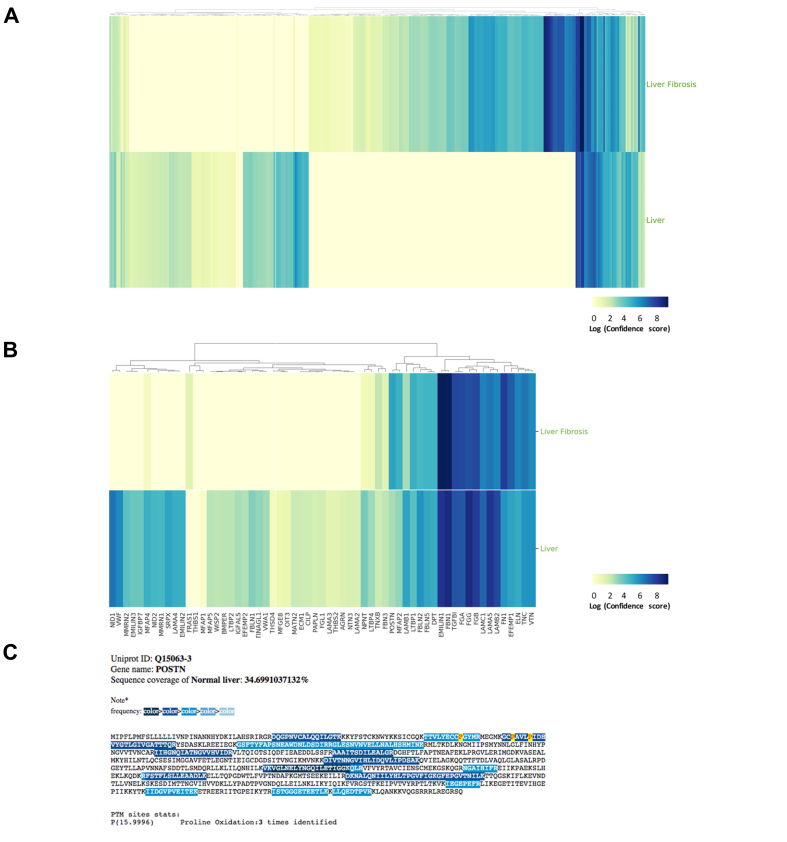
To illustrate how MatrisomeDB can be used to further our understanding of the roles of the ECM in liver diseases, we interrogated MatrisomeDB by selecting “Liver” and “Liver fibrosis” in the “Tissues” option box and “Human” in the “Species” option box (for more information on how to use MatrisomeDB, please refer to our online tutorial). This search returns a heatmap where the colour intensity reflects the confidence with which each protein is detected and which correlates to a certain extent with protein abundance (Fig. 6A). A more focused search can be performed by, for example, selecting “ECM Glycoproteins” in the “Matrisome Categories” option box. This search retrieves a confidence-score-based heatmap of all ECM glycoproteins detected in normal liver and fibrotic liver samples (Fig. 6B). If users wish to obtain detailed information on a given protein, they can further select it from the list found under the heatmaps. In particular, by clicking on the entries listed under the “Protein description” column, users can access a peptide coverage map depicting the actual peptides found in any given samples for a given protein.74 For illustrative purposes, we selected periostin (POSTN), an ECM glycoprotein that has been shown to play a role in the aetiology of liver fibrosis.86 The highlighted sequences of the coverage map correspond to the identified peptides in a particular sample and the colour code indicates the frequency with which each peptide was detected (Fig. 6C). Last, we indicated the presence, if detected experimentally, of any of the following post-translational modifications on the coverage map: lysine and proline hydroxylations; phosphorylation of serine, threonine, and tyrosine, and arginine citrullination, all of which play significant roles in the proper folding and function of ECM proteins. Here, isoform 3 of periostin (UniProt identifier: Q15063-3) was detected with 17 peptides in healthy human liver samples, covering 34% of the overall periostin sequence. In addition, 3 prolines (prolines 81, 93, and 97) were found to be hydroxylated (Fig. 6C).
Fig. 6.
Interrogating MatrisomeDB to study the ECM of the liver.
(A) Confidence-score-based heatmap generated upon selecting “Liver” and “Liver fibrosis” in the “Tissues” option box and “Human” in the “Species” option box in MatrisomeDB. Results show the tissue distribution all matrisome components detected. The colour code indicates the confidence score from high (dark blue) to low (light yellow). Clicking on the heatmap will open a detailed heatmap and a link to download the data in .csv format. (B) Confidence-score-based heatmap generated upon selecting “ECM Glycoproteins” in the “Categories” option box; “Liver” and “Liver fibrosis” in the “Tissue” option box and “Human” in the “Species” option box in MatrisomeDB. Results show the tissue distribution of all ECM glycoproteins detected. The colour code indicates the confidence score from high (dark blue) to low (light yellow). Clicking on the heatmap will open a detailed heatmap and a link to download the data in .csv format. (C) The peptide coverage map of POSTN in the normal human liver dataset.74 The colour code indicates the peptide-spectrum match frequency from high (dark blue) to low (light blue). Percent coverage and selected post-translational modifications detected are also indicated. ECM, extracellular matrix; POSTN, periostin.
Biomarker discovery opportunities
Chronic liver diseases are often clinically silent until very late stages, when the organ starts to decompensate. This is an especially important clinical need, as the potential reversibility of the disease decreases as disease severity progresses. There are various imaging (e.g., transient elastography) and scoring (e.g., FIB-4) approaches that have good negative predictive values (NPV; i.e., low false negative rate), but do not have such good positive predictive values (PPV; i.e., relatively high false positive rate) (e.g.,121). The end result is that although these approaches are good at predicting who is not at risk for severe liver disease, they do not accurately stratify inter-individual risk in the “at risk” group, limiting their ability to define cost-effective interventions.
As mentioned, the key feature of fibrogenesis is increased de novo synthesis of collagens. By extension, changes in biomarkers of collagen synthesis/deposition may serve as predictors of liver disease severity. For example, the precursor of Type III collagen (PRO-C3) has been identified to have areas under the receiver operating characteristics curve (AUROC) values for predicting liver disease severity that are superior to imaging and/or scoring approaches; the improved AUROCs were largely driven by better PPVs compared to the imaging and scoring approaches.122,123
Another possible source of new biomarkers is based on indices of ECM turnover. Even in cases where there is a net increase in ECM in the liver (e.g., fibrosis) overall turnover is also increased.124 The peptidome is defined as the population of low molecular weight biologic peptides (0.5–3 kDa), within the cells and biologic fluids. The study of the peptidome (i.e., ‘peptidomics’) is a discipline related to proteomics, but with significant methodological and analytical differences.[125], [126], [127] The peptidome also contains fragments of proteins degraded by normal and/or abnormal processes (i.e., ‘degradome’).128,129 The latter subset of the peptidome has generated key interest in some areas of human health as possible (surrogate) biomarkers for disease. Degradomic analysis of cancer metastasis, and by extension overall patient outcome, has garnished significant interest.127,130,131 The rationale is that metastasis and tumour growth require significant remodelling of the normal and cancerous interstitial space, which can lead to alterations in the degradome profile in biological fluids. Similar approaches are beginning to be applied for liver diseases. For example, the peptidome has been shown to predict liver disease severity and outcome in HBV infection.132 Interestingly, increased enzymatic activity can be exploited to design novel diagnostic approaches. In a 2013 study, Kwong and collaborators reported the design of mass-encoded synthetic MMP and cathepsin substrates that, upon cleavage, release biomarkers detectable during a simple urine test.133 Using this technology in a mouse model of liver fibrosis, they further showed that these agents can non-invasively monitor liver fibrosis progression and resolution.133
Future directions and conclusions
The studies presented in this review highlight how mass spectrometry-based proteomics has emerged as a powerful method to characterise the hepatic ECM and have revealed a much more complex protein landscape than anyone had anticipated. However, because of the destructive nature of the solubilisation methods employed, and because, so far, matrisome studies have been conducted on whole tissues, the information regarding locoregional specification are lost; yet this information is of paramount importance to fully understand the roles of the hepatic ECM in liver diseases. Hence, proteomic studies should be complemented by methods that permit the visualisation of the expression pattern of ECM proteins detected by mass spectrometry134,135 and by methods that can shed light on the mechanical properties of the ECM, since they are intrinsically linked with ECM and organ functions.
Another area of investigation that remains poorly understood is the regulation of post-translational modifications of ECM proteins. These modifications regulate the formation of polymeric, helical structures and cross-linked complexes associated with several fibrillar proteins. For example, prolyl 4-hydroxylases catalyse the hydroxylation of proline residues and their main substrates are collagens, since hydroxyprolines have been shown to stabilise collagen triple helices.136 Recent studies indicate that lysyl oxidases and transglutaminases also contribute to ECM crosslinking.137,138 Although these events are important for stabilising proteins and preventing their degradation under normal conditions, their activation may contribute to excessive ECM accumulation in response to injury (e.g., fibrosis).137 Furthermore, although fibrosis is potentially reversible, highly crosslinked ECM may be resistant to resolution.139,140 Crosslinking of the ECM may be altered via non-enzymatic means[141], [142], [143]; for example the formation of advanced glycation end-products during diabetes is hypothesised to contribute to ECM crosslinking and increased ECM ‘aging’.144 As recently reported for lung fibrosis,145 it would be interesting to see mass spectrometry-based methods applied to study these PTMs in the context of liver diseases.
Last, and despite significant efforts from the scientific community,146,147 recapitulating cell culture conditions capable of faithfully modelling liver pathophysiology and supporting robust drug testing in vitro remains challenging. We thus propose that the comprehensive and quantitative characterisation of the hepatic matrisome can provide a novel basis for the design of more relevant in vitro systems to study liver diseases.
Financial support
This work was supported by a start-up fund from the Department of Physiology and Biophysics at the University of Illinois at Chicago (AN) and NIH grants R01AA021978 and P30DKDK120531 (GEA).
Authors' contributions
GEA and AN co-wrote the manuscript together.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work. Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgement
We thank Donna Stolz, PhD, from the University of Pittsburgh Center for Biologic Imaging (CBI) for supplying the electron micrograph depicting hepatic ECM.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2020.100115.
Contributor Information
Gavin E. Arteel, Email: gearteel@pitt.edu.
Alexandra Naba, Email: anaba@uic.edu.
Supplementary data
References
- 1.Hynes R.O., Naba A. Overview of the matrisome -an inventory of extracellular matrix constituents and functions. Cold Spring Harbor Perspect Biol. 2012;4:a004903. doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naba A., Clauser K.R., Hoersch S., Liu H., Carr S.A., Hynes R.O. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol Cell Proteomics. 2012;11 doi: 10.1074/mcp.M111.014647. M111.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hynes R.O. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rozario T., DeSimone D.W. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol. 2010;341:126–140. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sewry C.A., Muntoni F. Inherited disorders of the extracellular matrix. Curr Opin Neurol. 1999;12:519–526. doi: 10.1097/00019052-199910000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Mao J.R., Bristow J. The Ehlers-Danlos syndrome: on beyond collagens. J Clin Invest. 2001;107:1063–1069. doi: 10.1172/JCI12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coulombe P.A., Kerns M.L., Fuchs E. Epidermolysis bullosa simplex: a paradigm for disorders of tissue fragility. J Clin Invest. 2009;119:1784–1793. doi: 10.1172/JCI38177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo X.D., Johnson J.J., Kramer J.M. Embryonic lethality caused by mutations in basement membrane collagen of C. elegans. Nature. 1991;349:707–709. doi: 10.1038/349707a0. [DOI] [PubMed] [Google Scholar]
- 9.Bonnans C., Chou J., Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergmeier W., Hynes R.O. Extracellular matrix proteins in hemostasis and thrombosis. Cold Spring Harb Perspect Biol. 2012;4:a005132. doi: 10.1101/cshperspect.a005132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bloksgaard M., Lindsey M., Martinez-Lemus L.A. Extracellular matrix in cardiovascular pathophysiology. Am J Physiol Heart Circ Physiol. 2018;315:H1687–H1690. doi: 10.1152/ajpheart.00631.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watt F.M., Fujiwara H. Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harb Perspect Biol. 2011;3:a005124. doi: 10.1101/cshperspect.a005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ricard-Blum S., Baffet G., Théret N. Molecular and tissue alterations of collagens in fibrosis. Matrix Biol. 2018;68–69:122–149. doi: 10.1016/j.matbio.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Socovich A.M., Naba A. The cancer matrisome: from comprehensive characterization to biomarker discovery. Semin Cell Dev Biol. 2019;89:157–166. doi: 10.1016/j.semcdb.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Pickup M.W., Mouw J.K., Weaver V.M. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15:1243–1253. doi: 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun B.K., Siprashvili Z., Khavari P.A. Advances in skin grafting and treatment of cutaneous wounds. Science. 2014;346:941–945. doi: 10.1126/science.1253836. [DOI] [PubMed] [Google Scholar]
- 17.Huebener P., Schwabe R.F. Regulation of wound healing and organ fibrosis by toll-like receptors. Biochim Biophys Acta. 2013;1832:1005–1017. doi: 10.1016/j.bbadis.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harvey A., Montezano A.C., Lopes R.A., Rios F., Touyz R.M. Vascular fibrosis in aging and hypertension: molecular mechanisms and clinical implications. Can J Cardiol. 2016;32:659–668. doi: 10.1016/j.cjca.2016.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sessions A.O., Engler A.J. Mechanical regulation of cardiac aging in model systems. Circ Res. 2016;118:1553–1562. doi: 10.1161/CIRCRESAHA.116.307472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sacca S.C., Gandolfi S., Bagnis A., Manni G., Damonte G., Traverso C.E. From DNA damage to functional changes of the trabecular meshwork in aging and glaucoma. Ageing Res Rev. 2016;29:26–41. doi: 10.1016/j.arr.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Phillip J.M., Aifuwa I., Walston J., Wirtz D. The mechanobiology of aging. Annu Rev Biomed Eng. 2015;17:113–141. doi: 10.1146/annurev-bioeng-071114-040829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pérez-Tamayo R. Cirrhosis of the liver: a reversible disease? Pathol Annu. 1979;14 Pt 2:183–213. [PubMed] [Google Scholar]
- 23.Iredale J.P., Benyon R.C., Pickering J., McCullen M., Northrop M., Pawley S. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wanless I.R., Nakashima E., Sherman M. Regression of human cirrhosis. Morphologic features and the genesis of incomplete septal cirrhosis. Arch Pathol Lab Med. 2000;124:1599–1607. doi: 10.5858/2000-124-1599-ROHC. [DOI] [PubMed] [Google Scholar]
- 25.Dienstag J.L., Goldin R.D., Heathcote E.J., Hann H.W.L., Woessner M., Stephenson S.L. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124:105–117. doi: 10.1053/gast.2003.50013. [DOI] [PubMed] [Google Scholar]
- 26.Hadziyannis S.J., Tassopoulos N.C., Heathcote E.J., Chang T.-T., Kitis G., Rizzetto M. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131:1743–1751. doi: 10.1053/j.gastro.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Schiff E.R., Lee S.S., Chao Y.-C., Kew Yoon S., Bessone F., Wu S.-S. Long-term treatment with entecavir induces reversal of advanced fibrosis or cirrhosis in patients with chronic hepatitis B. Clin Gastroenterol Hepatol. 2011;9:274–276. doi: 10.1016/j.cgh.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 28.Campana L., Iredale J.P. Regression of liver fibrosis. Semin Liver Dis. 2017;37:1–10. doi: 10.1055/s-0036-1597816. [DOI] [PubMed] [Google Scholar]
- 29.Tacke F., Trautwein C. Mechanisms of liver fibrosis resolution. J Hepatol. 2015;63:1038–1039. doi: 10.1016/j.jhep.2015.03.039. [DOI] [PubMed] [Google Scholar]
- 30.Zoubek M.E., Trautwein C., Strnad P. Reversal of liver fibrosis: from fiction to reality. Best Pract Res Clin Gastroenterol. 2017;31:129–141. doi: 10.1016/j.bpg.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Friedman S.L. Stellate cell activation in alcoholic fibrosis--an overview. Alcohol Clin Exp Res. 1999;23:904–910. [PubMed] [Google Scholar]
- 32.Gressner A.M., Bachem M.G. Cellular sources of noncollagenous matrix proteins: role of fat-storing cells in fibrogenesis. Semin Liver Dis. 1990;10:30–46. doi: 10.1055/s-2008-1040455. [DOI] [PubMed] [Google Scholar]
- 33.Gressner O.A., Weiskirchen R., Gressner A.M. Evolving concepts of liver fibrogenesis provide new diagnostic and therapeutic options. Comp Hepatol. 2007;6:7. doi: 10.1186/1476-5926-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wight T.N., Potter-Perigo S. The extracellular matrix: an active or passive player in fibrosis? Am J Physiol Gastrointest Liver Physiol. 2011;301:G950–G955. doi: 10.1152/ajpgi.00132.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karsdal M.A., Nielsen S.H., Leeming D.J., Langholm L.L., Nielsen M.J., Manon-Jensen T. The good and the bad collagens of fibrosis - their role in signaling and organ function. Adv Drug Deliv Rev. 2017;121:43–56. doi: 10.1016/j.addr.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 36.Naba A., Clauser K.R., Ding H., Whittaker C.A., Carr S.A., Hynes R.O. The extracellular matrix: tools and insights for the “omics” era. Matrix Biol. 2016;49:10–24. doi: 10.1016/j.matbio.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ricard-Blum S., Miele A.E. Omic approaches to decipher the molecular mechanisms of fibrosis, and design new anti-fibrotic strategies. Semin Cell Dev Biol. 2020;101:161–169. doi: 10.1016/j.semcdb.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 38.Lin X.Z., Horng M.H., Sun Y.N., Shiesh S.C., Chow N.H., Guo X.Z. Computer morphometry for quantitative measurement of liver fibrosis: comparison with Knodell's score, colorimetry and conventional description reports. J Gastroenterol Hepatol. 1998;13:75–80. doi: 10.1111/j.1440-1746.1998.tb00549.x. [DOI] [PubMed] [Google Scholar]
- 39.Martinez-Hernandez A., Amenta P.S. The extracellular matrix in hepatic regeneration. FASEB J. 1995;9:1401–1410. doi: 10.1096/fasebj.9.14.7589981. [DOI] [PubMed] [Google Scholar]
- 40.Friedman S.L. Extracellular matrix. In: Dufour J.F., editor. Signaling Pathways in Liver Diseases. Springer; Berlin: 2010. pp. 93–104. [Google Scholar]
- 41.Cassiman D., Libbrecht L., Desmet V., Denef C., Roskams T. Hepatic stellate cell/myofibroblast subpopulations in fibrotic human and rat livers. J Hepatol. 2002;36:200–209. doi: 10.1016/s0168-8278(01)00260-4. [DOI] [PubMed] [Google Scholar]
- 42.Zeisberg M., Yang C., Martino M., Duncan M.B., Rieder F., Tanjore H. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem. 2007;282:23337–23347. doi: 10.1074/jbc.M700194200. [DOI] [PubMed] [Google Scholar]
- 43.Robertson H., Kirby J.A., Yip W.W., Jones D.E., Burt A.D. Biliary epithelial-mesenchymal transition in posttransplantation recurrence of primary biliary cirrhosis. Hepatology. 2007;45:977–981. doi: 10.1002/hep.21624. [DOI] [PubMed] [Google Scholar]
- 44.Omenetti A., Porrello A., Jung Y., Yang L., Popov Y., Choi S.S. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest. 2008;118:3331–3342. doi: 10.1172/JCI35875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wells R.G. Cellular sources of extracellular matrix in hepatic fibrosis. Clin Liver Dis. 2008;12:759–768, viii. doi: 10.1016/j.cld.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez-Hernandez A., Amenta P.S. The hepatic extracellular matrix. I. Components and distribution in normal liver. Virchows Arch A Pathol Anat Histopathol. 1993;423:1–11. doi: 10.1007/BF01606425. [DOI] [PubMed] [Google Scholar]
- 47.Launois B., Jamieson G.G. The importance of Glisson's capsule and its sheaths in the intrahepatic approach to resection of the liver. Surg Gynecol Obstet. 1992;174:7–10. [PubMed] [Google Scholar]
- 48.Buschmann R.J., Ryoo J.W. Hepatic structural correlates of liver fibrosis: a morphometric analysis. Exp Mol Pathol. 1989;50:114–124. doi: 10.1016/0014-4800(89)90061-0. [DOI] [PubMed] [Google Scholar]
- 49.He Y., Kang C.H., Xu S., Tuo X., Trasti S., Tai D.C.S. Toward surface quantification of liver fibrosis progression. J Biomed Opt. 2010;15:056007. doi: 10.1117/1.3490414. [DOI] [PubMed] [Google Scholar]
- 50.Xu S., Kang C.H., Gou X., Peng Q., Yan J., Zhuo S. Quantification of liver fibrosis via second harmonic imaging of the Glisson's capsule from liver surface. J Biophotonics. 2016;9:351–363. doi: 10.1002/jbio.201500001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borro P., Dellepiane S.G., Pellicano R., Gemme L., Fagoonee S., Testino G. Quantification of ultrasound imaging in the staging of hepatic fibrosis. Panminerva Med. 2018;60:44–51. doi: 10.23736/S0031-0808.18.03416-X. [DOI] [PubMed] [Google Scholar]
- 52.Morrissey M.A., Sherwood D.R. An active role for basement membrane assembly and modification in tissue sculpting. J Cell Sci. 2015;128:1661–1668. doi: 10.1242/jcs.168021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yurchenco P.D. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harbor Perspect Biol. 2011;3:a004911. doi: 10.1101/cshperspect.a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 55.Cordero-Espinoza L., Huch M. The balancing act of the liver: tissue regeneration versus fibrosis. J Clin Invest. 2018;128:85–96. doi: 10.1172/JCI93562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim T.H., Mars W.M., Stolz D.B., Petersen B.E., Michalopoulos G.K. Extracellular matrix remodeling at the early stages of liver regeneration in the rat. Hepatology. 1997;26:896–904. doi: 10.1002/hep.510260415. [DOI] [PubMed] [Google Scholar]
- 57.Christophi C., Harun N., Fifis T. Liver regeneration and tumor stimulation--a review of cytokine and angiogenic factors. J Gastrointest Surg. 2008;12:966–980. doi: 10.1007/s11605-007-0459-6. [DOI] [PubMed] [Google Scholar]
- 58.Karsdal M.A., Manon-Jensen T., Genovese F., Kristensen J.H., Nielsen M.J., Sand J.M.B. Novel insights into the function and dynamics of extracellular matrix in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2015;308:G807–G830. doi: 10.1152/ajpgi.00447.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mohammed F.F., Khokha R. Thinking outside the cell: proteases regulate hepatocyte division. Trends Cell Biol. 2005;15:555–563. doi: 10.1016/j.tcb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 60.Michalopoulos G.K. Advances in liver regeneration. Expert Rev Gastroenterol Hepatol. 2014;8:897–907. doi: 10.1586/17474124.2014.934358. [DOI] [PubMed] [Google Scholar]
- 61.Su A.I., Guidotti L.G., Pezacki J.P., Chisari F.V., Schultz P.G. Gene expression during the priming phase of liver regeneration after partial hepatectomy in mice. Proc Natl Acad Sci U S A. 2002;99:11181–11186. doi: 10.1073/pnas.122359899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.White P., Brestelli J.E., Kaestner K.H., Greenbaum L.E. Identification of transcriptional networks during liver regeneration. J Biol Chem. 2005;280:3715–3722. doi: 10.1074/jbc.M410844200. [DOI] [PubMed] [Google Scholar]
- 63.Fountoulakis M., Suter L. Proteomic analysis of the rat liver. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;782:197–218. doi: 10.1016/s1570-0232(02)00562-7. [DOI] [PubMed] [Google Scholar]
- 64.Mazza G., Al-Akkad W., Rombouts K., Pinzani M. Liver tissue engineering: from implantable tissue to whole organ engineering. Hepatol Commun. 2018;2:131–141. doi: 10.1002/hep4.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Massey V.L., Dolin C.E., Poole L.G., Hudson S.V., Siow D.L., Brock G.N. The hepatic “matrisome” responds dynamically to injury: characterization of transitional changes to the extracellular matrix in mice. Hepatology. 2017;65:969–982. doi: 10.1002/hep.28918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dolin C.E., Arteel G.E. The matrisome, inflammation, and liver disease. Semin Liver Dis. 2020;40:180–188. doi: 10.1055/s-0039-3402516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elliott K.C., Cheruvelil K.S., Montgomery G.M., Soranno P.A. Conceptions of good science in our data-rich World. Bioscience. 2016;66:880–889. doi: 10.1093/biosci/biw115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Müller-Wille S., Charmantier I. Natural history and information overload: the case of Linnaeus. Stud Hist Philos Biol Biomed Sci. 2012;43:4–15. doi: 10.1016/j.shpsc.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taha I.N., Naba A. Exploring the extracellular matrix in health and disease using proteomics. Essays Biochem. 2019;63:417–432. doi: 10.1042/EBC20190001. [DOI] [PubMed] [Google Scholar]
- 70.Randles M.J., Humphries M.J., Lennon R. Proteomic definitions of basement membrane composition in health and disease. Matrix Biol. 2017;57–58:12–28. doi: 10.1016/j.matbio.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 71.Raghunathan R., Sethi M.K., Klein J.A., Zaia J. Proteomics, glycomics, and glycoproteomics of matrisome molecules. Mol Cell Proteomics. 2019;18:2138–2148. doi: 10.1074/mcp.R119.001543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Naba A., Clauser K.R., Hynes R.O. Enrichment of extracellular matrix proteins from tissues and digestion into peptides for mass spectrometry analysis. J Vis Exp. 2015;101:e53057. doi: 10.3791/53057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Naba A., Pearce O.M.T., Del Rosario A., Ma D., Ding H., Rajeeve V. Characterization of the extracellular matrix of normal and diseased tissues using proteomics. J Proteome Res. 2017;16:3083–3091. doi: 10.1021/acs.jproteome.7b00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Naba A., Clauser K.R., Whittaker C.A., Carr S.A., Tanabe K.K., Hynes R.O. Extracellular matrix signatures of human primary metastatic colon cancers and their metastases to liver. BMC Cancer. 2014;14:518. doi: 10.1186/1471-2407-14-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Naba A., Hoersch S., Hynes R.O. Towards definition of an ECM parts list: an advance on GO categories. Matrix Biol. 2012;31:371–372. doi: 10.1016/j.matbio.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pozzi A., Yurchenco P.D., Iozzo R.V. The nature and biology of basement membranes. Matrix Biol. 2017;57–58:1–11. doi: 10.1016/j.matbio.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aumailley M., Bruckner-Tuderman L., Carter W.G., Deutzmann R., Edgar D., Ekblom P. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–332. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 78.Khoshnoodi J., Pedchenko V., Hudson B.G. Mammalian collagen IV. Microsc Res Tech. 2008;71:357–370. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol. 2011;3:a004978. doi: 10.1101/cshperspect.a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fitzgerald J., Holden P., Hansen U. The expanded collagen VI family: new chains and new questions. Connect Tissue Res. 2013;54:345–350. doi: 10.3109/03008207.2013.822865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kanta J. Elastin in the liver. Front Physiol. 2016;7:491. doi: 10.3389/fphys.2016.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Murphy-Ullrich J.E., Sage E.H. Revisiting the matricellular concept. Matrix Biol. 2014;37:1–14. doi: 10.1016/j.matbio.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Robertson I.B., Horiguchi M., Zilberberg L., Dabovic B., Hadjiolova K., Rifkin D.B. Latent TGF-β-binding proteins. Matrix Biol. 2015;47:44–53. doi: 10.1016/j.matbio.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fan W., Liu T., Chen W., Hammad S., Longerich T., Hausser I. ECM1 prevents activation of transforming growth factor β, hepatic stellate cells, and fibrogenesis in mice. Gastroenterology. 2019;157:1352–1367.e13. doi: 10.1053/j.gastro.2019.07.036. [DOI] [PubMed] [Google Scholar]
- 85.Sirica A.E., Almenara J.A., Li C. Periostin in intrahepatic cholangiocarcinoma: pathobiological insights and clinical implications. Exp Mol Pathol. 2014;97:515–524. doi: 10.1016/j.yexmp.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kumar P., Smith T., Raeman R., Chopyk D.M., Brink H., Liu Y. Periostin promotes liver fibrogenesis by activating lysyl oxidase in hepatic stellate cells. J Biol Chem. 2018;293:12781–12792. doi: 10.1074/jbc.RA117.001601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Iozzo R.V., Schaefer L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42:11–55. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tanaka Y., Tateishi R., Koike K. Proteoglycans are attractive biomarkers and therapeutic targets in hepatocellular carcinoma. Int J Mol Sci. 2018;19:3070. doi: 10.3390/ijms19103070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krasny L., Paul A., Wai P., Howard B.A., Natrajan R.C., Huang P.H. Comparative proteomic assessment of matrisome enrichment methodologies. Biochem J. 2016;473:3979–3995. doi: 10.1042/BCJ20160686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goddard E.T., Hill R.C., Barrett A., Betts C., Guo Q., Maller O. Quantitative extracellular matrix proteomics to study mammary and liver tissue microenvironments. Int J Biochem Cell Biol. 2016;81:223–232. doi: 10.1016/j.biocel.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pratt J.M., Simpson D.M., Doherty M.K., Rivers J., Gaskell S.J., Beynon R.J. Multiplexed absolute quantification for proteomics using concatenated signature peptides encoded by QconCAT genes. Nat Protoc. 2006;1:1029–1043. doi: 10.1038/nprot.2006.129. [DOI] [PubMed] [Google Scholar]
- 92.Beier J.I., Luyendyk J.P., Guo L., von Montfort C., Staunton D.E., Arteel G.E. Fibrin accumulation plays a critical role in the sensitization to lipopolysaccharide-induced liver injury caused by ethanol in mice. Hepatology. 2009;49:1545–1553. doi: 10.1002/hep.22847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gillis S.E., Nagy L.E. Deposition of cellular fibronectin increases before stellate cell activation in rat liver during ethanol feeding. Alcohol Clin Exp Res. 1997;21:857–861. [PubMed] [Google Scholar]
- 94.Thiele G.M., Duryee M.J., Freeman T.L., Sorrell M.F., Willis M.S., Tuma D.J. Rat sinusoidal liver endothelial cells (SECs) produce pro-fibrotic factors in response to adducts formed from the metabolites of ethanol. Biochem Pharmacol. 2005;70:1593–1600. doi: 10.1016/j.bcp.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 95.Poole L.G., Arteel G.E. Transitional remodeling of the hepatic extracellular matrix in alcohol-induced liver injury. Biomed Res Int. 2016;2016:3162670. doi: 10.1155/2016/3162670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Srivastava A., Jong S., Gola A., Gailer R., Morgan S., Sennett K. Cost-comparison analysis of FIB-4, ELF and fibroscan in community pathways for non-alcoholic fatty liver disease. BMC Gastroenterol. 2019;19:122. doi: 10.1186/s12876-019-1039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baiocchini A., Montaldo C., Conigliaro A., Grimaldi A., Correani V., Mura F. Extracellular matrix molecular remodeling in human liver fibrosis evolution. PLoS One. 2016;11:e0151736. doi: 10.1371/journal.pone.0151736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Klaas M., Kangur T., Viil J., Mäemets-Allas K., Minajeva A., Vadi K. The alterations in the extracellular matrix composition guide the repair of damaged liver tissue. Scientific Rep. 2016;6:27398. doi: 10.1038/srep27398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gherlan G.S. Liver ultrasound elastography: more than staging the disease. World J Hepatol. 2015;7:1595–1600. doi: 10.4254/wjh.v7.i12.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang J.-H. Application of ultrasound liver elastography to the diagnosis and monitoring of liver disease. J Med Ultrasound. 2019;27:1–2. doi: 10.4103/JMU.JMU_108_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Allen A.M., Shah V.H., Therneau T.M., Venkatesh S.K., Mounajjed T., Larson J.J. The role of three-dimensional magnetic resonance elastography in the diagnosis of nonalcoholic steatohepatitis in obese patients undergoing bariatric surgery. Hepatology. 2020;71:510–521. doi: 10.1002/hep.30483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Decaris M.L., Gatmaitan M., FlorCruz S., Luo F., Li K., Holmes W.E. Proteomic analysis of altered extracellular matrix turnover in bleomycin-induced pulmonary fibrosis. Mol Cell Proteomics. 2014;13:1741–1752. doi: 10.1074/mcp.M113.037267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schiller H.B., Fernandez I.E., Burgstaller G., Schaab C., Scheltema R.A., Schwarzmayr T. Time- and compartment-resolved proteome profiling of the extracellular niche in lung injury and repair. Mol Syst Biol. 2015;11:819. doi: 10.15252/msb.20156123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lai K.K.Y., Shang S., Lohia N., Booth G.C., Masse D.J., Fausto N. Extracellular matrix dynamics in hepatocarcinogenesis: a comparative proteomics study of PDGFC transgenic and Pten null mouse models. PLoS Genet. 2011;7:e1002147. doi: 10.1371/journal.pgen.1002147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Campbell J.S., Hughes S.D., Gilbertson D.G., Palmer T.E., Holdren M.S., Haran A.C. Platelet-derived growth factor C induces liver fibrosis, steatosis, and hepatocellular carcinoma. Proc Natl Acad Sci U S A. 2005;102:3389–3394. doi: 10.1073/pnas.0409722102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Horie Y., Suzuki A., Kataoka E., Sasaki T., Hamada K., Sasaki J. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J Clin Invest. 2004;113:1774–1783. doi: 10.1172/JCI20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mazza G., Telese A., Al-Akkad W., Frenguelli L., Levi A., Marrali M. Cirrhotic human liver extracellular matrix 3D Scaffolds Promote Smad-Dependent TGF-β1 epithelial mesenchymal transition. Cells. 2019;9:83. doi: 10.3390/cells9010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;133:571–573. [PubMed] [Google Scholar]
- 109.Obenauf A.C., Massagué J. Surviving at a distance: organ specific metastasis. Trends Cancer. 2015;1:76–91. doi: 10.1016/j.trecan.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mielgo A., Schmid M.C. Liver tropism in cancer: the hepatic metastatic niche. Cold Spring Harb Perspect Med. 2020;10:a037259. doi: 10.1101/cshperspect.a037259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schulz P.O., Ferreira F.G., Nascimento M.F.A., Vieira A., Ribeiro M.A., David A.I. Association of nonalcoholic fatty liver disease and liver cancer. World J Gastroenterol. 2015;21:913–918. doi: 10.3748/wjg.v21.i3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Robinson S.M., Wilson C.H., Burt A.D., Manas D.M., White S.A. Chemotherapy-associated liver injury in patients with colorectal liver metastases: a systematic review and meta-analysis. Ann Surg Oncol. 2012;19:4287–4299. doi: 10.1245/s10434-012-2438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vauthey J.-N., Pawlik T.M., Ribero D., Wu T.-T., Zorzi D., Hoff P.M. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 114.Pathak S., Pandanaboyana S., Daniels I., Smart N., Prasad K.R. Obesity and colorectal liver metastases: mechanisms and management. Surg Oncol. 2016;25:246–251. doi: 10.1016/j.suronc.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 115.Hebert J.D., Myers S.A., Naba A., Abbruzzese G., Lamar J., Carr S.A. Proteomic profiling of the ECM of xenograft breast cancer metastases in different organs reveals distinct metastatic niches. Cancer Res. 2020;80:1475–1485. doi: 10.1158/0008-5472.CAN-19-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hudson S.V., Dolin C.E., Poole L.G., Massey V.L., Wilkey D., Beier J.I. Modeling the kinetics of integrin receptor binding to hepatic extracellular matrix proteins. Sci Rep. 2017;7:12444. doi: 10.1038/s41598-017-12691-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hudson S.V., Miller H.A., Mahlbacher G.E., Saforo D., Beverly L.J., Arteel G.E. Computational/experimental evaluation of liver metastasis post hepatic injury: interactions with macrophages and transitional ECM. Sci Rep. 2019;9:15077. doi: 10.1038/s41598-019-51249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Deutsch E.W., Csordas A., Sun Z., Jarnuczak A., Perez-Riverol Y., Ternent T. The ProteomeXchange consortium in 2017: supporting the cultural change in proteomics public data deposition. Nucleic Acids Res. 2017;45:D1100–D1106. doi: 10.1093/nar/gkw936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Perez-Riverol Y., Csordas A., Bai J., Bernal-Llinares M., Hewapathirana S., Kundu D.J. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res. 2019;47:D442–D450. doi: 10.1093/nar/gky1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shao X., Taha I.N., Clauser K.R., Gao Y.T., Naba A. MatrisomeDB: the ECM-protein knowledge database. Nucleic Acids Res. 2020;48:D1136–D1144. doi: 10.1093/nar/gkz849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Udompap P., Sukonrut K., Suvannarerg V., Pongpaibul A., Charatcharoenwitthaya P. Prospective comparison of transient elastography, point shear wave elastography, APRI and FIB-4 for staging liver fibrosis in chronic viral hepatitis. J Viral Hepat. 2020;27:437–448. doi: 10.1111/jvh.13246. [DOI] [PubMed] [Google Scholar]
- 122.Daniels S.J., Leeming D.J., Eslam M., Hashem A.M., Nielsen M.J., Krag A. ADAPT: an algorithm incorporating PRO-C3 accurately identifies patients with NAFLD and advanced fibrosis. Hepatology. 2019;69:1075–1086. doi: 10.1002/hep.30163. [DOI] [PubMed] [Google Scholar]
- 123.Dold L., Nielsen M.J., Praktiknjo M., Schwarze-Zander C., Boesecke C., Schierwagen R. Circulating levels of PRO-C3 reflect liver fibrosis and liver function in HIV positive patients receiving modern cART. PLoS One. 2019;14:e0219526. doi: 10.1371/journal.pone.0219526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Roderfeld M. Matrix metalloproteinase functions in hepatic injury and fibrosis. Matrix Biol. 2018;68–69:452–462. doi: 10.1016/j.matbio.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 125.Schrader M., Schulz-Knappe P. Peptidomics technologies for human body fluids. Trends Biotechnol. 2001;19:S55–S60. doi: 10.1016/S0167-7799(01)01800-5. [DOI] [PubMed] [Google Scholar]
- 126.Menschaert G., Vandekerckhove T.T.M., Baggerman G., Schoofs L., Luyten W., Van Criekinge W. Peptidomics coming of age: a review of contributions from a bioinformatics angle. J Proteome Res. 2010;9:2051–2061. doi: 10.1021/pr900929m. [DOI] [PubMed] [Google Scholar]
- 127.Greening D.W., Kapp E.A., Simpson R.J. The peptidome comes of age: mass spectrometry-based characterization of the circulating cancer peptidome. Enzymes. 2017;42:27–64. doi: 10.1016/bs.enz.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 128.Eckhard U., Marino G., Butler G.S., Overall C.M. Positional proteomics in the era of the human proteome project on the doorstep of precision medicine. Biochimie. 2016;122:110–118. doi: 10.1016/j.biochi.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 129.Madzharova E., Kastl P., Sabino F., Auf dem Keller U. Post-translational modification-dependent activity of matrix metalloproteinases. Int J Mol Sci. 2019;20:3077. doi: 10.3390/ijms20123077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shen Y., Tolić N., Liu T., Zhao R., Petritis B.O., Gritsenko M.A. Blood peptidome-degradome profile of breast cancer. PLoS One. 2010;5:e13133. doi: 10.1371/journal.pone.0013133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Labots M., Schütte L.M., van der Mijn J.C., Pham T.V., Jiménez C.R., Verheul H.M.W. Mass spectrometry-based serum and plasma peptidome profiling for prediction of treatment outcome in patients with solid malignancies. Oncologist. 2014;19:1028–1039. doi: 10.1634/theoncologist.2014-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tian L., Wang Y., Xu D., Gao Y., Wen X., Tian Y. The differential diagnostic model for serous peptidomics in HBV carriers established by MALDI-TOF-MS analysis. Clin Biochem. 2014;47:56–62. doi: 10.1016/j.clinbiochem.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 133.Kwong G.A., von Maltzahn G., Murugappan G., Abudayyeh O., Mo S., Papayannopoulos I.A. Mass-encoded synthetic biomarkers for multiplexed urinary monitoring of disease. Nat Biotechnol. 2013;31:63–70. doi: 10.1038/nbt.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mayorca-Guiliani A.E., Willacy O., Madsen C.D., Rafaeva M., Elisabeth Heumüller S., Bock F. Decellularization and antibody staining of mouse tissues to map native extracellular matrix structures in 3D. Nat Protoc. 2019;14:3395–3425. doi: 10.1038/s41596-019-0225-8. [DOI] [PubMed] [Google Scholar]
- 135.Rickelt S., Hynes R.O. Antibodies and methods for immunohistochemistry of extracellular matrix proteins. Matrix Biol. 2018;71–72:10–27. doi: 10.1016/j.matbio.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 136.Myllyharju J., Kivirikko K.I. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004;20:33–43. doi: 10.1016/j.tig.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 137.Liu S.B., Ikenaga N., Peng Z.-W., Sverdlov D.Y., Greenstein A., Smith V. Lysyl oxidase activity contributes to collagen stabilization during liver fibrosis progression and limits spontaneous fibrosis reversal in mice. FASEB J. 2015;30:1599–1609. doi: 10.1096/fj.14-268425. [DOI] [PubMed] [Google Scholar]
- 138.Tatsukawa H., Furutani Y., Hitomi K., Kojima S. Transglutaminase 2 has opposing roles in the regulation of cellular functions as well as cell growth and death. Cell Death Dis. 2016;7:e2244. doi: 10.1038/cddis.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Issa R., Zhou X., Constandinou C.M., Fallowfield J., Millward-Sadler H., Gaca M.D. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology. 2004;126:1795–1808. doi: 10.1053/j.gastro.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 140.Schuppan D., Ashfaq-Khan M., Yang A.T., Kim Y.O. Liver fibrosis: direct antifibrotic agents and targeted therapies. Matrix Biol. 2018;68–69:435–451. doi: 10.1016/j.matbio.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 141.Gautieri A., Redaelli A., Buehler M.J., Vesentini S. Age- and diabetes-related nonenzymatic crosslinks in collagen fibrils: candidate amino acids involved in advanced glycation end-products. Matrix Biol. 2014;34:89–95. doi: 10.1016/j.matbio.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 142.Rodriguez-Pascual F., Slatter D.A. Collagen cross-linking: insights on the evolution of metazoan extracellular matrix. Sci Rep. 2016;6:1–7. doi: 10.1038/srep37374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rojas A., Añazco C., González I., Araya P. Extracellular matrix glycation and receptor for advanced glycation end-products activation: a missing piece in the puzzle of the association between diabetes and cancer. Carcinogenesis. 2018;39:515–521. doi: 10.1093/carcin/bgy012. [DOI] [PubMed] [Google Scholar]
- 144.Huijberts M.S., Schaper N.C., Schalkwijk C.G. Advanced glycation end products and diabetic foot disease. Diabetes Metab Res Rev. 2008;24(Suppl 1):S19–S24. doi: 10.1002/dmrr.861. [DOI] [PubMed] [Google Scholar]
- 145.Merl-Pham J., Basak T., Knüppel L., Ramanujam D., Athanason M., Behr J. Quantitative proteomic profiling of extracellular matrix and site-specific collagen post-translational modifications in an in vitro model of lung fibrosis. Matrix Biol Plus. 2019;1:100005. doi: 10.1016/j.mbplus.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Underhill G.H., Khetani S.R. Emerging trends in modeling human liver disease in vitro. APL Bioeng. 2019;3:040902. doi: 10.1063/1.5119090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bhatia S.N., Underhill G.H., Zaret K.S., Fox I.J. Cell and tissue engineering for liver disease. Sci Transl Med. 2014;6:245sr2. doi: 10.1126/scitranslmed.3005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.