Introduction
The role of the immune system in cancer development and progression has become an important focus for therapeutic development. Tumour evasion from immune destruction can occur through several mechanisms, including activation of endogenous immune checkpoint pathways to suppress anti-tumour immunity. The development of immune checkpoint inhibitors (ICIs), targeting the co-inhibitory receptor programmed death-1 (PD-1) and its ligand PD-L1, has led to improved survival and quality of life in patients with advanced non-small cell lung cancer (NSCLC) with a more favourable toxicity profile than chemotherapy (1,2).
Immune-related adverse events (irAEs) can be related to the direct effects of lymphocyte activation against self-antigens in normal tissue as well as indirect effects of disrupting immune tolerance. Tumour-associated PD-L1 facilitates apoptosis of activated T-cells, stimulates IL-10 production to mediate immune suppression, and induces T-cell dysfunction through various mechanisms. Therefore, by blocking the PD-1/PD-L1 interaction, ICIs counteract the negative regulatory effect of immune checkpoint proteins and restore anti-tumour immunity. However, the resulting disruption in immune homeostasis also promotes T-cell activation in normal tissue where cells express self-antigens (3,4). As a result, ICIs are associated with a unique set of side effects termed irAEs. The toxicity profile of ICIs differs from previous therapeutic agents, such as chemotherapy or kinase inhibitors, in lung cancer and requires careful evaluation and management in patients receiving therapy.
The development of irAEs appears to be associated with better outcomes in several trials of ICIs in various cancers (5-8). In this report, we review the association between irAEs and NSCLC patient outcomes from therapy.
Methods
This project was approved by the University Health Network Research Ethics Board (15-9246-CE). Stage IV NSCLC patients treated with ICIs at the Princess Margaret Cancer Centre between May 2013 to August 2016 were prospectively evaluated, and data captured include demographics, tumour and treatment characteristics, treatment response, duration, survival, and adverse events. The relationship between treatment outcomes (response, duration, and survival) and occurrence of irAEs was examined. Toxicities were graded using the Common Terminology Criteria for Adverse Events version 4.0. Events were deemed immune-mediated based on investigator assessment (9).
Treatment response was assessed using the Response Evaluation Criteria in Solid Tumours (RECIST 1.1) at week 8 and beyond to include delayed responses (best overall treatment response) (10). Treatment duration was defined as the time from the first dose of checkpoint inhibitor therapy until the end of the last treatment cycle. Survival was defined as the time from the first dose until death.
Statistics
Association of categorical variables was tested by Chi-square or Fisher’s exact test. The Kaplan-Meier method was used to estimate the probability of overall survival and log-rank test was used to investigate significance between groups. Statistical significance was chosen at a two-sided P value of <0.05. SAS version 9.3 and R version 3.1.3 were used for statistical analysis.
Results
Patient characteristics and response
Ninety-seven patients with advanced NSCLC received ICIs during the study period. Most, 81%, received anti-PD-1 agents, 17% received anti-PD-L1 agents and 2% received combination anti-PD-L1 plus anti-CTLA-4 (cytotoxic T-lymphocyte associated antigen 4) therapy. Tumour PD-L1 expression by immunohistochemistry was confirmed as positive (any staining) in 35 (36%) patients, negative in 11% and unknown in 53%. Patients had received a median of 2 lines of prior systemic therapy, including platinum doublet chemotherapy. Twenty-two percent of patients previously received maintenance pemetrexed, and 57% received prior radiotherapy. Median follow-up for the cohort was 5.1 months (0.3–38.1 months) from treatment start. Baseline characteristics were balanced between the groups. Additional demographic and tumour characteristics are shown in Table 1.
Table 1. Patient characteristics.
| Characteristic | All patients, N=97 (%) | No irAE, N=48 (%) | Grade 1/2 irAE, N=42 (%) | Grade ≥3 irAE, N=7 (%) | P value |
|---|---|---|---|---|---|
| Median age (range) | 63.8 (31.1–80.9) years | 63.0 (31.1–80.9) years | 63.8 (42.0–80.3) years | 72.1 (63.1–75.6) years | 0.190 |
| Sex | 1.000 | ||||
| Male | 48 [50] | 24 [50] | 21 [50] | 3 [43] | |
| Female | 49 [50] | 24 [50] | 21 [50] | 4 [57] | |
| Histology | 0.302 | ||||
| Adenocarcinoma | 74 [76] | 36 [75] | 34 [81] | 4 [57] | |
| Squamous cell carcinoma | 15 [16] | 9 [19] | 5 [12] | 1 [14] | |
| Large cell, other | 8 [8] | 3 [6] | 3 [7] | 2 [29] | |
| EGFR mutation | 0.339 | ||||
| Positive | 12 [12] | 8 [17] | 4 [10] | 0 | |
| Negative | 71 [73] | 31 [64] | 33 [78] | 7 [100] | |
| Unknown | 14 [15] | 9 [19] | 5 [12] | 0 | |
| ALK rearrangement | 0.141 | ||||
| Positive | 1 [1] | 1 [2] | 0 | 0 | |
| Negative | 79 [82] | 35 [73] | 37 [88] | 7 [100] | |
| Unknown | 17 [17] | 12 [25] | 5 [12] | 0 | |
| PD-L1 expression | 0.372 | ||||
| Positive (any) | 35 [36] | 13 [27] | 18 [43] | 4 [57] | |
| Negative | 11 [11] | 6 [13] | 5 [12] | 0 | |
| Unknown | 51 [53] | 29 [60] | 19 [45] | 3 [43] | |
| Smoking status | 0.936 | ||||
| Current | 11 [11] | 6 [13] | 4 [10] | 1 [14] | |
| Past | 62 [64] | 29 [60] | 28 [67] | 5 [72] | |
| Never | 24 [25] | 13 [27] | 10 [23] | 1 [14] | |
| Prior lines of therapy | 0.103 | ||||
| 0 | 13 [13] | 3 [6] | 6 [14] | 4 [58] | |
| 1 | 25 [26] | 14 [29] | 10 [24] | 1 [14] | |
| 2 | 26 [27] | 13 [27] | 12 [29] | 1 [14] | |
| 3 or more | 33 [34] | 18 [38] | 14 [33] | 1 [14] | |
| Therapy type | 0.49 | ||||
| Anti-PD-1 | 79 [81] | 40 [83] | 34 [81] | 5 [71] | |
| Anti-PD-L1 | 16 [17] | 6 [13] | 8 [19] | 2 [29] | |
| Anti-PD-L1 & anti-CTLA-4 | 2 [2] | 2 [4] | 0 | 0 |
irAE, immune-related adverse event; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; PD-L1, programmed death-ligand 1; CTLA-4, cytotoxic T-lymphocyte associated antigen 4.
The overall response rate to checkpoint inhibitor therapy was 23% (22/97). Most responses (73% or 16/22) occurred within 8 weeks of starting therapy. One patient’s response was not evaluable as follow-up imaging was not performed after the patient’s single dose of therapy.
irAEs
IrAEs of any grade occurred in half of patients (49/97, 51%), with grade ≥3 irAEs occurring in 7%. One patient had grade 4 pneumonitis and none experienced grade 5 toxicity. Frequency and median time of onset of irAEs are shown in Table 2, with the majority occurring before the 8-week response assessment. The most commonly observed irAEs were arthralgia (13%), diarrhea/colitis (12%), and skin rash (11%). Infusion reactions and pyrexia were the earliest irAEs to occur during treatment, both with median onset of less than 2 weeks after just one dose of therapy. In contrast, hypothyroidism and pneumonitis were diagnosed a median of 12.0 and 16.9 weeks after treatment start, respectively. Discontinuation of treatment due to irAEs occurred in 10% of patients, half of whom experienced grade ≥3 irAEs.
Table 2. Frequency of immune-related adverse events and time of onset.
| Immune-related adverse event | Any grade, N (%) | Median onset, weeks (range) | Grade 3-4, N (%) | Median onset, weeks (range) |
|---|---|---|---|---|
| Arthralgia | 13 (13.4) | 3.0 (1.4–34.8) | 1 (1.0) | 3.0 |
| Diarrhea/colitis | 12 (12.4) | 7.9 (3.0–76.0) | 1 (1.0) | 8.9 |
| Hepatotoxicity* | 7 (7.2) | 6.1 (2.7–11.9) | 2 (2.1) | 5.0 (3.7–6.3) |
| Hypersensitivity | 3 (3.1) | 8.1 (2.4–12.3) | 0 (0.0) | – |
| Hyperthyroidism | 4 (4.1) | 3.4 (1.6–5.9) | 0 (0.0) | – |
| Hypothyroidism | 9 (9.3) | 12 (5.9–15.9) | 0 (0.0) | – |
| Infusion reaction | 6 (6.2) | 1.9 (0–2.4) | 1 (1.0) | 2.0 |
| Nephritis | 1 (1.0) | 11.9 | 0 (0.0) | – |
| Pneumonitis | 5 (5.2) | 16.9 (0.6–72.3) | 1 (1.0) | 0.6 |
| Pruritis | 10 (10.3) | 6.4 (0–66.0) | 0 (0.0) | – |
| Pyrexia | 5 (5.2) | 1.9 (0.7–2.0) | 0 (0.0) | – |
| Skin rash** | 11 (11.3) | 5.7 (0–84.0) | 2 (2.1) | 61.8 (39.6–84.0) |
*, including transaminitis, hepatitis, hyperbilirubinemia; **, including psoriasis.
Patients with grade ≥3 irAEs were more likely to have response to treatment than those with no or low grade irAEs, 68% vs. 20%, P=0.023 (Table 3). Three patients that responded to therapy with grade ≥3 irAEs required temporary treatment discontinuation with successful re-initiation of therapy after steroid treatment and one patient was taken off treatment permanently. Among the three patients that re-initiated therapy, two recovered with high dose steroids. The third with grade 3 rash recovered after a short course of oral steroids. The fourth had ongoing immune-mediated arthralgias, elevated lipase, and severe pneumonitis with repeat hospital admissions. Although these resolved with high dose steroids, it was decided not to rechallenge as the patient continued to respond off therapy. Median time to onset of grade ≥3 irAEs for the patients with response was 7.6 weeks (range, 3–84 weeks). When exploring patients with any grade irAE versus none, there was no difference in response rates. Median duration of treatment was numerically longer in patients with grade ≥3 irAEs compared to patients with no or low grade irAEs, 4.5 vs. 2.5 months, P=0.39. In this single arm study, median survival was not reached in those with grade ≥3 irAEs, compared to 15.7 months in patients with grade 1/2 irAEs and 7.4 months in those with no irAE, P=0.16 (Figure 1). More than half of patients with grade ≥3 irAEs were still alive and median survival could not be evaluated. Smoking status was not associated with differences in response rate nor the frequency of irAEs.
Table 3. Relationship between immune-related adverse events and response to treatment.
| irAE | All patients, N (%) | PD + SD, N (%) | PR, N (%) | P value |
|---|---|---|---|---|
| None | 48 (49.5) | 37 (77.1) | 11 (22.9) | 1.000 |
| Any grade* | 48 (49.5) | 37 (77.1) | 11 (22.9) | |
| Grade | ||||
| <3 | 90 (92.8) | 72 (80.0) | 18 (20.0) | 0.023 |
| ≥3* | 6 (6.2) | 2 (33.3) | 4 (67.7) | |
*, response was non-evaluable in one patient and was excluded from response analysis. irAE, immune-related adverse event; PD, progressive disease; SD, stable disease; PR, partial response.
Figure 1.
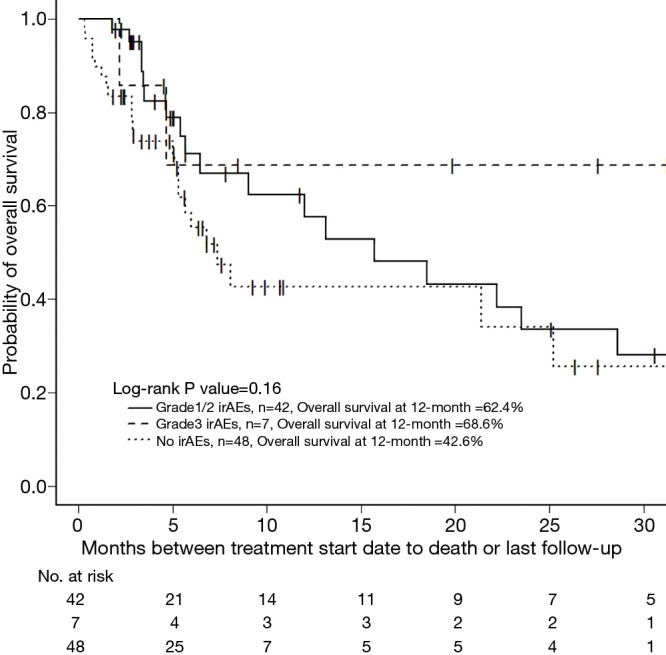
Overall survival of patients by immune-related adverse events (none, grade 1-2, or grade ≥3). One year survival was highest patients with grade ≥3 irAEs (68.6%), compared to those with grade1/2 irAEs (62.4%) or with no irAEs (42.6%). irAE, immune-related adverse event.
Discussion
Similar to the trend observed in recent studies of irAEs in various cancers (5-8), we demonstrated an association between the development of severe irAEs and response to treatment with ICIs in patients with advanced NSCLC. For most patients, this was not related to the duration of treatment exposure. In addition, patients with irAEs were found to have numerically longer survival in this non-comparative single arm study. Through modifying the balance of immune regulation, ICIs can reduce the degree of tumour-mediated immunosuppression and effectively restore and promote anti-tumour immune activity (11). However, achieving the optimal balance between self-tolerance and anti-tumour immune response represents one of the major challenges associated with the use of immunotherapeutic agents.
In studies of melanoma and renal cell cancer, the induction of irAEs was also found to be associated with improved survival or response in patients treated with ICIs (5,6,12). In two recent studies of patients with metastatic NSCLC treated with nivolumab, there was a positive correlation between treatment response and the development of irAEs (7), in particular skin irAEs (8). While PD-L1 status is a predictor of treatment response in advanced NSCLC patients receiving immunotherapy, not all patients with high PD-L1 tumour expression will benefit from therapy (13). Patients that develop early onset irAEs—particularly severe irAEs—during immunotherapy may represent a distinct group more prone to derive benefit. Thus, the development of early autoimmune manifestations due to reduced tolerance to self-antigens following treatment with ICIs may indicate heightened activation of the endogenous anti-tumour immune response and represent an important factor in evaluating potential benefit from therapy.
Limitations of the study include the small sample size and potential confounders including smoking status and duration of exposure to therapy. The sample size of our study is similar to those of several other recent investigations which have also suggested a positive correlation between tumour response and irAE (7,8). Response rates to PD-1 axis inhibitors have been shown to be higher among current or former smokers compared to never smokers (14). However, in our study, the frequency of irAEs was similar between these two groups. We believe the development of early onset irAEs, similar to clinical predictors such as smoking status, is associated with response. Another potential confounder is the association of clinical benefit with longer treatment exposure, which could also lead to an increase in irAEs. In our study, we selected an 8-week response assessment period to minimize this effect and most patients developed irAEs early on during treatment.
Furthermore, multiple studies have described patients who discontinued treatment early for irAEs yet maintained sustained responses (15-17). The mechanism for irAEs in association with response remains unclear. It is presumed that these are related to bystander effects from T cell activation. If patients are responding to therapy, they may be more likely to have irAEs as their immune system is more competent or if there is cross-reactivity between tumour and normal tissues, i.e., common antigens. Some have postulated a response-independent effect, where organs may have subclinical inflammation which progresses when PD-1 axis inhibition is introduced (17). Interestingly, a study of patients treated with anti-PD-1 agents that developed skin toxicity found that patients had shared T cell clones in both skin and tumour (18). The composition of the gut microbiome remains of interest, with an association with certain bacteria, e.g., Faecalibacterium, with tumour response and colitis (19). Other hypotheses include the potential to generate autoantibodies with checkpoint inhibition and the potential for T-cell “homing” to induce pituitary inflammation (20). Whether specific cytokines are important remains unclear, with a recent study suggesting that IL-6 may be upregulated in colitis biopsies and non-responding tumours, but not in responding tumours (21). Thus, the mechanism or mechanisms of this clinically important association requires further study.
ICIs have become the standard of care in the current treatment landscape for NSCLC. In this study, the occurrence of high grade irAEs was associated with better tumour response in advanced NSCLC patients treated with ICIs, suggesting a link between treatment efficacy and degree of immune activation. Further understanding of the role of irAEs and management in predicting anti-tumour immune activity may help guide response evaluation, dosing strategies including re-challenge and treatment monitoring in the clinical setting. These data add to the growing literature in this area, and will be strengthened by larger studies.
Supplementary
The article’s supplementary files as
Acknowledgments
Abstract previously presented at the 2018 International Association for the Study of Lung Cancer (IASLC) 19th World Conference on Lung Cancer (WCLC), Toronto, ON. September 23–26, 2018.
Funding: This study was supported by the Princess Margaret Cancer Foundation (NBL: OSI Pharmaceuticals Foundation Chair).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.04.30). AZ reports grants and personal fees from BMS, MSD, Roche, and AZ, outside the submitted work. LK reports she was previously (in the last 2 years) an employee of AstraZeneca plc. This was conducted independently from the submitted work. CL reports personal fees from Merck, Brystol-Myers Squibb, Astra Zeneca, and Pfizer, outside the submitted work. GL reports Honoraria from Merck, Pfizer, Novartis, Takeda, Bristol Myers Squibb, EMD Serano, Roche, Abbvie, AstraZeneca, Bayer. PB reports Honoraria from Abbvie, Lilly, Merck, Pfizer, advisory boards Abbvie, BI. NL reports institutional research funding outside submitted work from Roche, AZ, Array, Guardant; honoraria and/or travel for independent CME lectures outside submitted work from AZ, BMS, MSD, Roche, Pfizer; Advisor (compensated) for Excovery. The other authors have no conflicts of interest to declare.
References
- 1.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. 10.1016/S0140-6736(16)00587-0 [DOI] [PubMed] [Google Scholar]
- 2.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 3.Cadranel J, Canellas A, Matton L, et al. Pulmonary complications of immune checkpoint inhibitors in patients with nonsmall cell lung cancer. Eur Respir Rev 2019;28:190058. 10.1183/16000617.0058-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest 2015;125:3384-91. 10.1172/JCI80011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin Cancer Res 2016;22:886-94. 10.1158/1078-0432.CCR-15-1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang JC, Hughes M, Kammula U, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother 2007;30:825-30. 10.1097/CJI.0b013e318156e47e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connolly E, Mallesara G, Nordman I. 115P Immune related adverse events (irAE) and disease response with nivolumab in pre-treated advanced non-small cell lung cancer (NSCLC). Ann Oncol 2017;28: ii41-2. 10.1093/annonc/mdx091.035 [DOI] [Google Scholar]
- 8.Hasan Ali O, Diem S, Markert E, et al. Characterization of nivolumab-associated skin reactions in patients with metastatic non-small cell lung cancer. Oncoimmunology 2016;5:e1231292. 10.1080/2162402X.2016.1231292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Kane GM, Labbé C, Doherty MK, et al. Monitoring and Management of Immune-Related Adverse Events Associated With Programmed Cell Death Protein-1 Axis Inhibitors in Lung Cancer. Oncologist 2017;22:70-80. 10.1634/theoncologist.2016-0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 11.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lal A, Sahu K, Jindal V, et al. Role of immunotherapy in metastatic renal cell cancer: past, present and future. Ann Transl Med 2019;7:S349. 10.21037/atm.2019.09.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 2016;17:e542-e51. 10.1016/S1470-2045(16)30406-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the Treatment of Non-Small-Cell Lung Cancer. N Engl J Med 2015;372:2018-28. 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 15.Tachihara M, Negoro S, Inoue T, et al. Efficacy of anti-PD-1/PD-L1 antibodies after discontinuation due to adverse events in non-small cell lung cancer patients (HANSHIN 0316). BMC Cancer 2018;18:946. 10.1186/s12885-018-4819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iivanainen S, Koivunen JP. Early PD-1 Therapy Discontinuation in Responding Metastatic Cancer Patients. Oncology 2019;96:125-31. 10.1159/000493193 [DOI] [PubMed] [Google Scholar]
- 17.Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer 2019;7:306. 10.1186/s40425-019-0805-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berner F, Bomze D, Diem S, et al. Association of Checkpoint Inhibitor -Induced Toxic Effects with Shared Cancer and Tissue Antigens in Non-Small Cell Lung Cancer. JAMA Oncol 2019;5:1043-7. 10.1001/jamaoncol.2019.0402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaput N, Lepage P, Coutzac C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol 2017;28:1368-79. 10.1093/annonc/mdx108 [DOI] [PubMed] [Google Scholar]
- 20.Iwama S, De Remigis A, Callahan MK, et al. Pituitary Expression of CTLA-4 Mediates Hypophysitis secondary to administration of CTLA-R blocking antibody. Sci Transl Med 2014;6:230ra45. 10.1126/scitranslmed.3008002 [DOI] [PubMed] [Google Scholar]
- 21.Johnson DH, Hailemichael Y, Foo WC, et al. Interleukin-6 is potential target to de-couple checkpoint inhibitor-induced colitis from antitumor immunity. J Clin Oncol 2019;37:S2616. 10.1200/JCO.2019.37.15_suppl.2616 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


