Abstract
Background
There has been interest in using the non-invasive, home-based quantitative faecal immunochemical test (FIT) to rule out colorectal cancer (CRC) in high-risk symptomatic patients.
Aim
To elicit public preferences for FIT versus colonoscopy (CC) and its delivery in primary care.
Design & setting
A cross-sectional online survey in England.
Method
A total of 1057 adults (without CRC symptoms and diagnosis) aged 40–59 years were invited from an English online survey panel. Responders were asked to imagine they had been experiencing CRC symptoms that would qualify them for a diagnostic test. Participants were presented with choices between CC and FIT in ascending order of number of CRCs missed by FIT (from 1–10%). It was measured at what number of missed CRCs responders preferred CC over FIT.
Results
While 150 participants did not want either of the tests when both missed 1% CRCs, the majority (n = 741, 70.0%) preferred FIT to CC at that level of accuracy. However, this preference reduced to 427 (40.4%) when FIT missed one additional cancer. Women were more likely to tolerate missing CRC when using FIT. Having lower numeracy and perceiving a higher level of risk meant participants were less likely to tolerate a false negative test. Most of those who chose FIT preferred to return it by mail (62.2%), to be informed about normal test results by letter (42.1%), and about abnormal test results face to face (32.5%).
Conclusion
While the majority of participants preferred FIT over CC when both tests had the same sensitivity, tolerance for missed CRCs was low.
How this fits in
Hypothetical vignette studies have demonstrated a strong preference for CRC testing in low-risk scenarios. This was the first study to investigate users’ attitudes towards different CRC tests to rule out significant colorectal diseases when they experience symptoms.
This study showed that while most people with symptoms would prefer FIT over CC if both tests miss 1% of CRCs, a large proportion of participants switched their preference to CC as soon as FIT missed more CRCs than CC. A smaller but important subgroup of people exhibited a strong preference for non-invasive testing (that is, using FIT over CC) even if the test missed 10% of CRCs. Preferences for FIT rollout were not universal but varied by demographic background, and test result.
Introduction
CRC is the fourth most common cancer in the UK and the second biggest cause of cancer deaths.1 The majority of CRCs are still diagnosed among symptomatic patients when outcomes tend to be worse in terms of survival.2,3 There is strong impetus to promote early symptomatic presentation and prompt referral. One of the biggest barriers to prompt diagnosis is that symptoms of CRC are common and non-specific. CRC symptoms regarded as high-risk, such as rectal bleeding, only have a 3–4% positive predictive value.
In England, patients with symptoms suggestive of CRC are referred urgently via the 2-week wait (2WW) pathway (see Box 1 for a more detailed description of qualifying symptoms for different age groups).4,5 The majority of these patients complete a CC, which is the gold standard test to rule out CRC, but the procedure carries risks and opportunity costs for patients, as well as costs to the health service. As a result, there has been interest in using a non-invasive FIT to rule out CRC in patients with symptoms qualifying for 2WW referral in a much safer (avoiding the 0.03–0.7% risk of bowel perforation with CC) and more cost-effective way (the average price of colonoscopy is £372 compared with £5 per FIT kit).6,7
Box 1. Criteria for 2-week-wait referral.
National Institute for Health and Care Excellence (NICE)guidance 4
1.3.1 Refer adults using a suspected cancer pathway referral (for an appointment within 2 weeks) for colorectal cancer if:
they are aged ≥40 years with unexplained weight loss and abdominal pain or
they are aged ≥50 years with unexplained rectal bleeding or
-
they are aged ≥60 years with:
iron‑deficiency anaemia or
changes in their bowel habit, or
tests show occult blood in their faeces (new 2015)
1.3.2 Consider a suspected cancer pathway referral (for an appointment within 2 weeks) for colorectal cancer in adults with a rectal or abdominal mass (new 2015)
1.3.3 Consider a suspected cancer pathway referral (for an appointment within 2 weeks) for colorectal cancer in adults aged <50 years with rectal bleeding and any of the following unexplained symptoms or findings:
abdominal pain
change in bowel habit
weight loss
iron‑deficiency anaemia (new 2015)
1.3.4 This recommendation has been replaced by diagnostics guidance on quantitative faecal immunochemical tests to guide referral for colorectal cancer in primary care. The diagnostics guidance recommends tests for occult blood in faeces, for people without rectal bleeding but with unexplained symptoms that do not meet the criteria for a suspected cancer pathway referral inrecommendations 1.3.1 to 1.3.3.
Previous studies have reported that a negative FIT test could rule out CRC, with a negative predictive value of 96–100%, and free-up CC capacity, but a small proportion of patients with bowel cancer could receive false negative results, which could raise concerns.8–11 This is particularly relevant when the prevalence of disease is low, such as in patients attending primary care. A recent survey indicated that just over one-third of GPs preferred to use a FIT as a rule-out test over 2WW referral, but showed confusion over the optimal use of FIT in symptomatic patients.12 GPs who were relatively more likely to make urgent referrals (more than 10 referrals per year) were less willing to use FIT. Conversely, believing that FIT was highly accurate was the strongest positive predictor of wanting to use FIT as a triage test.
In addition to considering acceptability among relevant healthcare providers, it is also important to understand the attitudes of patients towards completing the test: only if users find the FIT process acceptable are they likely to complete it satisfactorily and follow the associated investigative pathway.13,14 Previous research suggests that asymptomatic participants in the bowel cancer screening programme share GPs' concerns about the accuracy of FIT to detect CRC.15–17 The only study in the symptomatic context used a hypothetical vignette study to investigate preferences for diagnostic testing for cancer (including CRC). Participants expressed a clear preference for diagnostic testing at all risk levels (including a risk as low as 1%).18 However, no research has been carried out investigating users’ attitudes towards different CRC tests to rule out significant colorectal diseases when they experience symptoms.
The present study aimed to test how public preferences varies between two diagnostics tests (FIT versus CC) at different missed cancer rates using a series of hypothetical choice scenarios. Additionally, the study wanted to elicit preferences for FIT-specific test procedures, such as mode of returning the test kit and preferred way of communicating test results.
Method
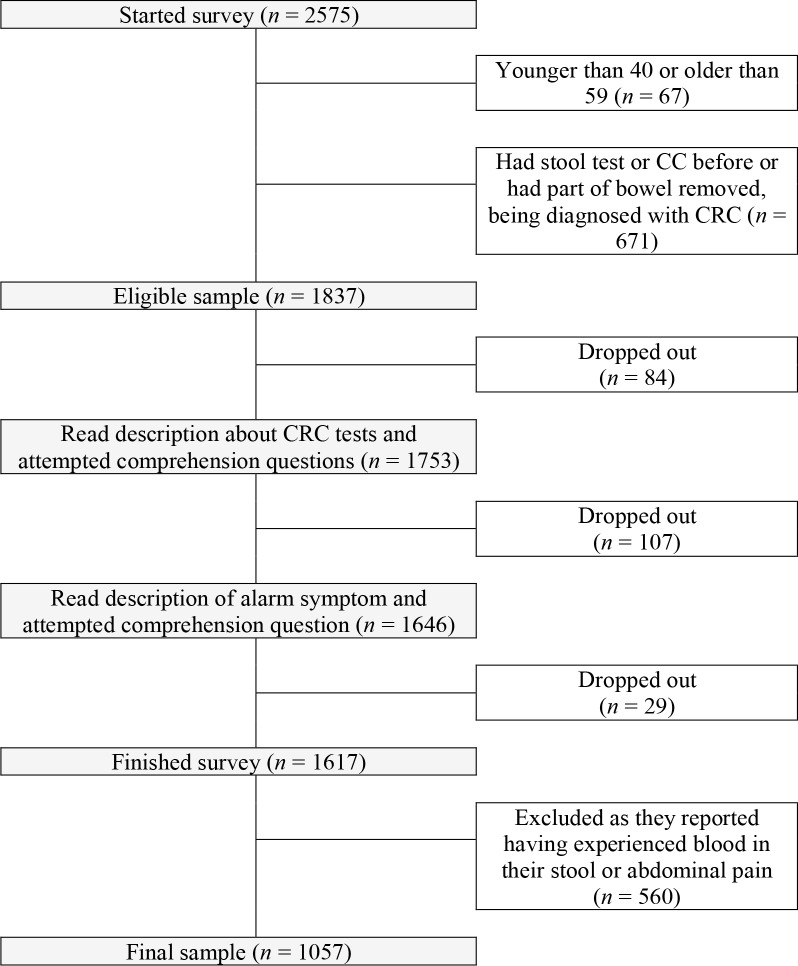
A total of 2508 men and women aged 40–59 years living in England were invited to participate from an English online survey panel (Survey Sampling International, now known as Dynata) in November 2018. Figure 1 outlines the various points at which people were excluded from or dropped out of the study. In total, 671 (26.8%) were excluded because they had previously completed a CC or stool test and had been diagnosed with CRC or had parts of their bowel removed.
Figure 1. Flow through the survey.
Those who were eligible to become participants (n = 1837) were provided with a description of CC and FIT and were asked to imagine they had been experiencing CRC symptoms (40–49-year-olds: unexplained weight loss and abdominal pain; 50–59-year-olds: unexplained rectal bleeding) that would qualify them for 2WW referral as per the National Institute for Health and Care Excellence (NICE) guideline NG12 (Box 1).4
Before participants completed the survey, they were asked to answer a few pre-screening questions in order to ensure that they understood the survey instructions. Those who could not correctly recall their communicated symptom(s) or did not correctly answer four comprehension questions about CC and FIT were asked to repeat the question until they had chosen the correct answer (see questions in the online depository: https://osf.io/ta4bc/).
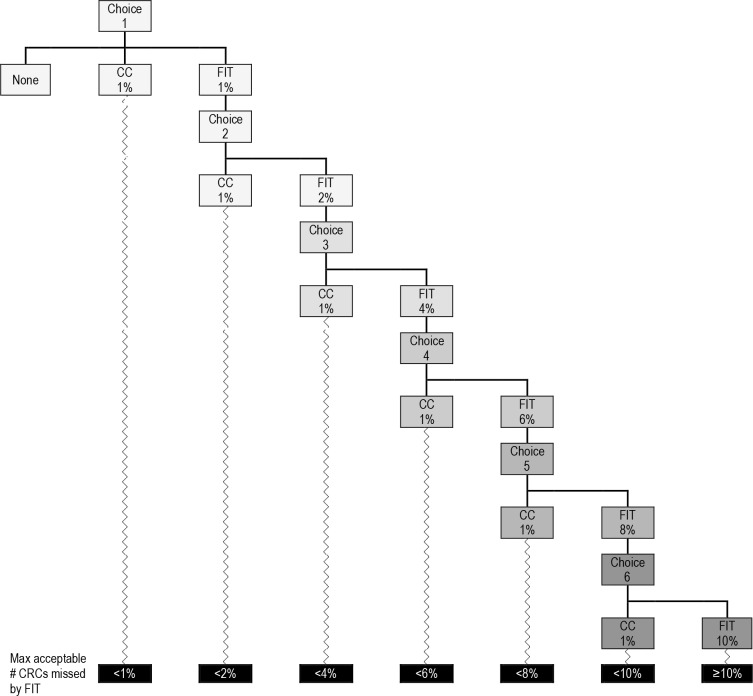
Once people successfully recalled the hypothetical symptoms, participants were asked to choose between the two tests to investigate their hypothetical symptoms. Specifically, a dynamic forced-choice staircase method was used to present participants with a series of binary comparisons between CC and FIT (see Figure 2).19,20 The staircase method has been extensively used in psychophysics19 to establish a threshold level. Its main advantage is its efficiency as it requires the presentation of many fewer stimuli than other traditional methods; in this case, a discrete choice experiment was used because it enabled the authors to focus on varying a single attribute (namely cancer miss rate), as previous evidence had identified this as the primary concern in patients and healthcare providers.12,15–17
Figure 2. Dynamic forced-choice staircases.
CC = colonoscopy. CRC = colorectal cancer. FIT = faecal immunochemical test. Max = maximum.
CC was reported to miss 1% of CRCs in all scenarios while the CRCs missed by FIT were reported in ascending order ranging from 1–10%. Those who either chose CC in the first scenario or stated that they did not want either test were asked to indicate the rationale for their choice by choosing from a list of reasons. Furthermore, participants who preferred CC by default were asked what else they would do about their hypothetical symptom(s). Possible answer options included going back to the GP; monitoring or managing symptom(s); avoiding thinking about the symptom(s); nothing; and something else. For all remaining responders, the point at which they switched from choosing FIT to CC provided the maximum acceptable proportion of missed CRCs (or the minimum acceptable sensitivity for FIT).
Preferences of FIT procedure
Participants who chose FIT in at least one comparison were asked several questions about the procedure. These included whether they would prefer to return the FIT in person to their GP or by freepost; how and by whom they would like to be informed about normal and abnormal test results; and what further tests they would want to have in case of a normal FIT result.
Numeracy skills
Numeracy was assessed using the item: ‘Which of the following numbers represents the biggest risk of getting a disease? 1 in 100, 1 in 1000, or 1 in 10’.21 Participants were scored as either correct or incorrect.
Demographics and previous symptoms
Information was collected about the participants’ sociodemographic characteristics such as participant’s age, ethnic group (‘white British’ or ‘other white’, versus ‘black’ or ‘Asian’ or ‘mixed’ and ‘other’), marital status (‘married’ or ‘cohabiting’, versus ‘single’ or ‘divorced’ or ‘separated’ or ‘widowed’), having obtained at least A-levels (‘yes’ versus ‘no’), being in paid employment (‘yes’ versus ‘no’), and self-perceived risk of developing CRC. The latter was measured on a 5-point Likert scale ranging from ‘much lower than average’, ‘a little lower than average’, ‘about the same’, ‘a little higher than average’, to ‘much higher than average’. Participants were also asked to indicate how frequently they had experienced, in real life, common CRC-related symptoms such as constipation, diarrhoea, abdominal pain, bowel incontinence, blood in stool, and indigestion in the last 3 months: ‘not at all’, ‘occasionally’, or ‘frequently’.
Statistical analysis
All statistical analysis was conducted with Stata/SE (version 15.1). The main outcome variable was the number of CRCs missed by FIT at which participants preferred CC over FIT. An adjusted ordered logistic regression was used to identify the potential factors associated with an individual's minimal acceptable sensitivity of FIT. A separate logistic regression analysed factors explaining not wanting to have CC or FIT if both miss 1% of CRCs. Owing to low frequencies, responses to the self-perceived risk questions were reclassified into three groups: (1) 'much lower than average’ combined with ‘a little lower than average’; (2) ‘about the same’; and (3) ‘a little higher than average’ combined with ‘much higher than average’. Similarly, the answers to the CRC-related symptoms were reclassified into two groups, (1) ‘not at all’ versus (2) ‘occasionally’ and ‘frequently’. For both multivariate analyses, adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were reported and a significance level of P<0.05 was used.
Results
Population characteristics
A total of 1837 responders were eligible to start the survey. Of those, 220 (11.98% of the total sample) dropped out. Of these 220 responders, 84 (38.2%) dropped out even before reading the vignette description, as shown in Figure 1. It can also be seen that 107/220 (48.6%) responders dropped out while reading the vignette and answering the associated comprehension question (see Supplementary file). A final 29/220 (13.2%) responders dropped out while answering the second comprehension question about the alarm symptom. In total, 1617 responders successfully finished the survey, of which 560 (34.6%) had at least one of the alarm symptoms, leaving a sample of 1057 responders for the final analysis. Table 1 gives an overview of the participants’ characteristics.
Table 1. Study sample‘s sociodemographic variables and symptoms in the last three weeks (n = 1057).
| Aged 40–49 years, n (%) | Aged 0–59 years, n (%) | Total, n (%) | P value | ||
|---|---|---|---|---|---|
| Sex | |||||
| Men | 281 (54.5) | 283 (52.3) | 564 (53.4) | 0.484 | |
| Women | 235 (45.5) | 258 (47.7) | 493 (46.6) | ||
| Marital status | |||||
| Single or divorced or separated | 166 (32.2) | 196 (36.2) | 362 (34.2) | 0.165 | |
| Married or cohabiting | 350 (67.8) | 345 (63.8) | 695 (65.8) | ||
| Education | |||||
| No A-levels | 170 (32.9) | 225 (41.6) | 395 (37.4) | 0.004 | |
| A-levels | 346 (67.1) | 316 (58.4) | 662 (62.6) | ||
| Paid employment | |||||
| No | 109 (21.1) | 201 (37.2) | 310 (29.3) | <0.001 | |
| Yes | 407 (78.9) | 340 (62.8) | 747 (70.7) | ||
| Ethnic group | |||||
| White | 461 (89.3) | 510 (94.3) | 971 (91.9) | 0.003 | |
| BAME | 55 (10.7) | 31 (5.7) | 86 (8.1) | ||
| Numeracy question | |||||
| Wrong | 227 (44.0) | 241 (44.5) | 468 (44.3) | 0.856 | |
| Correct | 289 (56.0) | 300 (55.5) | 589 (55.7) | ||
| Own perceived risk | |||||
| Lower | 87 (16.9) | 91 (16.8) | 178 (16.8) | 0.436 | |
| Same | 369 (71.5) | 400 (73.9) | 769 (72.8) | ||
| Higher | 60 (11.6) | 50 (9.2) | 110 (10.4) | ||
| Constipation | |||||
| Not at all | 429 (83.1) | 424 (78.7) | 853 (80.9) | 0.065 | |
| Occ/frequ | 87 (16.9) | 115 (21.3) | 202 (19.1) | ||
| Diarrhoea | |||||
| Not at all | 411 (80.0) | 430 (79.6) | 841 (79.8) | 0.893 | |
| Occ/frequ | 103 (20.0) | 110 (20.4) | 213 (20.2) | ||
| Bowel incontinence | |||||
| Not at all | 500 (97.3) | 521 (96.5) | 1021 (96.9) | 0.459 | |
| Occ/frequ | 14 (2.7) | 19 (3.5) | 33 (3.1) | ||
| Indigestion | |||||
| Not at all | 533 (79.8) | 491 (73.9) | 1024 (76.8) | 0.024 | |
| Occ/frequ | 245 (20.2) | 270 (26.1) | 515 (23.2) | ||
BAME = black and minority ethnic. Occ/frequ = occassionally or frequently
Preferences for FIT and CC
Preference for neither of the diagnostic tests
A total of 150 (14.2%) stated they would not want either test. Reasons for not wanting either test included perceiving both tests as insufficiently accurate (n = 61, 40.7%); being too scared of a bowel cancer diagnosis (n = 41, 27.3%); being afraid of test risks (n = 37, 24.3%); not needing the tests (n = 7, 4.7%); perceiving the tests as unpleasant, uncomfortable, and embarrassing (n = 5, 3.3%); or ‘other’ reasons (n = 14, 9.3%).
A total of 907 participants (85.8%) indicated either a preference for CC or FIT when both missed 1% of CRCs. The logistic model in Table 2 shows that the likelihood of not wanting either test was positively associated with having a black or Asian minority ethnic (BAME) background (OR 3.49; 95% confidence intervals [CI] = 2.07 to 5.89), having lower numeracy skills (OR 2.54; 95% CI = 1.74 to 3.71), and having a higher self-perceived CRC risk (OR 2.35; 95% CI = 1.20 to 4.62).
Table 2. Multivariate models explaining not wanting to have CC or FIT and switching point.
| Logistic model: not wanting any test | Ordinal model: switching point | |||
|---|---|---|---|---|
| Odds ratio | 95% CI | Odds ratio | 95% CI | |
| Sex | ||||
| Male | Ref | Ref | ||
| Female | 0.813 | 0.555 to 1.192 | 1.413 | 1.109 to 1.800a |
| Age, years | ||||
| 40–49 | Ref | Ref | ||
| 50–59 | 0.821 | 0.564 to 1.194 | 1.008 | 0.792 to 1.282 |
| Education | ||||
| No A-levels | Ref | Ref | ||
| A-levels | 0.864 | 0.586 to 1.274 | 1.222 | 0.950 to 1.572 |
| Paid employment | ||||
| Yes | Ref | Ref | ||
| No | 1.294 | 0.865 to 1.938 | 1.208 | 0.925 to 1.579 |
| Marital status | ||||
| Single or divorced or separated | Ref | Ref | ||
| Married or cohabiting | 0.937 | 0.640 to 1.373 | 0.807 | 0.629 to 1.035 |
| Ethnic group | ||||
| White | Ref | Ref | ||
| BAME | 3.492 | 2.071 to 5.888a | 0.676 | 0.402 to 1.134 |
| Numeracy question | ||||
| Correct | Ref | Ref | ||
| Wrong | 2.539 | 1.737 to 3.712a | 0.736 | 0.576 to 0.940b |
| Own perceived risk | ||||
| Lower | Ref | Ref | ||
| Same | 1.178 | 0.700 to 1.981 | 0.983 | 0.719 to 1.343 |
| Higher | 2.352 | 1.199 to 4.617b | 0.546 | 0.336 to 0.887b |
| Constipation | ||||
| Not at all | Ref | Ref | ||
| Occasionally | 1.056 | 0.637 to 1.751 | 0.848 | 0.614 to 1.169 |
| Diarrhoea | ||||
| Not at all | Ref | Ref | ||
| Occasionally | 0.856 | 0.514 to 1.424 | 0.939 | 0.691 to 1.277 |
| Bowel incontinence | ||||
| Not at all | Ref | Ref | ||
| Occasionally | 0.396 | 0.087 to 1.810 | 0.588 | 0.296 to 1.166 |
| Indigestion | ||||
| Not at all | Ref | Ref | ||
| Occasionally | 0.769 | 0.476 to 1.242 | 1.572 | 1.178 to 2.097a |
| 1048 | 903 |
a P<0.01. b P<0.05.
BAME = black and minority ethnic. CI = confidence intervals. Ref = reference.
Alternative actions
Participants reported that they would go back to their GP (n = 80, 53.3%), monitor their symptom(s) (n = 53, 35.3%), try to manage the symptoms on their own (n = 26, 17.3%), do nothing (n = 22, 14.7%), avoid thinking about their symptom(s) (n = 15, 10%), or take ‘another’ action (n = 3, 2.0%) instead of having either an FIT or CC.
Reasons for preferring CC
The main reasons for choosing CC over FIT when both tests miss 1% of CRCs were: perceiving CC to be more thorough (n = 98, 59.0%), better at finding polyps (n = 62, 37.4%), or more able to remove polyps (n = 49, 29.5%); to avoid uncertainty associated with an abnormal FIT result (n = 34, 20.5%); being more familiar with CC (n = 34, 20.5%); not wanting to handle stool (n = 19, 11.5%); lack of trust in FIT (n = 14, 8.4%); or ‘other’ reasons (n = 7, 4.2%).
Preference for FIT
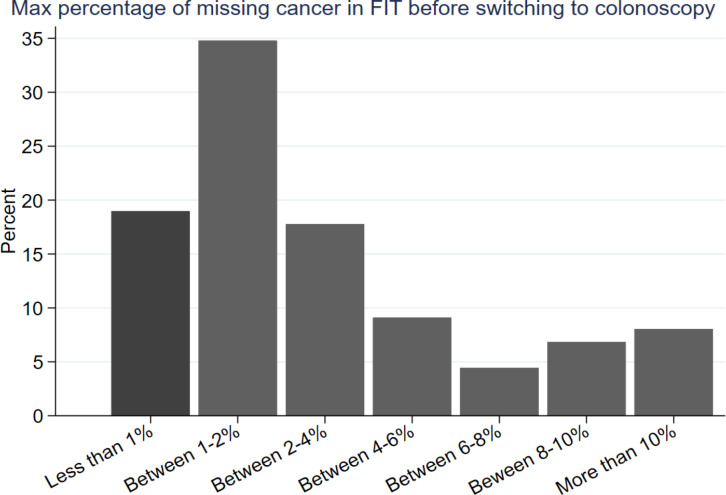
Figure 3 shows at what threshold of missed CRCs participants preferred CC over FIT. The majority (n = 741, 70.1%) preferred FIT over CC when both tests missed 1% of CRCs. This reduced to 427 (40.4%) when FIT missed 2% of CRCs. Only 7.1% (n = 75) preferred FIT when it was described to miss 10% of CRCs. Tolerating a higher level of CRCs missed by FIT was associated with being female (OR 1.41; 95% CI = 1.11 to 1.80) and suffering occasionally or frequently from indigestion (OR 1.57; 95% CI = 1.18 to 2.10). Conversely, having lower numeracy skills (OR 0.74; 95% CI = 0.58 to 0.94) and having a higher self-perceived CRC risk (OR 0.55; 95% CI = 0.34 to 0.89) were associated with switching sooner to CC.
Figure 3. Switching point for responders’ faecal immunochemical test (FIT) preference over colonoscopy (CC) (n = 1057).
Those who preferred FIT over CC in choice one were asked to choose between a CC that misses 1% of colorectal cancers (CRCs) and an alternative FIT that misses 2% of CRCs in choice 2. Participants who kept choosing FIT over CC were presented with new scenarios in which the number of CRCs missed by FIT increased constantly by 2%. In the final scenario, FIT was reported to miss 10% of CRCs. The method let the authors infer the maximum acceptable number of CRCs missed by FIT.
Preferences of communicating FIT result
The majority of participants who selected FIT at least once in the choice task (n = 461, 62.2%) would prefer to send it to a laboratory using freepost instead of returning it to their local GP practice in person (n = 280, 37.9%). If the FIT result was normal, most participants would prefer to be informed by letter (n = 312, 42.1%) or online (n = 226, 30.5%). Conversely, if the test result was abnormal, most participants would prefer to be informed face to face (n = 241, 32.5%) rather than by a letter (n =2 09, 28.2%) or over the phone (n = 167, 22.6%). Additionally, the majority would like to discuss the abnormal result with a specialist (n = 410, 55.3%) instead of their GP (n = 326, 44.0%).
Discussion
Summary
This study showed that while most responders would prefer FIT over CC if both tests miss 1% of CRCs, a large proportion of participants switched their preference to CC as soon as FIT missed more CRCs. This was particularly the case for participants who reported lower numeracy skills and among those who perceived their risk of CRC to be higher. Females and those occasionally or frequently suffering from indigestion were more likely to accept a larger number of false negative FIT tests. A smaller but important subgroup of people exhibited a strong preference for non-invasive testing (that is, using FIT over CC) even if the test missed 10% of CRCs. Out of the 75 who always chose FIT, most were female (54.7%), married or cohabiting (61.3%), white (92.0%), had at least A-levels (68.0%), were in paid employment (60.0%), and got the numeracy question right (60.0%).
Conversely, it was concerning that 150 participants (14.2%) reported that they would not undergo either of the tests to rule out CRC. The reasons for this were diverse and, in some cases, counterintuitive, showing the need to explore reasons for declining investigate testing, especially as many of the groups (for example, those with low literacy, and those from a minority ethnic background) who were overrepresented have low engagement rates with cancer screening programmes.22
Comparison with existing literature
Many of the findings are in line with a previous study showing that most patients would be willing to undergo a CC when they perceived their risk of CRC to be more than 1%.18 They also indicate that prospective patients — like GPs,12 and people invited for CRC screening15–17 — have concerns about the ability of FIT to detect CRC accurately. Furthermore, as reported in studies looking at uptake of screening tests for CRC, fear of the result was one of the most commonly reported reasons by responders who did not want to have either CC or FIT.23 There are also strong parallels with studies looking at preferences for CC versus FIT in the symptomatic context.24,25 Furthermore, there is the observation that women had a higher tolerance for missed cancers before switching to CC. This finding was in line with previous research demonstrating that women report more pain during CC26 and have a stronger preference for non-invasive CRC diagnostic modalities.27 Similarly, it was not surprising that responders with higher perceived CRC risk were more likely to switch sooner to CC given previous evidence showing that high-risk patients are more likely to prefer CC compared with FIT.24 In contrast, the finding that responders with low numeracy switched sooner to CC was novel and requires further investigation.
With regards to numeracy, it was important to note that only 55% of responders answered the numeracy question correctly; this was in line with a previous study using a comparable sample of UK adults in a similar age range,28 which may have to do with specific characteristics of the sample population. However, it also demonstrates the need to review specifically the way that information about risk (often presented in numerical form) is communicated to the public.
Strengths and limitations
This was the first study that aimed to elicit public preferences for FIT versus CC in the symptomatic context and to determine the point at which patients would prefer a more invasive procedure to avoid potentially missing CRC. These findings are important because of the urgent need to reduce the diagnostic burden on CC by using less resource-intensive modalities such as FIT. Furthermore, the investigation of preferences for the process of delivering FIT is timely given the current implementation of this test in primary care. Here the preference for accuracy level could be reflected directly with the choice of referral threshold for FIT. The findings indicate that, to meet patient preferences, the referral threshold of FIT should be low to reassure patients that the test will be no more likely than CC to miss CRC. The information about demographic antecedents of these preferences should help local authorities and commissioners provide services that are tailored to their specific population.
The major limitation of this study was that choices were focused to understand preferences for testing in relation to missed cancers, or to the sensitivity of FIT versus CC. The next step would be to conduct a discrete choice experiment in which several test attributes were manipulated, including test specificity. Previous studies have used this design to identify preferences for different CRC screening modalities and service specification within a screening modality.29
Another limitation was that this study focused on intention, which may not translate into real behaviour (that is, people who indicated that they prefer a FIT that misses 10% of CRCs over a CC that only misses 1% may choose differently if they find themselves in the real situation).30 Relatedly, the scenario setting was such that only FIT efficacy was variable, while the assumption was that CC is highly effective; it is known, however, that CC could miss up to 10% of cancers,31 so the follow-up survey should investigate whether the outcomes are similar when CC efficacy varies while FIT remains the same. Finally, it is important to note that responders who had recently experienced alarm symptoms were excluded. One may argue that these are the very people this study was aimed at; it was important, however, to ensure that responders were not influenced by their own experiences, but that every responder would make a choice based on the symptoms presented to them in the vignette. However, future research could be selectively sample responders with recent alarm symptoms to examine their preferences based on their recent symptoms and experiences.
Implications for practice
This study highlights important insights into people’s preferences of how to return their test kit, and how to receive and follow-up its results, which is in line with preferences expressed in CRC screening programmes. In the English CRC screening programme, people are asked to return the test kit in the mail and receive their results as part of a letter. People who receive an abnormal test result are then invited to attend an appointment with a screening practitioner to discuss follow-up testing. It was notable that in the surveyed cohort with imagined symptoms, most responders would prefer to return their kit by mail and receive a normal test return by letter but want to be informed about an abnormal test result face to face by a specialist rather than their GP. It should also be noted, however, that there was no universal preference or clearly dominating options, meaning that a one-size-fits-all approach might disenfranchise a subsection of the population. Local commissioners should, therefore, be encouraged to tailor implementation and service delivery to their local demographic.
Perhaps most interesting is the observation that participants’ ‘switching point’, or the tolerance for false negatives, was associated with being female and a previous history of indigestion. It is particularly interesting that the combination was specific to indigestion rather than any other symptom. The role of other comorbidities could be an important moderator. Previous studies have indicated that comorbidities can act as a prompt or barrier to help-seeking.32 In the case of indigestion, people may already have had previous investigation and, therefore, have a stronger preference for a non-invasive testing modality.
This study found that, as long as FIT can offer the same level of reassurance as CC in identifying patients who are highly unlikely to have CRC, it will be highly acceptable and generally preferred. It was also found, however, that the majority of people switched their preference back to the gold standard test as soon as FIT started missing even one additional cancer; thus, for the implementation of FIT to be successful, it will be crucial that FIT has a high accuracy level and that implementation plans will take into account local population preferences.
Funding
This project, as part of the qFIT pilot research study (IRAS 213710), was funded by the NHS England Cancer Vanguard Programme and delivered by the UCLH Cancer Collaborative. YH, SS and WV are funded by a Cancer Research UK Programme grant awarded to Prof Jane Wardle (C1418/A14134). BDN is funded by Macmillan as the GP Facilitator for Oxfordshire.
Ethical approval
This research project was approved by the University College London's research ethics committee (approval number: 13439/006).
Survey questionnaire, data, and Stata codes for the survey are available via OSF: https://osf.io/ta4bc/.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors declare that no competing interests exist.
References
- 1.Cancer Research UK Bowel cancer incidence statistics. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer/incidence. 2018 13 Jan 2020.
- 2.Redaniel MT, Ridd M, Martin RM, et al. Rapid diagnostic pathways for suspected colorectal cancer: views of primary and secondary care clinicians on challenges and their potential solutions. BMJ Open. 2015;5(10):e008577. doi: 10.1136/bmjopen-2015-008577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Research UK Routes to diagnosis of bowel cancer. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer/diagnosis-and-treatment#heading-Zero. 2016 13 Jan 2020.
- 4.National Institute for Health and Care Excellence Suspected cancer: recognition and referral. NICE guideline [NG12] https://www.nice.org.uk/guidance/ng12. 2017 13 Jan 2020. [PubMed]
- 5.Rodríguez-Alonso L, Rodríguez-Moranta F, Ruiz-Cerulla A, et al. An urgent referral strategy for symptomatic patients with suspected colorectal cancer based on a quantitative immunochemical faecal occult blood test. Dig Liver Dis. 2015;47(9):797–804. doi: 10.1016/j.dld.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Tiwari A, Sharma H, Qamar K, et al. Recognition of extraperitoneal colonic perforation following colonoscopy: a review of the literature. Case Rep Gastroenterol. 2017;11(1):256–264. doi: 10.1159/000475750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NHS England Home-testing kits that detect bowel cancer could almost halve invasive examinations by 2020. https://www.england.nhs.uk/2017/09/home-testing-kits-that-detect-bowel-cancer-could-almost-halve-invasive-examinations-by-2020/ 13 Jan 2020.
- 8.Högberg C, Söderström L, Lilja M. Faecal immunochemical tests for the diagnosis of symptomatic colorectal cancer in primary care: the benefit of more than one sample. Scand J Prim Health Care. 2017;35(4):369–372. doi: 10.1080/02813432.2017.1397255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mowat C, Digby J, Strachan JA, et al. Faecal haemoglobin and faecal calprotectin as indicators of bowel disease in patients presenting to primary care with bowel symptoms. Gut. 2016;65(9):1463–1469. doi: 10.1136/gutjnl-2015-309579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godber IM, Todd LM, Fraser CG, et al. Use of a faecal immunochemical test for haemoglobin can aid in the investigation of patients with lower abdominal symptoms. Clin Chem Lab Med. 2016;54(4):595–602. doi: 10.1515/cclm-2015-0617. [DOI] [PubMed] [Google Scholar]
- 11.Widlak MM, Thomas CL, Thomas MG, et al. Diagnostic accuracy of faecal biomarkers in detecting colorectal cancer and adenoma in symptomatic patients. Aliment Pharmacol Ther. 2017;45(2):354–363. doi: 10.1111/apt.13865. [DOI] [PubMed] [Google Scholar]
- 12.von Wagner C, Stoffel S, Freeman M, et al. Attitudes towards faecal immunochemical testing in patients at increased risk of colorectal cancer: an online survey of GPs in England. Br J Gen Pract. 2018;68(676):e757–e764. doi: 10.3399/bjgp18X699413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin JL, Barnett J. Integrating the results of user research into medical device development: insights from a case study. BMC Med Inform Decis Mak. 2012;12(1):74. doi: 10.1186/1472-6947-12-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res. 2017;17(1):88. doi: 10.1186/s12913-017-2031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghanouni A, Halligan S, Taylor SA, et al. Evaluating patients' preferences for type of bowel preparation prior to screening CT colonography: convenience and comfort versus sensitivity and specificity. Clin Radiol. 2013a;68(11):1140–1145. doi: 10.1016/j.crad.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Ghanouni A, Smith SG, Halligan S, et al. Public preferences for colorectal cancer screening tests: a review of conjoint analysis studies. Expert Rev Med Devices. 2013;10(4):489–499. doi: 10.1586/17434440.2013.811867. [DOI] [PubMed] [Google Scholar]
- 17.Ghanouni A, Halligan S, Plumb A, et al. Non- or full-laxative CT colonography vs. endoscopic tests for colorectal cancer screening: a randomised survey comparing public perceptions and intentions to undergo testing. Eur Radiol. 2014;24(7):1477–1486. doi: 10.1007/s00330-014-3187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banks J, Hollinghurst S, Bigwood L, et al. Preferences for cancer investigation: a vignette-based study of primary-care attendees. Lancet Oncol. 2014;15(2):232–240. doi: 10.1016/S1470-2045(13)70588-6. [DOI] [PubMed] [Google Scholar]
- 19.Cornsweet TN. The staircrase-method in psychophysics. Am J Psychol. 1962;75(3):485–491. doi: 10.2307/1419876. [DOI] [PubMed] [Google Scholar]
- 20.Hardisty DJ, Thompson KF, Krantz DH, Weber EU. How to measure time preferences: an experimental comparison of three methods. Judgm Decis Mak. 2013;8(3):236–249. [Google Scholar]
- 21.Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21(1):37–44. doi: 10.1177/0272989X0102100105. [DOI] [PubMed] [Google Scholar]
- 22.Von Wagner C, Verstraete W, Stoffel S. Psychological aspects of cancer screening. https://oxfordre.com/psychology/view/10.1093/acrefore/9780190236557.001.0001/acrefore-9780190236557-e-130. 2019. 24 Jan 2019.
- 23.Quaife SL, Waller J, von Wagner C, Vrinten C. Cancer worries and uptake of breast, cervical, and colorectal cancer screening: a population-based survey in England. J Med Screen. 2019;26(1):3–10. doi: 10.1177/0969141318796258. [DOI] [PubMed] [Google Scholar]
- 24.Bowyer HL, Vart G, Kralj-Hans I, et al. Patient attitudes towards faecal immunochemical testing for haemoglobin as an alternative to colonoscopic surveillance of groups at increased risk of colorectal cancer. J Med Screen. 2013;20(3):149–156. doi: 10.1177/0969141313503953. [DOI] [PubMed] [Google Scholar]
- 25.Atkin W, Cross AJ, Kralj-Hans I, et al. Faecal immunochemical tests versus colonoscopy for post-polypectomy surveillance: an accuracy, acceptability and economic study. Health Technol Assess. 2019;23(1):1–84. doi: 10.3310/hta23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holme O, Bretthauer M, de Lange T, et al. Risk stratification to predict pain during unsedated colonoscopy: results of a multicenter cohort study. Endoscopy. 2013;45(9):691–696. doi: 10.1055/s-0033-1344239. [DOI] [PubMed] [Google Scholar]
- 27.Bonello B, Ghanouni A, Bowyer HL, et al. Using a hypothetical scenario to assess public preferences for colorectal surveillance following screening-detected, intermediate-risk adenomas: annual home-based stool test vs. triennial colonoscopy. BMC Gastroenterol. 2016;16(1):113. doi: 10.1186/s12876-016-0517-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoffel ST, Yang J, Vlaev I, von Wagner C. Testing the decoy effect to increase interest in colorectal cancer screening. PLoS One. 2019;14(3):e0213668. doi: 10.1371/journal.pone.0213668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghanouni A, Halligan S, Taylor SA, et al. Quantifying public preferences for different bowel preparation options prior to screening CT colonography: a discrete choice experiment. BMJ Open. 2014;4(4):e004327. doi: 10.1136/bmjopen-2013-004327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheeran P. Intention — behavior relations: a conceptual and empirical review. Eur Rev Soc Psychol. 2002;12(1):1–36. doi: 10.1080/14792772143000003. [DOI] [Google Scholar]
- 31.Lüning TH, Keemers-Gels ME, Barendregt WB, et al. Colonoscopic perforations: a review of 30,366 patients. Surg Endosc. 2007;21(6):994–997. doi: 10.1007/s00464-007-9251-7. [DOI] [PubMed] [Google Scholar]
- 32.Renzi C, Kaushal A, Emery J, et al. Comorbid chronic diseases and cancer diagnosis: disease-specific effects and underlying mechanisms. Nat Rev Clin Oncol. 2019;16(12):746–761. doi: 10.1038/s41571-019-0249-6. [DOI] [PubMed] [Google Scholar]