Abstract
Medical thoracoscopy is a commonly used endoscopic technique for the diagnosis and treatment of respiratory diseases. As an invasive technique, it is mainly used for pleural effusions and pleural diseases that cannot be diagnosed by non-invasive methods. It is also of great application in the diagnosis and treatment of certain other diseases. Any technical operation requires special skills. There must be a learning process for mastering these skills. Although internal thoracoscopic surgery is simple, especially for respiratory specialists who have undergone training for thoracentesis or closed drainage, there are discrepancies in thoracoscopic diagnosis and treatment in hospitals in China; furthermore, the surgical methods are not uniform, and some even lead to serious complications. Therefore, the thoracoscopic diagnostic and treatment technology in China needs to be standardized. The Respiratory Professional Committee of the Integrated Medical Branch of the Chinese Medical Doctor Association invited relevant Chinese experts to formulate this standard after several rounds of discussion.
Keywords: Medical thoracoscopy, guidelines, China
Introduction
Medicine thoracoscopy is a commonly used endoscopic technique in the diagnosis and treatment of respiratory diseases. As an invasive technique, it is mainly used for pleural effusions and pleural diseases that cannot be diagnosed by non-invasive methods. Medical thoracoscopy can be performed under local anesthesia (or additional intravenous sedative anesthesia); it generally does not require general anesthesia and can be performed in the endoscopy room. The electronic thoracoscope with a flexible front end allows for biopsy and treatment under direct vision. Therefore, compared with video-assisted thoracoscopic surgery (VATS), the trauma is smaller, the medical cost is lower, and the diagnosis and treatment are more efficient with less complications, and it has been widely used in clinical practice.
Any technical operation requires special skills. There must be a learning process for mastering these skills. Although internal thoracoscopic surgery is simple, especially for respiratory specialists who have undergone training for thoracentesis or closed drainage, there are discrepancies in thoracoscopic diagnosis and treatment in hospitals in China; furthermore, the surgical methods are not uniform, and some even lead to serious complications. Therefore, the thoracoscopic diagnosis and treatment technology in China needs to be standardized. The Respiratory Professional Committee of the Integrated Medical Branch of the Chinese Medical Doctor Association invited relevant Chinese experts to formulate this standard after several rounds of discussion.
Material content
The concept of medical thoracoscopy and the distinction between medical thoracoscopy and VATS
Thoracoscopy was first performed by an Irishman, Francis-Richard Cruise in 1866, but the recognized “father of thoracoscopy” is Haus-Christian Jacobaeus, a Swedish physician from Copenhagen. In 1910, Jacobaeus published a paper on laparoscopy and thoracoscopic surgery (1,2). Jacobaeus defines the three key steps of thoracoscopic operation in Germany as follows: (I) delivering the puncture sheath into the pleural cavity without causing visceral injury and excessive pain; (II) introducing a transparent medium into the thoracic cavity (Jacobaeus used filtered air at the time); and (III) an endoscope with a small diameter that is accessible through the sheath. These three key steps are still effective today (3). Jacoboeus realized that thoracoscopic surgery can not only diagnose pleural diseases but also can be used for the treatment of pleural diseases. In 1913, Jacobaeus used thoracoscopy to loosen the adhesion zone with the parietal pleura visceral layer to create a thorough artificial pneumothorax (APT), so that this technique is applied to the collapse therapy of tuberculosis, which is called “Jacobaeus surgery” (4). This surgery was widely used for the treatment of tuberculosis before the appearance of antibiotics. Until the 1950s, with the widespread use of anti-tubercular drugs, “Jacobaeus surgery” was largely withdrawn as the treatment for tuberculosis. At the same time, for the treatment of complex respiratory diseases, respiratory physicians need to be familiar with medical thoracoscopy. Moreover, thoracoscopic surgery is gradually being applied to the diagnosis and treatment of many other lung diseases (5-7).
In the early 1990s, combined with the experience of general surgeons in the field of laparoscopic surgery, thoracic surgeons introduced “surgical thoracoscopic surgery” and “video-assisted thoracoscopic surgery (VATS)” (3,8). To clarify the difference between the two types of thoracoscopic surgery, the word “medical thoracoscopy” came into being. However, the term “thoracoscopy” refers to both the internal and surgical operations, resulting in some uncertainty between internal medicine and surgery. In 2010, Loddenkemper made a specific distinction in the indications for internal thoracic surgery in the book “Traditional Thoracoscopy” (Table 1) (9).
Table 1. Indications of medical thoracoscopy and VATS.
| Medical thoracoscopy | Medical thoracoscopy or VATS | VATS |
|---|---|---|
| Pleural effusion | Spontaneous pneumothorax | Pulmonary surgery |
| Unexplained pleural effusion | Lung cancer staging | Lung biopsy |
| Lung cancer staging | Pleurodesis (talc spray) | Pulmonary lobectomy |
| Diffuse malignant pleural mesothelioma staging | Empyema (phase I or phase II) | Pneumonectomy |
| Pleurodesis (talc or other hardener) | Drainage | Decortication |
| Diffuse pulmonary disease, local lesions, chest wall, diaphragmatic lesions | Lung Volume Reduction Surgery | |
| Pleural surgery | ||
| Pleurectomy (pneumothorax) | ||
| Drainage or decortication (phase III of empyema) | ||
| Esophageal surgery | ||
| Cyst and benign tumors excision | ||
| Esophagectomy, anti-reflux operation and mediastinal surgery | ||
| Mediastinal tumor resection | ||
| Lung conduit ligation | ||
| Partial pericardial excision | ||
| Sympathectomy |
Medical thoracoscopy was widely used in Europe in the 1960s, mainly for the diagnosis of pleural disease and for staging and the evaluation of efficacy and prognosis in lung cancer and malignant mesothelioma. Pleural atresia was performed by thoracoscopy for the treatment of malignant pleural effusion and intractable pneumothorax, intrathoracic foreign body extraction, hemothorax hemostasis, and empyema debridement. In the late 1970s, the thoracoscopic illumination system made a breakthrough, from the original direct illumination of the bulb to the quartz crystal fiber-conducting source, which greatly improved the field of view and clarity. With the development of science and technology, the emergence of halogen lamps, especially cold light bulbs, the application of quartz crystal optical cable, and the development of miniature camera systems and high-definition imaging equipment, the thoracoscope can not only obtain high-definition images but also display the images in real time on a high-definition TV monitor for viewing by multiple people. The magnified image provides a clear view of the structural changes in the visceral pleura, costal pleura, and mediastinum. At the same time, all kinds of supporting instruments for thoracoscopic surgery are continuously being improved and updated with clinical needs, which further promotes the development of thoracoscopic surgery. Since the 1990s, thoracoscopic surgery has been rapidly promoted and applied around the world, especially in the United States, and has rapidly developed into a new discipline: video-assisted thoracoscopic surgery (10).
Thoracoscopic surgery was introduced relatively late in China. In the 1980s, some patients with pleural disease were diagnosed with fiberoptic bronchoscopy instead of thoracoscopy (11). It has gradually developed, especially in the past 10 years, with the birth of semi-rigid medical thoracoscopy, which greatly promoted diagnosis and treatment using medical thoracoscopy. However, there is a lack of specialist training programs for medical thoracoscopy. From 2002 to 2003, the American Association of Chest Physicians surveyed the American Respiratory and Critical Care Specialist Training Program, showing that only 12% of specialists were trained, and in China, there is no specialist training program for medical thoracoscopy.
Standard operating specification
Equipment and instruments
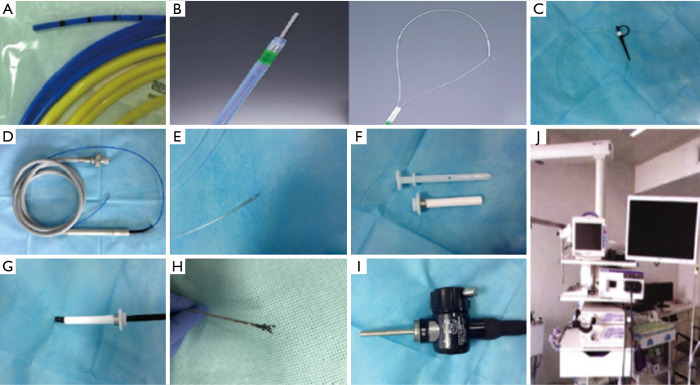
There are two main types of thoracoscopic lenses currently being used: rigid thoracoscope and semi-rigid thoracoscope (12). Necessary equipment for medical thoracoscopic techniques includes puncture sheath, thoracoscope, biopsy forceps, monopolar electrocoagulation forceps, light source, video system, suction system, incision suture device, chest tube and drainage system, tracheal intubation, monitoring system, and cardiopulmonary resuscitation equipment. At present, rigid thoracoscopic surgery is mostly used for surgery. Medical thoracoscopy involves the use of the semi-rigid thoracoscope (Olympus LTF-240) produced by Olympus Corporation of Japan. This semi-rigid medical thoracoscope is similar to a standard electronic bronchoscope and consists of an operating handle and an operating lever. The lever has an outer diameter of 7 mm and a length of 27 cm, which consists of two parts, a 22 cm long proximal hard section and a 5 cm long flexible front end. The handle has a switch to control the movement of the flexible front end, making it capable of two-way angle conversion (upward rotation of 160 degrees, down 130 degrees). There is also a 2.8-mm diameter working hole on the operating rod, which can be used to insert biopsy forceps, needle biopsy, and other attachments and is compatible with electrosurgery and laser operation. The device achieves a single-point puncture technique with the aid of a disposable plastic flexible puncture sheath (8 mm I.D.). The new model 240 mode of operation allows for autoclaving to avoid cross-infection with pathogens. Compared with rigid thoracoscopic, semi-rigid medical thoracoscopy also has a distinct advantage, it is compatible with Olympus’s compatible bronchoscope or gastrointestinal mirror operator and light source, so it will not bring additional costs, see Figure 1.
Figure 1.
The parameters of thoracoscope. (A) Semi-rigid thoracoscope (Olympus); the operating part is the same as the flexible bronchoscope. (B) Angled range: up 160/down 130. (C) Insertion section of the thoracoscope with an outer diameter of 7 mm and a working channel with a 2.8-mm diameter.
Operating environment, personnel, and monitoring requirements
Medical thoracoscopy can be performed in the operating room or in the endoscopy room. Four professionals required for thoracoscopic operation: 1 doctor for performing endoscopy, 1 doctor for assisting endoscopy, 1 nurse for taking necessary equipment outside the sterile area, and 1 doctor or nurse responsible for monitoring the vital signs of the patient. The medical thoracoscopy operation room should be equipped with devices for resuscitation, assisted ventilation, electrocardiogram, blood pressure monitoring, defibrillation, and oxygen source.
Patient preparation
(I) Preoperative 24-h X-ray, CT, or B-ultrasound to understand pleural effusion, gas accumulation, and pleural adhesion; (II) preoperative routine ECG, routine blood test, blood coagulation series, blood type, hepatitis series, syphilis, AIDS antibody, cardiopulmonary function, and blood gas analysis; (III) preoperative discussion and conversation: after completion of the preoperative examination, the subject director or professional team leader should assign relevant medical staff for preoperative discussion. According to the patient’s condition and the results of the examination, the patient should be evaluated for surgery. Estimate whether medical thoracoscopy or treatment can be performed. Once medical thoracoscopy operation is decided, the residents and attending physicians should conduct preoperative conversations with the patient and the patient’s family and obtain the patient’s and family’s consent; (IV) preoperative 24-h imaging (B-ultrasound, X-ray or CT) to locate the puncture point or to puncture the chest and pump water into the chest cavity to inject filtered air (300–500 mL or so to form an artificial pneumothorax), according to the situation; intramuscular injection of pethidine hydrochloride 50–100 mg; severe cough can be controlled by taking codeine solution 10 mL orally.
Patient anesthesia
Medical thoracoscopy usually uses local anesthesia with appropriate sedation (13). Medical thoracoscopy is different from VATS, and tracheal intubation is generally not required. However, some special indications may require general anesthesia, such as hypersensitivity to local anesthetics, excessive anxiety, inability to cooperate (such as children), or patients who need further operations (such as sympathectomy) (14). In addition to local anesthesia, the addition of sedatives and analgesics is extremely important. Sedatives can improve patient comfort, relieve pain, and create optimum conditions for physicians to operate, such as inhibiting patient activity and reducing cough reflexes. The most commonly used drug is propofol before or after surgery (15). The sedative effect of propofol is similar to that of midazolam, but it works faster and has a revitalizing effect (16). The use of analgesics can reduce pain and reduce the irritability and discomfort caused by pain. Analgesic drugs can be used including morphine, meperidine or fentanyl (3,17).
During local anesthesia, the patient should be in the lateral position with the affected side facing up. The ECG monitor, the blood pressure cuff meter, and the pulse blood glucose meter should be connected and the venous channel should be established in the non-operating arm. In addition, blood oxygen saturation should be recorded before sedation and before and after nasal inhalation.
The puncture point is selected in the vicinity of the midline of the axillary triangle. This area has less muscle and is easier to enter the chest. The front end of the area is adjacent to the lower edge of the pectoralis major muscle, the posterior margin is behind the latissimus dorsi, the iliac bulge is below, and the second intercostal space is touched by the tip. Puncture point determination depends on the following factors: (I) pleural effusion is most common between 5, 6, 7 intercostal, and metastatic tumor and diffuse malignant mesothelioma are more likely to invade the 6th and 7th intercostal space; (II) because pneumothorax usually occurs in upper lobe, therefore, in spontaneous pneumothorax, the puncture point should be selected in the 3rd or 4th intercostal space to create conditions for the examination of the apex of the lung; (III) for some special cases, based on clinical features, chest imaging or ultrasonography to determine the puncture point.
After selecting the puncture point, local anesthesia must be performed according to the procedure. Insert the needle between the lower edge of the upper rib and the upper edge of the lower rib, and the skin, subcutaneous tissue, intercostal muscle, and parietal pleura are sequentially penetrated, thereby anesthetizing the intercostal nerve. The periosteum of the ribs is prevented from touching the nearby intercostal arteries and veins by repeated attraction.
Thoracoscopic insertion path
The premise of medical thoracoscopy is that the thoracoscope can be freely inserted in the pleural cavity, and the puncture sheath and thoracoscope can be placed without damaging the lungs or other organs. In order to ensure that the thoracoscope enters the pleural cavity safely, the following aspects should be considered before the thoracoscopic surgery: (I) medical history: ask if there is pleurisy or if chest surgery was performed; (II) use chest imaging technique (X-ray, CT, MRI, ultrasound) to determine whether there is pleural adhesion; (III) if the existing pneumothorax >200 mL, successfully complete the thoracoscopic examination; (IV) pneumothorax with pleural adhesion, should be carried out under the guidance of chest imaging technology; (V) if there is a large amount of pleural cavity liquid, puncture sheath directly inserted or inserted under imaging guidance; (VI) if there is a small amount pleural effusion, special pneumatic brooch can be used to induce pneumothorax under pressure control; (VII) if there is no pleural effusion or pneumothorax, blunt distraction or pneumothorax induction techniques should be used to create artificial pneumothorax (see Table 2).
Table 2. Method of inserting a thoracoscope in various situations.
| Clinical situation | Method of inserting a thoracoscope |
|---|---|
| Pneumothorax | At least 100–200 mL of pleural space; puncture sheath should be placed directly |
| Large amount of pleural effusion | Directly insert the puncture sheath |
| Small amount of pleural effusion | Place the puncture sheath using ultrasound or fluoroscopy guidance; note that air can enter the chest through the puncture sheath. If the pleural effusion suction is difficult, pneumothorax can be induced using a special gas brooch under pressure control |
| Pleural adhesions lead to difficulty in artificial pneumothorax | Can be bluntly separated using Kelly forceps and fingers |
| Complete occlusion of the pleural cavity | Prohibition of medical thoracoscopy |
Operational techniques
(I) physician and assistant physician or nurse clean hands after following the standard surgical cleaning technique and wear sterile surgical gowns and sterile gloves. The skin of patients from the sternum to the clavicle, through the armpit to the scapula, and through the spinous process down to the bottom of the thorax needs to be prepared and disinfected; (II) the patient is placed in a lateral position; after routine skin disinfection, a sterile sheet is placed, the endoscopic doctor faces the patient, and the assistant is opposite the console. Two percent lidocaine is used to perform local infiltration anesthesia on puncture point, the skin is cut 1–2 cm along the intercostal direction, the subcutaneous muscle is separated layer by layer till the chest cavity using a vascular clamp, and then, the puncture sheath handle is grasped with the palm of the hand in a spiral movement. It is inserted until a feeling of falling. The operator can use the extended thumb to limit the depth of insertion into the pleural cavity to prevent lung injury; (III) the puncture sheath can be removed after entering the pleural cavity, and the cannula should be fixed by the assistant after continuing to penetrate the chest cavity by 1–3 cm. The thoracoscope is then placed along the cannula under direct vision. Most pleural effusions can be aspirated through the suction tube or working channel as needed, but aspiration should not be too fast to prevent pulmonary edema after re-expansion. Air can enter the pleural cavity and replace the original space to maintain normal intrathoracic pressure; (IV) thoracoscopic operation is performed under direct or TV-assisted vision. After thoracoscopic surgery, the dorsal thoracic cavity can be observed, and the diaphragm and rib angle can be directly viewed. After the pleural effusion is exhausted, the thoracic cavity can be manipulated to explore the pleural cavity. If the adhesive tape and the wrapped effusion are directly separated by biopsy forceps, the electrosurgical knife (Figure 2) or the argon gas knife can also be used for cutting and separating; (V) the anatomical structure in the thoracic cavity should be distinguished during the operation. On the right, you can find a joint between the three lobes where the oblique and horizontal splits are located, and the left side can be positioned by the oblique split. The diaphragm is identified by respiratory motion. The top of the lung is narrower, like a cone, and various adhesion bands between the visceral layer and the parietal pleura can be observed. (VI) Under the microscope, the surface of the normal lung is pink and soft, and the network is distributed with lung lobules. Carbon black pigmentation is scattered on the surface of the lung; the clear atelectasis on the pleural surface of the visceral layer is purplish red with clear boundaries; tuberculous pleurisy can be seen with congestion, edema, and diffuse miliary distribution of small nodules; malignant nodules and other typical pathological changes are very easy to identify: emphysema or pulmonary blebs are easy to find as they highlight the surface of the lungs (see Figure 3); (VII) suspicious lesions can be biopsied, brushing through the thoracic working hole. It is usually necessary to have a multi-site biopsy. In order to prevent bleeding, gas leakage, and other complications of lung laceration, the biopsy site mainly composed of parietal pleura is chosen, usually to avoid blood vessels. If there is bleeding, local hemoglobin can be perfused 2–4 u. The biopsy organization should be 3–6 pieces. If there are special requirements, such as genetic testing, it can be increased to 10–12 pieces. For unexplained pleural effusions, biopsy should be performed on small lesions in different areas, such as the anterior chest wall, posterior thoracic cavity, and diaphragm. If infection is considered, tissues and secretions should be retained for routine bacterial culture. Mycobacterial culture should be performed if tuberculosis is suspected. If there is an obvious lesion on the surface of the lung, biopsy can also be performed, but it is necessary to prevent excessive pulling and tearing of the lung; (VIII) an autofluorescence and narrow-spectrum imaging bronchoscope can also be introduced through the chest with the cannula, which helps to identify benign and malignant conditions; (IX) thoracoscopy, in addition to the diagnosis, can also be used for the treatment of a variety of diseases, such as tuberculous pleurisy and empyema in patients with adhesions; electrotomy, argon knife, freezing, laser and photodynamic therapy for benign and malignant diseases; radioactive ion implantation for pleural malignant disease; thoracoscopic pulmonary bleb puncture drainage; and intractable pneumothorax treatment and pleurodesis; (X) after completion of surgery, withdraw the thoracoscopy and other ancillary equipment. The thoracic puncture site is sutured, covered with sterile gauze, and the drainage tube is sealed at the puncture point to drain the residual air and liquid, thereby re-expanding the lung. The indications for chest tube removal vary from person to person. Under normal circumstances, the drainage tube can be removed when there is no gas discharge in the chest tube and the fluid stops flowing. (XI) Specimen evaluation: cytology tumor markers, molecules genetic testing, and detection and culture of infectious pathogens should be performed for pleural effusion. Biopsy tissue should be diagnosed by a physician with experience in pathology. If tuberculosis or other infections are suspected, the tuberculous tissue and fibrin tissue of the parietal pleura should be cultured for Mycobacterium tuberculosis, fungi, and/or anaerobic microorganisms. Specimens used for electron microscopy should be placed in a cooled glutaraldehyde fixative.
Figure 2.
Main equipments for thoracoscopic surgery. (A) Argon gas knife (direct injection). (B) Electrosurgical knife (needle-shaped trapped electrosurgical knife). (C) Laser. (D) Frozen. (E) Puncture needle. (F) Thoracoscopic hard puncture sheath needle and outer sleeve with an outer diameter of 10 mm. (G) Flexible thoracoscope, through the outer sheath of the sheath into. (H) Alligator-nose pliers with fixed needles. (I) Special waterproof cover, used to protect LTF-240 thoracoscope electrical plugs during high temperature and high-pressure sterilization. (J) Light source, operator, and monitor for bronchoscope.
Figure 3.
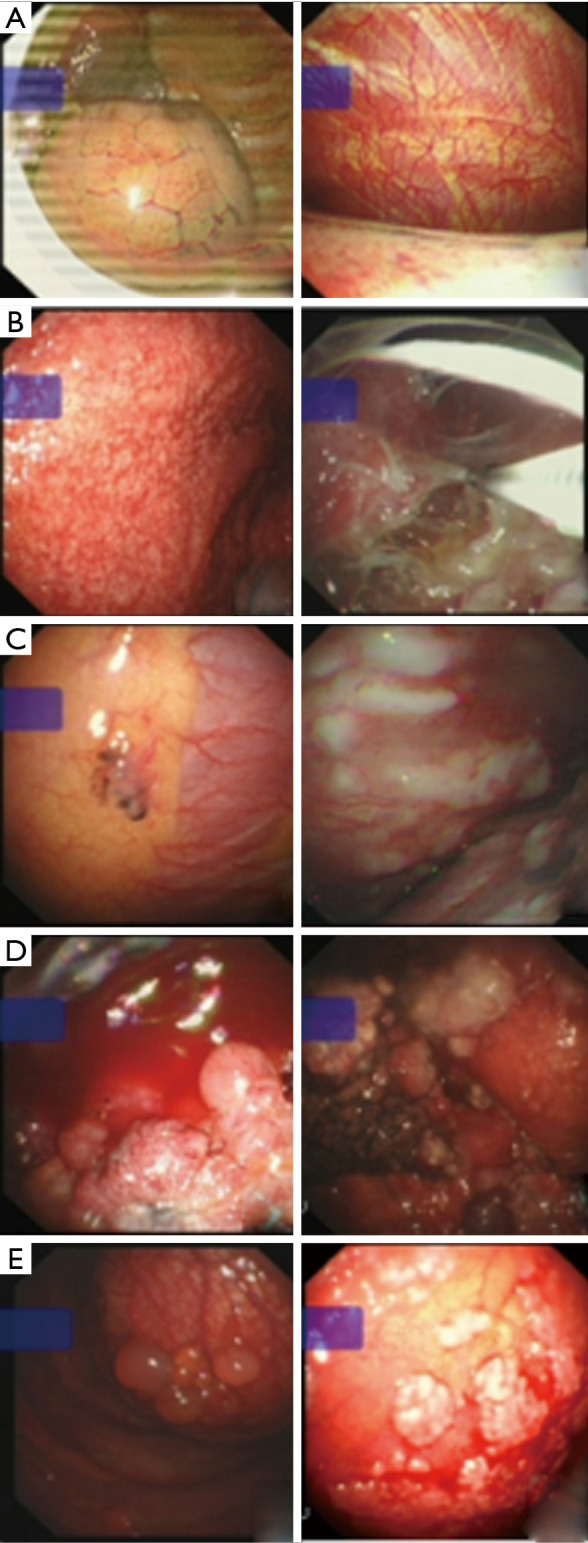
The representative images of different lesions captured by a thoracoscope. (A) Normal lung surface, visible in the visceral and parietal pleura. (B) Tuberculous pleurisy. (C) Pleural mesothelioma. (D) Pleural metastasis of primary lung cancer. (E) Other metastatic cancer seen during pleural endoscopy.
Indication
Medical thoracoscopy is primarily used as a diagnostic procedure, but it can also be used for therapeutic purposes, as shown in Table 1 (18). Medical thoracoscopy, as the “gold standard”, provides an excellent tool for the diagnosis and treatment of pleural effusions and refractory pneumothorax. The indications mainly include: diagnosis: (I) unexplained pleural effusion; (II) diffuse malignant pleural mesothelioma (MPM) and lung cancer staging; treatment: (I) malignant or recurrent pleural effusion; (II) early empyema; (III) spontaneous intractable pneumothorax (19). The advantages of medical thoracoscopic diagnosis of pleural disease are as follow: (I) rapid and accurate biopsy diagnosis (such as M. tuberculosis culture and hormone receptor detection); (II) applicability to not only parietal pleural biopsy, but also to diaphragm, lung, and mediastinal biopsy; (III) diagnosis and staging of lung cancer and diffuse pleural mesothelioma; and (IV) exclusion of malignant lesions and suspected tuberculosis. The advantages of medical thoracoscopic treatment of pleural diseases are as follows: (I) complete and rapid exclusion of pleural effusion; (II) assessment of compartmentalization (pulmonary tuberculosis and lung cancer); (III) assessment of lung recruitment potential; (IV) uniform spraying of talcum powder (6–10 mL) under direct vision, which is the gold standard for non-surgical treatment; (V) early initiation of drug treatment; and (VI) better guidance for chemotherapy.
For unexplained pleural effusions, the main diagnostic value of medical thoracoscopy is that it can rule out suspected malignant diseases or tuberculosis (20-22). Endoscopic evidence can also be used for some undiagnosed pleural effusions, such as rheumatic pleural effusion, pleural effusion caused by cirrhosis or pancreatitis or some rare causes such as amyloidosis or sarcoidosis (23,24). For the treatment of recurrent pleural effusion with certain non-malignant etiologies, such as chylothorax, hepatic effusion, cardiogenic, or systemic lupus erythematosus (SLE), thyroid powder spray fixation can be performed by medical thoracoscopy (25).
Contraindication
Medical thoracoscopy is a safe procedure with few absolute and relative contraindications (Table 3).
Table 3. Absolute and relative contraindications of medical thoracoscopy.
| Absolute contraindications | Relative contraindication |
|---|---|
| No pleural space | Cannot tolerate lateral position |
| Late empyema | Unstable cardiodynamics and hemodynamics |
| Unexplained pleural thickening | Severe hypoxemia that can only be corrected by oxygen therapy |
| Suspected mesothelioma (synechia and fusion of visceral pleura and parietal pleura) | Tendency of hemorrhage |
| Pulmonary hypertension | |
| Refractory cough | |
| Drug allergy | |
| Short expected survival and poor general condition |
Its absolute contraindications are as follows: extensive pleural adhesions, such as pleural fibrosis; post-infection or previous pleurodesis, resulting in pleural occlusion; and lack of space for medical thoracoscopy. For a medical thoracoscopy, the patient needs at least 300 mL of local pneumothorax or a 2–4-cm-deep pleural space.
Coagulopathy is usually only a relative contraindication. The patient’s platelet count should be >60×109/L and the international normalization rate should be less than 1.2. Coagulopathy that does not meet these two conditions must be corrected before medical thoracoscopy. Aspirin and clopidogrel do not increase the risk of bleeding. However, in patients with renal insufficiency, blood urea nitrogen (BUN) levels (>30 mg/dL) or creatinine levels (>3 mg/dL) significantly increase the risk of bleeding (26,27).
Patients with hypoxemia, especially those with hypercapnia, should be carefully evaluated. The risk of surgery should be determined according to the severity of respiratory insufficiency. Severe hypoxemia (PaO2 <50 mmHg) should be an absolute contraindication. If the patient has a large amount of pleural effusion or tension pneumothorax with severe hypoxemia, a thoracoscope can be used to quickly re-expand the lungs and improve breathing. Medical thoracoscopy is also not recommended for patients who cannot be placed in a lateral position. Thoracoscopy and treatment can be performed safely for patients with respiratory failure and hypoxemia using mechanical ventilation.
In cases of sustained cough, fever, or unstable heart conditions, thoracoscopic operation should be delayed. Patients with tension pneumothorax, severe hypoxemia, severe arrhythmia, and newly diagnosed myocardial infarction should not undergo medical thoracoscopy. Lung biopsy is contraindicated in patients with suspected pulmonary venous fistulas, hemangiomas, and hydatid cysts.
Complications and prevention
Standard medical thoracoscopic procedures are safe and effective for the diagnosis and treatment of pleural and pulmonary diseases (28). Although the risk is low, the risk-benefit ratio should be considered for each patient. Therefore, the patient’s condition and indications and contraindications for medical thoracoscopic surgery must be carefully evaluated. The safety of medical thoracoscopic surgery depends on a careful assessment of the patient’s condition, adequate training of thoracoscopic physicians, careful consideration of contraindications, and prevention of complications. At the same time, the recommended technique should be used during surgery and the heart and hemodynamic parameters as well as blood oxygen saturation should be monitored. Similar to all preoperative preparations for patients undergoing conscious sedation and anesthesia, fasting and water deprivation for 6–8 hours medical thoracoscopy are required to reduce the risk of aspiration (29). Complications can occur at any step of preoperative preparation, as well as at any intraoperative and postoperative stage (common complications; see Table 4). Embolism, especially gas embolism, is the most serious complication of artificial pneumothorax. However, it rarely occurs (<0.1%) and can be prevented by applying appropriate measures. Pain: The patient will experience short-term pain when the puncture sheath penetrates the parietal pleura or when the local anesthetic reaches this region and when extensive pleural adhesions are removed. When talcum powder is fixed, more severe pain will be experienced. When spraying talcum powder, patients should be given painkillers or lidocaine injections to the chest. Hypoxemia: Anesthesia-induced respiratory depression or pulmonary collapse caused by pneumothorax during operation can result in hypoxemia. During surgery, the patient should be given oxygen through the nasal cannula. Hypoventilation: Excessive sedation may lead to hypoventilation. ECG and oxygen saturation and PaCO2 monitoring should be performed during the operation. Arrhythmia: occasionally, a mild increase in sinus heartbeat peed may occur, but arrhythmia is relatively rare. Hypotension: massive drainage of pleural effusion can lead to fluid loss and a drop in blood pressure. Atropine is recommended to inhibit vasovagal reflexes. However, routine use of atropine is not recommended before surgery. Bleeding: this is one of the most concerning complications for medical thoracoscopic operators. Thus, surgery should be considered as an alternative. Superficial hemorrhage at the puncture site can be prevented by placing a puncture tube after compression. If the bleeding does not stop or the puncture biopsy accidentally injures the intercostal blood vessels, bleeding should be force-stopped or electrocoagulation should be used to stop bleeding. Damage to the lungs or other organs: lung tissue is most likely to tear, especially when two layers of the chest wall are adhered or visceral pleural biopsy is performed, resulting in pneumothorax or hemothorax.
Table 4. Common complications of medical thoracoscopy.
| Preoperative complication | Intraoperative complication | Postoperative complications |
|---|---|---|
| Air embolism, subcutaneous emphysema, and pain during artificial pneumothorax | Pain | Re-expansion pulmonary edema |
| Hypoventilation and dyspnea after artificial pneumothorax | Hypoxemia | Pain |
| Allergic reaction to local anesthesia | Hypoventilation | Fever |
| Arrhythmia | Incision infection | |
| Hypotension | Hypotension | |
| Hemorrhage | Empyema | |
| Injury of the lungs or other organs | Subcutaneous emphysema | |
| Lasting pneumothorax | ||
| Long time gas leakage | ||
| Lasting pleural effusion | ||
| Complications after talc spraying | ||
| Chest wall dissemination of tumor cell | ||
| Death |
The mortality rate of medical thoracoscopic surgery is extremely low, and deaths are rare. It has been reported that 1 out of 8,000 patients die (case fatality rate is 0.01%) (30). In order to prevent the occurrence of complications of thoracoscopic surgery, the standards of thoracoscopic operation must be strictly observed. The preoperative examination must be performed carefully, and the indications and contraindications should be clearly understood. During the operation, the nasal catheter is to be oxygenated, and blood gas analysis and cardiopulmonary monitoring are to be performed. If the bleeding is greater than 200 mL, electrocoagulation should be used to stop the bleeding, and the thoracic drainage tube should be retained until there is no gas overflow (31). On the day of medical thoracoscopy, the insufflation of the lungs can prevent atelectasis. Drainage of pleural effusion or pneumatosis should be carried out slowly to avoid pulmonary edema after recruitment maneuvers.
Conclusions
In summary, medical thoracoscopy is an endoscopic technique commonly used for the diagnosis and treatment of respiratory diseases. Medical thoracoscopy is simple, has few complications, and has high utility in clinical diagnosis and treatment. However, the risk-benefit ratio in each individual patient should be taken into account. Therefore, every respiratory endoscopic physician must carefully evaluate the patient’s condition and the indications and contraindications for medical thoracoscopy, and strictly follow the specifications to ensure patient safety. With the improvement in the skills and experience of an increasing number of well-educated and trained respiratory endoscopic surgeons in China, future medical thoracoscopic techniques will certainly yield substantial results.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This study was supported by the National Public Welfare Industry Research Project (Grant No. 201402024) and Tangdu Hospital Science and Technology Innovation Development Fund (Grant No. 2014LCYJ002).
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Once medical thoracoscopy operation is decided, the residents and attending physicians should conduct preoperative conversations with the patient and the patient’s family and obtain the patient’s and family’s consent.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-19-2276). YS serves as an unpaid editorial board member of Journal of Thoracic Disease from Mar 2012 to Mar 2022. The other authors have no conflicts of interest to declare.
References
- 1.Hoksch B, Birken-Bertsch H, Muller JM. Thoracoscopy before Jacobaeus. Ann Thorac Surg 2002;74:1288-90. 10.1016/S0003-4975(02)03676-7 [DOI] [PubMed] [Google Scholar]
- 2.Jacobaeus HC. Uber die Moglichkeit, die Zystoskopie bei Untersuchung seroser Hohlungen anzuwenden. Munch Med Wschr 1910;40:2090-2. [Google Scholar]
- 3.Lee P, Mathur PN, Colt HG. Advances in thoracoscopy: 100 years since Jacobaeus. Respiration 2010;79:177-86. 10.1159/000268617 [DOI] [PubMed] [Google Scholar]
- 4.Wittmoser R. Surgical thoracoscopy. Langenbecks Arch Chir Suppl II Verh Dtsch Ges Chir 1990;II:1325-31. [PubMed] [Google Scholar]
- 5.Mathur PN, Loddenkemper R. Medical thoracoscopy. Role in pleural and lung diseases. Clin Chest Med 1995;16:487-96. [PubMed] [Google Scholar]
- 6.Loddenkemper R. Thoracoscopy--state of the art. Eur Respir J 1998;11:213-21. 10.1183/09031936.98.11010213 [DOI] [PubMed] [Google Scholar]
- 7.Bhatnagar R, Maskell NA. Medical pleuroscopy. Clin Chest Med 2013;34:487-500. 10.1016/j.ccm.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 8.Landreneau RJ, Mack MJ, Hazelrigg SR, et al. Video-assisted thoracic surgery: basic technical concepts and intercostal approach strategies. Ann Thorac Surg 1992;54:800-7. 10.1016/0003-4975(92)91040-G [DOI] [PubMed] [Google Scholar]
- 9.Loddenkemper R MPN, Noppen M, et al. Medical Thoracoscopy / Pleuroscopy: Manual and Atlas. New York: Thieme Publishing Group, 2010. [Google Scholar]
- 10.Loddenkemper R, Mathur PN, Lee P, et al. History and clinical use of thoracoscopy/pleuroscopy in respiratory medicine. Breathe 2011;8:144-55. 10.1183/20734735.011711 [DOI] [Google Scholar]
- 11.Zehong J. Fiberoptic bronchoscopy was used into thoracoscopy for diagnosis of pleural effusion. Int J Respir 1986. [Google Scholar]
- 12.Casal RF, Eapen GA, Morice RC, et al. Medical thoracoscopy. Curr Opin Pulm Med 2009;15:313-20. 10.1097/MCP.0b013e32832b8b2d [DOI] [PubMed] [Google Scholar]
- 13.Astoul P, Maldonado F. Anesthetic drugs managed by pulmonologists during medical thoracoscopy: one size does not fit all! Respiration 2014;88:265-7. 10.1159/000365663 [DOI] [PubMed] [Google Scholar]
- 14.Liping Z. Thoracic Sympathectomy by the pleura videoscope with a single skin incision through the anterior medastinum for palmar hyperhidrosis. Zhe Jiang University 2013. [Google Scholar]
- 15.Vorster MJ, Bruwer JW, Frank W, et al. The use of propofol for sedation in medical thoracoscopy. Respiration 2015;89:435. 10.1159/000371451 [DOI] [PubMed] [Google Scholar]
- 16.Grendelmeier P, Tamm M, Jahn K, et al. Propofol versus midazolam in medical thoracoscopy: a randomized, noninferiority trial. Respiration 2014;88:126-36. 10.1159/000362797 [DOI] [PubMed] [Google Scholar]
- 17.Liu R, Wu Q, Chen X, et al. Diagnosis and treatment of pleural effusion: primary comparison between the medical thoracoscopy and video assisted thoracic surgery. China Journal of Endoscopy 2008;(11):1177-9.
- 18.Faguang J. The current status and clinical application of medical thoracoscopy. Chinese Journal of Practical Internal Medicine 2013;33:113-5. [Google Scholar]
- 19.Faguang J. Application of medical thracoscopy in diagnosis and treatment of pleural diseases. Chinese Journal of Lung Diseases (Electronic Edition) 2011;4:163-6. [Google Scholar]
- 20.Liu W, Jin F, Fu E, et al. Medical thoracoscope in diagnosis of large pleural effusion. Journal of Clinical Pulmonary Medicine 2010;15:175-6. [Google Scholar]
- 21.Fu E, Mu D, Jin F, et al. The diagnostic value of fiberoptic bronchoscopy instead of thoracoscopy and medical thoracoscopy for unexplained pleural effusion Chinese Journal of Clinicians (Electronic Edition) 2011;5:4867-9.
- 22.Sun R, Jin F, Xie Y, et al. Clinical observed by medical thoracoscope in diagnosis of unexplained pleural effusion. Chinese Journal of Lung Diseases (Electronic Edition) 2011;4:179-82. [Google Scholar]
- 23.Jin F, Li W, Fu E, et al. Evaluation of safety and value by using medical thoracoscopy to diagnose pleural effusion in unknown origin. Chinese Journal of Lung Diseases (Electronic Edition) 2010;3:86-9. [Google Scholar]
- 24.Li W, Chen X, Pan L, et al. A case of sarcoidosis with extensive pleural invasion and literature review. Chinese Journal of Lung Diseases (Electronic Edition) 2017;10:237-8. [Google Scholar]
- 25.Yan Z. Diagnosis and treatment of recurrent pleural effusion with thoracoscopic surgery. Bulletin Jinling Hospital 1992. [Google Scholar]
- 26.Tong Z, Wang Z, Wang C, et al. The Clinical application of medical thoracoscopy. Zhonghua Jie He He Hu Xi Za Zhi 2007;30:220-2. [PubMed] [Google Scholar]
- 27.Skalski JH, Astoul PJ, Maldonado F. Medical thoracoscopy. Semin Respir Crit Care Med 2014;35:732-43. 10.1055/s-0034-1395796 [DOI] [PubMed] [Google Scholar]
- 28.Brims FJ, Arif M, Chauhan AJ. Outcomes and complications following medical thoracoscopy. Clin Respir J 2012;6:144-9. 10.1111/j.1752-699X.2011.00254.x [DOI] [PubMed] [Google Scholar]
- 29.ZHAO Wenping DS. Nursing experience of medical thoracoscopy Chinese Community Doctors 2009.
- 30.Blanc FX, Atassi K, Bignon J, et al. Diagnostic value of medical thoracoscopy in pleural disease: a 6-year retrospective study. Chest 2002;121:1677-83. 10.1378/chest.121.5.1677 [DOI] [PubMed] [Google Scholar]
- 31.YE Yuming LJ, et al. Common adverse reactions and treatment of medical thoracoscopy (analysis of 175 cases). China Journal of Endoscopy 2012.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as