Abstract
Chronic obstructive pulmonary disease (COPD) is а highly prevalent, complex and heterogeneous clinical condition which is associated with significant concomitant diseases. COPD and cardiovascular diseases (CVDs) often coexist due to the high prevalence of each of these pathological conditions separately as well as the common risk factors (particularly smoking), mechanisms of interaction and influence of systemic inflammation. In addition, decreased pulmonary function in COPD is closely associated with an increased risk of congestive CVDs. One of the most important pathophysiological markers of COPD—lung hyperinflation—plays a significant role in the appearance of functional limitations of the pumping function of the heart, creating unfavorable conditions by exerting a compression effect on the heart muscle. The latter was confirmed by significant correlation between the COPD severity according to GOLD classification and the basic dimensions of the heart chambers. Several decades ago, the term “microcardia” was commonly used and indicated a radiological sign of emphysema. Some studies demonstrated a close relationship between the chance of occurrence of CVD and the severity of pulmonary dysfunction. Such an association has been demonstrated for the whole spectrum of CVD—including cerebrovascular disease, congestive heart failure (CHF) and rhythm disturbances—and was detected in the early stages of the disease. A large proportion of patients with mild and moderate COPD die due to CVD, which is much more likely than deaths in the same group due to respiratory insufficiency. COPD patients have a higher rate of hospitalization and death, the cause of which are coronary heart disease (CHD), stroke and CHF. Treatment of COPD today is mainly determined by national and international clinical guidelines, which should pay more attention to the problems of the treatment of COPD patients with comorbid conditions.
Keywords: Chronic obstructive pulmonary disease (COPD), cardio-vascular comorbidity, coronary heart disease (CHD), chronic heart failure, rhythm disturbances, atrial fibrillation (AF)
Chronic respiratory diseases (CRDs) cause enormous burden to the society. In 2016, these diseases were responsible for 3.8 million deaths or 7% of all global deaths. Seventy-five percent of these deaths occurred in those aged 30–69 years, demonstrating that CRDs are not solely a problem for the elderly (1).
Chronic obstructive pulmonary disease (COPD) is a major CRD characterized by high prevalence and mortality, and by a persistent and usually progressive airflow limitation associated with different risk factors, the main of which being tobacco smoking and air pollution. Based on multiple large-scale epidemiological studies, the global prevalence of COPD was estimated at 11.7% in 2010, with 384 million cases (2). There are around three million COPD deaths every year (3) making this disease the third leading cause of death worldwide. Numbers are increasing (4). With the growing prevalence of smoking in developing countries and population ageing in high-income countries, the prevalence of COPD is expected to rise over the next 30 years and, by 2030, there might be more than 4.5 million deaths per year from COPD and related conditions (5,6). In most patients, COPD is associated with significant concomitant disease/conditions, which increase morbidity and mortality (7). COPD is therefore a major public health problem globally in subjects over 40 years of age (8). Pharmacological therapy is used to reduce symptoms as well as the frequency and severity of exacerbations, and to improve health status and exercise tolerance (9).
In recent years, we have a greater understanding of the heterogeneity and complexity of this disease in terms of integrated clinical assessment of the severity, pathophysiology, and the relationship with other pathological conditions. New approaches to disease classification and assessment of severity and activity have appeared in recent international and national guidelines, based on the fact that the level of airflow limitation is not sufficiently interconnected with important characteristics of the disease such as exacerbations, quality of life, and risk of mortality (10-12).
COPD is a disease that occurs in the second half of life. A typical COPD patient suffers from an average of 4 or more comorbidities, and, every day, about one third of the patients take 5 to 10 different drugs (13). In this regard, it is important to determine the significance of a particular comorbidity for the treatment of COPD.
Concomitant disease can be considered significant for the course of the underlying pathological condition (COPD) if: (I) the disease affects the course and prognosis of the COPD and vice versa (14), (II) the frequency and impact on mortality from the concomitant disease exceeds the expected rate in the general population of COPD patients (15), or (III) the presence of the concomitant disease is part of a specific COPD phenotype (16).
The extent of the problem of COPD and the coexistence of cardiovascular diseases (CVDs) is determined by the prevalence of each of these pathological conditions separately and the fact that there are many similarities between these diseases. For COPD and CVD, there are common risk factors, the most important of which being smoking.
Mechanisms of interaction between COPD and CVD. Influence of systemic inflammation
Although the lungs are considered to be the main target organ for smoking, it is well known that other systems are also affected by this risk factor, particularly the cardiovascular system (17-19). In addition, decreased pulmonary function is closely associated with an increased risk of congestive heart failure (CHF) (20-22), myocardial infarction (MI) (23), and atrial fibrillation (AF) (24). Other common risk factors for COPD and CVD are age and healthy lifestyle violation (physical activity, nutrition, etc.).
Increased levels of inflammatory markers, such as C-reactive protein (CRP) and various chemokines, play a significant role in the development of atherosclerosis, coronary heart disease (CHD), CHF and AF (25). The content of the same inflammatory markers is increased in many patients with COPD (26). Moreover, the frequency of exacerbations in COPD is associated with a more pronounced inflammation and increased risk of MI (27). However, the development of any chronic disease is a complex process—dependent on many factors—and is difficult to explain by one unique mechanism. In addition, there is insufficient evidence today that suppressing inflammation can prevent COPD in combination or without association with other diseases (Box 1).
Individuals susceptible to damage caused by pollutants and smoking can suffer from both heart and lung disease. Just as pulmonary disease can contribute to heart disease, cardiac illness can itself contribute to pulmonary disease.
Effect of lung hyperinflation
Increased air volume in the lungs is one of the most important pathophysiological markers of COPD, manifested by a decrease in exercise tolerance and an increased risk of death (28,29).
Hyperinflation and air trapping lead to flattening of the diaphragm domes, its dysfunction, and impairment of the breathing mechanics. It is documented by an increase in the level of static volume indicators during the bodyplethysmographic study (functional residual capacity, residual volume, total lung capacity) and a decrease of their surrogate marker—inspiratory capacity (30,31).
The increase in the size of the lungs due to hyperinflation has a compression effect on the heart muscle, creating unfavorable conditions for its activity and hindering the implementation of its pumping function. A number of studies have shown that an increase in the severity of COPD and the appearance of emphysema are associated with a decrease in the size of the heart during X-ray examination (32-34). Almost 40 years ago, the term “microcardia” was widely used, and was considered a radiological sign of emphysema (33).
COPD patients with a ratio of inspiratory capacity/total lung capacity <0.25 were characterized by an impaired left ventricle (LV) function which was closely correlated with a decrease in exercise tolerance during the cardio-respiratory exercise test and a decrease in oxygen pulse indices, confirming the cardiac mechanism of exercise limitation (35). Later, it was shown that the main reason for the decrease in exercise tolerance was an impairment of the LV diastolic filling (36). The close correlation of COPD severity according to the GOLD classification and the basic dimension of the heart chambers has also been demonstrated: areas of the right and left atria, right ventricle diameter, left ventricular end-diastolic diameter (36).
Role of bronchial obstruction
Bronchial obstruction or narrowing of the bronchial tree lumen, functionally manifested by a limitation of airflow, is a mandatory criterion for documenting the diagnosis of COPD.
In a longitudinal population-based study and a meta-analysis of literature, it has been shown that a low lung function marker—decreased FEV1—is associated with an increased risk of cardiovascular mortality (37). In the NHANES (National Health and Nutrition Examination Survey) epidemiological study, patients with CHD and low FEV1 levels had a 5-fold higher risk of death. Moreover, a systematic review of large cohort studies (>500 participants) that reported on the relationship between FEV1 and cardiovascular mortality (12 studies; n=83,880 participants) showed that for every 10% reduction in FEV1, CVD mortality increased by 28%, indicating a close link between cardiovascular mortality and low pulmonary function. Data from two other cohort studies involving more than 5,000 participants showed that the odds ratio (OR) of CVD presence reached 1.7 in individuals diagnosed with COPD (GOLD1). The OR index increased by up to 2.2 and 2.4 in GOLD2 and GOLD3–4 COPD patients respectively. These data demonstrate a close relationship between the possibility of occurrence of CVD and the severity of pulmonary dysfunction. It is important to note that such an association has been demonstrated for the whole spectrum of CVD, including cerebrovascular disease, CHF and rhythm disturbances, and can already be seen in the early stages of the disease (38,39).
For example, heart failure (HF) was observed in 12.1% of participants with COPD in the NHANES study [1998–2008], compared to 3.9% without a COPD diagnosis, and in 7% of participants in other large cohort studies (40).
As part of a large multicenter observational study conducted in 17 cardiac clinics in Japan, it was shown that the prevalence of spirometry markers of airflow limitation suitable for the definition of COPD was detected in 995 cardiac patients with a history of smoking (41). The overall prevalence of fixed bronchial obstruction (confirmed by the ratio of postbronchodilator spirometric ratio FEV1/FVC <0.7) was 27.0%. Eighty-seven point seven percent of these patients had not previously been diagnosed with COPD (Box 2).
The prevalence of CVD among COPD patients
In COPD patients, the risk of CVD is on average 2–3 times higher than in persons of comparable age in the general population, even when taking into account the risk of smoking (42).
Previously, it was shown that about 40% of patients with mild and moderate COPD died due to CVD, which is 8–10 times more likely than deaths in the same group due to respiratory insufficiency (40,42). During the 5 years of follow-up, COPD patients had a higher rate of hospitalization and death, the cause of which was CHD, stroke and CHF.
One of the clear visual forms systematizing and reflecting the prevalence of various comorbid conditions in COPD—as well as their impact on the prognosis—is the so-called “comorbidome”, presented by Divo et al. (Figure 1) (15).
Figure 1.
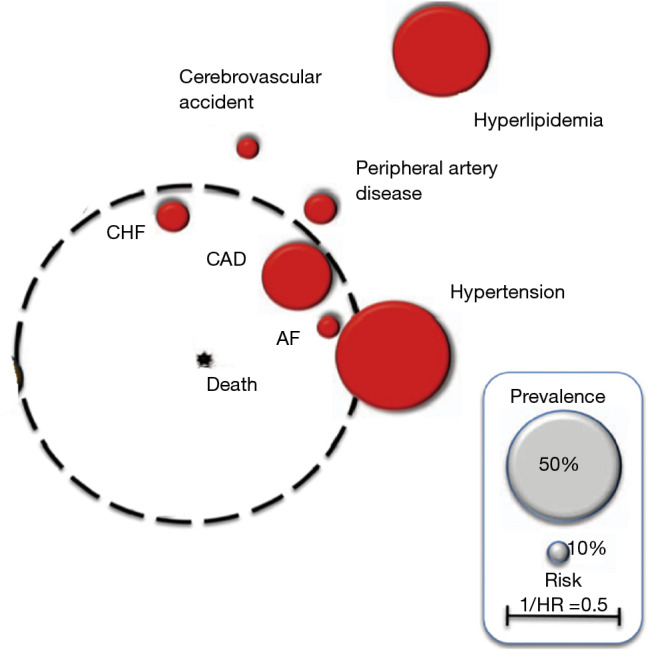
The “comorbidome” is a graphic expression of comorbidities with a prevalence of more than 10% in the entire cohort, and with the strongest relationship with mortality (HR: 0.1; 95% CI: 0.1; P<0.05). Adapted with permission from Am J Respir Crit Care Med. HR, hazard ratio; CI, confidence interval; AF, atrial fibrillation; CAD, coronary artery disease; CHF, congestive heart failure.
In this adapted diagram, the associated CVDs have a spherical image of varying magnitude.
The size of the circles reflects the prevalence of each disease in the population of COPD patients. The distance from the center of the diagram reflects the influence of this disease on the probability of death, expressed in the form of 1/hazard ratio (HR).
As a guideline for prevalence, grey circles are presented, the size of which reflects the prevalence of 50% (large) and 10% (small), respectively.
The above scheme clearly demonstrates that the most common comorbid conditions in COPD, conditionally related to the cardiovascular group, are hyperlipidemia and hypertension. At the same time, they play a smaller role in increasing the likelihood of death when compared to the less common CHD, CHF and AF. These last 3 comorbidities are the most significant from the point of view of the prognosis of COPD and deserve a separate more detailed discussion, as they have accumulated the greatest amount of evidence (Box 3).
CHD
Between COPD and CHD, there is a fairly strong epidemiological relationship, which persists regardless of statistical adjustment for the common risk factors (smoking index, cholesterol level, etc.). Both diseases are characterized by systemic inflammation, oxidative stress and coagulopathy.
The main marker of inflammation is CRP, the increased level of which simultaneously contributes to an increase in the level of bronchial resistance and an increase in the risk of CHD (43,44). At the same time, the activation of immune cells in atheromatous plaques, which occurs in CHD, stimulates the production of cytokines (interferon γ, interleukin-1, tumor necrosis factor α and interleukin-6), as well as inflammatory proteins of the acute phase (fibrinogen, CRP), which are involved in the inflammatory reaction of the airways in COPD (43,44).
Despite the close relationship, the question of the connection between the severity of COPD and the frequency of accompanying CHD remains important. It was shown that a particularly high level of angiographically-confirmed coronary artery disease (59%) was observed in patients with severe COPD awaiting pulmonary transplantation (45).
At the same time, the prevalence of COPD is high in patients with CHD, but, as in patients with CHF in cardiological practice, there is a high level of underdiagnosis of COPD (46-48). A recent study showed that airflow limitation was documented in 30.5% of patients with confirmed CHD (49).
If we compare stable COPD patients with accompanying CHD and patients without comorbidity, patients with comorbidity are characterized by: older age, of male sex, higher smoking index, lower quality of life and tolerance of physical activity, increased intensity of shortness of breath and a longer recovery period after an exacerbation (50,51).
In the acute period of the disease, patients with comorbidity may have manifestations of both diseases, and coronary events may be combined with exacerbations of COPD (52-55).
Concomitant COPD increases morbidity and mortality among patients with coronary artery disease. Signs of myocardial and endothelial damage in COPD are not always detected, which leads to the underdiagnosis of CHD. Even the presence of symptoms of simple chronic bronchitis increases the risk of death from coronary episodes by 50% (56). In addition, during the exacerbation of COPD, the risk of myocardial damage in patients with concomitant CHD increases. The presence of concomitant COPD may, in some cases, complicate the recognition of MI and contribute to its delayed diagnosis (57).
Detection of COPD in patients with CHD requires spirometric studies to identify and document airflow limitation (9). At the same time, spirometric examination should not be performed in patients with an unstable condition of the cardiovascular system: within 1 week (or 1 month) after MI (58). Despite the simplicity of diagnostic approaches, the underdiagnosis of COPD in patients with CHD and the associated lack of appropriate therapy remains a major problem.
According to published data, approximately 1 in 12 patients with severe or very severe airflow limitation hospitalized with COPD exacerbation had chest pain and/or ECG changes in dynamics that met the MI criteria (59). Moreover, available data demonstrate that increased cardiac troponin during COPD exacerbations is an independent prognostic marker of all-cause mortality (60,61). These data suggest that episodes of exacerbation may be associated with damage to the myocardium of varying degrees, which in turn may play a role in future cardiac events (62).
It remains an open question as to whether the risk of COPD increases as the severity of CVD worsens: according to the classification of GOLD, there is a significant heterogeneity of cardiovascular risk, even in cases of one category of severity (28).
Arrhythmias (AF)
AF is the most common supraventricular arrhythmia in the general population and in patients with COPD (20). Despite the fact that the largest number of publications is devoted to the combination of COPD and AF, there are also studies that investigated the combination of COPD with other rhythm disturbances: atrial tachycardia, atrial flutter, ventricular tachycardia and conduction disorders (63,64). The prevalence of AF in patients with stable COPD ranges from 4.7% to 15% (65), with a significant increase in patients with very severe COPD (20–30%) (16).
Moreover, the frequency of AF is closely related to the major spirometric indicator of the degree of bronchial obstruction—FEV1 (66).
On the other hand, the prevalence of COPD among patients with AF ranges from 10–15%, reaching 65% in patients older than 65 years (67,68). Exacerbations of COPD can be a trigger factor that causes AF, and, in turn, AF can cause exacerbations of COPD. The latter is a risk factor of sudden cardiac death, both in cohorts of CVD patients and in the general population.
AF in various studies was a negative prognostic factor in COPD patients with respect to quality of life and health status (69), risk of hospitalizations (45) and mortality from all causes (16,70-72).
AF in COPD with the lack of control of the ventricular contraction frequency can be accompanied by shortness of breath and pulmonary edema and is often attributed to exacerbations of COPD (54). On the other hand, impaired gas exchange, hypoxia and hypercapnia, as well as oxidative stress in exacerbations of COPD may be the causes of arrhythmias (73).
HF
According to the recent definition of the European Society of Cardiology, HF is determined as a syndrome in which patients have typical symptoms (shortness of breath, swelling of ankles, fatigue) and signs (elevated jugular pressure, crackling, peripheral edema), caused by disruption of the structure and/or function of the heart that leads to a decrease in cardiac output and/or increased intracardiac pressures at rest or during exercise (74).
The prevalence of HF among COPD patients is significantly higher (10–30%) compared to the general population (1–2%), with an estimated annual incidence of 3.7% and an OR of 2.57 [95% confidence interval (CI): 1.90–3.47; P<0.0001] (11,31). COPD is also a frequent concomitant disease in patients with HF and occurs, according to different studies, in 13–39% of patients (75,76).
In addition, according to a recent meta-analysis, COPD increases the risk of mortality in patients with HF (OR: 1.24–1.7).
Patients with stable COPD and HF are slightly older than patients with COPD and lack of HF, include a higher percentage of men, have more apparent symptoms and more concomitant diseases (15,77).
Exacerbations of COPD, as well as acute HF, are caused by different trigger factors: e.g., respiratory infections and pollutants in COPD and arrhythmias; acute coronary syndrome and hypertension in acute HF (78,79). At the same time, the mechanisms of pulmonary-cardiac interaction are quite complex, and respiratory symptoms often have both pulmonary and cardiac origins (52,59). In many cases, chronic HF is not detected in patients hospitalized for exacerbation of COPD, and is a cause that makes it difficult to cancel respiratory support and worsens the overall prognosis (80,81).
Thus, the differential diagnosis of COPD in patients with HF and vice versa may be difficult, especially in elderly patients and in smokers, with severe shortness of breath as the main symptom. In this case, spirometry can provide significant help (9,82). However, conducting a correct interpretation of the spirometric study in patients with CHF may be difficult, since spirometry cannot be performed in a patient with rapidly increasing decompensation and there is a certain risk of overdiagnosis of COPD (83). In addition, even in a stable and euvolemic state, patients with low ejection fraction (EF) can experience a 20% decrease in both FEV1 and FVC compared to the control group. However, the FEV1/FVC ratio does not change and remains at a high level, allowing the exclusion of bronchial obstruction (84). Body plethysmography is an important additional diagnostic tool that allows the correct identification of COPD in patients with low EF (85). Thus, the clinical decision, which requires identification of COPD in this category of patients, should be based on risk factors and spirometry conducted in the stable phase of the disease, supplemented, in certain cases, by data of body plethysmography.
On the other hand, the diagnosis of HF in COPD presents the same difficulties due to the commonality of risk factors and similar clinical manifestations (84,86,87). Reduced EF is the main criteria for the diagnosis of HF in COPD patients indicated in the literature (88).
In the absence of a decrease in EF, besides the clinical picture, it is recommended to use the following criteria:
elevated levels of natriuretic peptides: brain natriuretic peptide (BNP) >35 PG/mL and/or its N-terminal precursor (NT-proBNP) >125 PG/mL;
objective confirmation of structural changes in the heart: increase in LV myocardial mass index or left atrium size, or diastolic dysfunction detected by echocardiography (75).
At the same time, COPD itself can have an independent effect on cardiac function, thereby influencing the results of diagnostic tests. For example, a direct relationship between NT-proBNP and FEV1 was established in elderly individuals without HF (21).
Echocardiography remains the most important method in the diagnosis of HF. However, a significant problem in the application of this method is a significant reduction in the size of the acoustic window due to hyperinflation in emphysema in COPD patients, leading to a poor quality of the images obtained in 10–50% of patients (87) (Box 4).
Treatment of COPD with concomitant CVD in pulmonological practice
The COPD management in clinical practice of recent years is guided by national and international clinical recommendations, which are currently undergoing major changes (9). Unfortunately, none of these documents pay enough attention to the problems related to the treatment of COPD patients with comorbid conditions. These problems are often faced by doctors of both specialties (cardiologists and pulmonologists).
The basis of therapy for stable COPD is (I) smoking cessation, (II) administration of short- and long-acting bronchodilators (BD) (β2-agonists and anticholinergics), both individually and in the form of free and fixed combinations, (III) the use of combinations of inhaled corticosteroids (ICS) and long-acting β2-agonists (LABA), and more recently (IV) triple therapy in a single inhaler.
Cardiovascular safety of BD remains a debatable subject, especially in concomitant CVD. Whether BD increases the risk of developing HF in COPD patients or whether patients with COPD and concomitant HF have an increased risk of side effects remains an open question. For example, the results of most clinical studies indicate that LABA has a satisfactory cardiovascular safety profile (89). However, this cannot be fully transferred to patients with HF, in whom a change in the β-receptor system may be observed (for example, regulation by the type of negative feedback of β1 receptors while maintaining the unchanged level of β2 receptors) (90), which may lead to greater sensitivity to inotropic effects. Thus, in patients taking LABA, hospitalization or emergency care for HF may be more likely, especially in the first 2–3 weeks (91). However, in other studies, data on the increased risk of cardiovascular events and deaths in patients with HF treated by LABA were not confirmed (92,93).
Similar discussions have been conducted over the past 10 years on the safety profile of anticholinergics (94,95). Short-acting antimuscarinic agents (SAMA), such as ipratropium bromide, may slightly increase the risk of HF (96). However, with the use of long-acting antimuscarinic agents (LAMA), such as tiotropium, glycopyronium, aclidinium, umeclidinium and even a combination of LAMA and LABA, no data on an increase in the risk of HF were obtained (97-103).
In studies on the safety of phosphodiesterase-4 inhibitor (roflumilast) and ICS, no data were obtained on the increase in the risk of HF (104-106). A recently published landmark study among COPD patients with increased cardiovascular risk—SUMMIT—confirmed the cardiovascular safety of the combination of ICS/LABA (fluticasone furoate/vilanterol) (107). However, it should be noted that patients with severe HF and low FV (<30%) were excluded from the study.
Thus, it seems that LAMA are more preferable than LABA for the treatment of COPD and HF patients with low EF (108). Careful observation of COPD patients with HF could also be recommended during the first weeks of BD treatment (91), especially those with low EF. However, to date, there is no direct evidence that COPD patients with concomitant HF should be treated differently (9).
Regarding COPD patients with concomitant CHD, it was shown that the main classes of medications (LABA, LAMA and combinations of ICS/LABA) are effective and safe (89,109,110). In addition, in the FLAME study, the incidence of fatal MI, unstable angina and coronary revascularization was practically identical between groups of patients treated with a combination of LABA-LAMA (indacaterol-glycopyronium) and ICS-LABA (fluticasone-salmeterol) (102). The SUMMIT study demonstrated that the incidence of MI and unstable angina did not differ significantly in groups of COPD patients with increased cardiovascular risk treated with a combination of ICS-LABA (fluticasone-vilanterol), monocomponents and placebo (107).
The presence of AF in COPD patients should not have a serious impact on the therapy of the underlying disease. For many years, BD have been considered as having a potential arrhythmogenic effect. However, at present, there is a sufficient amount of evidence confirming the acceptable safety profile of LABA, LAMA and ICS (111-113). Nevertheless, short-acting β2-agonists and theophylline should be administered with caution: if they aggravate AF, and/or impair the control of ventricular contraction frequency (114-116), they should be stopped as soon as possible.
Oral macrolides, which are often prescribed for COPD exacerbations, can increase the duration of QT interval as well as the risk of arrhythmias and sudden cardiac death (117,118).
In conclusion, it should be emphasized that COPD patients today are a heterogeneous group of subjects with a complex multimorbidity, requiring a differentiated approach. Current available data cannot answer all the questions faced by doctors of different specialities, who encounter such patients in their daily practice. At the same time, it becomes obvious that only an integrated holistic multidisciplinary approach will achieve success in the understanding and management of this group of patients (Box 5).
Box 1 Clinical notes I: note of COPD and comorbidities interrelationship.
| Patients with COPD and comorbidities require an integrated clinical assessment of the severity, pathophysiology, and relationship with other pathological conditions |
| The comorbid condition affects the course and prognosis of COPD and vice versa |
| Pulmonary disease can contribute to heart disease, and, on the other hand, a cardiac pathologic condition can itself contribute to pulmonary disease |
COPD, chronic obstructive pulmonary disease.
Box 2 Clinical notes II: note of the influence of lung function on cardiovascular comorbidity.
| Decreased pulmonary function is closely associated with an increased risk of CVD |
| Hyperinflation can render a compression effect on the heart, creating unfavorable conditions for myocardial function |
| Association between lung function and possibility of disease occurrence has been demonstrated for the whole spectrum of CVD, including cerebrovascular disease |
CVD, cardiovascular disease.
Box 3 Clinical notes III: note of typical features of COPD patients with cardiovascular comorbidities.
| In COPD patients, the risk of CVD is higher than in persons of comparable age in the general population |
| In patients with CVD, there is a high level of underdiagnosis of COPD and vice versa |
| Patients with cardiovascular comorbidity are usually older, more often male, have a higher smoking index, a lower quality of life and exercise tolerance, a higher dyspnea score and a longer recovery period after an exacerbation |
COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease.
Box 4 Clinical notes IV: note of COPD exacerbations and cardiovascular events.
| During exacerbation, patients with COPD and CVD may have manifestations of both diseases and cardiac events may combine with exacerbations of COPD |
| Exacerbations of COPD can be a trigger factor that causes arrhythmias, and, in turn, rhythm disturbances can cause COPD acute exacerbations |
COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease.
Box 5 Clinical notes V: note of cardiovascular safety of basic COPD management.
| It is important to determine the significance of a particular comorbidity for the treatment of COPD |
| The results of most clinical studies indicate that basic COPD pharmacotherapy (LAMA, LABA, LAMA-LABA and their combinations with ICS, as well as phosphodiesterase-4 inhibitor-roflumilast) has a satisfactory cardiovascular safety profile |
COPD, chronic obstructive pulmonary disease; LAMA, long-acting antimuscarinic agents; LABA, long-acting β2-agonists; ICS, inhaled corticosteroids.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editors (Yousser Mohammad, Alvaro Cruz) for the series “GARD Section” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.60). The series “GARD Section” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
References
- 1.World Health Organization. Noncommunicable diseases country profiles 2018. Geneva: World Health Organization, 2018. Available online: https://www.who.int/nmh/publications/ncd-profiles-2018/en
- 2.Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. J Glob Health 2015;5:020415. 10.7189/jogh.05.020415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385:117-71. 10.1016/S0140-6736(14)61682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burney PG, Patel J, Newson R, et al. Global and regional trends in COPD mortality, 1990-2010. Eur Respir J 2015;45:1239-47. 10.1183/09031936.00142414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J 2006;27:397-412. 10.1183/09031936.06.00025805 [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Projections of mortality and causes of death, 2016 to 2060. Geneva: World Health Organization. Available online: http://www.who.int/healthinfo/global_burden_disease/projections/en/ (accessed 14 October 2018).
- 7.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J 2009;33:1165-85. 10.1183/09031936.00128008 [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach. Geneva: World Health Organization, 2007. Available online: http://www.who.int/gard/publications/GARD_Manual/en/
- 9.Global Initiative for Chronic Obstructive Lung Disease. 2020 Gold reports. 2020 Global strategy for prevention, diagnosis and management of COPD. Available online: https://goldcopd.org/gold-reports/
- 10.Miller J, Edwards LD, Agustí A, et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir Med 2013;107:1376-84. 10.1016/j.rmed.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 11.Müllerová H, Landis SH, Aisanov Z, et al. Health behaviors and their correlates among participants in the Continuing to Confront COPD International Patient Survey. Int J Chron Obstruct Pulmon Dis 2016;11:881-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miravitlles M, Roche N, Cardoso J, et al. Chronic obstructive pulmonary disease guidelines in Europe: a look into the future. Respir Res 2018;19:11. 10.1186/s12931-018-0715-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barr RG, Celli BR, Mannino DM, et al. Comorbidities, patient knowledge, and disease management in a national sample of patients with COPD. Am J Med 2009;122:348-55. 10.1016/j.amjmed.2008.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mannino DM, Thorn D, Swensen A, et al. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J 2008;32:962-9. 10.1183/09031936.00012408 [DOI] [PubMed] [Google Scholar]
- 15.Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012;186:155-61. 10.1164/rccm.201201-0034OC [DOI] [PubMed] [Google Scholar]
- 16.Martinez CH, Han MK. Contribution of the environment and comorbidities to chronic obstructive pulmonary disease phenotypes. Med Clin North Am 2012;96:713-27. 10.1016/j.mcna.2012.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The health consequences of smoking—50 years of progress: a report of the surgeon general. Atlanta: Centers for Disease Control and Prevention (US), 2014. [PubMed] [Google Scholar]
- 18.Vanfleteren LE, Spruit MA, Groenen M, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;187:728-35. 10.1164/rccm.201209-1665OC [DOI] [PubMed] [Google Scholar]
- 19.Müllerova H, Agusti A, Erqou S, et al. Cardiovascular comorbidity in COPD: systematic literature review. Chest 2013;144:1163-78. 10.1378/chest.12-2847 [DOI] [PubMed] [Google Scholar]
- 20.Agarwal SK, Heiss G, Barr RG, et al. Airflow obstruction, lung function, and risk of incident heart failure: the Atherosclerosis Risk in Communities (ARIC) study. Eur J Heart Fail 2012;14:414-22. 10.1093/eurjhf/hfs016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wannamethee SG, Shaper AG, Papacosta O, et al. Lung function and airway obstruction: associations with circulating markers of cardiac function and incident heart failure in older men-the British Regional Heart Study. Thorax 2016;71:526-34. 10.1136/thoraxjnl-2014-206724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simbirtseva AS, Rylova NV. Prognostic role of clinical phenotypes and verified flora in patients with pneumonia associated with decompensated CHF. Russian Heart Failure Journal 2015;16:331-8. 10.18087/rhfj.2015.6.2174 [DOI] [Google Scholar]
- 23.Tockman MS, Pearson JD, Fleg JL, et al. Rapid decline in FEV1. A new risk factor for coronary heart disease mortality. Am J Respir Crit Care Med 1995;151:390-8. 10.1164/ajrccm.151.2.7842197 [DOI] [PubMed] [Google Scholar]
- 24.Li J, Agarwal SK, Alonso A, et al. Airflow obstruction, lung function, and incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2014;129:971-80. 10.1161/CIRCULATIONAHA.113.004050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pai JK, Pischon T, Ma J, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med 2004;351:2599-610. 10.1056/NEJMoa040967 [DOI] [PubMed] [Google Scholar]
- 26.Gan WQ, Man SF, Senthilselvan A, et al. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004;59:574-80. 10.1136/thx.2003.019588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel AR, Kowlessar BS, Donaldson GC, et al. Cardiovascular risk, myocardial injury, and exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;188:1091-9. 10.1164/rccm.201306-1170OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J 2004;23:832-40. 10.1183/09031936.04.00116004 [DOI] [PubMed] [Google Scholar]
- 29.Casanova C, Cote C, de Torres JP, et al. Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005;171:591-7. 10.1164/rccm.200407-867OC [DOI] [PubMed] [Google Scholar]
- 30.De Troyer A. Effect of hyperinflation on the diaphragm. Eur Respir J 1997;10:708-13. [PubMed] [Google Scholar]
- 31.Krieger BP. Hyperinflation and intrinsic positive end-expiratory pressure: less room to breathe. Respiration 2009;77:344-50. 10.1159/000192790 [DOI] [PubMed] [Google Scholar]
- 32.Thurlbeck WM, Simon G. Radiographic appearance of the chest in emphysema. AJR Am J Roentgenol 1978;130:429-40. 10.2214/ajr.130.3.429 [DOI] [PubMed] [Google Scholar]
- 33.Wigh RE. On defining microcardia: application in pulmonary emphysema. South Med J 1978;71:150-4. 10.1097/00007611-197802000-00020 [DOI] [PubMed] [Google Scholar]
- 34.Hutsebaut J, Scano G, Garcia-Herreros P, et al. Hemodynamic characteristics in chronic obstructive lung disease as related to cardiac size. Respiration 1981;41:25-32. 10.1159/000194355 [DOI] [PubMed] [Google Scholar]
- 35.Vassaux C, Torre-Bouscoulet L, Zeineldine S, et al. Effects of hyperinflation on the oxygen pulse as a marker of cardiac performance in COPD. Eur Respir J 2008;32:1275-82. 10.1183/09031936.00151707 [DOI] [PubMed] [Google Scholar]
- 36.Watz H, Waschki B, Meyer T, et al. Decreasing cardiac chamber sizes and associated heart dysfunction in COPD: role of hyperinflation. Chest 2010;138:32-8. 10.1378/chest.09-2810 [DOI] [PubMed] [Google Scholar]
- 37.Sin DD, Man SF. Chronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortality. Proc Am Thorac Soc 2005;2:8-11. 10.1513/pats.200404-032MS [DOI] [PubMed] [Google Scholar]
- 38.Lindberg A, Larsson LG, Rönmark E, et al. Co-morbidity in mild-to-moderate COPD: comparison to normal and restrictive lung function. COPD 2011;8:421-8. 10.3109/15412555.2011.629858 [DOI] [PubMed] [Google Scholar]
- 39.Chen W, Thomas J, Sadatsafavi M, et al. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med 2015;3:631-9. 10.1016/S2213-2600(15)00241-6 [DOI] [PubMed] [Google Scholar]
- 40.Schnell K, Weiss CO, Lee T, et al. The prevalence of clinically-relevant comorbid conditions in patients with physician-diagnosed COPD: a cross-sectional study using data from NHANES 1999-2008. BMC Pulm Med 2012;12:26. 10.1186/1471-2466-12-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Onishi K, Yoshimoto D, Hagan GW, et al. Prevalence of airflow limitation in outpatients with cardiovascular diseases in Japan. Int J Chron Obstruct Pulmon Dis 2014;9:563-8. 10.2147/COPD.S59962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vanfleteren LEGW, Spruit MA, Wouters EFM, et al. Management of chronic obstructive pulmonary disease beyond the lungs. Lancet Respir Med 2016;4:911-24. 10.1016/S2213-2600(16)00097-7 [DOI] [PubMed] [Google Scholar]
- 43.Brusselle GG, Joos GF, Bracke KR. New insights into the immunology of chronic obstructive pulmonary disease. Lancet 2011;378:1015-26. 10.1016/S0140-6736(11)60988-4 [DOI] [PubMed] [Google Scholar]
- 44.Papaioannou AI, Mazioti A, Kiropoulos T, et al. Systemic and airway inflammation and the presence of emphysema in patients with COPD. Respir Med 2010;104:275-82. 10.1016/j.rmed.2009.09.016 [DOI] [PubMed] [Google Scholar]
- 45.Curkendall SM, DeLuise C, Jones JK, et al. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol 2006;16:63-70. 10.1016/j.annepidem.2005.04.008 [DOI] [PubMed] [Google Scholar]
- 46.Reed RM, Eberlein M, Girgis RE, et al. Coronary artery disease is under-diagnosed and under-treated in advanced lung disease. Am J Med 2012;125:1228.e13-22. 10.1016/j.amjmed.2012.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arnett DK, Goodman RA, Halperin JL, et al. AHA/ACC/HHS strategies to enhance application of clinical practice guidelines in patients with cardiovascular disease and comorbid conditions: from the American Heart Association, American College of Cardiology, and US Department of Health and Human Services. Circulation 2014;130:1662-7. 10.1161/CIR.0000000000000128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kozlova LI, Aĭsanov ZR, Chuchalin AG. What is the danger of long-term application of beta blockers in patients with ischemic heart disease and concomitant chronic obstructive pulmonary disease? Ter Arkh 2005;77:18-23. [PubMed] [Google Scholar]
- 49.Franssen FM, Soriano JB, Roche N, et al. Lung function abnormalities in smokers with ischemic heart disease. Am J Respir Crit Care Med 2016;194:568-76. 10.1164/rccm.201512-2480OC [DOI] [PubMed] [Google Scholar]
- 50.Rodríguez LA, Wallander MA, Martín-Merino E, et al. Heart failure, myocardial infarction, lung cancer and death in COPD patients: a UK primary care study. Respir Med 2010;104:1691-9. 10.1016/j.rmed.2010.04.018 [DOI] [PubMed] [Google Scholar]
- 51.Patel ARC, Donaldson GC, Mackay AJ, et al. The impact of ischemic heart disease on symptoms, health status, and exacerbations in patients with COPD. Chest 2012;141:851-7. 10.1378/chest.11-0853 [DOI] [PubMed] [Google Scholar]
- 52.MacDonald MI, Shafuddin E, King PT, et al. Cardiac dysfunction during exacerbations of chronic obstructive pulmonary disease. Lancet Respir Med 2016;4:138-48. 10.1016/S2213-2600(15)00509-3 [DOI] [PubMed] [Google Scholar]
- 53.Campo G, Pavasini R, Malagù M, et al. Chronic obstructive pulmonary disease and ischemic heart disease comorbidity: overview of mechanisms and clinical management. Cardiovasc Drugs Ther 2015;29:147-57. 10.1007/s10557-014-6569-y [DOI] [PubMed] [Google Scholar]
- 54.Roca M, Verduri A, Corbetta L, et al. Mechanisms of acute exacerbation of respiratory symptoms in chronic obstructive pulmonary disease. Eur J Clin Invest 2013;43:510-21. 10.1111/eci.12064 [DOI] [PubMed] [Google Scholar]
- 55.Donaldson GC, Hurst JR, Smith CJ, et al. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest 2010;137:1091-7. 10.1378/chest.09-2029 [DOI] [PubMed] [Google Scholar]
- 56.Onishi K. Total management of chronic obstructive pulmonary disease (COPD) as an independent risk factor for cardiovascular disease. J Cardiol 2017;70:128-34. 10.1016/j.jjcc.2017.03.001 [DOI] [PubMed] [Google Scholar]
- 57.Rothnie KJ, Smeeth L, Herrett E, et al. Closing the mortality gap after a myocardial infarction in people with and without chronic obstructive pulmonary disease. Heart 2015;101:1103-10. 10.1136/heartjnl-2014-307251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cooper BG. An update on contraindications for lung function testing. Thorax 2011;66:714-23. 10.1136/thx.2010.139881 [DOI] [PubMed] [Google Scholar]
- 59.McAllister DA, Maclay JD, Mills NL, et al. Diagnosis of myocardial infarction following hospitalisation for exacerbation of COPD. Eur Respir J 2012;39:1097-103. 10.1183/09031936.00124811 [DOI] [PubMed] [Google Scholar]
- 60.Pavasini R, d'Ascenzo F, Campo G, et al. Cardiac troponin elevation predicts all-cause mortality in patients with acute exacerbation of chronic obstructive pulmonary disease: systematic review and meta-analysis. Int J Cardiol 2015;191:187-93. 10.1016/j.ijcard.2015.05.006 [DOI] [PubMed] [Google Scholar]
- 61.Neukamm AM, Høiseth AD, Hagve TA, et al. High-sensitivity cardiac troponin T levels are increased in stable COPD. Heart 2013;99:382-7. 10.1136/heartjnl-2012-303429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee HM, Lee J, Lee K, et al. Relation between COPD severity and global cardiovascular risk in US adults. Chest 2012;142:1118-25. 10.1378/chest.11-2421 [DOI] [PubMed] [Google Scholar]
- 63.att SP, Dransfield MT. Chronic obstructive pulmonary disease and cardiovascular disease. Transl Res 2013;162:237-51. 10.1016/j.trsl.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 64.Goudis CA, Konstantinidis AK, Ntalas IV, et al. Electrocardiographic abnormalities and cardiac arrhythmias in chronic obstructive pulmonary disease. Int J Cardiol 2015;199:264-73. 10.1016/j.ijcard.2015.06.096 [DOI] [PubMed] [Google Scholar]
- 65.Buch P, Friberg J, Scharling H, et al. Reduced lung function and risk of atrial fibrillation in the Copenhagen City Heart Study. Eur Respir J 2003;21:1012-6. 10.1183/09031936.03.00051502 [DOI] [PubMed] [Google Scholar]
- 66.Konecny T, Park JY, Somers KR, et al. Relation of chronic obstructive pulmonary disease to atrial and ventricular arrhythmias. Am J Cardiol 2014;114:272-7. 10.1016/j.amjcard.2014.04.030 [DOI] [PubMed] [Google Scholar]
- 67.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1-76. 10.1016/j.jacc.2014.03.022 [DOI] [PubMed] [Google Scholar]
- 68.Knuiman M, Briffa T, Divitini M, et al. A cohort study examination of established and emerging risk factors for atrial fibrillation: the Busselton Health Study. Eur J Epidemiol 2014;29:181-90. 10.1007/s10654-013-9875-y [DOI] [PubMed] [Google Scholar]
- 69.Miyazaki M, Nakamura H, Chubachi S, et al. Analysis of comorbid factors that increase the COPD assessment test scores. Respir Res 2014;15:13. 10.1186/1465-9921-15-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Atzema CL, Lam K, Young C, et al. Patients with atrial fibrillation and an alternative primary diagnosis in the emergency department: a description of their characteristics and outcomes. Acad Emerg Med 2013;20:193-9. 10.1111/acem.12078 [DOI] [PubMed] [Google Scholar]
- 71.Steer J, Gibson J, Bourke SC. The DECAF Score: predicting hospital mortality in exacerbations of chronic obstructive pulmonary disease. Thorax 2012;67:970-6. 10.1136/thoraxjnl-2012-202103 [DOI] [PubMed] [Google Scholar]
- 72.Ekström MP, Jogréus C, Ström KE. Comorbidity and sex-related differences in mortality in oxygen-dependent chronic obstructive pulmonary disease. PLoS One 2012;7:e35806. 10.1371/journal.pone.0035806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Terzano C, Romani S, Conti V, et al. Atrial fibrillation in the acute, hypercapnic exacerbations of COPD. Eur Rev Med Pharmacol Sci 2014;18:2908-17. [PubMed] [Google Scholar]
- 74.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129-200. 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 75.Mentz RJ, Kelly JP, von Lueder TG, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol 2014;64:2281-93. 10.1016/j.jacc.2014.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kwon BJ, Kim DB, Jang SW, et al. Prognosis of heart failure patients with reduced and preserved ejection fraction and coexistent chronic obstructive pulmonary disease. Eur J Heart Fail 2010;12:1339-44. 10.1093/eurjhf/hfq157 [DOI] [PubMed] [Google Scholar]
- 77.Dal Negro RW, Bonadiman L, Turco P. Prevalence of different comorbidities in COPD patients by gender and GOLD stage. Multidiscip Respir Med 2015;10:24. 10.1186/s40248-015-0023-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet 2007;370:786-96. 10.1016/S0140-6736(07)61382-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nieminen MS, Brutsaert D, Dickstein K, et al. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J 2006;27:2725-36. 10.1093/eurheartj/ehl193 [DOI] [PubMed] [Google Scholar]
- 80.Abroug F, Ouanes-Besbes L, Nciri N, et al. Association of left-heart dysfunction with severe exacerbation of chronic obstructive pulmonary disease: diagnostic performance of cardiac biomarkers. Am J Respir Crit Care Med 2006;174:990-6. 10.1164/rccm.200603-380OC [DOI] [PubMed] [Google Scholar]
- 81.Yu TC, Zhou H, Suh K, et al. Assessing the importance of predictors in unplanned hospital readmissions for chronic obstructive pulmonary disease. Clinicoecon Outcomes Res. 2015;7:37-51. 10.2147/CEOR.S74181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Regan EA, Lynch DA, Curran-Everett D, et al. Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med 2015;175:1539-49. 10.1001/jamainternmed.2015.2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Minasian AG, van den Elshout FJ, Dekhuijzen PR, et al. Serial pulmonary function tests to diagnose COPD in chronic heart failure. Transl Respir Med 2014;2:12. 10.1186/s40247-014-0012-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Güder G, Brenner S, Störk S, et al. Chronic obstructive pulmonary disease in heart failure: accurate diagnosis and treatment. Eur J Heart Fail 2014;16:1273-82. 10.1002/ejhf.183 [DOI] [PubMed] [Google Scholar]
- 85.Brenner S, Güder G, Berliner D, et al. Airway obstruction in systolic heart failure--COPD or congestion? Int J Cardiol 2013;168:1910-6. 10.1016/j.ijcard.2012.12.083 [DOI] [PubMed] [Google Scholar]
- 86.Gorelik IL, Kalmanova EN, Aisanov ZR, et al. Functional and structural changes of the heart in chronic obstructive pulmonary disease in combination with coronary heart disease. Pulmonology 2010;1:100-5. [Google Scholar]
- 87.Songara A, Pasari N, Ajmera A, et al. Int J Res Med Sci 2016;4:2564-8. [Google Scholar]
- 88.Sacks CA, Jarcho JA, Curfman GD. Paradigm shifts in heart-failure therapy--a timeline. N Engl J Med 2014;371:989-91. 10.1056/NEJMp1410241 [DOI] [PubMed] [Google Scholar]
- 89.Lahousse L, Verhamme KM, Stricker BH, et al. Cardiac effects of current treatments of chronic obstructive pulmonary disease. Lancet Respir Med 2016;4:149-64. 10.1016/S2213-2600(15)00518-4 [DOI] [PubMed] [Google Scholar]
- 90.Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res 2003;93:896-906. 10.1161/01.RES.0000102042.83024.CA [DOI] [PubMed] [Google Scholar]
- 91.Gershon A, Croxford R, Calzavara A, et al. Cardiovascular safety of inhaled long-acting bronchodilators in individuals with chronic obstructive pulmonary disease. JAMA Intern Med 2013;173:1175-85. 10.1001/jamainternmed.2013.1016 [DOI] [PubMed] [Google Scholar]
- 92.Dong YH, Chang CH, Gagne JJ, et al. Comparative cardiovascular and cerebrovascular safety of inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a population-based cohort study. Pharmacotherapy 2016;36:26-37. 10.1002/phar.1684 [DOI] [PubMed] [Google Scholar]
- 93.Bermingham M, O'Callaghan E, Dawkins I, et al. Are beta2-agonists responsible for increased mortality in heart failure? Eur J Heart Fail 2011;13:885-91. 10.1093/eurjhf/hfr063 [DOI] [PubMed] [Google Scholar]
- 94.Anthonisen NR, Connett JE, Enright PL, et al. Hospitalizations and mortality in the Lung Health Study. Am J Respir Crit Care Med 2002;166:333-9. 10.1164/rccm.2110093 [DOI] [PubMed] [Google Scholar]
- 95.Ogale SS, Lee TA, Au DH, et al. Cardiovascular events associated with ipratropium bromide in COPD. Chest 2010;137:13-9. 10.1378/chest.08-2367 [DOI] [PubMed] [Google Scholar]
- 96.Tashkin DP, Leimer I, Metzdorf N, et al. Cardiac safety of tiotropium in patients with cardiac events: a retrospective analysis of the UPLIFT® trial. Respir Res 2015;16:65. 10.1186/s12931-015-0216-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Verhamme KM, Afonso AS, van Noord C, et al. Tiotropium Handihaler and the risk of cardio- or cerebrovascular events and mortality in patients with COPD. Pulm Pharmacol Ther 2012;25:19-26. 10.1016/j.pupt.2011.10.004 [DOI] [PubMed] [Google Scholar]
- 98.D'Urzo AD, Kerwin EM, Chapman KR, et al. Safety of inhaled glycopyrronium in patients with COPD: a comprehensive analysis of clinical studies and post-marketing data. Int J Chron Obstruct Pulmon Dis 2015;10:1599-612. 10.2147/COPD.S81266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kerwin EM, D'Urzo AD, Gelb AF, et al. Efficacy and safety of a 12-week treatment with twice-daily aclidinium bromide in COPD patients (ACCORD COPD I). COPD 2012;9:90-101. 10.3109/15412555.2012.661492 [DOI] [PubMed] [Google Scholar]
- 100.Trivedi R, Richard N, Mehta R, et al. Umeclidinium in patients with COPD: a randomised, placebo-controlled study. Eur Respir J 2014;43:72-81. 10.1183/09031936.00033213 [DOI] [PubMed] [Google Scholar]
- 101.Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med 2016;374:2222-34 . 10.1056/NEJMoa1516385 [DOI] [PubMed] [Google Scholar]
- 102.Calzetta L, Rogliani P, Matera MG, et al. A systematic review with meta-analysis of dual bronchodilation with LAMA/LABA for the treatment of stable COPD. Chest 2016;149:1181-96. 10.1016/j.chest.2016.02.646 [DOI] [PubMed] [Google Scholar]
- 103.White WB, Cooke GE, Kowey PR, et al. Cardiovascular safety in patients receiving roflumilast for the treatment of COPD. Chest 2013;144:758-65. 10.1378/chest.12-2332 [DOI] [PubMed] [Google Scholar]
- 104.Macie C, Wooldrage K, Manfreda J, et al. Cardiovascular morbidity and the use of inhaled bronchodilators. Int J Chron Obstruct Pulmon Dis 2008;3:163-9. 10.2147/COPD.S1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Macie C, Wooldrage K, Manfreda J, et al. Inhaled corticosteroids and mortality in COPD. Chest 2006;130:640-6. 10.1378/chest.130.3.640 [DOI] [PubMed] [Google Scholar]
- 106.Vestbo J, Anderson JA, Brook RD, et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet 2016;387:1817-26. 10.1016/S0140-6736(16)30069-1 [DOI] [PubMed] [Google Scholar]
- 107.Jara M, Lanes SF, Wentworth C, 3rd, et al. Comparative safety of long-acting inhaled bronchodilators: a cohort study using the UK THIN primary care database. Drug Saf 2007;30:1151-60. 10.2165/00002018-200730120-00007 [DOI] [PubMed] [Google Scholar]
- 108.Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008;359:1543-54. 10.1056/NEJMoa0805800 [DOI] [PubMed] [Google Scholar]
- 109.Wedzicha JA, Calverley PM, Seemungal TA, et al. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med 2008;177:19-26. 10.1164/rccm.200707-973OC [DOI] [PubMed] [Google Scholar]
- 110.Wise RA, Anzueto A, Cotton D, et al. Tiotropium respimat inhaler and the risk of death in COPD. N Engl J Med 2013;369:1491-501. 10.1056/NEJMoa1303342 [DOI] [PubMed] [Google Scholar]
- 111.Calverley PM, Anderson JA, Celli B, et al. Cardiovascular events in patients with COPD: TORCH study results. Thorax 2010;65:719-25. 10.1136/thx.2010.136077 [DOI] [PubMed] [Google Scholar]
- 112.Singh S, Loke YK, Enright P, et al. Pro-arrhythmic and pro-ischaemic effects of inhaled anticholinergic medications. Thorax 2013;68:114-6. 10.1136/thoraxjnl-2011-201275 [DOI] [PubMed] [Google Scholar]
- 113.Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest 2004;125:2309-21. 10.1378/chest.125.6.2309 [DOI] [PubMed] [Google Scholar]
- 114.Wilchesky M, Ernst P, Brophy JM, et al. Bronchodilator use and the risk of arrhythmia in COPD: part 1: Saskatchewan cohort study. Chest 2012;142:298-304. 10.1378/chest.10-2499 [DOI] [PubMed] [Google Scholar]
- 115.Sessler CN, Cohen MD. Cardiac arrhythmias during theophylline toxicity. A prospective continuous electrocardiographic study. Chest 1990;98:672-8. 10.1378/chest.98.3.672 [DOI] [PubMed] [Google Scholar]
- 116.Ohta K, Fukuchi Y, Grouse L, et al. A prospective clinical study of theophylline safety in 3810 elderly with asthma or COPD. Respir Med 2004;98:1016-24. 10.1016/j.rmed.2004.02.020 [DOI] [PubMed] [Google Scholar]
- 117.U.S. Food and Drug Administration. FDA Drug Safety Communication: Azithromycin (Zithromax or Zmax) and the risk of potentially fatal heart rhythms. Available online: http://www.fda.gov/drugs/drugsafety/ucm341822.htm
- 118.Guo D, Cai Y, Chai D, et al. The cardiotoxicity of macrolides: a systematic review. Pharmazie 2010;65:631-40. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


