Abstract
Background
Elastofibroma dorsi (ED) is a benign soft-tissue tumor of the chest wall located near the tip of the scapula. Clinical presentation includes swelling, pain and impairment of shoulder movements. The present literature relies only on few small case series. The aim of this study was to analyze the surgical management of ED, focusing on the debated topics regarding preoperative evaluation, operative technique, post-operative outcome and follow-up.
Methods
We conducted a single-center retrospective cohort analysis of patients operated for ED between 2003 and 2018. Diagnostic techniques were ultrasonography (US), computed tomography (CT-scan) and magnetic resonance imaging (MRI). CT-scan represented our preferred imaging study for preoperative assessment. Surgery was proposed for symptomatic and/or large lesions. Marginal excision through a muscle-sparing approach was performed. An open-door follow-up policy was adopted. All clinical, radiological, perioperative and pathological variables were matched in a univariate analysis. A multivariate analysis was performed to investigate risk factors for postoperative complications. Correlations analysis between radiological and pathological measurements of elastofibroma was conducted.
Results
Seventy elastofibromas were excised in 59 patients. Mean age was 59 years and female prevalence was 59%. All elastofibromas were completely resected with no recurrence. Postoperative complications rate was 17%. Complications were mild in most cases. At the univariate analysis, patients with body mass index (BMI) >25 had a longer operative time (P=0.048), patients on antiplatelet medications experienced a prolonged drainage time (P=0.006) and a higher rate of complications (P=0.038); the occurrence of complications resulted in prolonged drainage time (P=0.047) and length of stay (P=0.023). A BMI ≤25 was the only independent risk factor for postoperative morbidity (OR 8.71, P=0.024). CT-scan showed the highest correlation with pathological size (r=0.819), US the lowest (r=0.421).
Conclusions
Marginal resection through a muscle-sparing approach is safe and effective for the treatment of ED. CT-scan can be adequate for preoperative assessment. Giving the benign nature of the lesion and the absence of recurrence after complete resection, an open-door follow-up may be appropriate.
Keywords: Subscapular mass, elastofibroma dorsi (ED), benign tumor, surgery
Introduction
Elastofibroma dorsi (ED) is a soft tissue tumor of the chest wall. It typically presents as an ill-defined mass adjacent to the inferior pole of the scapula, laying over the periosteum of the sixth to eighth rib, underneath the muscles of the thoracic wall. ED is a benign slow-growing tumor and can be bilateral in a variable rate between 10% and 66% (1,2). ED has been traditionally described as rare (3), but it is now considered more common than it was previously thought: several studies based on CT-evaluation (4,5) and autopsy series (6,7) revealed a prevalence of 1.6% to 16.5%. ED is most frequently encountered in elderly patients and it is more common in females than in males (2,5).
Repetitive mechanical friction between the tip of the scapula and the thoracic wall has been advocated as the main pathogenetic mechanism for the development of ED (8), although familiarity (2) and genetic alterations (9,10) have also been reported.
Local pain and functional limitation of the shoulder girdle are the most common symptoms associated with ED (11). Clinical examination and imaging assessment lead to the diagnosis of ED with a high degree of confidence (12), thus biopsy is usually deemed unnecessary (13). Ultrasonography (US), computed tomography (CT-scan) and magnetic resonance imaging (MRI) have been proposed for the imaging assessment of ED (14,15) and they are adopted in different combinations in the clinical practice. Nevertheless, at present no univocal diagnostic algorithm has been reported in the literature (12) and which imaging technique represents the best diagnostic strategy for ED is still debated.
Surgical treatment is indicated for symptomatic and/or large ED and marginal resection is commonly accepted as the surgical technique of choice, with an acceptable rate of minor complications and a very low risk of local recurrence (12).
Follow-up for patients who underwent surgical excision of ED is still a matter of debate. Some authors supported a prolonged follow-up strategy based on clinical and radiological evaluation (8,11,12), while others proposed an open-door follow-up policy, in which the patient asks for a consultation only in case of concern (3).
Available information on ED relies on limited number of case series and case reports. In this study we retrospectively reviewed our series of patients operated for ED, with the aim to evaluate the diagnostic, surgical and postoperative aspects, especially focusing on the most debated topics. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-649) (16).
Methods
This is a monocentric retrospective analysis based on the data of a prospectively-maintained database with their medical records in our institution. We reviewed medical information of patients who consecutively underwent surgical excision of ED at our institution, between January 2003 and December 2018. All available data regarding patient characteristics and surgical outcomes were collected for further analysis.
The present study was approved by the local Ethics Committee (CE number 901/2019) and conformed to the principles of Helsinki Declaration.
Preoperative assessment
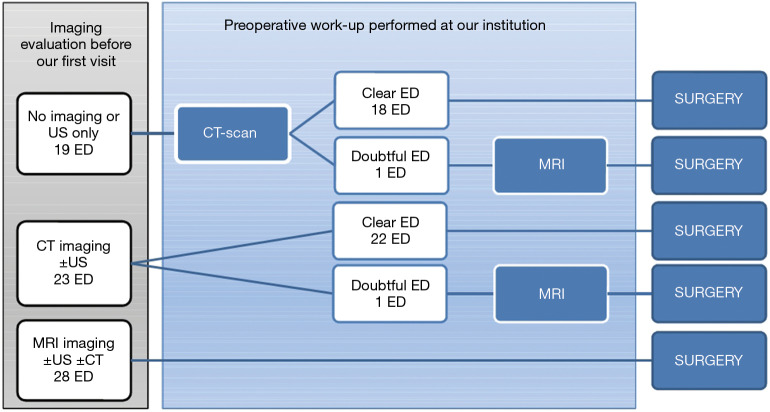
All patients were referred to our institution for the presence of a palpable mass in the subscapular region, suspected for ED. When patients came to our attention, the majority of them had been already evaluated by one or more imaging studies. We consider both CT-scan and MRI as adequate to select patients for surgery, though CT-scan represents our preferred imaging technique. Conversely, we do not deem US imaging alone sufficient for preoperative assessment. Therefore, if CT-scan and/or MRI were already available, we did not prescribe any further imaging assessment, whereas in case of patients with no examination or US only, we prescribed a CT-scan. We further requested MRI only when clinical and CT-scan features did not provide a confident diagnosis of ED (i.e., suspect of malignancy). This algorithm of imaging assessment is reported in Figure 1.
Figure 1.
The flowchart represents preoperative work-up according to the algorithm adopted in our Institution.
It is our policy to propose surgical excision for patients who fulfill one or more of the following criteria: (I) presence of symptoms; (II) large lesion (usually more than 5 cm in maximum diameter); (III) atypical clinical or radiological presentation. Furthermore, because ED is a benign disease, patient preference was largely taken into account, and in every case informed consent for surgery was obtained after appropriate counseling.
Surgical technique
All patients were operated under general anesthesia. Patients with bilateral ED underwent sequential two-stage excision. The patient was positioned in a lateral decubitus, with the arm on the operated side extended forward and the scapula protracted anteriorly. This position allowed optimal exposure of the lesion, which usually lays under the scapular body. An oblique or transverse skin incision was performed over the mass, usually below the tip of the scapula. A muscle-sparing technique was adopted to gain access to the lesion: latissimus dorsi muscle was incised along the fibers and splitted, serratus anterior muscle was then accessed and its fibers were splitted as well. ED usually appeared as a whitish, hard and ill-defined mass, firmly attached to the serratus anterior fibers and to the periosteum of the ribs. The lesion was resected by marginal excision, which consisted in the dissection all around the mass, through the peritumoral reactive tissue, using scissors and electrocautery. A surgical margin of 3–5 mm was considered acceptable for this type of resection. A tubular suction drainage was placed and the integrity of the muscular layers was restored with interrupted resorbable sutures. Local ice application was maintained for the first 24–36 hours after the operation and a compression bandage was applied and maintained for 7–10 days. No particular restriction to shoulder movements was adopted in the postoperative period, other than avoidance of extreme abduction of the upper limb and heavy activities involving the operated side. Drain was kept in place for at least 24 hours, depending on the amount of fluid leak. In case of prolonged drainage time, the patient was discharged with the drain in place.
Data collection and follow-up
Clinical, operative and pathological data were retrieved from clinical records, databases and imaging storage system of the institution. The following variables were analyzed: age at operation, sex, body mass index (BMI), patient’s occupation, antiplatelet medications, American Society of Anesthesiologist (ASA) Scale Classification, presence of symptoms, type of preoperative imaging study (US, CT-scan and MRI), maximum ED diameter on preoperative imaging tests, operative time, drainage time, length of hospital stay, postoperative complications and maximum ED pathological diameter. Regarding patients’ occupation, manual/heavy activities either currently of formerly, were differentiated from sedentary works; hobbies were also taken into account. We usually perform an active follow-up based on clinical examination for a few months after discharge, to evaluate surgical results. After this period, we adopt an open-door policy for follow-up, in which patients and their general practitioners are encouraged to contact the institution if they suffer from any complaints. For the purposes of the study, information about recurrence was obtained by telephone interviews or from outpatient clinical visits if needed.
Statistical analysis
Statistical analysis was conducted using the SPSS statistical software 25.0 (SPSS Inc., Chicago, IL, USA). Descriptive analysis was expressed in terms of frequency, mean, median, standard deviation and range. All variables were matched to each other in a univariate analysis. Categorical variables were compared with the Chi-square test and the Fisher’s exact test for small samples. Continuous variables were compared using the t-test and the analysis of variance, while ordinal variables were compared using nonparametric tests (Kruskal-Wallis or Mann-Whitney). When necessary, continuous variables were dichotomized using the median as cut-off point. A multivariate analysis was performed to investigate the risk factors for postoperative complications; logistic regression was used and covariates odds ratios with 95% confidence intervals were reported. Linear regression was used to analyze the correlation between each imaging measure and its corresponding pathological measurement; the correlation level was expressed using the Pearson’s correlation coefficient (r). Statistical significance was defined as P<0.05.
Results
A total of 59 patients underwent surgical excision of 70 ED. Eleven patients (18.6%) presented with bilateral lesions, 24 (40.7%) with left-sided and 24 (40.7%) with right-sided lesions. Demographic and clinical characteristics of patients are summarized in Table 1.
Table 1. Characteristics of the 59 patients.
| Variables | Number |
|---|---|
| Age (years) | 59.6 [33–81] |
| Sex, N [%] | |
| Male | 24 [41] |
| Female | 35 [59] |
| Body mass index | 25.9 [17.2–39.7] |
| Manual/heavy activities, N [%] | |
| Yes | 27 [46] |
| No | 32 [54] |
| Antiplatelet therapy, N [%] | |
| Yes | 8 [14] |
| No | 51 [86] |
| ASA score, N [%] | |
| I-II | 50 [85] |
| III | 9 [15] |
Continuous variables are expressed as mean and range.
ED was symptomatic in 56 cases (80%) and symptoms were local pain and/or functional impairment of the shoulder movement in all cases. Overall, preoperative imaging evaluation included US for 56 lesions (80%), CT-scan for 47 lesions (67%) and MRI for 30 (43%), performed in various combinations. Figure 1 illustrates the preoperative work-up undertaken for the 70 lesions. In no case we considered US alone as suitable to candidate a patient for surgical excision, according to our strategy of preoperative assessment. CT-scan made a confident diagnosis of ED in 40/42 cases (95%), and no further investigation was requested; in the two remaining cases, MRI was required in addition to CT-scan to better define the lesion before the operation. In 28 cases (40%) MRI was already available at the time of our visit, thus no further examination was requested.
All lesions were completely removed by marginal excision, with no macroscopic residual tumor at surgical exploration. At histological examination, the diagnosis of ED was confirmed in all cases, as well as the complete excision of the lesions. The maximum ED pathological diameter was 79.4±18.9 mm on average (range, 50–130 mm). Surgical excision achieved complete resolution of symptoms in all patients. Perioperative features and complications of the 70 surgical procedures are reported in Table 2. Complications were managed conservatively in 9/12 cases (75%). One patient was re-operated on the first postoperative day for hematoma and two patients underwent late surgical revision for wound infection. In case of bilateral lesions, the mean interval between the two procedures was 24±11 months (range, 11–47 months). In all patients both lesions were already detectable at the time of our first visit.
Table 2. Perioperative features and complications of the 70 surgical procedures.
| Variables | Number |
|---|---|
| Operative time (min) | 90.2 [25–150] |
| Complications, N [%] | 12 [17] |
| Seroma | 7 |
| Hematoma | 2 |
| Infection | 2 |
| Prolonged impairment of shoulder movement | 1 |
| Drainage time (days) | 3.0 [1–17] |
| Length of hospital stay (days) | 2.0 [1–9] |
| Discharge with drain in place, N [%] | 13 [19] |
Continuous variables are expressed as median and range, except for operative time (mean and range).
Mean follow-up was 87 months (range, 7–196 months). No recurrence was observed.
The following significant associations were found at the univariate analysis. Male patients were more frequently involved in manual/heavy activities compared to females (71% and 30% respectively, OR 2.32, P=0.001); patients in ASA III group were older than those in ASA I-II (mean age 68.7 and 57.8 years respectively, P=0.01); patients with a BMI >25 had a longer operative time compared to those with a BMI ≤25 (mean time 96.8 and 83.8 minutes respectively, P=0.048). The occurrence of complications resulted in prolonged drainage time (mean 4.2 versus 2.9 days in the absence of complications, P=0.047) and prolonged length of stay (mean 4.2 versus 2.8 days in the absence of complications, P=0.023). Patients on antiplatelet medications experienced a prolonged drainage time compared to those without antiplatelet medications (5.2 versus 2.8 days respectively, P=0.006) and a higher rate of complications (40% versus 13.3% respectively, OR 4.33, P=0.038). In the multivariate analysis (Table 3), a low BMI was found as the only independent risk factor for postoperative complications (OR 8.71, P=0.024).
Table 3. Multivariate analysis. Risk factors for postoperative complications.
| Covariates | Complications, yes vs. no | ||
|---|---|---|---|
| OR | 95% CI | P | |
| Age (≤59 vs. >59 years) | 0.77 | 0.15–3.99 | 0.757 |
| BMI (≤25 vs. >25) | 8.71 | 1.33–57.14 | 0.024 |
| Manual/heavy activities (yes vs. no) | 1.90 | 0.44–8.20 | 0.390 |
| Antiplatelet therapy (yes vs. no) | 3.76 | 0.67–21.20 | 0.133 |
| ASA score (I-II vs. III) | 0.49 | 0.02–8.35 | 0.625 |
| Side (right vs. left) | 1.72 | 0.38–7.89 | 0.485 |
| Operative time (>90 vs. ≤90 minutes) | 2.61 | 0.55–12.34 | 0.227 |
| Pathological diameter (>80 vs. ≤80 mm) | 2.00 | 0.38–10.70 | 0.417 |
BMI, body mass index; ASA, American Society of Anesthesiologist; OR, odds ratio; CI, confidence intervals.
The correlations between the diameters measured by each imaging technique and the corresponding pathological diameters are reported in Figure 2. The regression analysis found a poor positive correlation between US and pathological dimensions (r=0.421, P=0.008; Figure 2A), a moderate positive correlation between MRI and pathological dimensions (r=0.624, P<0.001; Figure 2B) and a high positive correlation between CT and pathological dimensions (r=0.819, P<0.001; Figure 2C).
Figure 2.
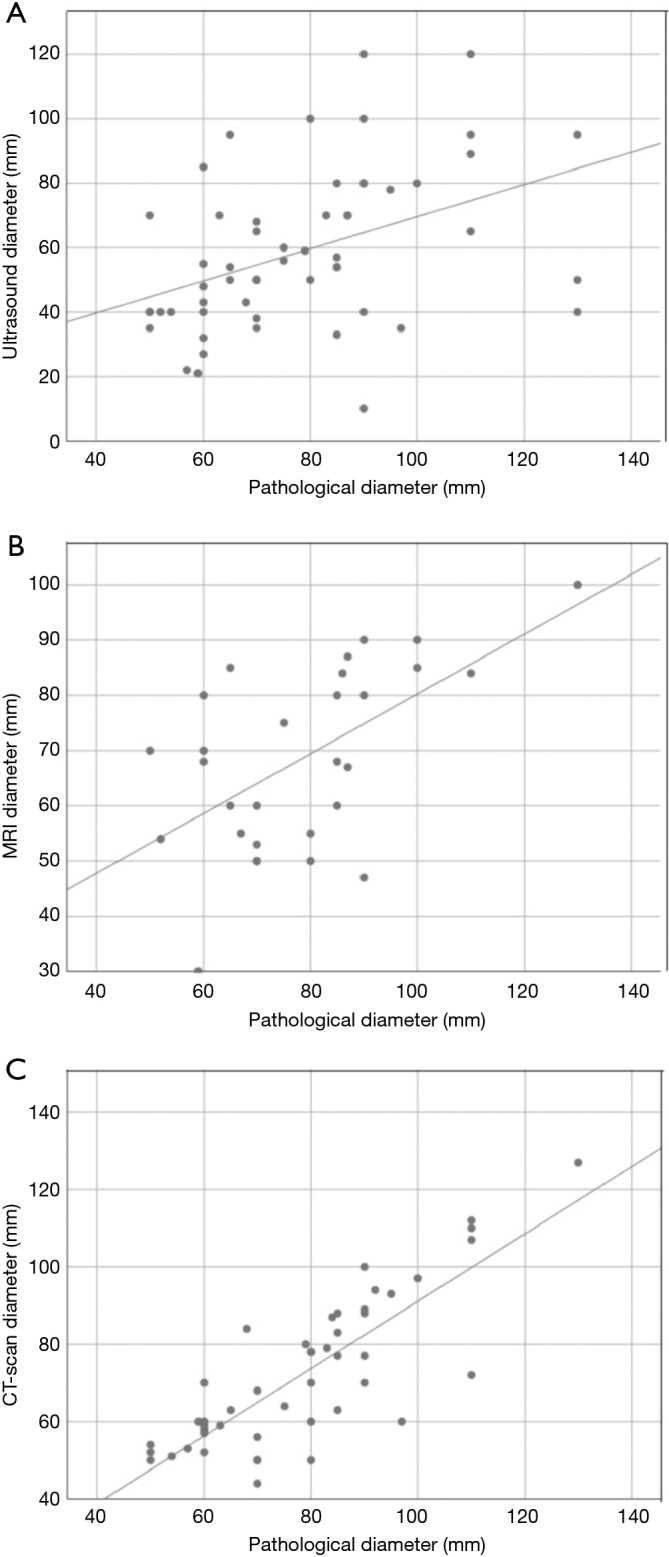
Graphics with plotted points and regression lines show the correlation between elastofibromas diameter measured by ultrasonography and pathology (A), MRI and pathology (B), CT-scan and pathology (C).
The differences between the measures obtained with each imaging method and the corresponding pathological measurements were also investigated. The results are reported in Table 4. CT-scan achieved the closest measures to the actual pathological size (mean absolute difference of 8.4 mm). All three techniques underestimated the actual dimension of the lesions in the majority of cases.
Table 4. Absolute differences between radiological and pathological diameters, and frequencies of underestimated and overestimated measurements by each imaging technique.
| Imaging technique | Difference between radiological and pathological diameters (mm) | Underestimated measurements | Overestimated measurements |
|---|---|---|---|
| Ultrasonography (n=56) | 25.0±18.7 | 48 (86%) | 8 (14%) |
| CT-scan (n=47) | 8.4±9.9 | 36 (77%) | 11 (23%) |
| MRI (n=30) | 16.7±11.6 | 24 (80%) | 6 (20%) |
Differences are expressed as means ± standard deviation.
Discussion
ED is a benign soft-tissue tumor of the chest wall, located in the deep dorsal region between the ribs and the inferomedial border of the scapula, deeper to the latissimum dorsi and serratus anterior muscles (17). ED is considered a rare disease, but the real prevalence is probably underestimated because small lesions are often asymptomatic (18). In fact, studies based on CT-scan revealed a prevalence of 1.6–2.7% (4,5), which increased up to 13–16% in autopsies series (6,7). Because our study is based on a surgical series, prevalence cannot be investigated. Data reported in the literature showed a predominance of ED in older patients and in females (2,4). In our series we observed a similar distribution, with a F:M ratio of 1.5:1 and a mean age of 59 years.
In 2013 WHO classified elastofibroma as a benign fibroblast/myofibroblast tumor (19). However, its etiology is regarded as multifactorial (1,8). Familiar occurrence (2) and identification of chromosomal changes (9,10,20) suggest that this lesion may be considered as a true neoplastic disease. On the other hand, ED has been traditionally considered as a pseudotumor, originating from periosteal fibroblasts stimulated by repetitive friction between the tip of the scapula and the thoracic wall (1,21). The typical anatomic location of ED in the parascapular region is consistent with this theory. Because forceful and repetitive movements of the shoulder girdle seem to be involved in ED pathogenesis (1,22), the relationship between manual/heavy activities and the development of ED has been investigated, and a variable correlation was found in 15% to 95% of patients (2,3). In our series, 46% of patients were manual laborers, either currently of formerly. This prevalence increased to 70.8% among men, which were more frequently involved in heavy activities compared to women (P=0.001). However, 25/35 women (71.4%) had never been involved in heavy/manual activities. Therefore, from our data we cannot fully support the pathogenetic hypothesis of the response to repetitive micro-traumas. On the other hand, no patients in our study reported a family history of ED, as already observed by others authors (2,9,13).
Patients usually refer a long clinical course of a slow-growing mass in the typical subscapular location. Bilateral ED is reported between 10% and 66% of cases (1,2). In our series, bilaterality was found in 18.6% of patients. The lesion becomes appreciable as a solid nontender mass when it grows beyond the subscapular region and it usually appears firmly adherent to the deeper planes and mobile with respect to the superficial soft tissues (15). Symptoms are generally mild to moderate, and include local discomfort or pain and functional limitation in shoulder movements. Findikcioglu found an association between ED size and the presence of symptoms (22). In our series the majority of the lesions were symptomatic (80%), but we did not find a significant correlation between tumor size and symptoms, maybe because the number of asymptomatic cases was too small (14 lesions).
Clinical history and features, typical location and/or bilaterality suggest the diagnosis of ED (14,18). The differential diagnosis is with other soft-tissue tumors of the chest wall, especially sarcomas (15,23). Therefore, at least one imaging study must be performed to confirm the diagnosis. US, CT-scan and MRI are all indicated for the radiological evaluation of clinically suspected ED (Table 5). All these imaging techniques reflect the histological features of elastofibroma with a satisfying degree of confidence (closely packed strands of collagen and elastic fibrils with interspersed adipose tissue) (2,8,17), thus avoiding the need of histological confirmation (13,23,24). Although MRI is considered the most accurate test, some authors suggest US examination as sufficient for diagnostic purposes (12,15,25).
Table 5. Imaging features of ED on US, CT-scan and MRI and their relative pros and cons in the diagnostic assessment process.
| Characteristics | Ultrasonography | CT-scan | MRI |
|---|---|---|---|
| Main features | Solid oval lesion | Solid lenticular lesion | Solid lenticular lesion |
| Ill-defined margins on superficial and deep planes | Well-defined margins on superficial planes and ill-defined margins on deep ones | Well-defined margins on superficial and deep planes | |
| No evidence of intralesional vascularization at color-Doppler evaluation | Variable and non specific enhancement pattern after i.v. administration of iodinate contrast agent | Variable and non specific enhancement pattern after i.v. administration of gadolinium-based contrast agent | |
| Pros | Easily accessible; inexpensive; quick procedure | Easily available; moderately expensive; rapid time of execution; bone integrity assessment | Optimal identification of the fibrous and adipose components of ED |
| Cons | Unable to identify the subscapular component of ED; influenced by deep artifacts and patient's body constitution | Poor evaluation of the adipose component of ED | Limited availability; expensive; longer time of execution; poor evaluation of undelaying bone structures |
ED, elastofibroma dorsi.
The assessment to select patients for surgery should add further information about the extension of the lesion and the relationship with the surrounding tissues (5,26). Lack of agreement exists regarding which is the imaging test of choice. As a consequence, in the routine clinical practice, multiple exams are often performed in the same patient, increasing time and costs (15,26). US is not able to determine the precise dimension of the lesion (26) and many authors did not consider US alone as sufficient for patient candidate to surgery (8,13). CT-scan images give precise information about the extension of the lesion (18), but has limited ability to detect thin adipose layers, thus it may be difficult to distinguish the deepest margin of the lesion from the periosteum of the ribs (8). However, the integrity of the underlying bone is easily assessed (15). MRI pattern reflects better than any other imaging test the fibrous and fatty components of ED (27), and the lesion appears well delimitated because the thin adipose layer all around is easily detectable (8,15). However, MRI does not seem to add significant information to CT-scan when performed for preoperative assessment purposes (8). In the study of Minarro, CT achieved the closest measures to reality and fewer errors compared with MRI (26).
At the time of our first visit, no prior imaging was available in 5 cases (7%); one imaging study was already available in 22 cases (31%), two imaging studies in 39 (56%), and US, CT and MRI had all been performed in 4 cases (6%). In most patients, these exams were requested by the general practitioner. US was the imaging test most frequently prescribed (56/70, 80%) especially as the first diagnostic step, and this is reasonable because it is non-invasive, quick and inexpensive. However, MRI was also prescribed in a significant number of cases (28/70, 40%), of which 22 already had US. This is consistent with the accepted notion that MRI is the exam of choice for soft-tissue tumors. Following our policy of preoperative assessment, we never prescribed US, we did not request any further exam when the patient already had CT or MRI (50/70, 71%) and we prescribed CT in the 19 cases who had no examination or US only. Finally, we requested MRI only in 2 cases showing doubtful CT-scan features, to rule out malignancy. Therefore, in 40 out of 42 cases, CT-scan alone allowed an adequate preoperative evaluation and in all these cases the diagnosis of ED was confirmed on histological examination. Moreover, CT-scan showed the highest accuracy in the measurement of the lesions, even better than MRI, while US showed the lowest one (Figure 2A,B,C and Table 4). These results are in line with the findings reported by Minarro (26). Nonetheless, MRI still represents an irreplaceable technique when malignancy is suspected.
Because there is no evidence of malignant transformation (7,27), resection of ED is not mandatory. Commonly accepted indications for surgical excision are the presence of symptoms and large lesions, usually more than 5 cm (8,11). Patient preference is also important, and the decision to operate should be taken jointly by the surgeon and the patient. Preoperative biopsy or resection with frozen section are recommended only if clinical and radiological findings are doubtful for malignancy (23,28). In our series all patients fulfilled one or both the aforementioned criteria for surgical excision. We had no case of atypical presentation, therefore preoperative biopsy or frozen section were never performed. Surgical resection usually achieves optimal resolution of local symptoms (11,13) and confirms the diagnosis of ED, as also observed in our series.
General consensus exists about performing the surgical procedure under general anesthesia (8,22,24,28,29). The location of ED is deep, and sharp dissection is often necessary due to the lack of a capsule, making the excision sometimes long and laborious. Thus, we agree that local anesthesia is not adequate to provide optimal pain control during the procedure. Interestingly, we found that overweight patients (BMI >25) experienced a prolonged operative time (P=0.048). Probably the fat tissue made more difficult the identification of the dissection plane along the pseudocapsule.
Patient positioning during operation is key to achieve better exposure of the mass. Some authors prefer a prone position (3,22), but a lateral decubitus position is the most frequently adopted (8,13,28). We also prefer a lateral decubitus position, which is more familiar and comfortable for the thoracic surgeon and less demanding for the anesthesia management (30). Anterior protraction of the ipsilateral arm allows to unmask the subscapular portion of the mass. A muscle-sparing approach is recommended: it reduces postoperative muscular impairment, still allowing optimal access to the lesion (22). Marginal resection represents the standard surgical treatment for ED, because it has been proved to be sufficient to achieve complete excision with free margins (23). All our patients underwent marginal resection through a muscle-sparing approach and a complete resection was achieved in all 70 lesions.
The most common postoperative complications after excision of ED are seroma and hematoma (8,24). Wound infection and prolonged chronic pain have also been reported (13,15,22,24). Morbidity rate reaches a prevalence of 40–44% in some case series (13,22,29), whereas larger series report a lower morbidity rate of 11–13%. (8,11,24). In our series, complications rate was 17%, therefore comparable with larger series. Seromas and hematomas accounted for 75% of these complications (9/12). Three different strategies can be adopted to reduce the rate of postoperative seromas/hematomas: positioning of a suction drainage (8,22), application of a compressive bandage (9,24) and immobilization of the shoulder on the operated side (11,22,29). The positioning of a drain and the compressive bandage are widely accepted as standard practice. Accordingly, we left a drain in place and we applied a bandage in all cases. Precise indications about the criteria for drainage removal are not clearly stated (11,24). In our patients the drain was left in place for at least 24 hours and the time for removal was dictated by the quality and quantity of fluid collected. We considered 50 mL or less in 24 hours a good cut-off for drainage removal. Postoperative mobilization of the operated shoulder remains a matter of debate. Authors’ recommendations vary among no immobilization (8,24), immobilization for one week (22,29), up to one month (11). It is definitely not clear if this practice can have beneficial effects on postoperative outcome. On the other hand, immobilization can lead to residual stiffness, chronic pain and functional impairment, especially in older patients. For these reasons, it is our practice to never impose any shoulder immobilization. In our experience, the above-mentioned drainage and bandage strategy contributed to keep the rate of seroma/hematoma acceptable. Moreover, most postoperative complications are mild and can be managed conservatively, in an outpatient setting (8,11,29). Also in our experience, all seromas were treated conservatively by fine needle aspiration, with rapid resolution. Therefore, we believe that excision of ED should not be discouraged by the possibility of complications, provided that patients candidate to surgery are adequately informed.
Some authors reported that larger lesions showed higher rate of complications (8,29). We did not observe this correlation in our study. We found that patients treated with antiplatelet medications experienced a prolonged drainage time and a higher rate of complications. Multivariate analysis did not confirm the association between antiplatelet treatment and postoperative complications, likely because of the small number of events in both variables. However, we believe that close attention should be paid when operating patients on antiplatelet therapy. In our study, a low BMI was found as a factor independently affecting postoperative morbidity, especially the occurrence of seroma/hematoma. Because seroma and hematoma are detected as collections of fluid, it is likely that they could be easily palpable in thin patients, while they remain clinically occult in patients with more fatty tissue.
Recurrence after complete excision of ED is virtually absent. Recurrences were described only in cases of incomplete resection, and even in these cases they were uncommon and occurred late. Parratt reported only one case of recurrence upon seven cases of incomplete excision of ED, 8 years after surgery (13). Battaglia reported a 2% recurrence rate after incomplete resection (15) and Mariño a 10%, still due to residual tissue after excision, with a mean time to recurrence of 47 months (23).
There is no consensus in the literature about the strategy for long-term follow-up in patients operated for ED. Only few studies described methods and timing of follow-up. Lococo operated a follow-up prolonged up to 5 years (8), while Deveci conducted follow-up for the first 1.5 years after the operation (11). El Hammoumi reported that patients were followed-up once in a month for the first six months and then at intervals of 3 months (24). Findikcioglu reported a mean follow-up of 38 months (22), Karakurt 58.4 months (28) and Mariño 7.8 years (23), but none of these authors described their policy. Conversely, Smith proposed a free follow-up, in which the patient contacted the physician only in cases of complaints (3). We also adopted this strategy and no patient has ever contacted our institution after the end of the active postoperative follow-up. For the purpose of this study, all patients were recently contacted and no recurrence was referred. Because recurrence of ED is exceedingly rare and was observed only in cases of incomplete resection, and because malignant transformation has never been described, we believe that an open-door follow-up may be adequate for operated patients, provided that a complete removal of the lesion has been achieved. Most authors agree that a simple clinical examination is sufficient and that radiological exams should be performed only in cases of suspicion of recurrence (3,11). We agree with this method of follow-up.
The retrospective and monocentric nature represent the main limitations of this study. However, this is one of the large series of resected ED. Strong points of our study are also that it thoroughly investigated the debated topics of preoperative assessment, and explored the overlooked subject of follow-up.
Conclusions
ED is strongly suspected based on history and typical clinical presentation but radiological assessment should be performed to confirm the diagnosis and for preoperative work-up. From our results, CT-scan seems to be adequate for both these purposes, and MRI could be reserved only when atypical features are present. Marginal excision allows complete resection and resolution of symptoms. Giving the benign nature of ED and the absence of recurrence after complete excision, we believe that an open-door follow-up may be adequate. These strategies for preoperative assessment and follow-up can make the management of this benign condition more time-saving and cost-effective, especially in an era in which optimization of medical resources is of outmost importance.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments. The present study was approved by the local Ethics Committee (CE number 901/2019), and in every case informed consent for surgery was obtained after appropriate counseling.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-649.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-649). The authors have no conflicts of interest to declare.
References
- 1.Kara M, Dikmen E, Kara SA, et al. Bilateral elastofibroma dorsi: proper positioning for an accurate diagnosis. Eur J Cardiothorac Surg 2002;22:839-41. 10.1016/S1010-7940(02)00475-X [DOI] [PubMed] [Google Scholar]
- 2.Nagamine N, Nohara Y, Ito E. Elastofibroma in Okinawa. A clinicopathologic study of 170 cases. Cancer 1982;50:1794-805. [DOI] [PubMed] [Google Scholar]
- 3.Smith HG, Hannay JAF, Thway K, et al. Elastofibroma dorsi: The clunking tumour that need not cause alarm. Ann R Coll Surg Engl 2016;98:208-11. 10.1308/rcsann.2016.0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandser EA, Goree JC, El-Khoury GY. Elastofibroma dorsi: prevalence in an elderly patient population as revealed by CT. AJR. Am J Roentgenol 1998;171:977-80. 10.2214/ajr.171.4.9762978 [DOI] [PubMed] [Google Scholar]
- 5.Tepe M, Polat MA, Calisir C, et al. Prevalence of elastofibroma dorsi on CT: Is it really an uncommon entity? Acta Orthop Traumatol Turc 2019;53:195-8. 10.1016/j.aott.2019.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Järvi OH, Länsimies PH. Subclinical elastofibromas in the scapular region in an autopsy series. Additional notes on the aetiology and pathogenesis of elastofibroma pseudoneoplasm. Acta Pathologica Microbiologica Scandinavica Section A Pathology 1975;83:87-108. [DOI] [PubMed] [Google Scholar]
- 7.Giebel GD, Bierhoff E, Vogel J. Elastofibroma and pre-elastofibroma--a biopsy and autopsy study. Eur J Surg Oncol 1996;22:93-6. 10.1016/S0748-7983(96)91781-3 [DOI] [PubMed] [Google Scholar]
- 8.Lococo F, Mattei F, Petrone G, et al. Elastofibroma dorsi: clinicopathological analysis of 71 cases. Thorac Cardiovasc Surg 2013;61:215-22. 10.1055/s-0032-1328932 [DOI] [PubMed] [Google Scholar]
- 9.Nishio J, Isayama T, Iwasaki H, et al. Elastofibroma dorsi: diagnostic and therapeutic algorithm. J Shoulder Elbow Surg 2012;21:77-81. 10.1016/j.jse.2011.01.043 [DOI] [PubMed] [Google Scholar]
- 10.Hernández JLG, Rodríguez-Parets JO, Valero JM, et al. High-resolution genome-wide analysis of chromosomal alterations in elastofibroma. Virchows Archiv 2010;456:681-7. 10.1007/s00428-010-0911-y [DOI] [PubMed] [Google Scholar]
- 11.Deveci MA, Özbarlas HS, Erdoğan KE, et al. Elastofibroma dorsi: clinical evaluation of 61 cases and review of the literature. Acta Orthop Traumatol Turc 2017;51:7-11. 10.1016/j.aott.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartocci M, Dell'Atti C, Meacci E, et al. Clinical features, imaging findings, treatment aspects of elastofibroma dorsi and long-term outcomes after surgical resection. Eur Rev Med Pharmacol Sci 2017;21:2061-8. [PubMed] [Google Scholar]
- 13.Parratt MTR, Donaldson JR, Flanagan AM, et al. Elastofibroma dorsi: management, outcome and review of the literature. J Bone Joint Surg Br 2010;92:262-6. 10.1302/0301-620X.92B2.22927 [DOI] [PubMed] [Google Scholar]
- 14.Malghem J, Baudrez V, Lecouvet F, et al. Imaging study findings in elastofibroma dorsi. Joint Bone Spine 2004;71:536-41. 10.1016/j.jbspin.2004.04.006 [DOI] [PubMed] [Google Scholar]
- 15.Battaglia M, Vanel D, Pollastri P, et al. Imaging patterns in elastofibroma dorsi. Eur J Radiol 2009;72:16-21. 10.1016/j.ejrad.2009.05.024 [DOI] [PubMed] [Google Scholar]
- 16.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147:573-7. 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 17.Dinauer PA, Brixey CJ, Moncur JT, et al. Pathologic and MR imaging features of benign fibrous soft-tissue tumors in adults. Radiographics 2007;27:173-87 10.1148/rg.271065065 [DOI] [PubMed] [Google Scholar]
- 18.Kransdorf MJ, Meis JM, Montgomery E. Elastofibroma: MR and CT appearance with radiologic-pathologic correlation. AJR Am J Roentgenol 1992;159:575-9. 10.2214/ajr.159.3.1503030 [DOI] [PubMed] [Google Scholar]
- 19.Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. WHO Classification of Tumours of Soft Tissue and Bone. Pathology and Genetics of Tumours of Soft Tissue and Bone.4th ed. Lyon: IARC Press, 2013. [Google Scholar]
- 20.Hisaoka M, Hashimoto H. Elastofibroma: clonal fibrous proliferation with predominant CD34-positive cells. Virchows Archiv 2006;448:195-9. 10.1007/s00428-005-0053-9 [DOI] [PubMed] [Google Scholar]
- 21.Kumaratilake JS, Krishnan R, Lomax-Smith J, et al. Elastofibroma: disturbed elastic fibrillogenesis by periosteal-derived cells? An immunoelectron microscopic and in situ hybridization study. Hum Pathol 1991;22:1017-29. 10.1016/0046-8177(91)90010-M [DOI] [PubMed] [Google Scholar]
- 22.Findikcioglu A, Kilic D, Karadayi Ş, et al. A thoracic surgeon's perspective on the elastofibroma dorsi: a benign tumor of the deep infrascapular region. Thorac Cancer 2013;4:35-40. 10.1111/j.1759-7714.2012.00139.x [DOI] [PubMed] [Google Scholar]
- 23.Tamimi Mariño I.", Solis PS, Lara AP, et al. Sensitivity and positive predictive value of magnetic resonance imaging in the diagnosis of elastofibroma dorsi: review of fourteen cases. J Shoulder Elbow Surg 2013;22:57-63. 10.1016/j.jse.2012.02.005 [DOI] [PubMed] [Google Scholar]
- 24.El Hammoumi M, Qtaibi A, Arsalane A, et al. Elastofibroma dorsi: clinicopathological analysis of 76 cases. Korean J Thorac Cardiovasc Surg 2014;47:111. 10.5090/kjtcs.2014.47.2.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bianchi S, Martinoli C, Abdelwahab IF, et al. Elastofibroma dorsi: sonographic findings. AJR Am J Roentgenol 1997;169:1113-5. 10.2214/ajr.169.4.9308474 [DOI] [PubMed] [Google Scholar]
- 26.Minarro JC, Urbano-Luque MT, López-Jordan A, et al. Roman-Torres, The comparison of measurement accuracy among three different imaging modalities in evaluating elastofibroma dorsi. an analysis of 52 cases. Int Orthop 2015;39:1145-9. 10.1007/s00264-015-2740-8 [DOI] [PubMed] [Google Scholar]
- 27.Tsubakimoto M, Yamashiro T, Tsuchiya N, et al. MRI findings and demographics of elastofibroma dorsi: assessment of diffusion-weighted imaging and contrast enhancement patterns. Acta Radiologica 2018;59:709-15. 10.1177/0284185117732099 [DOI] [PubMed] [Google Scholar]
- 28.Karakurt O, Kaplan T, Gunal N, et al. Elastofibroma dorsi management and outcomes: review of 16 cases. Interact Cardiovasc Thorac Surg 2014;18:197-201. 10.1093/icvts/ivt442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagano S, Yokouchi M, Setoyama T, et al. Elastofibroma dorsi: Surgical indications and complications of a rare soft tissue tumor. Mol Clin Oncol 2014;2:421-4. 10.3892/mco.2014.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edgcombe H, Carter K, Yarrow S. Anaesthesia in the prone position. Br J Anaesth 2008;100:165-83. 10.1093/bja/aem380 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as