Abstract
Background
Non-small cell lung cancer (NSCLC) is one of the cancers with the highest morbidity and mortality among the world. Studies have shown that the invasion and metastasis of tumor are biological characteristics of lung cancer, and also the main cause of treatment failure and patient death. In-depth study of lung cancer invasion related genes will help to explore the etiology of lung cancer, molecular typing and individualized treatment of lung cancer. Studies have shown that CD276 molecules are closely related to the prognosis of tumors, but the exact mechanism remains to be unclear.
Methods
We used the UALCAN and KM-plotter databases to investigate the expression of CD276 in human NSCLC and adjacent normal tissues, and its correlation with clinicopathology. In addition, we analyzed the function of CD276 in NSCLC cell by suppressing the expression of CD276 in A549 and H460 cells.
Results
In this study, we found that CD276 expression was significantly up-regulated in NSCLC tissues, and its expression was positively correlated with tumor stage in NSCLC. Silencing in CD276 inhibited cell invasion and migration by reducing integrin-associated protein expression.
Conclusions
Our results indicate functional role of CD276 in the progression of NSCLC.
Keywords: CD276, non-small cell lung cancer (NSCLC), integrin signaling, invasion, migration
Introduction
Lung cancer is one malignant cancer of the highest incidence and mortality rates all around the world (1). About 80% of the patients are non-small cell lung cancer (NSCLC) patients (2). Most lung cancers are diagnosed in the advanced stage. Currently, only 30% of NSCLCs can be surgically removed, but the 5-year survival rate is low (3). Traditional radiotherapy and chemotherapy have not been effective in treating patients with advanced lung cancer, and a new method for seeking treatment of lung cancer has become an urgent problem. Tumor invasion and metastasis is a malignant marker of lung cancer, and it is also the main cause of treatment failure and death. In-depth study of genes involved in lung cancer invasion and metastasis can help to explore the etiology of lung cancer and provide a potential basis for molecular typing and individualized treatment of lung cancer.
CD276 serves as a new member of the B7 family of costimulatory molecules, and its receptor has been unknown (4). B7 superfamily molecules have the function of initiating and maintaining T lymphocyte-mediated immune responses (5). The human CD276 gene is located on chromosome 15q24.1. Nucleotide sequence analysis indicated that CD276 is 951 bases in length and encodes 316 amino acids (6). The protein has a signal peptide at the amino terminus, including immunoglobulin-like variable regions. The extracellular segment of the constant region and the transmembrane region and the 45 amino acid cytoplasmic region belong to the immunoglobulin superfamily and are type I transmembrane proteins.
CD276 is specifically expressed in a variety of tumors, and the expression is closely related to clinical prognosis. In ovarian cancer, CD276 positively expressed ovarian tumor blood vessels are often associated with short survival and high cancer recurrence rates (7). In colon cancer, 54.3% of cancer patients had high expression of CD276, and the expression intensity of CD276 was positively correlated with tumor grade (8). In addition, the high expression of CD276 in cancer tissues was negatively related with the number of tumor infiltrating lymphocytes, and positively correlated with lymph node metastasis and tumor malignancy (9). Recent evidence shows that, although CD276 is considered an immune co-stimulator, it also plays a role in suppressing T cells and immune evasion, leading to tumor development (10). In addition, CD276 can play a certain role in the progression of cancer invasion and migration, angiogenesis and other processes, including its epigenetic effects through gene modification and regulation. Its selective expression on tumor cells makes it an attractive target.
In summary, the high expression of CD276 in tumor makes it a new diagnostic marker for tumors or as a new indicator reflecting the immune status and prognosis of patients. However, the function of CD276 in the processing of lung cancer remain unclear. In this paper, we researched the function of CD276 in lung cancer cell by abrogating the expression of CD276 in A549 and H460 cells.
Methods
Cells and reagents
The human lung cancer cell lines A549 and H460 were purchased from the Shanghai Cell Bank of the Chinese Academy of Science (Shanghai, China). The lung cancer cells were cultured in high glucose medium DMEM containing 10% fetal bovine serum (Gibco, USA). All cells were incubated at 37 °C in a 5% CO2 incubator.
Western blotting
Cells were harvested and lysed by using RIPA buffer, then mixed with 1x loading buffer, samples were separated by 10% SDS-PAGE gel electrophoresis and transferred to PVDF membranes (Millipore, Billerica, MA, USA). The membrane was blocked with 5% skim milk for 1 hour at room temperature, incubated with primary antibody overnight at 4 °C, and then incubated with secondary antibody for 1 hour at room temperature. Protein bands were visualized by SuperSignal chemiluminescent substrate (Millipore, Billerica, MA, USA). GAPDH is used as a load internal reference.
Quantitative RT-PCR (qRT-PCR)
Total RNA isolation and cDNA preparation were carried out as previously described (11). PCR was carried out using SYBR Premix Ex Taq II (RR820A, Takara, Japan). The specific primers were used for amplification. The relative quantification of gene expression for each sample was conducted using the ΔΔCT method. Each experiment was performed in triplicate.
Transwell assay
A Transwell chamber with an 8 µm aperture was used. Forty-eight hours after cell transfection, the cell suspension was transferred to the upper chamber coated with Matrigel. A medium containing 20% fetal calf serum was added to the lower chamber. The cells in the upper chamber were fixed in cold methanol for 30 minutes, and then stained with 1% crystal violet for 30 minutes. The number of cells on the underside of the filter was counted under a microscope. Different fields of view were randomly selected and then an average count was performed.
Scratch wound healing assay
The density of seed cells entering a 12-well tissue culture plate reached a confluence of about 70–80% monolayer after 24 hours of growth. The center of the hole was gently stroke with a new 10 µL pipette tip and the single layer was gently scratched. After the scratch, the medium was gently washed twice with a medium to remove the separated cells. The cells were incubated for an additional 48 hours and then the stained monolayer photographs were taken under a microscope.
Cell counting kit-8 (CCK-8) assay
Cell density was adjusted at 20,000 cells/mL by culture medium supplemented with 10% FBS. One hundred µL of cell suspension (20,000 cells/mL) was dispensed into a 96-well plate. According to the manufacturer’s instructions, cell proliferation was detected with a CCK-8 (MCE, USA) at 0, 24, 48, 72, 96 h for A549 cell and at 0, 12, 36, 48, 60 h for H460 cell. Optical density values were measured with a microplate reader at an absorbance of 450 nm.
Colony formation assay
The cells were collected and plated into 6-well plate, 500 cells per well, and three replicate wells were set. Cells was incubated for a few hours in a CO2 incubator at 37 °C and attached to the plate. The cells were incubated for 15 days at 37 °C in a CO2 incubator until the cells in the control plates formed colonies of significantly better size (50 cells per colony is the smallest score). The colonies were stained with Giemsa staining solution.
EdU proliferation staining
The proliferative capacity of tumor cells was determined using a Click-iT EdU cell proliferation assay (Thermo Fisher Scientific, C10337). Five hundred µL of Click-iT reaction mixture was added to each well and the plate was shaken briefly to ensure thorough mixing. The cells were stored in the dark for 30 minutes at room temperature. The reaction mixture was discarded and each well was washed once with 1 mL of PBS containing 3% BSA. The washings were discarded and the nuclei were stained with DAPI for 5 minutes at room temperature.
Results
CD276 is highly expressed in lung cancer and correlated with prognosis
We performed an analysis about CD276 expression in lung cancer tissues by using UALCAN database (http://ualcan.path.uab.edu). Statistical analysis in the UALCAN database revealed that CD276 was highly expressed in lung cancer compared with the normal group, and the difference was statistically significant (P<0.05), as shown in Figure 1A. Further analysis found that the expression of CD276 was higher in lung cancer based on individual cancer stages than that in normal tissues, and the difference was statistically significant (P<0.05), as shown in Figure 1B. To further clarify the relationship between CD276 expression level and the prognosis of patients with lung cancer, KM-plotter database analysis showed two survival curves of patients with high CD276 expression and low expression group (different probe), and found that the level of CD276 has a significant impact on overall survival of patients (Figure 1C,D).
Figure 1.
CD276 is up-regulated in lung cancer and correlated with prognosis. (A) High CD276 expression in lung cancer compared with the normal group; (B) high CD276 expression of was higher in lung cancer based on individual cancer stages; (C) relationship between CD276 (1552914_a_at probe) expression level and prognosis of patients with lung cancer; (D) relationship between CD276 (224859_at probe) expression level and survival of patients with lung cancer.
The abrogation of CD276 expression suppresses the migration and invasive ability of lung cancer cells
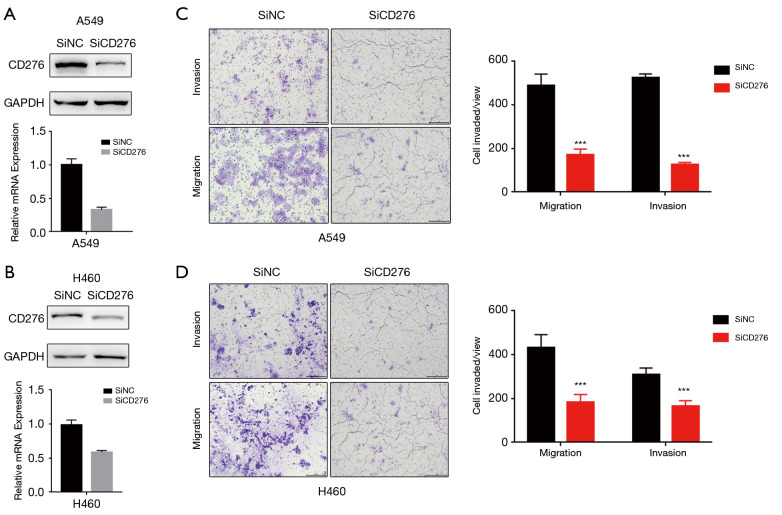
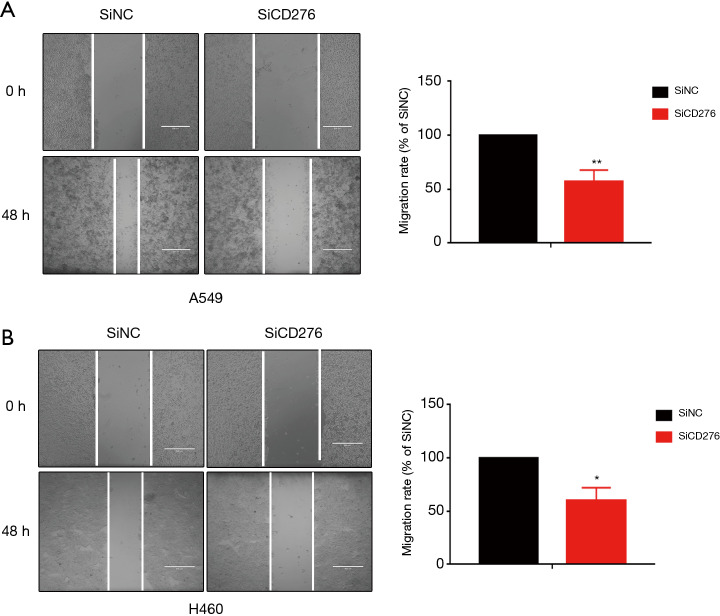
Western blot and qRT-PCR assaies revealed that CD276 expression obviously reduced by transfected CD276 siRNA into A549 and H460 cells (Figure 2A,B). To investigate the effects of CD276 on lung cancer cell migration and invasion, we performed a Transwell assay. As shown in Figure 2C,D, compared with the negative control siRNA transfected cells, depletion of CD276 in A549 and H460 cells showed a weak invasion and migration abilities (P<0.05). The wound healing assay revealed that the knockdown of CD276 suppressed significantly the migration of A549 and H460 cells (Figure 3A,B).
Figure 2.
The abrogation of CD276 expression suppresses the migration and invasive ability of lung cancer cells. (A) Western blot and qRT-PCR assaies revealed that CD276 expression obviously reduced by transfected CD276 siRNA into A549 cells; (B) Western blot and qRT-PCR results showed that CD276 expression obviously damaged after transfection with CD276 siRNA in H460; (C) the representative microscopic fields of invasion and migration A549 cells transfected with CD276 siRNA and negative control siRNA, respectively. Abrogation of CD276 significantly inhibited invasion and migration abilities of A549 cells; (D) the representative microscopic fields of invasion and migration H460 cells transfected with CD276 siRNA and negative control siRNA, respectively. Abrogation of CD276 significantly inhibited invasion and migration abilities of H460 cells. ***, P<0.001. qRT-PCR, quantitative RT-PCR.
Figure 3.
The depletion of CD276 suppressed the migration ability of lung cancer cell. (A) Wound healing assay demonstrated that knockdown of CD276 suppressed migration in A549 cell; (B) wound healing assay demonstrated that knockdown of CD276 suppressed migration in H460 cell. *, P<0.05; **, P<0.01.
CD276 promotes cell proliferation, colony formation in A549 and H460 cells
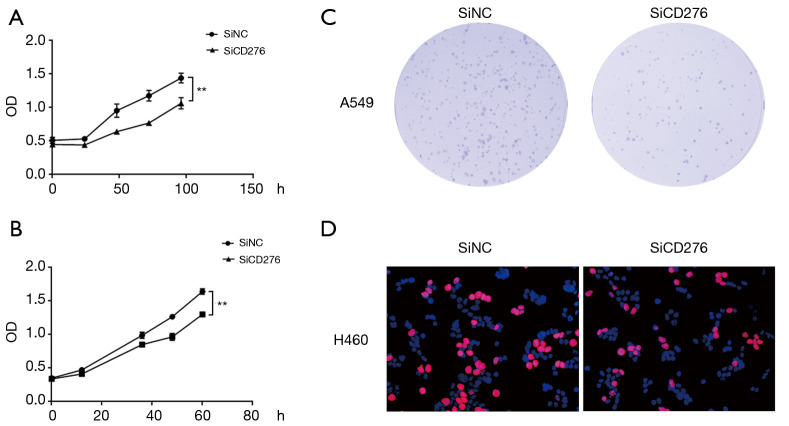
To test the effect of CD276 on the proliferation of lung cancer cells, we performed a CCK-8 experiment. Abrogation of CD276 significantly inhibited cell proliferation in A549 and H460 cells compared to the negative control group (Figure 4A,B). Besides, we performed colony formation and EdU assay. The results showed inhibition of CD276 reduced colony numbers in A549 cells (Figure 4C). The EdU assay showed depletion of CD276 reduced cell proliferation (Figure 4D).
Figure 4.
CD276 improves cell proliferation, colony formation in A549 and H460 cells. (A) Cell growth curves exhibited that the proliferation ability of A549 cell was abrogated by transfecting CD276 siRNA; (B) cell growth curves exhibited that the proliferation ability of the CD276-abrogated H460 cell; **, P<0.01; (C) assessment of clonogenic potential of the CD276-depleted A549 cell; (D) EdU assay revealed that the proliferation ability of H460 cell was reduced after transfection with CD276 siRNA.
The depletion of CD276 reduced the expression of integrin pathway associated proteins
We found that the expression of integrin-associated protein was related with the expression of CD276 by using GEPIA database (Figure 5A). Accordingly, we speculated whether the depletion of CD276 inhibited the integrin signaling. We performed the qPCR assay and found that the inhibition of CD276 downregulated the expression of many integrin-associated proteins in A549 and H460 cells (Figure 5B,C). The depletion of CD276 led to inhibition of cell migration and invasion, which may be caused by down-regulation of integrin pathway-associated proteins.
Figure 5.
The depletion of CD276 reduced the expression of integrin pathway associated proteins. (A) The expression level of integrin associated protein was related with the expression of CD276 by using GEPIA database; (B) qRT-PCR analysis of integrin pathway associated proteins in A549 with CD276 depletion; (C) qRT-PCR analysis of integrin pathway associated proteins in H460 with CD276 depletion. *, P<0.05; **, P<0.01. qRT-PCR, quantitative RT-PCR.
Discussion
Lung cancer is one of the most common cancers with the highest morbidity and mortality in the whole world. Studies have shown that tumor invasion and metastasis are malignant markers and biological characteristics of lung cancer, and are also the main cause of treatment failure and death in patients. And to explore the molecular mechanism related to it has become a very urgent issue.
More and more research results show that CD276 plays an important role in tumor evolution and distant metastasis. CD276 is overexpressed in tumor tissues, and several human malignant tumors with high expression of CD276 are positively correlated with the malignancy of these diseases (12), and the expression level of CD276 is invasive with tumor tissue or the clinical stage of tumor (13). There was a significant correlation between the probability of recurrence and death or the survival time of the patient (8,14,15). Chavin et al. found that in patients with prostate cancer treated with new hormone-assisted therapy (NHT), the higher the expression of CD276, the more prone to prostate cancer cells to undergo bone metastasis (15). The studies have revealed that the mRNA and protein levels of CD276 are increased in human gastric cancer tissues, and CD276 is positively correlated with postoperative survival rate (16). In colon cancer, 54.3% of cancer patients have high expression of CD276, and the expression intensity of CD276 is positively correlated with tumor grade (8). In ovarian cancer, the ovarian tumor blood vessels owning positive CD276 expression are often associated with short survival and high cancer recurrence rates in patients (7). Through immunohistochemical analysis of clinical specimens, the researchers find that the expression of CD276 is closely related to lymph node metastasis in patients with lung cancer, pancreatic cancer, breast cancer, and hypopharyngeal squamous cell carcinoma (10,17,18). The level of CD276 is closely related to lymph node metastasis and distant metastasis in lung cancer patients (19). The specific mechanism of CD276 in tumor metastasis is unclear.
Integrin is a transmembrane heterodimer composed of two subunits, α and β. The α and β subunits have long extracellular, transmembrane and short intracellular regions. The extracellular region of integrin is connected to the extracellular matrix, while the intracellular region is connected to the cytoskeleton, forming focal adhesions, and mediating the transmission of intracellular and extracellular signals. The focal adhesion composed of extracellular matrix-integrin-cytoskeleton protein is the structural basis of integrin signal transduction. Many signal proteins play a role in integrin-mediated signal transduction by binding to focal adhesion, so that adjust cell adhesion and migration. In our research, we found that CD276 can regulate the expression of multiple integrin-related proteins. Because CD276 is also a membrane protein, we speculate that CD276 can bind to integrin-related proteins, which in turn activates integrin signals and promotes tumor cell migration. However, this needs further experimental confirmation.
Our results showed that higher CD276 levels in NSCLC cancer tissues was associated with tumor stage. We also investigated the impacts of siRNA-mediated CD276 silencing in the lung cancer cell lines, A549 and H460. CCK-8 assay results showed difference between CD276-silenced A549 and H460 cells and controls (P<0.05). In addition, wound healing and Transwell assays showed that CD276 silencing reduced cancer cell migration and invasion. Ours is the first report that CD276 silencing down-regulates integrin-associated proteins. Recent studies have reported that integrin-mediated cell adhesion pathway induces the migration and invasion of cancer cells (20). CD276 may activate integrin signaling, although this requires further study.
In summary, we investigated the role of CD276 in lung cancer cell migration and invasion, proliferation. We showed that CD276 silencing downregulated integrin-associated proteins. Our findings suggest that CD276 and/or its associated molecules, including integrin, may be novel targets for NSCLC therapeutics.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.04.41). The authors have no conflicts of interest to declare.
References
- 1.Huang N, Zhu J, Liu D, et al. Overexpression of Bcl-2-associated death inhibits A549 cell growth in vitro and in vivo. Cancer Biother Radiopharm 2012;27:164-8. 10.1089/cbr.2011.1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Billmeier SE, Ayanian JZ, Zaslavsky AM, et al. Predictors and outcomes of limited resection for early-stage non-small cell lung cancer. J Natl Cancer Inst 2011;103:1621-9. 10.1093/jnci/djr387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.David EA, Clark JM, Cooke DT, et al. The role of thoracic surgery in the therapeutic management of metastatic non-small cell lung cancer. J Thorac Oncol 2017;12:1636-45. 10.1016/j.jtho.2017.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun J, Mao Y, Zhang YQ, et al. Clinical significance of the induction of macrophage differentiation by the costimulatory molecule B7-H3 in human non-small cell lung cancer. Oncol Lett 2013;6:1253-60. 10.3892/ol.2013.1586 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Lu B, Chen L, Liu L, et al. T-cell-mediated tumor immune surveillance and expression of B7 co-inhibitory molecules in cancers of the upper gastrointestinal tract. Immunol Res 2011;50:269-75. 10.1007/s12026-011-8227-9 [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Yang X, Wu Y, et al. B7-H3 promotes gastric cancer cell migration and invasion. Oncotarget 2017;8:71725-35. 10.18632/oncotarget.17847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zang X, Sullivan PS, Soslow RA, et al. Tumor associated endothelial expression of B7-H3 predicts survival in ovarian carcinomas. Mod Pathol 2010;23:1104-12. 10.1038/modpathol.2010.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun J, Chen LJ, Zhang GB, et al. Clinical significance and regulation of the costimulatory molecule B7-H3 in human colorectal carcinoma. Cancer Immunol Immunother 2010;59:1163-71. 10.1007/s00262-010-0841-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu YH, Zhang GB, Wang JM, et al. B7-H3 and CD133 expression in non-small cell lung cancer and correlation with clinicopathologic factors and prognosis. Saudi Med J 2010;31:980-6. [PubMed] [Google Scholar]
- 10.Arigami T, Narita N, Mizuno R, et al. B7-h3 ligand expression by primary breast cancer and associated with regional nodal metastasis. Ann Surg 2010;252:1044-51. 10.1097/SLA.0b013e3181f1939d [DOI] [PubMed] [Google Scholar]
- 11.Hui L, Zhang S, Wudu M, et al. CBLL1 is highly expressed in non-small cell lung cancer and promotes cell proliferation and invasion. Thorac Cancer 2019;10:1479-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ling V, Wu PW, Spaulding V, et al. Duplication of primate and rodent B7-H3 immunoglobulin V- and C-like domains: divergent history of functional redundancy and exon loss. Genomics 2003;82:365-77. 10.1016/S0888-7543(03)00126-5 [DOI] [PubMed] [Google Scholar]
- 13.Lutz AM, Bachawal SV, Drescher CW, et al. Ultrasound molecular imaging in a human CD276 expression-modulated murine ovarian cancer model. Clin Cancer Res 2014;20:1313-22. 10.1158/1078-0432.CCR-13-1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crispen PL, Sheinin Y, Roth TJ, et al. Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin Cancer Res 2008;14:5150-7. 10.1158/1078-0432.CCR-08-0536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chavin G, Sheinin Y, Crispen PL, et al. Expression of immunosuppresive B7-H3 ligand by hormone-treated prostate cancer tumors and metastases. Clin Cancer Res 2009;15:2174-80. 10.1158/1078-0432.CCR-08-2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu CP, Jiang JT, Tan M, et al. Relationship between co-stimulatory molecule B7-H3 expression and gastric carcinoma histology and prognosis. World J Gastroenterol 2006;12:457-9. 10.3748/wjg.v12.i3.457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamato I, Sho M, Nomi T, et al. Clinical importance of B7-H3 expression in human pancreatic cancer. Br J Cancer 2009;101:1709-16. 10.1038/sj.bjc.6605375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katayama A, Takahara M, Kishibe K, et al. Expression of B7-H3 in hypopharyngeal squamous cell carcinoma as a predictive indicator for tumor metastasis and prognosis. Int J Oncol 2011;38:1219-26. 10.3892/ijo.2011.949 [DOI] [PubMed] [Google Scholar]
- 19.Zhang G, Xu Y, Lu X, et al. Diagnosis value of serum B7-H3 expression in non-small cell lung cancer. Lung Cancer 2009;66:245-9. 10.1016/j.lungcan.2009.01.017 [DOI] [PubMed] [Google Scholar]
- 20.Yu WM, Hawley TS, Hawley RG, et al. Role of the docking protein Gab2 in beta(1)-integrin signaling pathway-mediated hematopoietic cell adhesion and migration. Blood 2002;99:2351-9. 10.1182/blood.V99.7.2351 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as