Abstract
Background
Previous studies have already established that low platelet count is related to adverse outcomes in patients with type A acute aortic dissection (AAAD). However, there are yet limited studies investigating the association of platelet count and the risk of postoperative pneumonia in AAAD patients.
Methods
This retrospective cohort study was conducted in Xiangya Hospital of Central South University from January 2014 to May 2019. Clinical and laboratory data were collected. The correlation between platelet count and postoperative pneumonia was analyzed using multivariate logistic regression and the area under the receiver operating characteristic curve (AUC) was used to assess the predictive power of platelet count on pneumonia.
Results
A total of 268 patients with AAAD were enrolled. The overall incidence of pneumonia was 36.94% (n=99). Multivariate logistic regression revealed that platelet count was negatively associated with the risk of postoperative pneumonia (OR 0.93; 95% CI: 0.88–0.98) after adjusting for the confounders. Compared to the lowest platelet count tertile (T1), medium platelet count (T2) and highest platelet count (T3) had a lower risk of postoperative pneumonia after adjusting for the confounders (OR 0.80, 95% CI: 0.40–1.60; OR 0.30, 95% CI: 0.13–0.66; respectively). A similar trend was observed when the platelet count was handled as categorical variables (tertiles). The area under the ROC curve was 0.635 (95% CI: 0.565–0.707), with a sensitivity of 76.77%, a specificity of 50.89% and an accuracy of 60.45%.
Conclusions
Our findings indicate that low platelet count is an independent risk factor of postoperative pneumonia in patients with AAAD and has a specific predictive power on the risk of postoperative pneumonia.
Keywords: Platelet count, postoperative pneumonia, type A acute aortic dissection (AAAD), predictor
Introduction
According to previous studies, 50% of patients with type A acute aortic dissection (AAAD) die within 48 hours (1). While, a good operative strategy and precise emergency surgery for AAAD can increase the rate of survival, also commonly results in postoperative morbidity (2). Postoperative pneumonia is a severe complication of cardiac surgery. Most commonly, it occurs during the first week after surgery, with an estimated mortality rate ranging from 20% to 50% (3). It poses a substantial risk to the morbidity and mortality by causing pulmonary dysfunction or multiple organ failure (4).
Platelets are crucial components involved in the coagulation process. Additionally, platelet activation could lead to unstable hemodynamics and plays an essential role in the development of AAD, which reduces the platelet count in patients with AAD (5,6). However, the impact of reduced platelet count on the prognosis in patients with AAAD remains subtle yet. A study conducted by Chen et al. indicated that a low preoperative platelet count in patients with AAAD was independently associated with the occurrence of healthcare-associated infections (2). Also, Huang et al. reported that a low admission platelet count increased the in-hospital mortality rates in patients with AAAD (7). However, few studies have focused on the association between platelet count and postoperative pneumonia in patients with AAAD.
Therefore, the purpose of this study was to explore the association of platelet count with the risk of postoperative pneumonia and to assess the predictive value of platelet count for the risk of postoperative pneumonia in Chinese AAAD patients.
Methods
Patients selection
This was a retrospective cohort study based on the medical records of patients who underwent surgery for AAAD from January 1, 2014 to May 30, 2019 in Xiangya Hospital of Central South University. All patients underwent surgery within 48 hours from admission by the identical experienced surgical team. The diagnosis for AAAD was based on computed tomography scanning and echocardiography. Participants’ inclusion criteria were: all surgical patients diagnosed with AAAD and age ≥18 years old. Exclusion criteria were: death during operation, death within 24 hours after operation, severe organ dysfunction before operation and suffering from pneumonia before operation. The study was conducted in accordance with the guidelines for good clinical practice and the principles of the Declaration of Helsinki. It was approved by the Medical Ethics Committee of Xiangya Hospital of Central South University (ethical number: 2019010038). Owing to the retrospective study, individual consent was waived.
Data collection
Demographic, laboratory, clinical, intra- and perioperative data were collected by record review. Clinical and laboratory data were recorded throughout the hospitalization course by trained staff. Demographic data included age and gender. Laboratory data included neutrophil, lymphocyte, hemoglobin, platelet count, activated partial thromboplastin time (APTT), and prothrombin time (PT). Clinical data included history of smoking, drinking, Marfan syndrome, hypertension, diabetes mellitus, hemopericardium, chronic renal failure, cerebrovascular disease, coronary artery disease, and Penn class. Intraoperative data included autologous blood transfusion, ventilator time, operative time, cardiopulmonary bypass (CPB) time, and surgery type. Perioperative data included hospital stay length, intensive care unit (ICU) stay length, perioperative blood product transfusion and in-hospital mortality.
The platelet count referred to the lowest in-hospital values measured during 48 hours before the operation which was obtained by counting the whole blood cells.
Outcomes
The outcome variable was the development of pneumonia after surgery. Pneumonia was diagnosed according to the Society of Thoracic Surgeons criteria, based on laboratory findings (e.g., positive sputum culture results from transtracheal fluid and/or bronchial washings) and/or radiological evidence (e.g., chest radiograph diagnostic of pulmonary infiltrates). Fever and white blood cell count were also considered as evidence of pneumonia (8).
Statistical analysis
Quantitative parameters were expressed as means ± standard deviation or medians with ranges, and qualitative parameters were expressed as frequencies and percentages. Continuous variables were compared using the t-test (for normal distribution) or Mann-Whitney’s U test (for skewed distribution). The chi-squared test or Fisher’s exact test was applied to compare the categorical variables. Missing values for autologous blood transfusion was 5.6%, ventilator time 2.24%. Missing data for these two continuous variables were handled via median imputation. There was no multicollinearity between the independent variables. Univariate logistic regression analysis was performed to evaluate the risk factors associated with postoperative pneumonia in patients with AAAD. Non-adjusted and adjusted multiple logistic regression model was applied to evaluate the effect of the platelet count on postoperative pneumonia occurrence. Whether the covariances were adjusted determined by the principle that the variables added to this model, changed the matched odds ratio by at least 10% (9). The predictive capability of platelet count for postoperative pneumonia was assessed using the ROC analysis.
All analyses were performed with the statistical software packages R (http://www.R-project.org, The R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc, Boston, MA, USA). A two-tailed P value below 0.05 was considered as statistically significant.
Results
The selection of participants
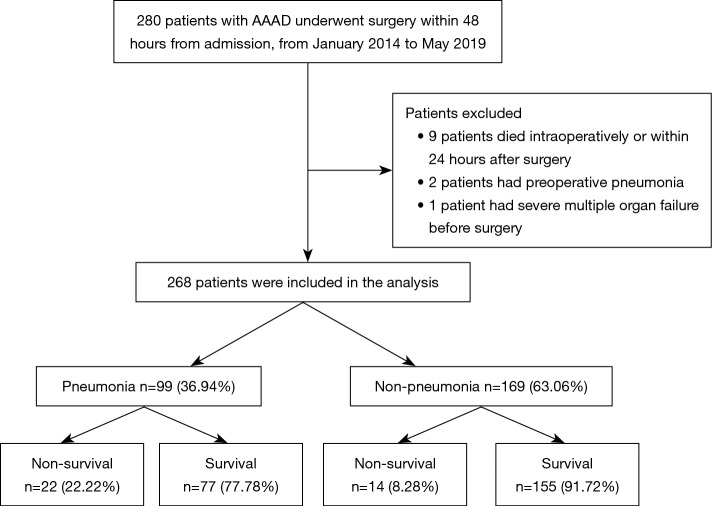
Among the 280 patients whose records were reviewed, nine patients died intraoperatively or within 24 hours after surgery, two had preoperative pneumonia and one had severe multiple organ failure before surgery. The remaining 268 patients with AAAD were enrolled in the present study (Figure 1); 99 (36.94%) of the 268 patients developed postoperative pneumonia and the in-hospital mortality rate was 22.22% among these patients.
Figure 1.
Flow chart of the study. AAAD, A acute aortic dissection.
Patients’ baseline characteristics
The baseline characteristics are presented by platelet count tertiles in Table 1. Of all these total participants, the highest incidence (48.28%) of postoperative pneumonia was in patients with the lowest platelet count tertiles (P<0.001). Compared with high level (T3) of platelet count group, patients in other groups (T1 and T2) had a significantly lower lymphocyte (P<0.001), higher APTT (P=0.020), PT (P=0.009), operative time (P=0.023), plasma transfusion (P=0.008), platelet transfusion (P=0.047) and ICU stay length (P=0.003). The remaining variables showed no statistically significant differences among the groups.
Table 1. Baseline clinical characteristics of all patients with AAAD.
| Variable | Platelet count tertiles (×109/L) | P value | |||
|---|---|---|---|---|---|
| T1 (<136) (n=87) | T2 (136 to 184) (n=91) | T3 (>184) (n=90) | |||
| Age (year) | 51.21±9.71 | 50.46±11.16 | 48.21±12.25 | 0.174 | |
| Gender (male) | 69 (79.31) | 62 (68.13) | 58 (64.44) | 0.079 | |
| Laboratory examination pre-operation | |||||
| Neutrophil (×109/L) | 9.64±3.44 | 9.04±3.62 | 9.09±4.29 | 0.510 | |
| Lymphocyte (×109/L) | 1.08±0.57 | 1.18±0.54 | 1.44±0.60 | <0.001* | |
| Hemoglobin (g/L) | 124.40±20.59 | 121.88±18.80 | 119.37±22.69 | 0.273 | |
| APTT (sec) | 36.83±7.02 | 34.53±5.74 | 34.44±6.37 | 0.020* | |
| PT (sec) | 14.83±1.93 | 14.17±2.07 | 14.03±1.46 | 0.009* | |
| Complications | |||||
| Smoking | 41 (47.13) | 42 (46.15) | 40 (44.44) | 0.936 | |
| Drinking | 31 (35.63) | 35 (38.46) | 29 (32.22) | 0.680 | |
| Marfan syndrome | 3 (3.45) | 7 (7.69) | 7 (7.78) | 0.403 | |
| Hypertension | 66 (75.86) | 60 (65.93) | 59 (65.56) | 0.245 | |
| Diabetes mellitus | 6 (6.90) | 5 (5.49) | 2 (2.22) | 0.330 | |
| Hemopericardium | 9 (10.34) | 12 (13.19) | 4 (4.44) | 0.120 | |
| Chronic renal failure | 6 (6.90) | 5 (5.49) | 1 (1.11) | 0.150 | |
| Cerebrovascular disease | 6 (6.90) | 2 (2.20) | 6 (6.67) | 0.279 | |
| Coronary artery disease | 7 (8.05) | 7 (7.69) | 9 (10.00) | 0.838 | |
| Penn class | 0.600 | ||||
| Class Aa | 52 (59.77) | 51 (56.04) | 47 (52.22) | ||
| Non class Aa | 35 (40.23) | 40 (43.96) | 43 (47.78) | ||
| Intra-operative variables | |||||
| Autologous blood transfusion (unit) | 0 (0 to 45) | 0 (0 to 30) | 0 (0 to 30) | 0.448 | |
| Ventilator time (hour) | 6 (0 to 192) | 10 (0 to 960) | 8 (0 to 1,176) | 0.165 | |
| Operative time (hour) | 11.07±3.35 | 11.40±4.31 | 10.18±3.41 | 0.023* | |
| CPB time (min) | 173.86±74.77 | 167.42±70.16 | 147.08±86.45 | 0.057 | |
| Surgery type | 0.174 | ||||
| AAR + TAR (TAVR) + FET | 44 (50.57) | 49 (53.85) | 39 (43.33) | ||
| Bentall + TAR (TAVR) + FET | 14 (16.09) | 25 (27.47) | 19 (21.11) | ||
| David + TAVR + FET | 4 (4.60) | 2 (2.20) | 4 (4.44) | ||
| Combine others | 25 (28.74) | 15 (16.48) | 28 (31.11) | ||
| Perioperative blood transfusion | |||||
| RBCs (unit) | 10.14±7.76 | 9.00±7.47 | 8.29±8.04 | 0.071 | |
| Plasma (unit) | 15.01±10.31 | 12.06±9.28 | 10.45±9.71 | 0.008* | |
| Platelet (therapeutic dose) | 1.57±1.17 | 1.35±1.18 | 1.31±1.45 | 0.047* | |
| Cryoprecipitate (therapeutic dose) | 1.55±1.18 | 1.41±1.27 | 1.28±1.35 | 0.359 | |
| Hospital stay length (day) | 17.52±8.35 | 16.46±8.38 | 17.57±8.84 | 0.617 | |
| ICU stay length (day) | 7.82±6.02 | 7.74±5.97 | 7.01±9.24 | 0.003* | |
| Pneumonia | 42 (48.28) | 38 (41.76) | 19 (21.11) | <0.001* | |
| In-hospital mortality | 14 (16.09) | 14 (15.38) | 8 (8.89) | 0.297 | |
Results are expressed as n (%) or mean ± SD or median (min to max). *, P<0.05. APTT, activated partial thromboplastin time; PT, prothrombin time; CPB, cardiopulmonary bypass; AAR, ascending aorta replacement; TAR, total arch replacement; TAVR, total aortic vascular replacement; FET, frozen elephant trunk; RBC, red blood cell; ICU, intensive care unit.
Univariate analysis risk factors associated with postoperative pneumonia
Table 2 shows the results of the univariate analyses. Using the univariate binary logistic regression analysis, we found that the platelet count was negatively associated with the risk of postoperative pneumonia. In contrast, age, ventilator time, ICU and hospital stay length, RBCs, plasma, platelet and cryoprecipitate transfusion were positively correlated with the risk of postoperative pneumonia. Other variables were not found to be significantly associated with the risk of postoperative pneumonia.
Table 2. Univariate analysis of risk factors associated with pneumonia in patients with AAAD.
| Variable | No pneumonia (n=169) | Pneumonia (n=99) | OR (95% CI) | P value |
|---|---|---|---|---|
| Age (year) | 48.91±11.36 | 51.72±10.55 | 1.02 (1.00, 1.05) | 0.048* |
| Gender (male) | 117 (69.23) | 72 (72.73) | 1.19 (0.68, 2.05) | 0.545 |
| Neutrophil (×109/L) | 8.95±3.91 | 9.77±3.56 | 1.06 (0.99, 1.13) | 0.091 |
| Lymphocyte (×109/L) | 1.28±0.60 | 1.15±0.56 | 0.68 (0.43, 1.05) | 0.083 |
| Hemoglobin (g/L) | 120.54±19.45 | 124.10±22.79 | 1.01 (1.00, 1.02) | 0.176 |
| Platelet count (×109/L) | 184.60±76.72 | 150.32±55.98 | 0.99 (0.98, 0.99) | <0.001* |
| APTT (sec) | 35.44±6.94 | 34.92±5.55 | 0.99 (0.95, 1.03) | 0.518 |
| PT (sec) | 14.19±1.86 | 14.60±1.86 | 1.13 (0.98, 1.29) | 0.090 |
| Smoking | 81 (47.93) | 42 (42.42) | 0.80 (0.49, 1.32) | 0.383 |
| Drinking | 60 (35.50) | 35 (35.35) | 0.99 (0.59, 1.67) | 0.980 |
| Marfan syndrome | 14 (8.28) | 3 (3.03) | 0.35 (0.10, 1.24) | 0.102 |
| Hypertension | 114 (67.46) | 71 (71.72) | 1.22 (0.71, 2.11) | 0.467 |
| Diabetes mellitus | 8 (4.73) | 5 (5.05) | 1.07 (0.34, 3.37) | 0.907 |
| Hemopericardium | 15 (8.88) | 10 (10.10) | 1.15 (0.50, 2.68) | 0.739 |
| Chronic renal failure | 5 (2.96) | 7 (7.07) | 2.50 (0.77, 8.09) | 0.127 |
| Cerebrovascular disease | 6 (3.55) | 8 (8.08) | 2.39 (0.80, 7.10) | 0.117 |
| Coronary artery disease | 16 (9.47) | 7 (7.07) | 0.73 (0.29, 1.84) | 0.500 |
| Penn class | ||||
| Class Aa | 91 (53.85) | 59 (59.60) | Ref | |
| Non class Aa | 78 (46.15) | 40 (40.40) | 0.79 (0.48, 1.31) | 0.361 |
| Autologous blood transfusion (unit) | 0 (0 to 30) | 0 (0 to 45) | 1.04 (1.00, 1.08) | 0.059 |
| Ventilator time (hour) | 8 (0 to 192) | 14 (0 to 1,176) | 1.01 (1.00, 1.01) | 0.009* |
| Operative time (hour) | 10.61±3.58 | 11.34±4.00 | 1.05 (0.99, 1.12) | 0.128 |
| CPB time (min) | 160.96±80.76 | 165.61±73.32 | 1.00 (1.00, 1.00) | 0.638 |
| Surgery type | ||||
| AAR + TAR (TAVR) + FET | 79 (46.75) | 53 (53.54) | Ref | |
| Bentall + TAR (TAVR) + FET | 43 (25.44) | 15 (15.15) | 0.52 (0.26, 1.03) | 0.061 |
| David + TAVR + FET | 7 (4.14) | 3 (3.03) | 0.64 (0.16, 2.58) | 0.529 |
| Others | 40 (23.67) | 28 (28.28) | 1.04 (0.58, 1.89) | 0.889 |
| RBCs (unit) | 8.14±7.09 | 10.82±8.58 | 1.04 (1.01, 1.08) | 0.008* |
| Plasma (unit) | 11.18±9.19 | 14.69±10.72 | 1.04 (1.01, 1.06) | 0.007* |
| Platelet (therapeutic dose) | 1.27±1.12 | 1.65±1.47 | 1.26 (1.03, 1.54) | 0.023* |
| Cryoprecipitate (therapeutic dose) | 1.26±1.14 | 1.67±1.44 | 1.29 (1.05, 1.57) | 0.014* |
| Hospital stay length (day) | 15.57±6.17 | 19.91±10.97 | 1.06 (1.03, 1.10) | <0.001* |
| ICU stay length (day) | 5.46±3.19 | 11.03±10.26 | 1.22 (1.14, 1.32) | <0.001* |
Results are expressed as n (%) or mean ± SD or median (min to max). *, P<0.05. OR, odds ratio; CI, confidence interval; Ref, reference; APTT, activated partial thromboplastin time; PT, prothrombin time; CPB, cardiopulmonary bypass; AAR, ascending aorta replacement; TAR, total arch replacement; TAVR, total aortic vascular replacement; FET, frozen elephant trunk; RBC, red blood cell; ICU, intensive care unit.
Association of the platelet count with the risk of postoperative pneumonia
In this study, we constructed four models to analyze the independent effects of the platelet count on the risk of postoperative pneumonia (Table 3). In non-adjusted model II, the platelet count showed a negative correlation with postoperative pneumonia (OR 0.92, 95% CI: 0.88–0.96). Furthermore, a rise of 10×109/L in the platelet count was associated with an 8% decrease in the risk of pneumonia. In model II (adjusted for age and gender), the result remained significant (OR 0.92, 95% CI: 0.88–0.97). Multiple variables, including blood component transfusion, length of hospital and ICU stay, and postoperative pneumonia may affect each other. In order to eliminate the interference of these factors, we applied model III and model IV to evaluate the relationship between platelet count and postoperative pneumonia. In model III (adjusted for age, gender, autologous blood transfusion, neutrophil, lymphocyte, PT, ventilator time and surgery type), the result did not have obvious changes (OR 0.91, 95% CI: 0.87–0.96) and a rise of 10×109/L in the platelet count was associated with a 9% decrease in the risk of pneumonia. In model IV (adjusted covariates in model III plus the length of hospital and ICU stay, RBCs, plasma, cryoprecipitate and platelet transfusion), the result did not change obviously (OR 0.93, 95% CI: 0.88–0.98). For the purpose of sensitivity analysis, we handled the continuous variable (platelet count) as three equal categorical variables, and the P value for the trend of platelet count was consistent with the result when the platelet count was considered as a continuous variable.
Table 3. Multivariable regressions analysis of association between platelet count (per 10×109/L) and postoperative pneumonia.
| Variable | Model I (OR, 95% CI) | Model II (OR, 95% CI) | Model III (OR, 95% CI) | Model IV (OR, 95% CI) |
|---|---|---|---|---|
| Platelet count | 0.92 (0.88, 0.96)* | 0.92 (0.88, 0.97)* | 0.91 (0.87, 0.96)* | 0.93 (0.88, 0.98)* |
| Platelet count tertiles | ||||
| T1 | Ref | Ref | Ref | Ref |
| T2 | 0.77 (0.43, 1.39) | 0.79 (0.43, 1.44) | 0.78 (0.41, 1.48) | 0.80 (0.40, 1.60) |
| T3 | 0.29 (0.15, 0.55)* | 0.31 (0.16, 0.60)* | 0.25 (0.12, 0.53)* | 0.30 (0.13, 0.66)* |
| P value for trend | <0.001 | <0.001 | <0.001 | 0.003 |
*, P<0.05. Model I, we adjusted none; Model II, we adjusted age and gender; Model III, we adjusted age, gender, autologous blood transfusion, neutrophil, lymphocyte, PT, ventilator time and surgery type; Model IV, we adjusted covariates in model III plus the length of hospital and ICU stay, RBCs, plasma, cryoprecipitate and platelet transfusion. OR, odds ratio; CI, confidence interval; Ref, reference.
ROC analysis
Table 4 shows the results of the ROC analyses of the platelet count used to identify postoperative pneumonia in patients with AAAD. The AUC of the ROC curve was 0.635 (95% CI: 0.565–0.707), with the optional cut-off value 173.5×109 as well as 76.77% for sensitivity, 50.89% for specificity and 60.45% for accuracy.
Table 4. Area under the ROC curves and sensitivity analysis of platelet count.
| Test | AU-ROC | 95% CI | Cut off (×109/L) | Specificity | Sensitivity | Accuracy |
|---|---|---|---|---|---|---|
| Platelet count | 0.635 | 0.565 to 0.707 | 173.5 | 0.5089 | 0.7677 | 0.6045 |
AU-ROC, area under the receiver operating characteristic; CI, confidence interval.
Discussion
In this study, we found a significant association between platelet count and postoperative pneumonia after adjustment for potential confounders. A 10×109/L decrease of platelet count was associated with a 7% increase in the risk of pneumonia. Previously, few studies investigated the association between platelet count and postoperative pneumonia in patients with AAAD. However, the association between platelet count and postoperative pneumonia has been evaluated in the context of other diseases and procedures. In a study of 212 cases liver transplantation, the researchers found that preoperative low platelet count was related to postoperative pneumonia (10), which is consistent with our result. The findings in other studies also supported our results. Chen et al. reported a low preoperative platelet count would significantly increase the risk of healthcare-associated infections (including pneumonia) after surgical arch replacement in a sample of 210 participants (2). Kelly et al. found that low platelet count was associated with postoperative morbidity (including pneumonia) in patients who underwent distal pancreatectomy (11). Besides, when platelet count was divided into three tertiles in this study. Compared with the lowest platelet count tertile (T1), the risk of postoperative pneumonia decreased by 20% in medium platelet count tertile (T2) and by 70% in the highest platelet count tertile (T3). Similar trends were observed whether we handled platelet count as a categorical variable or a continuous variable. It further illustrates the robustness of our results and that low platelet count is a risk factor of postoperative pneumonia in AAAD patients. Besides, other risk factors have also been reported to be related to postoperative pneumonia, such as hypertension, chronic renal failure (12), age >70 years (13), perioperative transfusions (14), type of surgery, weight loss, chronic obstructive pulmonary disease, general anesthesia, smoking and alcohol use (15). Therefore, early intervention for these risk factors might be helpful to prevent postoperative pneumonia. Postoperative pneumonia is considered as a preventable complication (16) and is closely associated with prolonged length of hospital and ICU stay (17) and in-hospital mortality (18). Similarly, in this study, we found that the mortality rate was significantly higher in patients who suffered from postoperative pneumonia (22.22%) than in those without postoperative pneumonia (8.28%). Also, the length of hospital and ICU stay was significantly longer in patients with pneumonia than those without pneumonia. Therefore, the low platelet count in AAAD patients could become a predictor for early clinical intervention to prevent the occurrence of postoperative pneumonia. Also, it might aid to shorten the length of hospital and ICU stay effectively, further reduce the financial burden on patients and even reducing in-hospital mortality.
Moreover, we evaluated the predictive value of platelet count on postoperative pneumonia in patients with AAAD in this study. The ROC curve demonstrated that platelet count might be a predictor of postoperative pneumonia (AUC: 0.635). The sensitivity and specificity were 76.77% and 50.89%, respectively. The result shows the platelet count has a specific predictive power as a single predictor of postoperative pneumonia. To enhance the predictive value, a prediction model based on a combination of parameters should be developed, for it is unlikely that a single parameter can faithfully represent all possible patient conditions. The concept of combining multiple parameters is considered to be the best alternative to overcome the limitations of a single parameter and is likely to maximize their clinical usefulness. In Nomura et al.’s study, single predictor such as preoperative white blood cells, C-reactive protein or angiotensin-converting enzyme 2, had limited ability to predict postoperative pneumonia in patients with esophagostomy. The AUC of each predictor was 0.567, 0.571 and 0.652, respectively (19). Strobel et al. combined 17 preoperative variables to predict postoperative pneumonia in patients after coronary artery bypass grafting, and the AUC reached 0.74 (20). Takesue et al. used an 18-parameters model to predict postoperative pneumonia in patients after gastroenterological surgery, and the AUC reached as high as 0.822 (21). In addition, through the ROC curve analysis, we found the optimal cut-off value of the admission platelet count was 173.5×109 /L, which was rather close to that reported by Chen et al. (171×109 /L) (2). This result could hopefully be useful for future clinical prediction models of postoperative pneumonia.
The underlying mechanism of the association between a low admission platelet count and postoperative pneumonia in patients with AAAD is not well understood so far. One possible explanation could be the platelet activation. Platelet consumption (low platelet count) possibly due to platelet activation, which is associated with arterial dissection occurrence (5,22). Activated platelets may secrete proinflammatory cytokines to initiate inflammatory responses (23). These pro-inflammatory molecules could exacerbate neutrophil rolling, adhesion and recruitment, thereby promoting the formation of platelet-neutrophil complexes. Both complexes and proinflammatory molecules could promote lung inflammation and increase the severity of lung infection (24). Another possible explanation is bleeding, which consumes massive platelets, later leads to low platelet count. Low platelet count was associated with transfusion (25,26) and we also found that patients with lower platelet count received more blood product transfusion in this study. However, transfusion itself has been linked with a variety of postoperative adverse outcomes such as bacteremia, pneumonia and mortality (27,28). Transfusions increase the suppressor T cell activity and inhibit B cell differentiation and natural killer cell activity indirectly (29), which could further lead to a greater likelihood of infection in transfused patients. Also, fragments of banked blood deposited in the lung interstitial activating inflammation that could worsen lung damage may account for the development of pneumonia (2).
There were some limitations. First, this study was a retrospective study of a small sample. Owing to a retrospective cohort study, it cannot avoid a certain bias. However, we adjusted the potential confounding factors as much as possible in the data analysis to reduce the potential bias. Considering the power of the statistics was 95%, although the sample size was small, the results of this article had a certain credibility. Second, we hadn’t reviewed the results of thromboelastography, which might help us to explain the relationship between low platelet count and postoperative pneumonia. We will expect to expand the sample in the future study or conduct a prospective cohort study and incorporate thromboelastography into our indicators to prove that low platelet count is a risk factor for postoperative pneumonia in AAAD patients. Third, a lot of AAAD patients may also suffer from fever and elevated white blood cell count perioperatively because of stress response. Also, other factors like infection from other system or absorption of pleural effusion might present with similar symptoms and signs. They can be hardly classified as pneumonia.
Conclusions
In conclusion, a low platelet count is an independent risk factor of postoperative pneumonia in patients with AAAD. Platelet count has a specific predictive power as one of the predictors to predict high-risk populations. Early intervention might prevent postoperative pneumonia, thereby shorten the length of ICU and hospital stays and reduce in-hospital mortality.
Supplementary
The article’s supplementary files as
Acknowledgments
We thank Wenquan Liang, PhD (Department of General Surgery, Chinese PLA General Hospital) and Wenjing Li, PhD (Department of Periodontology, Peking University School and Hospital of Stomatology) for these helpful review and comments regarding the manuscript. We would like to thank Editage (www.editage.cn) for English language editing.
Funding: This work was supported by the National Natural Science Foundation of China (grant no. 81873574).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Medical Ethics Committee of Xiangya Hospital of Central South University (ethical number: 2019010038). Owing to the retrospective study, individual consent was waived.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.84). The authors have no conflicts of interest to declare.
References
- 1.Conzelmann LO, Weigang E, Mehlhorn U, et al. Mortality in patients with acute aortic dissection type A: analysis of pre- and intraoperative risk factors from the German Registry for Acute Aortic Dissection Type A (GERAADA). Eur J Cardiothorac Surg 2016;49:e44-52. 10.1093/ejcts/ezv356 [DOI] [PubMed] [Google Scholar]
- 2.Chen WS, Ni BQ, Li SQ, et al. Novel risk factors for the healthcare associated infections (HAIs) in patients with Stanford type A aortic dissection (TAAD). J Thorac Dis 2018;10:2135-41. 10.21037/jtd.2018.03.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bateman BT, Bykov K, Choudhry NK, et al. Type of stress ulcer prophylaxis and risk of nosocomial pneumonia in cardiac surgical patients: cohort study. BMJ 2013;347:f5416. 10.1136/bmj.f5416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topal AE, Eren MN. Risk factors for the development of pneumonia post cardiac surgery. Cardiovasc J Afr 2012;23:212-5. 10.5830/CVJA-2012-005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sbarouni E, Georgiadou P, Analitis A, et al. Significant changes in platelet count, volume and size in acute aortic dissection. Int J Cardiol 2013;168:4349-50. 10.1016/j.ijcard.2013.05.074 [DOI] [PubMed] [Google Scholar]
- 6.Qin C, Gu J, Qian H, et al. Potential Mechanism of Post-Acute Aortic Dissection Inflammatory Responses: The Role of mtDNA from Activated Platelets. Cardiology 2016;135:228-35. 10.1159/000446870 [DOI] [PubMed] [Google Scholar]
- 7.Huang B, Tian L, Fan X, et al. Low admission platelet counts predicts increased risk of in-hospital mortality in patients with type A acute aortic dissection. Int J Cardiol 2014;172:e484-6. 10.1016/j.ijcard.2014.01.001 [DOI] [PubMed] [Google Scholar]
- 8.Likosky DS, Harrington SD, Cabrera L, et al. Collaborative Quality Improvement Reduces Postoperative Pneumonia After Isolated Coronary Artery Bypass Grafting Surgery. Circ Cardiovasc Qual Outcomes 2018;11:e004756. 10.1161/CIRCOUTCOMES.118.004756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kernan WN, Viscoli CM, Brass LM, et al. Phenylpropanolamine and the risk of hemorrhagic stroke. N Engl J Med 2000;343:1826-32. 10.1056/NEJM200012213432501 [DOI] [PubMed] [Google Scholar]
- 10.Levesque E, Hoti E, Azoulay D, et al. Pulmonary complications after elective liver transplantation-incidence, risk factors, and outcome. Transplantation 2012;94:532-8. 10.1097/TP.0b013e31825c1d41 [DOI] [PubMed] [Google Scholar]
- 11.Kelly KJ, Greenblatt DY, Wan Y, et al. Risk stratification for distal pancreatectomy utilizing ACSNSQIP: preoperative factors predict morbidity and mortality. J Gastrointest Surg 2011;15:250-9. 10.1007/s11605-010-1390-9 [DOI] [PubMed] [Google Scholar]
- 12.Vera Urquiza R, Bucio Reta ER, Berríos Bárcenas EA, et al. Risk factors for the development of postoperative pneumonia after cardiac surgery. Arch Cardiol Mex 2016;86:203-7. 10.1016/j.acmx.2015.12.005 [DOI] [PubMed] [Google Scholar]
- 13.Hortal J, Giannella M, Pérez MJ, et al. Incidence and risk factors for ventilator-associated pneumonia after major heart surgery. Intensive Care Med 2009;35:1518-25. 10.1007/s00134-009-1523-3 [DOI] [PubMed] [Google Scholar]
- 14.Watanabe H, Horita N, Shibata Y, et al. Diagnostic test accuracy of D-dimer for acute aortic syndrome: systematic review and meta-analysis of 22 studies with 5000 subjects. Sci Rep 2016;6:26893. 10.1038/srep26893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arozullah AM, Khuri SF, Henderson WG, et al. Development and validation of a multifactorial risk index for predicting postoperative pneumonia after major noncardiac surgery. Ann Intern Med 2001;135:847-57. 10.7326/0003-4819-135-10-200111200-00005 [DOI] [PubMed] [Google Scholar]
- 16.Katsura M, Kuriyama A, Takeshima T, et al. Preoperative inspiratory muscle training for postoperative pulmonary complications in adults undergoing cardiac and major abdominal surgery. Cochrane Database Syst Rev 2015;10:CD010356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le CD, Lehman E, Aziz F. Development of Postoperative Pneumonia After Endovascular Aortic Aneurysm Repair is Associated with an Increased Length of Intensive Care Unit Stay. Cureus 2019;11:e4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett KM, Kent KC, Schumacher J, et al. Targeting the most important complications in vascular surgery. J Vasc Surg 2017;65:793-803. 10.1016/j.jvs.2016.08.107 [DOI] [PubMed] [Google Scholar]
- 19.Nomura S, Tsujimoto H, Aosasa S, et al. Impact of angiotensin-converting enzyme 2 levels on postoperative pneumonia after esophagectomy. J Surg Res 2018;224:200-6. 10.1016/j.jss.2017.12.023 [DOI] [PubMed] [Google Scholar]
- 20.Strobel RJ, Liang Q, Zhang M, et al. A Preoperative Risk Model for Postoperative Pneumonia After Coronary Artery Bypass Grafting. Ann Thorac Surg 2016;102:1213-9. 10.1016/j.athoracsur.2016.03.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takesue Y, Miyata H, Gotoh M, et al. Risk calculator for predicting postoperative pneumonia after gastroenterological surgery based on a national Japanese database. Ann Gastroenterol Surg 2019;3:405-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang S, Qian H, Yang Q, et al. Relationship between the extent of dissection and platelet activation in acute aortic dissection. J Cardiothorac Surg 2015;10:162. 10.1186/s13019-015-0351-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Semple JW, Italiano JE, Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol 2011;11:264-74. 10.1038/nri2956 [DOI] [PubMed] [Google Scholar]
- 24.Lê VB, Schneider JG, Boergeling Y, et al. Platelet Activation and Aggregation Promote Lung Inflammation and Influenza Virus Pathogenesis. Am J Respir Crit Care Med 2015;191:804-19. 10.1164/rccm.201406-1031OC [DOI] [PubMed] [Google Scholar]
- 25.Bronheim RS, Oermann EK, Cho SK, et al. Coagulation Profile as a Risk Factor for 30-Day Morbidity and Mortality Following Posterior Lumbar Fusion. Spine 2017;42:950-7. 10.1097/BRS.0000000000001935 [DOI] [PubMed] [Google Scholar]
- 26.Bloch EM, Ingram C, Hull J, et al. Risk factors for peripartum blood transfusion in South Africa: a case-control study. Transfusion 2018;58:2149-56. 10.1111/trf.14772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rohde JM, Dimcheff DE, Blumberg N, et al. Health care-associated infection after red blood cell transfusion: a systematic review and meta-analysis. JAMA 2014;311:1317-26. 10.1001/jama.2014.2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L, Liao Q, Zhang T, et al. Blood Transfusion is an Independent Risk Factor for Postoperative Serious Infectious Complications After Pancreaticoduodenectomy. World J Surg 2016;40:2507-12. 10.1007/s00268-016-3553-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplan J, Sarnaik S, Levy J. Transfusion-induced immunologic abnormalities not related to the AIDS virus. N Engl J Med 1985;313:1227. 10.1056/NEJM198511073131912 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as