Abstract
Background
Pulmonary hypertension (PH) is a chronic progressive disease characterized by increasing pulmonary vascular resistance, poor prognosis and high disability rate. Although many targeted drugs for PH have been put to clinical use, most patients still have poor exercise tolerance and quality of life. Exercise training is considered to further improve exercise capacity and quality of life in patients with PH, but it has not been fully studied and utilized. The aim of this systematic review and meta-analysis is to evaluate the effectiveness and safety of exercise training in patients with PH.
Methods
A search was conducted for the meta-analysis using the databases PubMed, Embase, Cochrane Library, including literature published before December 2018. The primary outcome of this meta-analysis was a change in the 6-minute walk distance (6MWD). In addition, peak oxygen uptake (PeakVO2), resting pulmonary arterial systolic pressure (PASPrest), resting heart rate (HRrest), peak exercise heart rate (HRpeak), oxygen uptake anaerobic threshold (VO2 at AT), maximum workload and quality of life (QoL) were also assessed.
Results
A total of 651 patients in 17 studies were included. A meta-analysis showed that exercise training was associated with significant improvement in the 6MWD [weighted mean difference (WMD): 64.75 m (95% CI: 53.19–76.31 m, P<0.001)], peakVO2 [WMD: 1.78 mL/min/kg (95% CI: 1.27–2.29 mL/min/kg, P<0.001)], HRpeak [WMD: 11.07 beats/min (95% CI: 8.04–14.11 beats/min, P<0.001)] and QoL measured by SF-36 questionnaire subscale scores. Furthermore, exercise training is well tolerated, and no major adverse event occurred related to exercise training.
Conclusions
Exercise training is associated with a significant improvement in exercise capacity, cardiorespiratory fitness and quality of life among patients with PH and proved to be safe for stable PH patients with optimization of medical therapy. However, more large-scale multicenter studies are needed to confirm the effectiveness and safety of exercise training in patients with PH.
Keywords: Pulmonary hypertension (PH), exercise training, exercise capacity, rehabilitation
Introduction
Pulmonary hypertension (PH) is a chronic progressive disease characterized by gradually increased pulmonary vascular resistance, which eventually leads to increased right heart load, right heart failure and even death. According to McGoon et al., the prevalence of adult pulmonary arterial hypertension (PAH) and idiopathic pulmonary arterial hypertension (IPAH) is at least 15 cases per million and 5.9 cases per million, respectively. The annual incidence of PAH is at least 2.4 cases per million, and IPAH accounts for 35–48% of PAH cases (1). PH has a high mortality and disability rate, and the prognosis is extremely poor. In the early years, the National Institute of health (NIH) in the United States found that the average survival period of IPAH patients was only 2.8 years, and the 1-, 3- and 5-year survival rates were 68%, 48% and 34%, respectively (2). By 2006, the 1-, 3- and 5-year survival rates of IPAH patients in China were 68%, 38.9% and 20.8%, respectively (3). In the past 20 years, pharmacotherapy targeting the three different mechanisms of PAH, including prostacyclin and its derivatives, endothelin receptor antagonists and 5-type phosphodiesterase inhibitors, has been gradually used in the clinical treatment of PH and has been proven to slow the progress of PAH and improve the survival rate (4-6). However, despite receiving standard targeted pharmacotherapy, the prognosis is still poor (7), most patients remain symptomatic and have reduced exercise capacity, quality of life (8,9). Therefore, to improve patients’ exercise tolerance and quality of life, it is necessary to explore adjunctive therapeutic strategies to further improve the prognosis of patients with PH.
Traditionally, exercise training and cardiopulmonary rehabilitation have been considered a contraindication for patients with PH due to safety concerns, such as the risk of sudden cardiac death, exacerbation of pulmonary vascular remodelling, and deterioration in right heart function. Therefore, in the past, most doctors recommended PH patients to avoid exercise. In patients with chronic heart failure and chronic obstructive pulmonary disease (COPD), exercise training has been proven to improve cardiopulmonary function and clinical outcomes (10,11). Considering that the pathophysiological changes of PH overlap with those of heart failure and COPD, some researchers hypothesize that exercise training may be beneficial to PH patients.
In recent years, several small clinical trials have assessed the value of exercise training as an adjunctive therapeutic strategy in patients with chronic PH. Although most of these studies were small and did not design clinical end points of mortality or hospitalizations related to PH, they have demonstrated a different extent of improvement in exercise tolerance and quality of life in response to training, especially the improvement of the 6-minute walking distance (6MWD) and quality of life (QoL) (8,12-19). However, there are some inconsistent findings. Martínez-Quintana et al. and de Man et al. reported that exercise training cannot improve the 6MWD or QoL (20,21). Therefore, as the efficacy of exercise training for PH patients is not yet clear, we conducted a systematic review and meta-analysis to evaluate the effectiveness and safety of exercise training for PH patients.
Methods
Search strategy and study selection
A comprehensive computerized literature search of the PubMed, Embase, Cochrane Library, was conducted using MeSH terms and keywords, including PH, pulmonary arterial hypertension, exercise, exercise training, and rehabilitation, for articles published before December 2018. From this search, we only included articles specifically addressing the effects of a supervised exercise training program in patients with PH. In addition, the reference lists from review articles were searched manually to identify other possible eligible studies.
Inclusion criteria: (I) prospective intervention studies, including randomized control trials, nonrandomized control trials and pre-post intervention studies; (II) age >18 years; (III) primary outcome was change in the 6MWD after exercise training, secondary outcomes were improvement in cardiopulmonary function, including peak oxygen uptake (peakVO2), systolic pulmonary artery pressure at rest (PASPrest), peak heart rate (HRpeak), and QoL which was indicated by the SF-36 questionnaire subscale scores. Exclusion criteria: (I) did not involve at least one of the above outcomes; (II) unavailable full text or accurate data extraction; (III) retrospective studies and case series studies. Outcomes: (I) primary outcomes: 6MWD; (II) secondary outcomes: PeakVO2, PASPrest, HRpeak and QoL (SF-36); (III) adverse events: syncope, infection, decline in blood oxygen saturation.
Data extraction
Two reviewers extracted data independently from eligible studies using standardized forms to verify consistency and accuracy. The following information was recorded for each study: author, year of publication, nature of study, baseline demographic and clinical characteristics, right heart catheterization data, pre and post exercise intervention measures of outcome variables (6MWD, PeakVO2, PASPrest, HRrest, HRpeak, VO2 at AT, Workloadmax, SF-36 score) and adverse events. The 6MWD was reported in all studies. Both fatal and nonfatal adverse events among the exercise-training patients were recorded.
Study quality assessment
Study quality was assessed independently by two reviewers. Disagreements were resolved by consensus. The Cochrane Bias Risk Assessment Tool recommended by the Cochrane Collaboration was used to evaluate the quality of the randomized controlled trials (RCTs). Nonrandomized controlled trials (non-RCTs) and pre-post intervention studies used Methodological index for non-randomized studies (MINORS items) for quality assessment.
Data synthesis and statistical analysis
Review Manager Software (RevMan5.3) was used for statistical analyses. According to different studies, the chi-square test was used to test the heterogeneity of the results. If I2<50%, then there was no significant statistical heterogeneity among the studies. A fixed-effect model was used to analyse the results. If I2>50%, then statistical heterogeneity existed among the studies, and a random-effect model was used to analyse the results. Continuity data were analysed by the weighted mean difference (WMD). If different measuring tools were used for the same variable, standardized mean difference (SMD) was used to analyse the data, and a 95% confidence interval (95% CI) was used to represent the effect of each observation outcome.
Results
Literature search
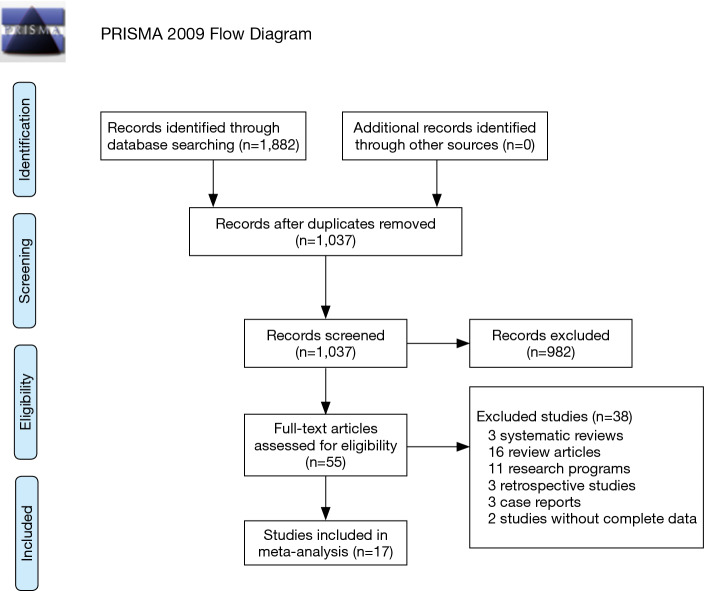
A flow diagram of the literature search and selection is presented in Figure 1.
Figure 1.
Flow diagram.
Characteristics of the participants and study designs
Seventeenth studies with 651 participants were finally selected in this meta-analysis, including 6 RCTs, 3 non-RCTs, and 8 pre-post intervention studies. Baseline demographic and clinical characteristics of the study participants are summarized in Table 1. All of the included studies provided information on the etiology of PH among the study participants. The main types of PH are class I and class IV, in which class I PH accounts for 48% and class IV PH accounts for 24%. All patients in the studies were already receiving optimized PH-targeted treatment and were stable for at least 2 months with no recent hospitalizations or medication changes. Specific inclusion and exclusion criteria are shown in Table S1. The exercise training methods adopted include low-load aerobic exercise (bicycle ergometer training, treadmill training), respiratory training and resistance training. In most studies, exercise intensity was controlled at 60–80% of peak exercise capacity. Because the safety of exercise training in PH is not clear, most of the exercise training adopted in the study was initially arranged in the inpatient department or outpatient department, followed by home-based exercise training and telephone follow-up. Only Fukui et al. (22) and Inagaki et al. (23) adopted home-based exercise training. The specific exercise training program is shown in Table S2.
Table 1. Baseline demographic and clinical characteristics of study participants.
| Author | Year | Research types | No. of participants (% women) | Mean age [year]* | Etiology of PAH | WHO functional class | PAH medications used | Baseline PeakVO2
(mL/min/kg)* |
Baseline 6-minute walk distance (m)* |
|---|---|---|---|---|---|---|---|---|---|
| Mereles et al. | 2006 | Randomized controlled trial | Ex T: 15; control: 15; women: 66.7% | 50 [13] | 80% IPAH; 20% CTEPH | 20% Class II; 73%Class III | ERA: 63%; PD5-I: 33% | Ex T: 13.2 (3.1); Control: 11.9 (3.1) | Ex T: 439 [82]; Control: 411 [86] |
| Ley et al. | 2013 | Randomized controlled trial | Ex T: 10; control: 10; women: 70% | 50 [11] | 55% IPAH; 20% CTEPH; 10% CTD |
20% Class II; 80% Class III | Mono: 25%; Dual: 60%; Triple: 15% | NA | Ex T: 449 [80]; Control: 423 [101] |
| Chan et al. | 2013 | Randomized controlled trial | Ex T: 10; control: 13; women: 100% | 54 [10] | 22% IPAH; 74% CTD; 4% drug induced | 91% Class II/III | Mono: 30%; Dual: 26%; Triple:39% | Ex T: 17.6 (5.7); Control: 14.7 (5.1) | Ex T: 411 [73]; Control: 377 [97] |
| Saglam et al. | 2015 | Randomized controlled trial | Ex T: 14; control: 15; women: 80.6% | 50 [12] | 26% IPAH; 23% CHD; 51% CTD | 52% Class II; 48% Class III | ERA: 32%; PD5-I: 10%; CCB: 16% | NA | Ex T: 427 [98]; Control: 357 [137] |
| Ehlken et al. | 2015 | Randomized controlled trial | Ex T: 38; control: 41; women: 54% | 56 [12] | 71% PAH; 29% CTEPH | 16% Class II; 76% Class III; 5% Class IV | Mono: 31%; Dual: 48%; Triple: 11.5% | Ex T: 13.3 (3.6); Control: 12.7 (4.0) | Ex T: 453 [91]; Control: 413 [95] |
| Laura González-Saiz et al. | 2017 | Randomized controlled trial | Ex T: 20; control: 20; women: 60% | 46 [11] | 38% IPAH; 10% CTEPH; 8% CHD; 15% CTD |
63% Class II; 14% Class III | Mono:40%; Combi: 37.5%; Mono + PGI: 25%; Combi + PGI: 17.5% | Ex T: 15.7 (3.3); Control: 19.8 (6.5) | Ex T: 500 [70]; Control: 546 [99] |
| Shigefumi Fukui et al. | 2016 | Non-randomized control trial | Ex T: 17; control: 24; women: 73% | 68 [9] | 100% CTEPH | 85% Class II; 12% Class III | Drug:61% | Ex T: 17.4 (2.6) Control: 17.8 (2.6) |
Ex T: 498 [96]; Control:468 [102] |
| Martínez-Quintana et al. | 2010 | Non-randomized control trial | Ex T: 4; control: 4; women: 62.5% | 28 [6] | 100% CHD | NA | ERA:87.5% | NA | Ex T: 364 [50]; Control:442 [193] |
| Fox et al. | 2011 | Non-randomized control trial | Ex T: 11; control: 11; women: 68% | 52 [19] | 45% IPAH; 9% CTEPH; 5% CHD; 41% CTD | NA | ERA: 63%; PD5-I: 45%; Mono: 54%; Combi: 45% | Ex T: 8.2 (1.9); Control: 11.6 (5.5) | Ex T: 353 [60]; Control:425 [80] |
| Grünig et al. | 2012 | Pre-post intervention study | Ex T: 21; women: 95% | 52 [18] | 100% CTD | 43% Class II; 33% Class III | Mono: 38%; Dual: 48%; Triple: 14% | Ex T: 11.8 (3.4) | Ex T: 386 [121] |
| Grünig et al. | 2012 | Pre-post intervention study | Ex T: 183; women: 69% | 53 [15] | 45% IPAH; 17% CTEPH; 8% CHD; 10% CTD |
14% Class II; 75% Class III | ERA: 59%; PD5-I: 58%; Mono: 44%; Combi: 51% | Ex T: 12.2 (3.5) | Ex T: 425 [106] |
| Nagel et al. | 2012 | Pre-post intervention study | Ex T: 35; women: 46% | 61 [15] | 100% CTEPH | 20% Class II; 74% Class III | ERA: 60%; PD5-I: 60%; Mono: 49%; Combi: 46% | Ex T: 12.1 (1.7) | Ex T: 408 [108] |
| Becker-Grünig et al. | 2013 | Pre-post intervention study | Ex T: 20; women: 80% | 48 [11] | 100% CHD | 30% Class II; 70% Class III | ERA: 70%; PD5-I: 60% | Ex T: 11.4 (2.2) | Ex T: 423 [90] |
| Kabitz et al. | 2014 | Pre-post intervention study | Ex T: 7; women: 57% | 60 [11] | 72% IPAH; 28% CTD | 86% Class III; 14% Class IV | ERA: 28%; PD5-I: 86% | NA | Ex T: 417 [51] |
| Grünig et al. | 2011 | Pre-post intervention study | Ex T: 58; women: 72% | 51 [12] | 64% IPAH; 10% CTEPH; 2% CHD; 4% CTD |
17% Class II; 76% Class III | Mono: 66%; Dual: 31%; Triple: 3% | Ex T: 12.5 (3.0) | Ex T: 440 [90] |
| Inagaki et al. | 2014 | Pre-post intervention study | Ex T: 8; women: 100% | 64 [12] | 100% CTEPH | 75% Class II; 25% Class III | ERA: 38%; PD5-I: 62%; Mono: 62%; Combi: 38% | NA | Ex T: 383 [91] |
| De man et al. | 2009 | Pre-post intervention study | Ex T: 19; women: 79% | 42 [13] | 100% IPAH | NA | Mono: 42%; Combi: 58% | Ex T: 15 (4.0) | Ex T: 496 [108] |
*, data are shown as mean (SD).
Table S1. Inclusion criteria and exclusion criteria.
| Author | Year | Inclusion criteria | Exclusion criteria |
|---|---|---|---|
| Mereles et al. | 2006 | • Severe chronic pulmonary hypertension, receiving targeted drug therapy, stable condition ≥3 months • WHO-FC II–IV • No recent syncope or skeletal muscle disorder |
Those who do not meet the inclusion criteria |
| Ley et al. | 2013 | • Age ≥18 years • Targeted drug therapy, stable condition≥3 months • WHO-FC II–III • No recent syncope, no skeletal muscle disorder |
• Age ≤18 years old • WHO-FC Class I or IV • Other factors that do not meet the inclusion criteria |
| Chan et al. | 2013 | • WHO group I PAH • Diagnosis of resting mPAP ≥25 mmHg by right heart catheter • The condition is stable ≥3 months, and has not participated in pulmonary rehabilitation training for nearly 6 months |
• WHO-FC Class I or IV • FEV1/FVC ≤65% • EF <40%, PCWP ≥18 mmHg • Serious mental illness • severe liver and kidney dysfunction, metabolic abnormalities • History of ischemic heart disease • Use of exercise-restrictive drugs, antivirals, drugs, smoking, pregnancy |
| Ehlken et al. | 2015 | • WHO-FC II–IV • Receive pulmonary hypertension target drugs, stable condition ≥2 months |
Those who do not meet the inclusion criteria |
| Saglam et al. | 2015 | • WHO-FC II–III • Receive pulmonary hypertension target drugs, stable condition ≥3 months |
Severe obstructive and restrictive pulmonary diseases, severe ischemic heart disease, left heart failure, pulmonary heart disease, cognitive impairment, infection of virus in nearly 6 months, bone and joint disorder |
| González-Saiz et al. | 2017 | • Age >18 years • Diagnosis of PAH or CTEPH by right cardiac catheterization • Targeted drug therapy, stable condition (>3 months) • No recent syncope, no musculoskeletal disorder • WHO-FC II–III |
Two people changed targeted drugs before starting exercise training |
| Fukui et al. | 2016 | • Inoperable CTEPH who underwent their final BPA with improved resting mean pulmonary arterial pressure of 24.7±5.5 mmHg and who suffered remaining exercise intolerance • WHO ≥II |
One person had skeletal and muscular disorder |
| Martínez-Quintana et al. | 2010 | • Age ≥14 years • No change in drug treatment regimen for pulmonary hypertension in the past 6 months • WHO-FC II–III |
Those who do not meet the inclusion criteria |
| Fox et al. | 2011 | • Right heart catheter resting mPAP>25mmHg, PCWP ≤15 mmHg, PVR ≥3 wood Units • Receive targeted drug therapy, stable treatment for ≥3 months • NYHA classification II–III |
• Level I or IV of NYHA, PAH due to CHD with a right-to-left shunt, left heart disease, chronic hypoxia or chronic lung disease (total lung volume/FEV1 < 60% predicted value) • Diseases requiring hospitalization occur during case screening • Any non-PAH medical condition likely to interfere with participation in or completion of the program • Participants in other rehabilitation programs within 6 months |
| Grünig/Marier et al. | 2012 | • PAH related to connective tissue diseases diagnosed by guidelines • WHO-FC II–IV • Targeted drugs for pulmonary hypertension and connective tissue disease were administered, and the condition was stable for more than 2 months. |
• Severe interstitial lung disease • One patient was excluded for respiratory infection on follow-up |
| Grünig/Lichtblau et al. | 2012 | • WHO-FC II–IV • Targeted drug therapy, stable condition ≥2 months • New diagnosis of pulmonary hypertension, 2–6 months after receiving new targeted drugs |
• Unstable clinical symptoms (6 people) • Gastrocnemius paralysis occurs after falling (1 person) • Family problems (2 people) • MRSA infection (1 person) • Peripheral arterial occlusion impairing 6MWD (1 person) |
| Nagel et al. | 2012 | • Patients with CTEPH during 06/2006 – 10/2011 • Targeted drug therapy, stable condition ≥ 2 months • WHO-FC II–IV |
• Change in targeted drugs 2–4 weeks before training (2 people) • Misdiagnosis (2 people) |
| Becker-Grünig et al. | 2013 | • Adult patients with invasively confirmed severe congenital heart disease with PAH during 09/2008 – 10/2011 • Receive targeted drug therapy, stable condition ≥2 months • Newly diagnosed pulmonary hypertension, 2-6 months after receiving new targeted drug therapy • WHO-FC II–III |
Those who do not meet the inclusion criteria |
| Kabitz et al. | 2014 | • WHO-FC II–IV • No recent syncope • Diagnosis of PAH based on current clinical classification criteria • Targeted drug therapy, stable condition ≥2 months |
Complicated with left heart disease, lung disease, rib cage abnormality, neuromuscular abnormality, cachexia, systemic steroid therapy |
| Grünig et al. | 2011 | • Severe chronic pulmonary hypertension and right heart failure diagnosed according to guidelines during 01/2003 – 04/2007 • WHO-FC II–IV • Targeted drug therapy, stable condition ≥3 months |
• Presence of underlying mitral stenosis as an etiology for PAH (1 person) • Change in targeted drugs (1 person) • Family reasons (1 person) |
| Inagaki et al. | 2014 | • Outpatients with inoperable or residual CTEPH • Receive targeted drug therapy, stable condition ≥3 months • Age 18–80 years old • WHO-FC II–IV |
Individuals with other unstable/severe pulmonary disease or cardiac, orthopedic, or neurological disorders limiting exercise performance |
| de Man et al. | 2009 | • Diagnosed with IPAH according to WHO criteria established by RHC • Stable clinical condition, defined as a change in 6-min walk distance (6MWD) of <10% in three consecutive measurements prior to inclusion (over a period of minimally 1 year), and no change in medical therapy for >3 months • Aged 18 years. or older • Living within 5 km of a rehabilitation center associated with the current study |
Those who do not meet the inclusion criteria |
Table S2. Characteristics of the exercise training programs.
| Author | Year | Design | Exercise training group intervention | Control group intervention | Duration | Result |
|---|---|---|---|---|---|---|
| Mereles et al. | 2006 | RCT | • Interval bicycle ergometer training 7 days/week at low workloads • Exercise intensity at 60% to 80% of PeakVO2 • 60 min of walking 5 days/week • 5 days/week of 30 min of resistance training • 30 min of respiratory training 5 days/week • 3 weeks in hospital supervised training followed by 12 weeks training at home |
• Common rehabilitation program based on healthy nutrition, physical therapy such as massages, inhalation, counselling, and muscular relaxation without exercise and respiratory training | 15 weeks | 6MWD ↑; QOL ↑; VO2 (peak+AT) ↑; Workload ↑; WHO FC ↑ |
| Ley et al. | 2013 | RCT | • Same as Mereles et al. 2006 | • Routine daily activities and no specific exercise intervention | 3 weeks | 6MWD ↑; MRI perfusion (pulmonary blood volume) ↑; peak flow↑ |
| Chan et al. | 2013 | RCT | • Aerobic training intervention 24–30 sessions of medically supervised treadmill walking for 30–45 min per session. • Target exercise intensity of 70% to 80% of each patient’s heart rate (HR) reserve obtained from the baseline. • Education intervention. |
• 1 hour of education intervention including lung disease processes, medication use, oxygen therapy, sleep disorders, panic control, relaxation techniques, breathing retraining, community resources etc. | 10 weeks | 6MWD ↑ QOL ↑ |
| Ehlken | 2015 | RCT | • In-hospital training for 3 weeks • Home-based exercise training for 12 weeks • Protocol same as Mereles et al. 2006 |
• Usual care | 15 weeks | peak VO2 ↑; 6MWD ↑; QOL ↑; cardiac index ↑; mPAP↓ |
| Saglam et al. | 2015 | RCT | • Inspiratory muscle training at 30% of the maximum inspiratory pressure which is measured each week • 30 min/day • 7 day/week • 6 weeks |
• Sham inspiratory muscle training at a fixed workload of 10% of the maximum inspiratory pressure; • 30 min/day • 7 day/week • 6 weeks |
6 weeks | 6MWD↑; maximum inspiratory pressure ↑; maximum expiratory pressure↑; FEV1, FVC↑ |
| González-Saiz et al. | 2017 | RCT | • Aerobic training: treadmill dynamometer, 20–40 minutes/session, 5 sessions/week (Monday to Friday), a total of 40 sessions, gradually increase the duration/intensity of each session according to personal situation • Resistance training: 3 sessions/week (Monday, Wednesday and Friday), a total of 24 sessions • Respiratory exercise: 2 sessions/day (one at the hospital in the morning, one at home in the evening), 6 day/week |
• Meet regularly with clinicians | 8 weeks | The improvement of 6MWD was not obvious; muscle strength ↑; PeakVO 2↑ |
| Shigefumi Fukui et al. |
2016 | Non-RCT | • Hospital training for 1 week: walking, bicycle ergometer, low-intensity resistance exercise in lower limbs • Outpatient training for 11 weeks: walking, 30–60 minutes/time, 4–5 times/week; low-intensity resistance exercise in lower limbs, 3 days/week • Patients recorded the time and times of exercise • Educational courses, including lifestyle guidance, counselling, psychological support |
• Maintenance of pulmonary hypertension targeted drug therapy | 12 weeks | 6MWD (−); PeakVO2 ↑; exercise load ↑; QOL↑; Quadriceps strength ↑; WHO FC↑ |
| Martínez-Quintana et al. | 2010 | Non-RCT | • 3 months in-hospital training: 2 days/week • Training sessions with 10 minutes of warming up, brief period of resistance exercise (1–2 kg), interval of bicycle ergometer training (10–25 weeks for 24 minutes, 20–50 weeks for 30 seconds) • 9 months of home training: walk on flat ground every day and do exercises similar to exercise training in hospital |
• Maintain daily activities without special exercise intervention | 12 months | 6MWD (−); QOL ↑; limb strength ↑; WHO FC↑ |
| Fox et al. | 2011 | Non-RCT | • Supervised 24 biweekly 1 hour sessions of exercise training in two 6-week blocks • Exercise intensity at 60% to 80% of peak VO2 In the first block, subjects did interval training with treadmill walking, cycling, and step climbing • In the second block, subjects performed longer periods of continuous aerobic exercise, with resistance training |
• Usual care with maintenance of routine daily activities and no specific exercise intervention | 12 weeks | 6MWD ↑; peak VO2↑ |
| Grünig/Maier et al. | 2012 | Pre-post intervention study | • 3 weeks of supervised training in hospital • 1.5 hours/day, 7 days/week, including interval bicycle ergometer training at low workload (10–60 W), single group muscle training—low workload dumbbell training (500–1,000 g), respiratory training 5 days/week; • 12-week home-based training: more than 30 minutes/day, 5 days/week |
— | 15 weeks | 6MWD ↑; QOL ↑; VO2↑; PASP ↓ (3 weeks) |
| Grünig/Lichtblau et al. | 2012 | Pre-post intervention study | Protocol same as Grünig/Maier et al. 2012 | — | 15 weeks | 6MWD ↑; QOL ↑; peak VO2 ↑; WHO FC ↑; activity tolerance↑ |
| Nagel et al. | 2012 | Pre-post intervention study | Protocol same as Grünig/Maier et al. 2012 | — | 15 weeks | 6MWD ↑; QOL ↑; peak VO2↑; NT-proBNP ↓; (3 weeks) |
| Becker-Grünig et al. | 2013 | Pre-post intervention study | Protocol same as Grünig/Maier et al. 2012. | — | 15 weeks | 6MWD ↑; QOL ↑ |
| Kabitz et al. | 2014 | Pre-post intervention study | Protocol same as Grünig/Maier et al. 2012 | — | 15 weeks | 6MWD ↑; TwPmo↑ |
| Grünig et al. | 2011 | Pre-post intervention study | • 3 weeks of supervised training in hospital • 1.5 hours/day, 7 days/week, including walking, single group muscle training - low workload dumbbell training (500–1,000 g), respiratory training 5 days/week • 24±12 months of Home-based training: a personal training manual, bicycle ergometer training |
— | 15 weeks | 6MWD ↑; QOL ↑; peak VO2 ↑; WHO FC ↑; HRrest ↓; workload↑ |
| Inagaki et al. | 2014 | Pre-post intervention study | • 12 week outpatient rehabilitation program with one in hospital class each week and home based rehabilitation 24–30 sessions over 10 weeks • Combination of strength, endurance and respiratory exercises • Endurance training at 60% of target heart rate |
— | 12 weeks | 6MWD ↑; quadriceps strength↑ |
| de Man et al. | 2009 | Pre-post intervention study | • The standardized exercise protocol adopted from the AHA guidelines for rehabilitation of CHF patients • Supervised exercise training consisted of cycling (based on VO2max assessed at baseline measurements) and quadriceps muscle training (based on repetition maximum assessed on the first day of training) • 3 times/week |
— | 12 weeks | 6MWD (−); peak VO2 (−); endurance improved; No. of capillaries per myocyte ↑; oxidative enzymes ↑ |
Quality assessment
The Cochrane Bias Risk Assessment Tool was used to evaluate the quality of the randomized controlled trials, as shown in Figure 2. Quality assessment of nonrandomized controlled trials and pre-post interventional studies have been detailed in Table 2 using MINORS items.
Figure 2.
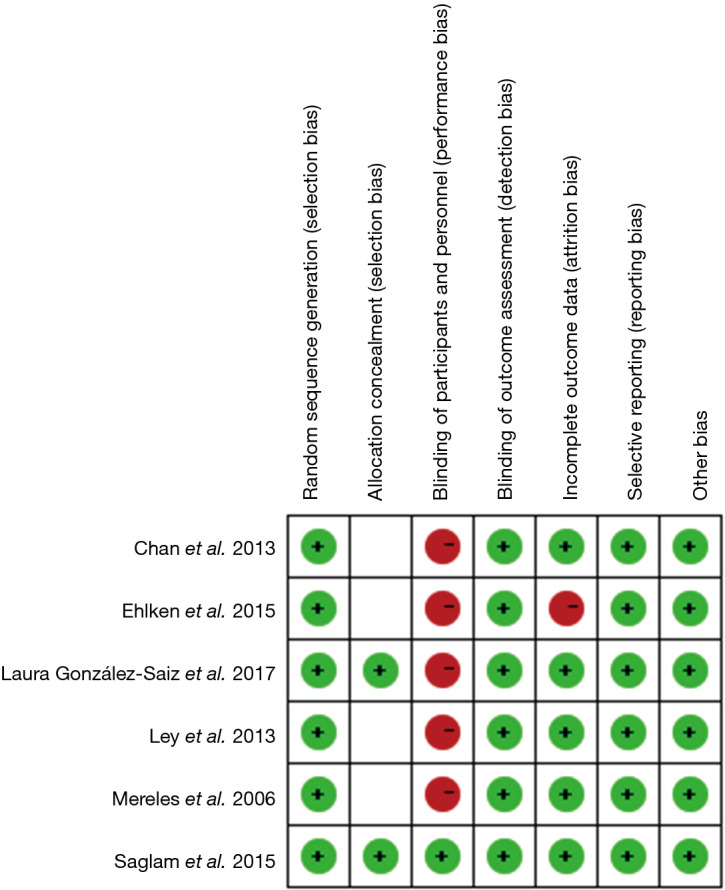
Quality assessment of RCTs. RCT, randomized controlled trial.
Table 2. Quality assessment of non-randomized controlled trials and pre-post interventional studies.
| Author | Design | MINORS items | In the case of comparative studies | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. A stated aim of the study | 2. Inclusion of consecutive patients | 3. Prospective collection of data | 4. Endpoint appropriate to the study aim | 5. Unbiased evaluation of endpoints | 6. Follow-up period appropriate to the major endpoint. | 7. Loss to follow up not exceeding 5% | 8. A control group having the gold standard intervention | 9. Contemporary groups | 10. Baseline equivalence of groups |
11. Prospective calculation of the sample size | 12. Statistical analyses adapted to the study design | |||
| Grünig/Maier et al. 2012 | Pre-post intervention study | Y | Y | Y | Y | Y | Y | N | N | |||||
| Grünig/Lichtblau et al. 2012 | Pre-post intervention study | Y | Y | Y | Y | Y | Y | N | N | |||||
| Nagel et al. 2012 | Pre-post intervention study | Y | Y | Y | Y | Y | Y | N | N | |||||
| Becker-Grünig et al. 2013 | Pre-post intervention study | Y | Y | Y | Y | Y | Y | N | N | |||||
| Kabitz et al. 2014 | Pre-post intervention study | Y | Y | Y | Y | Y | Y | Y | N | |||||
| Grünig et al. 2011 | Pre-post intervention study | Y | Y | Y | Y | Y | Y | Y | N | |||||
| Inagaki et al. 2014 | Pre-post intervention study | Y | Y | Y | Y | Y | Y | Y | N | |||||
| de Man et al. 2009 | Pre-post intervention study | Y | Y | Y | Y | Y | Y | N | N | |||||
| Martínez-Quintana et al. 2010 | Non-RCT | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | |
| Fox et al. 2011 | Non-RCT | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | |
| Shigefumi Fukui et al. 2016 | Non-RCT | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | |
The effect of exercise training on the 6MWD
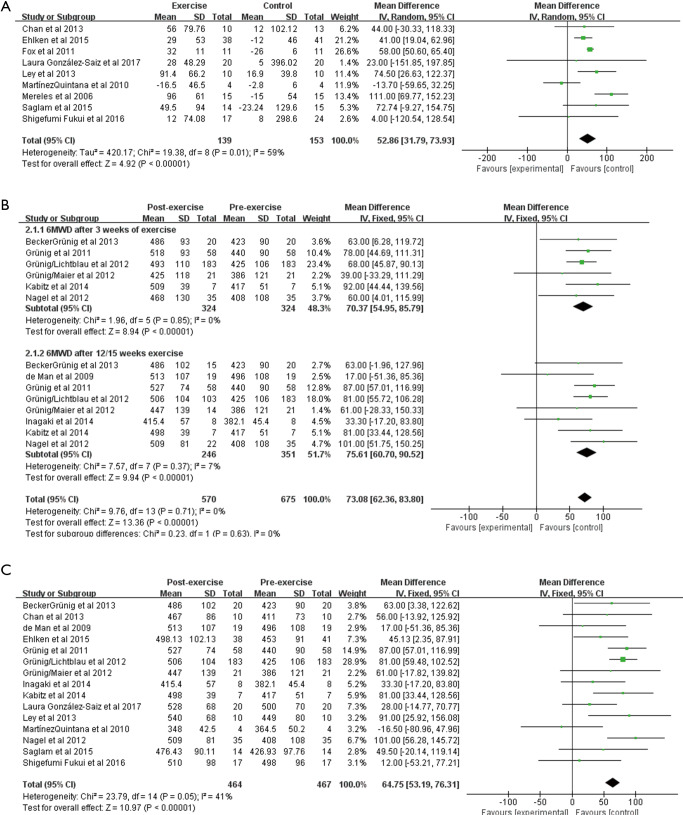
The 6MWD was reported in all the included studies (651 patients). There was moderate heterogeneity (I2=59%, P=0.01) in the analysis of all parallel intervention studies (RCTs and non-RCTs). We observed a significant improvement in the 6MWD from baseline to follow-up using random effects analysis [WMD: 52.86 m (95% CI 31.79–73.93 m), P<0.001; Figure 3A]. Analysis of the 8 pre-post intervention studies showed a significant improvement in the 6MWD after exercise training for 3 weeks [WMD: 70.37 m (95% CI: 54.95–85.79 m, P<0.001; Figure 3B], and no heterogeneity was observed (I2=0%, P=0.85). After exercise training for 12 or 15 weeks, the 6MWD increased to 75.61 m [95% CI: 60.70–90.52 m, P<0.001; Figure 3B], and the heterogeneity was small (I2=7%, P=0.37). We also conducted a combined analysis of parallel intervention studies and pre-post intervention studies. After exercise, the 6MWD increased 64.75 m [95% CI: 53.19–76.31 m, P<0.001; Figure 3C], and the heterogeneity was small (I2=41%, P=0.05).
Figure 3.
Forest plot showing effect of exercise training on 6MWD. 6MWD, 6-minute walk distance.
The effect of exercise training on peakVO2
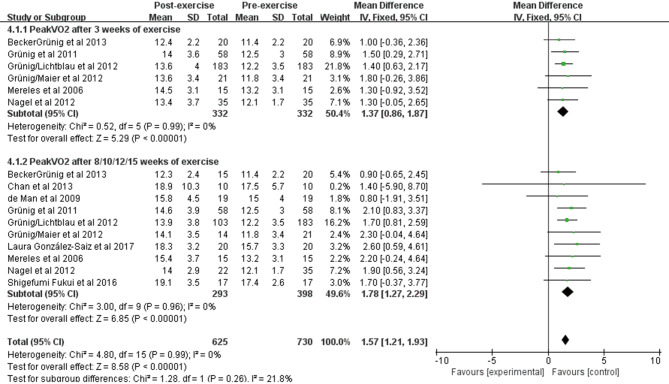
PeakVO2 was reported in 10 studies of 398 patients at baseline and after exercise training. We observed a significant improvement in PeakVO2 using fixed effects analysis after exercise training for 3 weeks [WMD: 1.37 mL/min/kg (95% CI: 0.86–1.87 mL/min/kg, P<0.001); Figure 4], and no heterogeneity was observed (I2=0%, P=0.99). After exercise training for 8/10/12/15 weeks, a greater improvement was observed [WMD: 1.78 mL/min/kg (95% CI: 1.27–2.29 mL/min/kg, P<0.001); Figure 4], and no heterogeneity was observed between studies (I2=0%, P=0.96).
Figure 4.
Forest plot showing effect of exercise training on PeakVO2.
The effect of exercise training on PASPrest
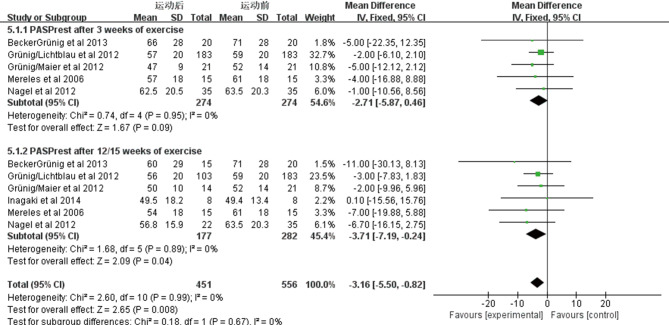
Six studies included 282 patients estimated the changes in resting pulmonary artery systolic blood pressure (PASPrest) before and after exercise training. We observed a marked improvement in PASPrest using fixed effects analysis after exercise training for 3 weeks [WMD: −2.71 mmHg (95% CI: −5.87–0.46 mmHg, P=0.09); Figure S1], and no heterogeneity was observed (I2=0%, P=0.95). An improvement also can be observed after exercise training for 12/15 weeks [WMD: −3.71 mmHg (95% CI: −7.19–−0.24 mmHg, P=0.04); Figure S1], and no heterogeneity was observed (I2=0%, P=0.89).
Figure S1.
Forest plot showing effect of exercise training on systolic pulmonary artery pressure at rest (PASPrest) on pooled analysis of all included studies.
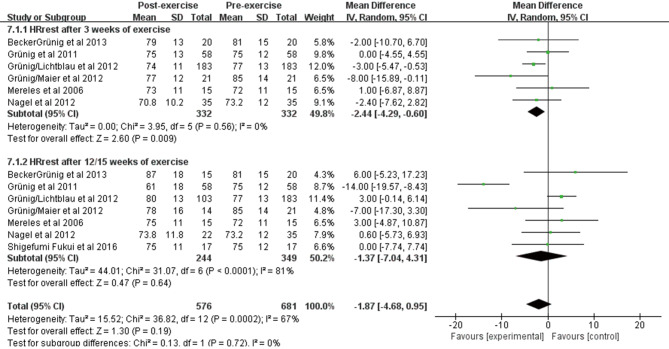
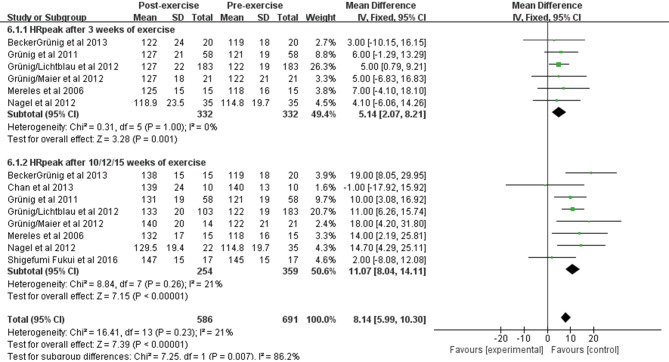
The effect of exercise training on HRrest and HRpeak
Seven studies included 349 patients who observed changes in resting heart rate and peak exercise heart rate before and after exercise training. Pooling across these studies showed that exercise training was associated with a significant improvement in HRrest after 3 weeks [WMD: −2.44 beats/min (95% CI: −4.29–−0.60 beats/min, P=0.009); Figure S2]; however, no significant change was observed after 12/15 weeks [WMD: −1.37 beats/min (95% CI: −7.04–4.31 beats/min, P=0.64); Figure S2]. In terms of HRpeak, we observed an obvious improvement after 3 weeks of exercise training [WMD: 5.14 beats/min (95% CI: 2.07–8.21 beats/min, P=0.001); Figure S3] and a significant increase after 10/12/15 weeks [WMD: 11.07 beats/min (95% CI: 8.04–14.11 beats/min, P<0.001); Figure S3]. The heterogeneity among studies was small (I2=21%, P=0.26).
Figure S2.
Forest plot showing effect of exercise training on heart rate at rest (HRrest) on pooled analysis of all included studies.
Figure S3.
Forest plot showing effect of exercise training on peak heart rate (HRpeak) on pooled analysis of all included studies.
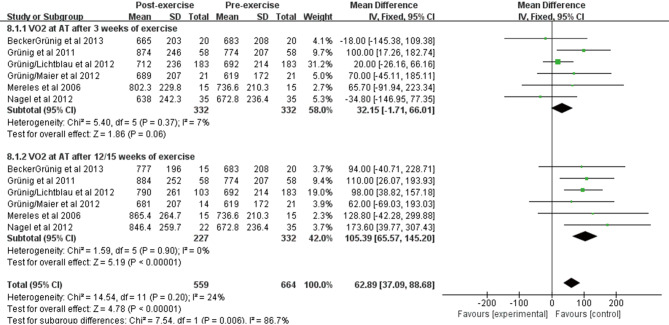
The effect of exercise training on VO2 at AT
VO2 at AT was reported in 6 studies of 332 patients at baseline and after exercise training. We observed a significant improvement in VO2 at AT using fixed effects analysis after exercise training for 3 weeks [WMD: 32.15 mL/min/kg (95% CI: −1.71–66.01 mL/min/kg, P=0.06); Figure S4], and the heterogeneity was small among studies (I2=7%, P=0.37). After exercise training for 12/15 weeks, a greater improvement was observed [WMD: 105.39 mL/min/kg (95% CI: 65.57–145.20 mL/min/kg, P<0.001); Figure S4], and no heterogeneity was observed among the studies (I2=0%, P=0.90).
Figure S4.
Forest plot showing effect of exercise training on oxygen uptake at the anaerobic threshold (VO2 at AT) on pooled analysis of all included studies.
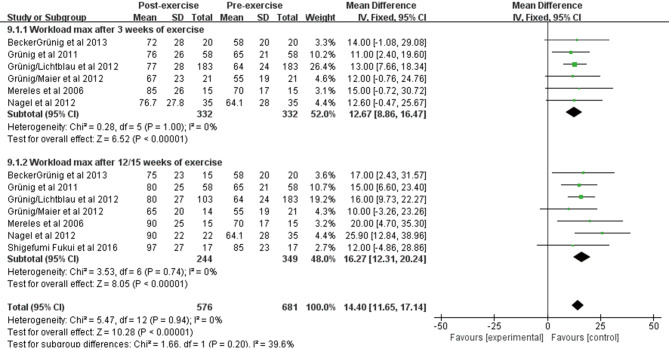
Effect of exercise training on Workloadmax
Workloadmax was reported in 7 studies of 349 patients at baseline and after exercise training. We observed a significant improvement in Workloadmax using fixed effects analysis after exercise training for 3 weeks [WMD: 12.67 weeks (95% CI: 8.86–16.47, P<0.001); Figure S5], and no heterogeneity was observed among studies (I2=0%, P=1.00). After exercise training for 12/15 weeks, a greater improvement was observed [WMD: 16.27 weeks (95% CI: 12.31–20.24, P<0.001); Figure S5], and no heterogeneity was observed (I2=0%, P=0.74).
Figure S5.
Forest plot showing effect of exercise training on maximal workload (workloadmax) on pooled analysis of all included studies.
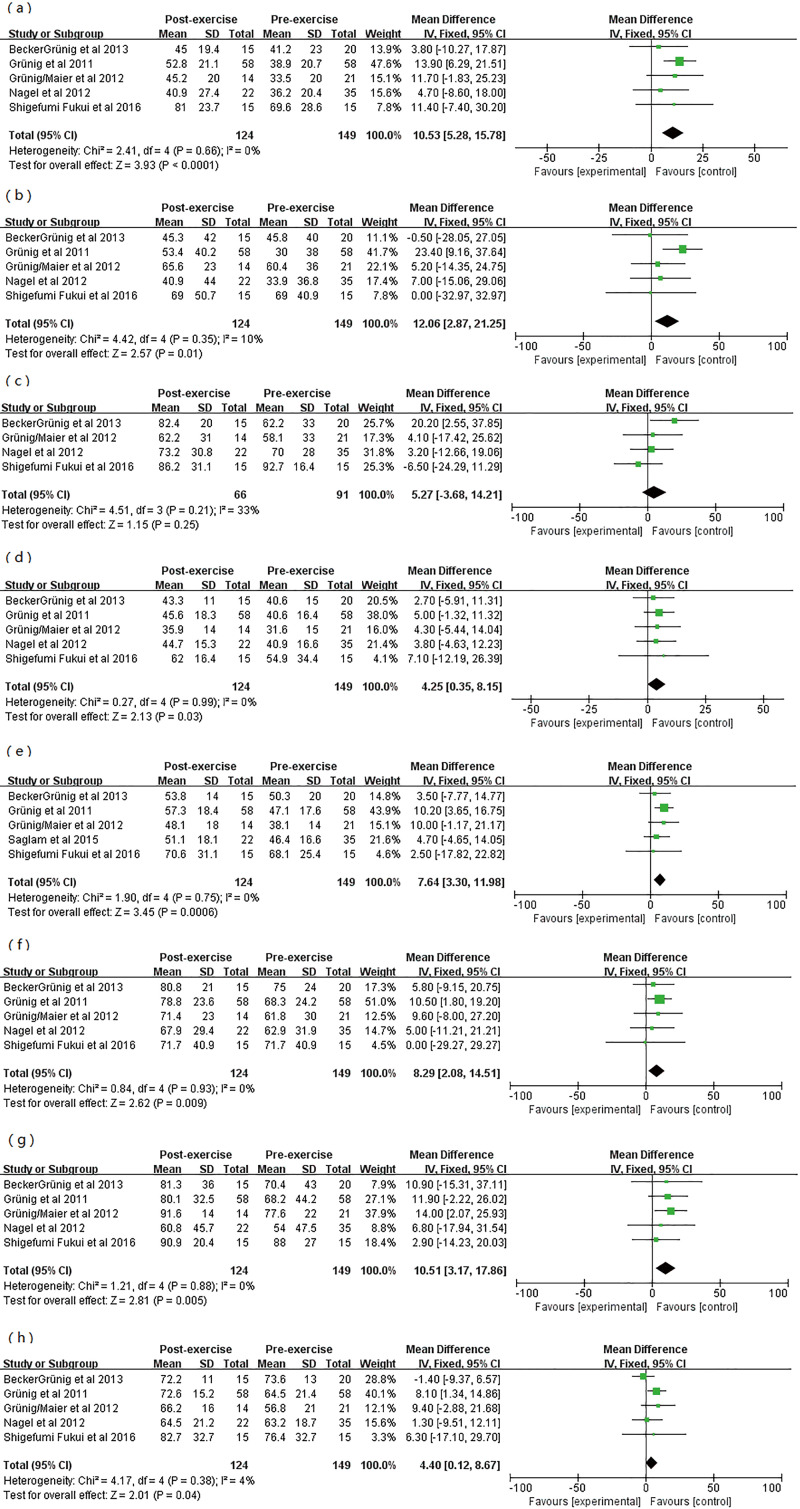
The effect of exercise training on QoL
QoL as measured by the SF-36 questionnaire was assessed in 5 studies of 149 patients, and 124 patients completed the SF-36 questionnaire at baseline and after exercise training. Pooled analysis across these five studies showed a significant improvement in quality of life measured by the SF-36 questionnaire subscale scores (Table S3, Figure S6).
Table S3. Pooled estimates for changes in quality of life subscale scores with exercise training among participants with pulmonary hypertension.
| SF-36 subscale | Studies (n) | WMD (95% CI) (points) | P value |
|---|---|---|---|
| Physical functioning | 5 | 10.53 (5.28–15.78) | <0.0001 |
| Role-physical | 5 | 12.06 (2.87–21.25) | 0.01 |
| Bodily pain | 4 | 5.27 (−3.68–14.21) | 0.25 |
| General health perception | 5 | 4.25 (0.35–8.15) | 0.03 |
| Vitality | 5 | 7.64 (3.30–11.98) | 0.0006 |
| Social functioning | 5 | 8.29 (2.08–14.51) | 0.009 |
| Role-emotional | 5 | 2.90 (−14.23–20.03) | 0.005 |
| Mental health | 5 | 6.30 (−17.10–29.70) | 0.04 |
Figure S6.
Forest plot showing effect of exercise training on quality of life (QoL) in pre-post studies. (A) Physical functioning score; (B) role physical score; (C) physical pain score; (D) general health score; (E) energy score; (F) social function score; (G) emotional function score; (H) mental health score.
Safety of exercise training
A total of 490 patients in 17 studies participated in exercise training, and 17 patients had adverse events (Table S4). The incidence of adverse events was 3.46%, including 2 cases of syncope, 1 case of presyncope, 7 cases of dizziness (1 case of hypoglycaemia), 3 cases of supraventricular tachycardia, 3 cases of cyanosis and 1 case of herpes zoster. Furthermore, no major adverse events, such as progression of symptoms, right heart failure, or death, were reported among the participants during the exercise training period.
Table S4. Adverse events related to exercise training.
| Author | Year | Number of exercise training participants | Adverse events related to exercise training |
|---|---|---|---|
| Mereles et al. | 2006 | 15 | Dizziness with training in 2 patients; oxygen saturation dropped in 1 patient |
| Ley et al. | 2013 | 10 | None |
| Chan et al. | 2013 | 10 | None |
| Ehlken | 2015 | 38 | Unrecorded |
| Saglam et al. | 2015 | 14 | None |
| González-Saiz et al. | 2017 | 20 | Atrioventricular nodal reentrant tachycardia during post-intervention CET in 1 patient; dizzZiness during aerobic training in 1 patient (hypoglycemia) |
| Shigefumi Fukui et al. | 2016 | 17 | Unrecorded |
| Martínez-Quintana et al. | 2010 | 4 | Exercise intolerance with cyanosis in 2 patients |
| Fox et al. | 2011 | 11 | None |
| Grünig/Maier et al. | 2012 | 21 | None |
| Grünig /Lichtblau et al. | 2012 | 183 | Syncope after training in 1 patient; presyncope after training in 1 patient; self-limiting SVT in 2 patients during exercise |
| Nagel et al. | 2012 | 35 | Syncope during exercise in 1 patient; herpes zoster in 1 patient |
| Becker-Grünig et al. | 2013 | 20 | None |
| Kabitz et al. | 2014 | 7 | None |
| Grünig et al. | 2011 | 58 | Dizziness with training in 2 patients |
| Inagaki et al. | 2014 | 8 | None |
| de Man et al. | 2009 | 19 | Dizziness during the quadriceps exercise in 2 patients |
Publication bias
The publication bias was evaluated for the two primary outcome indicators of the 6MWD and PeakVO2. The funnel chart is shown in Figure S7 and Figure S8, and no obvious publication bias was observed according to Egger’s test (P>0.05).
Figure S7.
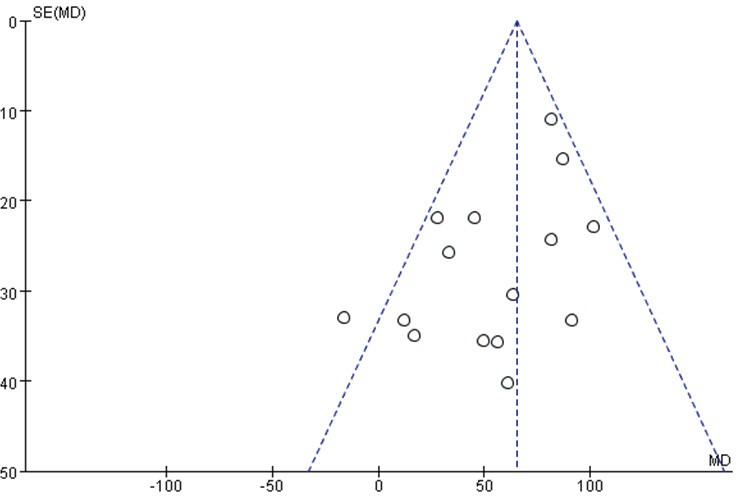
Funnel plot of 6MWD.
Figure S8.
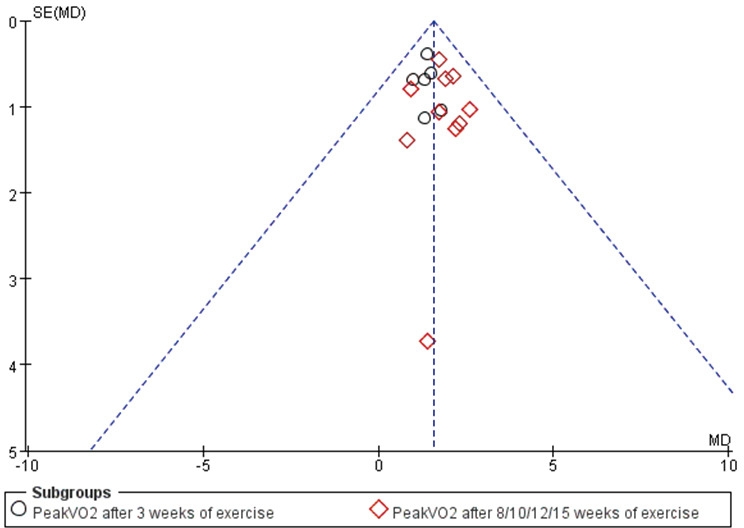
Funnel plot of PeakVO2.
Discussion
This meta-analysis included 17 studies from 2006 to 2017, including 651 patients with chronic PH, and 490 patients participated in exercise training. The results suggest that exercise training can significantly improve exercise capacity and cardiopulmonary function from baseline to follow-up. The main improvements included 6MWD (increased 64.75 m), peakVO2 (increased 1.78 mL/min/kg) and QoL measured by the SF-36 questionnaire. Other exercise capacity and cardiopulmonary function indicators, such as workloadmax, VO2 at AT and HRpeak, improved to different degrees. Moreover, exercise training is well tolerated in these patients with PH, with a low incidence of adverse events and no serious adverse consequences.
In the past, exercise training and cardiopulmonary rehabilitation therapy have been considered unsafe in patients with PH, but with the successful practical experience of rehabilitation in patients with heart failure, case series such as Mainguy et al. (24), Shoemaker et al. (25), and retrospective studies such as Uchi et al. (26) began to report the effectiveness and safety of exercise training in patients with chronic PH. In 2006, Mereles et al. (8) published a RCT of exercise training in patients with PH. The results showed that 6MWD in the exercise group increased 96±61 m after 15 weeks of exercise, which was 111 m higher than the control group, along with an improvement in the QoL. On account of the research findings in Mereles et al. and several pre-post intervention studies (13-15,17), the 2015 ESC/ERS guidelines for the diagnosis and treatment of PH proposed that monitored exercise training could be used as an adjuvant therapy for PAH patients with no relief after receiving optimal targeted pharmacotherapy (Class of Recommendation IIa, Level of Evidence B) (27).
In 2015 to 2017, 4 meta-analyses have concluded the efficacy and safety of exercise training in PH patients, and the results showed a remarkable improvement in 6MWD (57.7–72.5 m) and PeakVO2 (1.69–2.4 mL/min/kg) (28-31).
This meta-analysis, combined with several new studies from 2015 to 2017 which are not included in the above meta-analyses, further demonstrated the effectiveness and safety of exercise training as an adjunctive therapy for PH.
Effects of exercise training in PH
Effects on exercise capacity
Effects of exercise training in PH patients on exercise capacity have been verified by the previous 4 meta-analyses and this meta-analysis, including an improvement in 6MWD, peakVO2, and workloadmax.
The six-minute walk test is a submaximal exercise test used to evaluate the exercise capacity of PH patients, but it is more convenient than the cardiopulmonary exercise test (CPET) to evaluate the cardiopulmonary function of PH patients. Furthermore, Miyamoto et al. suggested that 6MWD has a strong, independent association with mortality (32). Therefore, in the clinical trials of PH, the improvement in 6MWD was considered an important outcome indicator.
In the pooled analysis of all types of studies, the 6MWD increased 64.75 m after exercise training. This distance is greater than the minimum clinically significant difference of 25–33 m (33). In terms of the PH specific pharmacotherapies, Channick et al. reported that the 6MWD increased 70 m after bosentan treatment for 12 weeks in patients with PH (34). Galie et al. reported that 6MWD increased 50 m in PAH patients after 12 weeks of treatment with sildenafil 80 mg/day (35). These findings suggest that exercise training may result in an improvement at least as great as that acquired from targeted pharmacotherapies. However, all the participants in exercise training in the included trials were receiving an optimal targeted pharmacotherapy on the premise of stable condition, so exercise training can be considered an effective adjunctive treatment for patients with stable PAH.
Most of the studies included in this meta-analysis showed that the 6MWD increased after exercise training in PH patients, except for three studies, Martínez-Quintana et al. (20), de Man et al. (21) and Fukui et al. (22). This is likely because Martínez-Quintana et al. focused on cycling and lower limb strength training and did not include walking and respiratory muscle exercise (20). Fukui et al. did not include upper limb muscle training or respiratory training, it focuses on the effects of exercise training on a single training component (lower limbs) rather than a mixture of different training components (22). However, improvements in PeakVO2, workloadmax, quadriceps strength and heart failure symptoms (22), and WHO functional class (20,22), were observed in these three studies suggesting lower strength training may be beneficial.
In addition to 6MWD, peakVO2 and workloadmax have a remarkable improvement after exercise training in the meta-analysis, and be similar to 6MWD, peakVO2 measured during a CPET also relates to survival in PH (36).
Effects on QoL
Another important outcome indicator is the QoL, which is considered to be one of the predictors of PAH prognosis (37). A study has shown that the decline of QoL in PAH patients is related to a decrease in exercise capacity, symptoms of cardiopulmonary failure, depression and anxiety. PeakVO2, 6MWD, anxiety, age, long-term oxygen therapy and right heart failure, which are all independent influencing factors of QoL. However, there was no significant correlation between the hemodynamic parameters and QoL score in resting hemodynamics (38). Five studies included in this meta-analysis analyzed the impact of exercise training on the quality of life in PH patients. The results showed that except for the physical pain score, the other seven dimensions of SF-36 improved in varying degrees.
Mechanisms of the improvements
Animal studies
The mechanism of improvement in exercise capacity, cardiopulmonary fitness and quality of life after exercise training in PH patients remains unclear. In a rat model induced by hypoxia, the study found that exercise training can prevent vascular remodeling caused by hypoxia and improve exercise capacity and hemodynamics in rats. Moreover, this study indicated that regular exercise training exerts an inhibiting effect on smooth muscle cell proliferation to a similar extent as pharmacological treatment. Although the signaling pathways underlying exercise-induced effects in hypoxic mice are unknown, regular exercise training did not affect the NO-sGC-PDE axis as targeted drugs, such as sildenafil (39). Therefore, this study suggests that exercise training may become a therapy for PAH in addition to targeting drugs through some mechanisms that have not yet been discovered. In another animal model, exercise training was conducted in PAH rats induced by monocrotaline. The results showed that exercise training could increase vascular density, decrease pulmonary artery diameter and right ventricular end-diastolic pressure (40).
Human studies
Change in skeletal muscle fiber type and increase in capillaries in muscle fiber
In human studies, Mainguy et al. assessed changes in skeletal muscle in IPAH patients after exercise training. This study suggested that although the skeletal muscle-type proportion is largely genetically determined, exercise training induced fiber-type shifting from type IIx to IIa and tended to increase the type I fiber surface. This less fatigable muscle profile may have resulted in a higher anaerobic threshold (24). de Man et al. also suggested that exercise training can increase the number of capillaries in skeletal muscle fibers, especially the capillaries of type I skeletal muscle fibers, and enhance oxidative enzyme activity, thereby increasing quadriceps strength and quadriceps endurance (21).
Improvement in peak oxygen consumption
Peak oxygen consumption is the highest amount of oxygen consumed by an individual undergoing CPET and is the best index of aerobic capacity and the gold standard for cardiorespiratory fitness (41). Many studies of exercise training have shown the improvement of peak oxygen consumption (8,12-14,16,19,22,42), and is possibly due to improvement of capillary density of skeletal muscle (19). Additionally, PeakVO2 is linearly associated with RV function (43).
Improvement in cardiac function
Right ventricular (RV) dysfunction is a crucial factor contributing to functional impairment and mortality, and the improvement of CI, CO, peakVO2/kg during exercise might improve RV function (19). Moreover, in this meta-analysis, HRpeak increase 11.07 beats/min. Taking in conjunction with improved PeakVO2, this suggests an improvement in cardiac function after exercise training (44).
Improvement in haemodynamics
Ley et al. assessed the pulmonary perfusion of PH patients after exercise training by magnetic resonance (MR). The results showed that the peak pulmonary flow velocity and perfusion of PH patients in the exercise group increased significantly after 3 weeks of exercise training (45). Several studies included in this meta-analysis have found that PASPrest of PH patients decreased after exercise training (8,14,15). In the pooled analysis of our meta-analysis, we also found an improvement in PASPrest from baseline to follow up. It is inferred that exercise training can reduce pulmonary vascular resistance, increase pulmonary circulation perfusion, and improve cardiopulmonary function.
Training modality
Exercise training intervention for PH patients consist of diversified training components, as shown in Table S2. In general, exercise training contains three kinds of exercises, resistance training, aerobic training and respiratory muscle training. The resistance training mainly consisted of dumbbell training of distinct muscle groups, such as dumbbell training with low weights (500 to 1,000 g). Aerobic training mainly consisted of ergometer training and treadmill walking. Training intensity of aerobic training was adjusted daily to the individual strengths and limitations, such as physical exertion, peak heart rate and oxygen saturation. The training intensity was low, and corresponding to 60% to 80% of the heart rate they had reached during peak oxygen uptake in the initial exercise test. Respiratory training was included in the training programme in 11 studies of this meta-analysis. Among these studies, Saglam et al. is a study only use inspiratory muscle training without resistance and aerobic training (46).
At present, there is no definite conclusion about the specific program of exercise training, such as frequency, exercise time, duration and intensity. In addition, the difference between interval and continuous exercise training in PH patients is not clear yet. The most commonly used exercise program is Mereles et al.’s exercise prescription (8). Studies using the exercise prescription similar to Mereles et al. showed that 6MWD was significantly improved (13-17,45,47). It may be related to the fact that the program includes aerobic training, resistance training, respiratory muscle training, and intensive training in the hospital for 3 weeks, which provides close supervision for exercise patterns, exercise intensity, and the correct technique for respiratory muscle training, followed by 12 weeks home-based exercise training.
In terms of outpatient training programme, only Fukui et al. and Inagaki et al. performed exercise training at home (22,23), whether home-based exercise training is safe and effective requires more large-scale clinical trials to confirm.
Safety
In terms of safety, the total incidence of adverse events in the included studies was only 3.46%, and there were no severe adverse events such as right heart failure, worsening of PH, and death during the exercise training. We observed a high degree of tolerance to exercise training in patients with PH. Grunig et al. reported that the 1- and 2-year survival rates of PH patients with exercise training were 100% and 95%, respectively, suggesting that exercise training under supervision is safe (13). Becker-Grunig et al. followed up patients for 21±14 months and found that the 1- and 2-year survival rates were 100%, while the 1- and 2-year survival rates without transplantation were 100% and 93%, respectively (17). Martínez-Quintana et al. followed up patients for 12 months, and no serious adverse events occurred. However, this is a small sample size study with only 4 participants (20). Other studies included in this meta-analysis only conducted 3 to 15 weeks of exercise training, so the long-term safety of exercise training remains unclear. More large clinical trials are needed to further confirm the long-term benefit in PH patients.
Exercise training and rehabilitation in China
In China, research on exercise training for PH patients is still lacking, and the exercise training guidance for PH patients is far from sufficient. The studies included in this meta-analysis mostly use bicycle ergometers or treadmills for exercise training, but it is difficult to perform exercise training under the supervision of doctors because bicycle ergometers are not widespread in China. To explore the appropriate exercise training program in China, Shimei et al. studied the exercise training methods of PAH patients. Twenty-six patients with PAH were randomized into two groups: one group took slow walking as the main exercise training method, and the other took fast walking as the main exercise training method. After the experiment, the fast walking group demonstrated an increase in the 6MWD (25±18 m) and suggested that fast walking exercise is better for Chinese patients with PH (48). The exercise training of PAH patients still needs more exploration in China.
Limitations
The studies on the effectiveness and safety of exercise training in PH patients are mostly small, single center studies, and there is a lack of large multicenter randomized controlled studies. The application of meta-analysis avoids the limitations of single small sample clinical trials. In this paper, 17 studies from 2006 to 2017 were systematically evaluated and statistically analyzed through a comprehensive search of multiple databases, providing evidence-based comprehensive treatment for PH patients. However, there are some limitations in this study. First, heterogeneity exists because of the different exercise training protocols and different populations among studies. Second, most of the included studies had a relatively short duration and follow-up, and had not evaluated clinical end-points, such as hospitalization events and mortality. Therefore, it is unable to assess the continuous impact of exercise training on these clinical endpoints. Third, most of the included studies are single-center and small sample trials. Finally, as with other meta-analyses, selective bias cannot be completely eliminated because articles can only be retrieved from published trials.
Conclusions
Exercise training is safe for patients with PH and can improve their exercise capacity and quality of life. However, more large-scale and multicenter studies are needed to further verify the long-term effectiveness and safety of exercise training and to evaluate the clinical endpoints, such as mortality and hospitalization, to provide evidence for the application of exercise training in the real world of patients with PH.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by Guangzhou Municipal Science and Technology Bureau (No. 201604020185).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.69). The authors have no conflicts of interest to declare.
References
- 1.McGoon MD, Benza RL, Escribano-Subias P, et al. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol 2013;62:D51-9. 10.1016/j.jacc.2013.10.023 [DOI] [PubMed] [Google Scholar]
- 2.Rich S, Dantzker DR, Ayres SM, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med 1987;107:216-23. 10.7326/0003-4819-107-2-216 [DOI] [PubMed] [Google Scholar]
- 3.Jing ZC, Xu XQ, Han ZY, et al. Registry and survival study in chinese patients with idiopathic and familial pulmonary arterial hypertension. Chest 2007;132:373-9. 10.1378/chest.06-2913 [DOI] [PubMed] [Google Scholar]
- 4.Lan NSH, Massam BD, Kulkarni SS, et al. Pulmonary Arterial Hypertension: Pathophysiology and Treatment. Disease 2018;6:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryerson CJ, Nayar S, Swiston JR, et al. Pharmacotherapy in pulmonary arterial hypertension: a systematic review and meta-analysis. Respir Res 2010;11:12. 10.1186/1465-9921-11-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sahni S, Ojrzanowski M, Majewski S, et al. Pulmonary arterial hypertension: a current review of pharmacological management. Pneumonol Alergol Pol 2016;84:47-61. 10.5603/PiAP.a2015.0084 [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin VV. Looking to the future: a new decade of pulmonary arterial hypertension therapy. Eur Respir Rev 2011;20:262-9. 10.1183/09059180.00006411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mereles D, Ehlken N, Kreuscher S, et al. Exercise and respiratory training improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension. Circulation 2006;114:1482-9. 10.1161/CIRCULATIONAHA.106.618397 [DOI] [PubMed] [Google Scholar]
- 9.Matura LA, McDonough A, Carroll DL. Cluster analysis of symptoms in pulmonary arterial hypertension: a pilot study. Eur J Cardiovasc Nurs 2012;11:51-61. 10.1177/1474515111429649 [DOI] [PubMed] [Google Scholar]
- 10.Davies EJ, Moxham T, Rees K, et al. Exercise training for systolic heart failure: Cochrane systematic review and meta-analysis. Eur J Heart Fail 2010;12:706-15. 10.1093/eurjhf/hfq056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beauchamp MK, Nonoyama M, Goldstein RS, et al. Interval versus continuous training in individuals with chronic obstructive pulmonary disease--a systematic review. Thorax 2010;65:157-64. 10.1136/thx.2009.123000 [DOI] [PubMed] [Google Scholar]
- 12.Fox BD, Kassirer M, Weiss I, et al. Ambulatory rehabilitation improves exercise capacity in patients with pulmonary hypertension. J Card Fail 2011;17:196-200. 10.1016/j.cardfail.2010.10.004 [DOI] [PubMed] [Google Scholar]
- 13.Grunig E, Ehlken N, Ghofrani A, et al. Effect of exercise and respiratory training on clinical progression and survival in patients with severe chronic pulmonary hypertension. Respiration 2011;81:394-401. 10.1159/000322475 [DOI] [PubMed] [Google Scholar]
- 14.Grunig E, Lichtblau M, Ehlken N, et al. Safety and efficacy of exercise training in various forms of pulmonary hypertension. Eur Respir J 2012;40:84-92. 10.1183/09031936.00123711 [DOI] [PubMed] [Google Scholar]
- 15.Grunig E, Maier F, Ehlken N, et al. Exercise training in pulmonary arterial hypertension associated with connective tissue diseases. Arthritis Res Ther 2012;14:R148. 10.1186/ar3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagel C, Prange F, Guth S, et al. Exercise training improves exercise capacity and quality of life in patients with inoperable or residual chronic thromboembolic pulmonary hypertension. PLoS One 2012;7:e41603. 10.1371/journal.pone.0041603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker-Grunig T, Klose H, Ehlken N, et al. Efficacy of exercise training in pulmonary arterial hypertension associated with congenital heart disease. Int J Cardiol 2013;168:375-81. 10.1016/j.ijcard.2012.09.036 [DOI] [PubMed] [Google Scholar]
- 18.Leighton Chan M, MPH., Lisa M. K. Chin P, Michelle Kennedy M, et al. Benefits of Intensive Treadmill Exercise Training on Cardiorespiratory Function and Quality of Life in Patients With Pulmonary Hypertension. Chest 2013;143:324-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehlken N, Lichtblau M, Klose H, et al. Exercise training improves peak oxygen consumption and haemodynamics in patients with severe pulmonary arterial hypertension and inoperable chronic thrombo-embolic pulmonary hypertension: a prospective, randomized, controlled trial. Eur Heart J 2016;37:35-44. 10.1093/eurheartj/ehv337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martínez-Quintana E, Miranda-Calderin G, Ugarte-Lopetegui A, et al. Rehabilitation program in adult congenital heart disease patients with pulmonary hypertension. Congenit Heart Dis 2010;5:44-50. 10.1111/j.1747-0803.2009.00370.x [DOI] [PubMed] [Google Scholar]
- 21.de Man FS, Handoko ML, Groepenhoff H, et al. Effects of exercise training in patients with idiopathic pulmonary arterial hypertension. Eur Respir J 2009;34:669-75. 10.1183/09031936.00027909 [DOI] [PubMed] [Google Scholar]
- 22.Fukui S, Ogo T, Takaki H, et al. Efficacy of cardiac rehabilitation after balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Heart 2016;102:1403-9. 10.1136/heartjnl-2015-309230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inagaki T, Terada J, Tanabe N, et al. Home-based pulmonary rehabilitation in patients with inoperable or residual chronic thromboembolic pulmonary hypertension: a preliminary study. Respir Investig 2014;52:357-64. 10.1016/j.resinv.2014.07.002 [DOI] [PubMed] [Google Scholar]
- 24.Mainguy V, Maltais F, Saey D, et al. Effects of a rehabilitation program on skeletal muscle function in idiopathic pulmonary arterial hypertension. J Cardiopulm Rehabil Prev 2010;30:319-23. 10.1097/HCR.0b013e3181d6f962 [DOI] [PubMed] [Google Scholar]
- 25.Shoemaker MJ, Wilt JL, Dasgupta R, et al. Exercise training in patients with pulmonary arterial hypertension: a case report. Cardiopulm Phys Ther J 2009;20:12-8. 10.1097/01823246-200920040-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uchi M, Saji T, Harada T. Feasibility of cardiopulmonary rehabilitation in patients with idiopathic pulmonary arterial hypertension treated with intravenous prostacyclin infusion therapy. J Cardiol 2005;46:183-93. [PubMed] [Google Scholar]
- 27.Galie N, Humbert M, Vachiery JL, et al. [2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension]. Kardiol Pol 2015;73:1127-206. 10.5603/KP.2015.0242 [DOI] [PubMed] [Google Scholar]
- 28.Buys R, Avila A, Cornelissen VA. Exercise training improves physical fitness in patients with pulmonary arterial hypertension: a systematic review and meta-analysis of controlled trials. BMC Pulm Med 2015;15:40. 10.1186/s12890-015-0031-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pandey A, Garg S, Khunger M, et al. Efficacy and Safety of Exercise Training in Chronic Pulmonary Hypertension: Systematic Review and Meta-Analysis. Circ Heart Fail 2015;8:1032-43. 10.1161/CIRCHEARTFAILURE.115.002130 [DOI] [PubMed] [Google Scholar]
- 30.Yuan P, Yuan XT, Sun XY, et al. Exercise training for pulmonary hypertension: a systematic review and meta-analysis. Int J Cardiol 2015;178:142-6. 10.1016/j.ijcard.2014.10.161 [DOI] [PubMed] [Google Scholar]
- 31.Morris NR, Kermeen FD, Holland AE. Exercise-based rehabilitation programmes for pulmonary hypertension. Cochrane Database Syst Rev 2017;1:CD011285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med 2000;161:487-92. 10.1164/ajrccm.161.2.9906015 [DOI] [PubMed] [Google Scholar]
- 33.Singh SJ, Puhan MA, Andrianopoulos V, et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J 2014;44:1447-78. 10.1183/09031936.00150414 [DOI] [PubMed] [Google Scholar]
- 34.Channick RN, Simonneau G, Sitbon O, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet 2001;358:1119-23. 10.1016/S0140-6736(01)06250-X [DOI] [PubMed] [Google Scholar]
- 35.Galie N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2005;353:2148-57. 10.1056/NEJMoa050010 [DOI] [PubMed] [Google Scholar]
- 36.Groepenhoff H, Vonk-Noordegraaf A, Boonstra A, et al. Exercise testing to estimate survival in pulmonary hypertension. Med Sci Sports Exerc 2008;40:1725-32. 10.1249/MSS.0b013e31817c92c0 [DOI] [PubMed] [Google Scholar]
- 37.Pepke-Zaba J, Beardsworth A, Chan M, et al. Tadalafil therapy and health-related quality of life in pulmonary arterial hypertension. Curr Med Res Opin 2009;25:2479-85. 10.1185/03007990903210066 [DOI] [PubMed] [Google Scholar]
- 38.Halank M, Einsle F, Lehman S, et al. Exercise capacity affects quality of life in patients with pulmonary hypertension. Lung 2013;191:337-43. 10.1007/s00408-013-9472-6 [DOI] [PubMed] [Google Scholar]
- 39.Weissmann N, Peters DM, Klopping C, et al. Structural and functional prevention of hypoxia-induced pulmonary hypertension by individualized exercise training in mice. Am J Physiol Lung Cell Mol Physiol 2014;306:L986-95. 10.1152/ajplung.00275.2013 [DOI] [PubMed] [Google Scholar]
- 40.Colombo R, Siqueira R, Becker CU, et al. Effects of exercise on monocrotaline-induced changes in right heart function and pulmonary artery remodeling in rats. Can J Physiol Pharmacol 2013;91:38-44. 10.1139/cjpp-2012-0261 [DOI] [PubMed] [Google Scholar]
- 41.American Thoracic S, American College of Chest P. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003;167:211-77. 10.1164/rccm.167.2.211 [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez-Saiz L, Fiuza-Luces C, Sanchis-Gomar F, et al. Benefits of skeletal-muscle exercise training in pulmonary arterial hypertension: The WHOLEi+12 trial. Int J Cardiol 2017;231:277-83. 10.1016/j.ijcard.2016.12.026 [DOI] [PubMed] [Google Scholar]
- 43.Lewis GD, Bossone E, Naeije R, et al. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation 2013;128:1470-9. 10.1161/CIRCULATIONAHA.112.000667 [DOI] [PubMed] [Google Scholar]
- 44.Chia KS, Wong PK, Faux SG, et al. The benefit of exercise training in pulmonary hypertension: a clinical review. Intern Med J 2017;47:361-9. 10.1111/imj.13159 [DOI] [PubMed] [Google Scholar]
- 45.Ley S, Fink C, Risse F, et al. Magnetic resonance imaging to assess the effect of exercise training on pulmonary perfusion and blood flow in patients with pulmonary hypertension. Eur Radiol 2013;23:324-31. 10.1007/s00330-012-2606-z [DOI] [PubMed] [Google Scholar]
- 46.Saglam M, Arikan H, Vardar-Yagli N, et al. Inspiratory muscle training in pulmonary arterial hypertension. J Cardiopulm Rehabil Prev 2015;35:198-206. 10.1097/HCR.0000000000000117 [DOI] [PubMed] [Google Scholar]
- 47.Kabitz HJ, Bremer HC, Schwoerer A, et al. The combination of exercise and respiratory training improves respiratory muscle function in pulmonary hypertension. Lung 2014;192:321-8. 10.1007/s00408-013-9542-9 [DOI] [PubMed] [Google Scholar]
- 48.Shimei Z, Yuanhua Y, Tuguang K, et al. Study of exercise training methods on patients with pulmonary hypertension. Int J Respi 2018;38:1250-5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as