Abstract
Aim
Short-stem total hip arthroplasty is designed to preserve proximal bone stock in case of eventual revision, potentially benefiting younger and more active patients. This prospective, single-center study assessed the safety and performance of the partially neck-sparing Nanos™ short-stem uncemented prosthesis at 24 months using clinical outcome scores and radiographic results.
Methods
Between April 2011 and February 2015, 52 subjects (mean age, 54.9 years) underwent total hip arthroplasty and were followed up at 3, 6, 12 and 24 months. The primary outcome was improvement in quality of life as measured by the Short-Form 36 Mental Component Score (SF-36 MCS). Secondary clinical outcomes included the Harris Hip Score, Hip Disability and Osteoarthritis Outcome Score, Postel Merle d’Aubigné-Score, Activity Level University of California, Los Angeles score, and Pain Visual Analogue Scale. Complications and radiographic images were also recorded at each follow-up.
Results
Mean SF-36 MCS score significantly improved from baseline to final follow up at 24 months (61.3 vs. 79.5, respectively; p < 0.001). All secondary clinical outcomes also showed significant improvement (p < 0.001) during this time period. Neutral stem positioning was achieved in 45 subjects (86.5%). Two subjects (3.8%) underwent revisions: one for a periprosthetic fracture unrelated to the study device and another due to a prosthetic joint infection. Intraoperatively, one fissure fracture of the acetabulum occurred.
Conclusion
Total hip arthroplasty with the Nanos short-stem led to significant clinical improvements and a high subjective satisfaction rate at 24 months. Further follow-up will determine whether these effects are sustained in the long term.
Keywords: Total hip arthroplasty, Short stem, Quality of life, Patient-reported outcomes, Uncemented
1. Introduction
As the number of total hip arthroplasty (THA) procedures being performed continued to rise over the last two decades, it became apparent that novel implant designs were required to meet the needs of a wider variety of patients.1 Short-stem hip implants were introduced in the late 1990s primarily for younger and more active patients, who are presumed to place higher demands on their implants than those who had traditionally undergone THA in earlier years. Short-stem implants were hypothesized to provide superior conditions for subsequent revision surgery, should it be required, by allowing for greater preservation of the proximal femur, more balanced bone remodeling, and enhanced physiologic load transfer.2,3
Despite their shared treatment goal, short-stem implants are far from homogeneous in their design or anchorage philosophy.2,4 The most widely applied method for classifying these stems is according to whether they preserve, partially spare, or resect the femoral neck.4 Given the number of unique designs within this class, there is a need for high-quality prospective studies to evaluate clinical and radiographic outcomes and patient safety for every available stem.
The Nanos is a short-stem uncemented prosthesis (OHST Medizintechnik AG, distributed by Smith & Nephew GmbH, Marl, Germany) available on the market since 2002. It is made of a plasma-coated titanium forged-alloy (Ti6Al4V), and has a porous calcium phosphate coating in the proximal part and a polished tip. The Nanos belongs to the partially neck-sparing subgroup of short stems and strives for a metaphyseal and partially diaphyseal anchorage.4
Good primary stability and osseointegration in 17 subjects who underwent radiostereometric analysis (RSA) for up to 24 months after implantation with the Nanos were previously reported.5 The current study aims to provide additional information on the safety and performance of this femoral implant through the reporting of clinical outcomes, radiographic outcomes, and complications among the full cohort of participants.
2. Methods
2.1. Study design and subject cohort
After obtaining approval from university's ethical review committee, 60 subjects scheduled to undergo primary THA were enrolled in this prospective, single-center study between April 2011 and February 2015. Inclusion criteria were age between 30 and 65 years at date of surgery and at least three months between surgical procedures in case of bilateral THA. Subjects were excluded if they met any of the following criteria: previous bone or soft tissue surgery of the affected hip except for arthroscopic surgery, a local or systemic infection, a previously diagnosed osteoporosis, a femoral neck angle of >145° or <125°, diseases of the cardiovascular system involving particularly reduced load capacity in the everyday life, American Society of Anaesthesiologists' (ASA) Score 3 or 4, a documented allergy to elements of the implanted device, a neurological disease with changed motor function, pregnancy, a body mass index >30, alcoholism or addictive disorders.
This study was conducted in accordance with the ethical standards of the responsible committee and with the ethical principles of the 1975 Declaration of Helsinki. The study was approved by the Ethical Committee (EC) of the Hannover Medical School on Apr 28, 2010, by the German Federal office for Radiation Protection on Sep 09, 2010, and by the German Federal Institute for Drugs and Medical Devices on Jan 19, 2011. The study was registered on ClinicalTrials.gov (NCT04172129; https://clinicaltrials.gov/ct2/show/NCT04172129). Written informed consent was obtained from all participating patients.
Eight subjects were excluded, with the remaining 52 subjects having a mean age of 54.9 years (range, 39–65) at surgery (Fig. 1). There were 16 (30.8%) males and 36 (69.2%) females with a mean body weight of 77.8 kg. Indications for THA were primary osteoarthritis (n = 39; 75.0%), dysplasia (n = 10; 19.2%), osteonecrosis (n = 2; 3.9%), and rheumatoid arthritis (n = 1; 1.9%).
Fig. 1.
Flowchart of subject availability.
Of the remaining 52 subjects, 4 were lost to follow-up due to revision of the implant (n = 2) or refusal to return due to absence of symptoms (n = 2). Full data analysis after 24 months is therefore available for 48 subjects.
The following protocol deviations were recorded throughout the study: early study termination (12 subjects), missed 3-month visit (1), missed 1-year visit (1), missed x-rays (2), patient refused x-rays (2), subject became pregnant (1), and informed consent used wrong footer (10). Additionally, 4 subjects did not receive a pregnancy test. However, all subjects reported not being pregnant, and a pregnancy test was not written as a specific requirement in the study protocol, just the patient information sheet. None of the protocol deviations required reporting to the institutional review board/ethics committee.
2.2. Surgical procedure
All subjects underwent primary THA with the Nanos short-stem uncemented prosthesis performed by the same senior surgeon. Nanos (sizes 2–8) was implanted in combination with a ceramic femoral head. Two subjects (3.9%) received a Plasmacup™ acetabular component (Aesculap AG, Tuttlingen, Germany) and the remaining 50 (96.2%) an EP-FIT Plus™ Cup (Smith & Nephew, Marl, Germany). The bearing couple was ceramic/ceramic in 35 subjects (67.3%), ceramic/standard polyethylene in 16 (30.8%), and ceramic/cross-linked polyethylene in 1 (1.9%). The surgical approach was either minimally invasive via an anterolateral approach (n = 23; 44.2%) or conventional via a direct lateral approach (n = 29; 55.8%). All subjects were treated with full load bearing and with a standardized physiotherapy protocol starting the day after surgery.
2.3. Outcomes
Outcomes data were collected prior to surgery and at 3, 6, 12 and 24 months after surgery.
The primary endpoint was the evaluation of changes in quality of life following THA by the use of the Short-Form 36 (SF-36) Mental Component Score (MCS). The SF-36 assesses eight health concepts: physical functioning, bodily pain, role limitations due to physical health problems, role limitations due to personal or emotional problems, general mental health/emotional well-being, social functions, energy/fatigue, and general health perceptions.6
Secondary clinical outcomes included the Harris Hip Score (HHS),7 Hip Disability and Osteoarthritis Outcome Score (HOOS),8 Postel Merle d’Aubigné-Score (PMA),9 University of California, Los Angeles (UCLA) scale,10 and pain Visual Analogue Scale (VAS).11
Radiographic evaluation was conducted directly postoperatively and at each follow-up point. The images were reviewed by the investigators and specific information regarding implant position, implant fixation, heterotopic ossifications (HO), radiolucencies, osteolysis, atrophy and hypertrophy were collected. HO was classified according to the Brooker Classification System.12
Complications were collected throughout the study and assessed for severity and possible relationship to the study device.
2.4. Statistical analysis
Statistical calculation of sample size was performed based on estimates of the effect size of the primary endpoint. A mean change in SF-36 MCS from baseline to 2 years of at least 4 points was assumed. A type I error (α) of 0.025 corresponding to the one-sided hypothesis test and a type II error (β) of 0.1 (or statistical power, 1-β of 0.9) were defined. With an expected change from baseline to 2 years in the SF-36 MCS of 7.9, a standard deviation (SD) of 8.5, and a minimal clinically relevant change of 4, it was estimated that at least 50 subjects would be required. Missing SF-36 MCS data from 3 months (first post-operative time point) through the final study visit at 24 months were imputed using the Multiple Imputation method.
Statistical analyses were performed using SAS version 9.4. Statistical significance was assumed when p-values associated with any test statistic used for any comparison was ≤0.05.
3. Results
The study's primary outcome, the mean SF-36 MCS score, significantly improved by 18.2 points (SD, 17.36; 95% confidence interval: 16.0–20; p < 0.001) from baseline (61.3) to final follow up (79.5). There was also improvement observed in all eight SF-36 sub-scores at the four consecutive follow-up visits (Table 1). All sub-scores except for general health and role limitations due to personal or emotional problems showed a significant improvement beginning at 3 months follow-up. The general health sub-score significantly improved beginning at 6 months follow-up compared to baseline through to the final follow-up at 24 months. The sub-score role limitations due to personal or emotional problems significantly improved beginning 12 months after surgery compared to baseline.
Table 1.
Summary of improvement in mean SF-36 sub-scores throughout the entire 24-month follow-up period.
| Outcome | Statistic | Baseline | 3 Months | 6 Months | 12 Months | 24 Months |
|---|---|---|---|---|---|---|
| SF-36 MCS score | N | 51 | 51 | 51 | 51 | 51 |
| Mean (SD) | 61.3 (19.7) | 76.1 (18.7) | 77.2 (18.6) | 80.9 (14.5) | 79.5 (14.5) | |
| P value for change from baseline | – | <0.001 | <0.001 | <0.001 | <0.001 | |
| SF-36 physical functioning | N | 52 | 49 | 48 | 48 | 48 |
| Mean (SD) | 30.6 (20.1) | 64.1 (25.7) | 73.0 (23.7) | 78.2 (21.4) | 75.5 (23.8) | |
| P value for change from baseline | – | <0.001 | <0.001 | <0.001 | <0.001 | |
| SF-36 Role limitations due to physical health problems | N | 52 | 50 | 48 | 48 | 48 |
| Mean (SD) | 26.0 (37.0) | 51.0 (44.0) | 75.0 (38.9) | 83.3 (30.7) | 82.8 (35.4) | |
| P value for change from baseline | – | 0.003 | <0.001 | <0.001 | <0.001 | |
| SF-36 Bodily Pain | N | 52 | 50 | 48 | 48 | 48 |
| Mean (SD) | 30.9 (19.0) | 68.7 (23.0) | 76.0 (22.3) | 81.3 (18.9) | 83.6 (17.9) | |
| P value for change from baseline | – | <0.001 | <0.001 | <0.001 | <0.001 | |
| SF-36 General Health | N | 52 | 50 | 48 | 48 | 48 |
| Mean (SD) | 63.5 (17.4) | 66.4 (20.1) | 70.4 (16.9) | 69.6 (17.4) | 68.1 (18.5) | |
| P value for change from baseline | – | 0.170 | 0.005 | 0.038 | 0.016 | |
| SF-36 Role limitations due to personal or emotional problemsa | N | 52 | 50 | 48 | 48 | 48 |
| Mean (SD) | 67.9 (41.7) | 76.0 (39.9) | 79.9 (35.6) | 91.0 (26.4) | 88.2 (28.8) | |
| P value for change from baseline | – | 0.307 | 0.148 | 0.002 | <0.001 | |
| SF-36 Energy/Fatiguea | N | 52 | 50 | 48 | 48 | 48 |
| Mean (SD) | 48.5 (18.3) | 65.0 (19.4) | 65.3 (19.9) | 68.5 (16.1) | 65.9 (16.2) | |
| P value for change from baseline | – | <0.001 | <0.001 | <0.001 | <0.001 | |
| SF-36 Emotional well-beinga | N | 51 | 50 | 48 | 48 | 48 |
| Mean (SD) | 65.5 (19.6) | 77.4 (18.4) | 78.9 (17.5) | 81.6 (12.8) | 80.6 (15.2) | |
| P value for change from baseline | – | <0.001 | <0.001 | <0.001 | <0.001 | |
| SF-36 Social Functioninga | N | 52 | 50 | 48 | 48 | 48 |
| Mean (SD) | 67.3 (25.1) | 86.5 (18.5) | 87.2 (18.7) | 93.5 (14.1) | 93.2 (12.6) | |
| P value for change from baseline | – | <0.001 | <0.001 | <0.001 | <0.001 |
Abbreviations: NA = not available; SD = standard deviation; SF-36 MCS = Short-Form 36 Mental Component Score.
Part of the Mental Component Score.
All other clinical outcomes showed significant improvements from baseline to 24-month follow-up (Table 2).
Table 2.
Summary of improvement in mean HHS, HOOS, PMA, UCLA, and VAS pain scores throughout the entire 24-month follow-up period.
| Outcome | Statistic | Baseline | 3 Months | 6 Months | 12 Months | 24 Months |
|---|---|---|---|---|---|---|
| HHS Total Score (0–100) | N | 52 | 50 | 49 | 48 | 48 |
| Mean (SD) | 54.5 (10.5) | 88.6 (14.1) | 94.0 (9.6) | 96.2 (7.7) | 96.6 (7.8) | |
| P value for change from baseline | – | <0.001 | <0.001 | <0.001 | <0.001 | |
| HOOS (0–96) | N | 52 | 50 | 48 | 48 | 48 |
| Mean (SD) | 54.4 (15.8) | 20.8 (14.0) | 16.3 (15.5) | 10.3 (9.8) | 10.4 (11.5) | |
| P value for change from baseline | – | <0.001 | <0.001 | <0.001 | <0.001 | |
| PMA (0–18) | N | 52 | 50 | 49 | 48 | 48 |
| Mean (SD) | 12.1 (1.9) | 16.0 (2.2) | 17.0 (1.4) | 17.2 (1.3) | 17.4 (1.3) | |
| P value for change from baseline | – | <0.001 | <0.001 | <0.001 | <0.001 | |
| UCLA activity level (0–10) | N | 52 | 50 | 49 | 48 | 48 |
| Mean (SD) | 5.2 (2.1) | 6.2 (1.8) | 6.9 (1.7) | 7.0 (1.7) | 6.9 (1.6) | |
| P value for change from baseline | – | 0.0132 | <0.001 | <0.001 | <0.001 | |
| VAS pain score (0–100) | N | 52 | 50 | 48 | 48 | 48 |
| Mean (SD) | 64.1 (21.5) | 19.7 (19.0) | 13.8 (16.8) | 10.1 (9.5) | 12.0 (13.4) | |
| P value for change from baseline | – | <0.001 | <0.001 | <0.001 | <0.001 |
Abbreviations: HHS = Harris Hip Score; HOOS = Hip Disability and Osteoarthritis Outcome; PMA = Postel Merle d’Aubigné-Score; SD = standard deviation; UCLA = University of California, Los Angeles scale; VAS = Visual Analogue Scale.
Neutral stem positioning was achieved in 45 subjects (86.5%). Two subjects showed a varus tilt of the stem of up to 5° and 5 subjects a valgus position of up to 5°. The stem position was unchanged in 46 subjects (97.9%) at 24 months. One subject experienced a periprosthetic fracture and initial subsidence of 11 mm, followed by secondary stabilization. This led to a revision surgery, as furtherly described below.
A cup positioning with an inclination angle of 40–50° and a neutral stem positioning was achieved in 45 subjects (86.5%), whereas six subjects showed an angle of <40° and 1 subject of >50°. Forty subjects (76.9%) had a good bone-cup contact in all zones (1–3), whereas a good stem-bone contact in all zones (1–7) was present in 49 subjects (94.2%).
At final 24-month follow-up, there was no sign of HO in 36 subjects (78.3%). HO of grade I was observed in six subjects (13.0%), grade II in three (6.5%), and grade III in one (2.2%).
At the 24-month follow-up, femoral radiolucent lines were present in nine hips (19.2%) in the distal area of the stem (Zones 3–6, Zones 10–12). However, no radiolucent lines >1 mm were reported. There was no femoral osteolysis in any subject. Sixteen subjects (34.0%) had femoral atrophy in any zone and 17 subjects (36.2%) had hypertrophy in any zone.
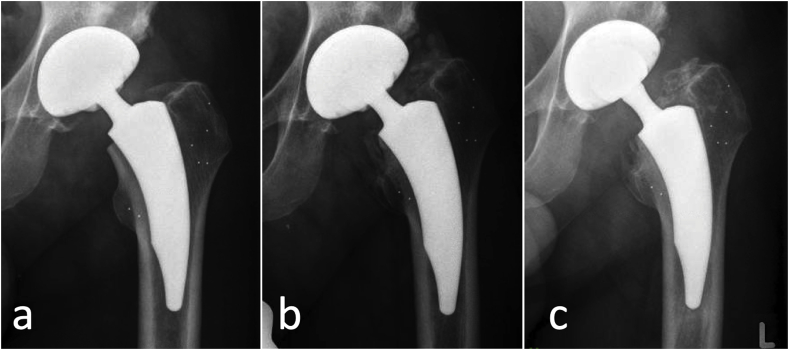
Complications occurred in 3 subjects (5.7%). An intraoperative complication occurred in one case (1.9%), in the form of intraoperative fissure of the acetabulum due to sclerotic bone. There were no early postoperative complications. One subject (1.9%) had a revision surgery of all implanted components two and a half months after surgery due to septic loosening and an infection. One other subject (1.9%) had a periprosthetic fracture identified at three month follow up, after the first x-ray taken after mobilization had revealed normal findings (Fig. 2). The patient reported load-dependent pain that had occurred after operative removal of renal calculus under general anaesthetic. Any traumatic event could not be remembered. The fracture was not considered related to the study device. Twenty months after initial surgery, the subject underwent revision of the femoral head to a longer neck due pain and gluteal insufficiency. The stem was osseously integrated and remained in situ. After revision surgery, the patient was satisfied and did not report any persistent pain.
Fig. 2.
Radiological course of a patient with a periprosthetic fracture. The x-ray taken after mobilization did not reveal any abnormal findings (a). The tantalum markers in the greater and lesser trochanters were implanted for radiostereometric analysis. Three months after surgery, a fracture of the lesser trochanter, ossifications and a stems subsidence of 11 mm were detected (b). Twenty months after surgery, the patient underwent revision surgery due to pain and gluteal insufficiency: ossifications were removed, the head was changed to a longer neck, while the stem was fully osseointegrated and remained in situ (c).
4. Discussion
This prospective, single-center study's primary aim was to investigate changes in clinical outcomes after THA with the Nanos short-stem prosthesis. Compared with baseline measurements, significant improvements were noted for all outcomes at 24-month follow-up. Clinical outcomes were consistent with those reported in the earlier subgroup analysis of 17 patients from this cohort.5
Because a wide variety of short-stem designs are available to surgeons and patients,4 there remains a need to report clinical findings with unique implants in order to gauge their relative safety and performance. There are several publications detailing the clinical performance of the Nanos.13, 14, 15, 16, 17, 18, 19 These studies uniformly reported beneficial clinical performance with this implant at follow-up times of up to 5 years. All studies included HHS as an outcome, reporting mean scores in the ≥90 range considered “excellent.” The only exception is the analysis by Czech et al.,16 who divided patients into cohorts of ≤60 and > 60 years of age, with the latter group reporting mean HHS of 85.3, a range nonetheless still identified as “good.” Amenabar et al.,18 Ettinger et al.,14 Kaipel et al.,13 and Stadler et al.15 reported mean final HHS scores >90 in subjects whose average age (range, 61.6–64 years) was notably higher than our own cohort (mean age, 54.9 years). Also noteworthy are the results from Capone et al.,19 who reported excellent clinical outcomes at 5.6 years in patients undergoing THA for osteonecrosis of the femoral head, for which traditional THA has been shown to result in worse outcomes and survivorship than other indications.20
To the best of our knowledge, ours is the first study to include SF-36 MCS and VAS pain as outcomes of interest with this particular implant. Short-stem designs in general have been proposed to confer comparable survivorship and functional improvements to conventional stems, with additional benefits such as decreased thigh pain.21 Our observation of significant improvements in multiple markers of post-THA quality of life, pain, function, and mobility provides further evidence that short-stem designs can meet key treatment goals of younger, active patients for whom short-stem prostheses are routinely offered as an alternative to conventional THA.18 Our results are consistent with positive clinical outcomes observed with other short-stem designs sharing similar design concepts.22, 23, 24 However, the wide variety of available short-stem implant precludes generalizing their potential impact to the design class as a whole.
In the early phase of their clinical use, the smaller surface and bone-implant-interface of short-stem implants raised concerns about whether they could achieve sufficient primary stability,25 although since then several studies have shown that short-stem THA is an efficient and safe procedure.13,14,22
Our prior publication detailing the RSA results of a subgroup of 17 initial patients from this cohort concluded that the Nanos exhibited only slight initial migration within three months after implantation, which subsequently led to secondary stabilization.5 This in line with other studies of Nanos’ migration patterns at follow-up durations of up to 5 years.13,17,19
In the current updated analysis, implantation with the Nanos resulted in neutral stem positioning for the majority of subjects, with the exception being two and five subjects with varus tilts of up to 5° and a valgus position of up to 5°, respectively. A 2017 bone remodeling analysis of the Nanos and Metha prostheses conducted by Brinkmann et al. concluded that varus/valgus positioning was negligible with these designs and unlikely to lead to stress shielding on a magnitude similar to conventional stems.26 This is a key proposed advantage, as stress shielding is an established contributor to early implant failure.27 Notably, stem angulation may be influenced by patient positioning.28
In comparison with older patients, younger patients undergoing THA have consistently been shown to have an increased risk of early and long-term revision.29 We observed a 98.1% stem survivorship at final follow-up. Other groups have reported 100% survivorship for Nanos at follow-up periods just over 5 years.14, 15, 16 The two revisions in our cohort were due to periprosthetic fracture (unrelated to study device) and aseptic loosening resulting from infection. Analyses of large data sets from implant registries have reported a 0.47% rate of periprosthetic fracture within 2 years for uncemented femoral stems30 and 0.9% for prosthetic joint infections for primary THA in general.31 Although our rate for these complications is comparatively higher, this is most likely due to the relatively small cohort employed.
This study has several limitations. Firstly, all THAs were performed by a single surgeon experienced with the implantation of this device, restricting the ability to extrapolate these results. Secondly, the study consisted of 52 subjects, which is relatively small compared with other trials evaluating the clinical and radiographic outcome of short-stem THA.21 Thirdly, the 24-month follow-up period can be considered as relatively brief, although the majority of studies of short stems to date have provided only short- and medium-term follow-up.21
5. Conclusion
This study indicates that the Nanos short-stem prosthesis results in promising clinical and radiographic outcomes after a follow-up of 24 months. Our results are in line with those previously reported for this device, and with those of short stems with similar design concepts. We observed a low complication rate and high rate of stem survivorship. These results are highly supportive of the proposed design advantages of short-stem prostheses. Longer-duration follow-up studies will determine whether these advantages are extended into the second decade of implantation.
Funding
Smith & Nephew Orthopaedics AG (Baar, Switzerland) acted as the sponsor of this study, granted financial compensation for expenses of the study and for presentation and publication of the data. The company did not take part in analysis and interpretation of data.
CRediT authorship contribution statement
Stefan Budde: Conceptualization, Investigation, Resources, Writing - original draft. Michael Schwarze: Formal analysis, Visualization. Thilo Floerkemeier: Data curation, Formal analysis, Resources. Jochen Plagge: Formal analysis. Nils Wirries: Formal analysis, Writing - review & editing. Henning Windhagen: Conceptualization, Supervision, Writing - review & editing. Fritz Thorey: Conceptualization, Investigation, Resources. Alexander Derksen: Writing - original draft.
Declaration of competing interest
Stefan Budde and Fritz Thorey received financial support from Smith & Nephew in relation to this study. Stefan Budde has a consulting contract with Artiqo GmbH, Germany. Henning Windhagen has a consulting contract and acts as a paid consultant for Aesculap, Tuttlingen, Germany. Alexander Derksen, Thilo Floerkemeier, Jochen Plagge, Michael Schwarze, and Nils Wirries have no disclosures to report.
Acknowledgements
None.
References
- 1.Lehil M.S., Bozic K.J. Trends in total hip arthroplasty implant utilization in the United States. J Arthroplasty. 2014;29:1915–1918. doi: 10.1016/j.arth.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 2.Jerosch J. [Is shorter really better? : philosophy of short stem prosthesis designs] Orthopä. 2011;40:1075–1083. doi: 10.1007/s00132-011-1848-9. [DOI] [PubMed] [Google Scholar]
- 3.Yan S.G., Weber P., Steinbruck A., Hua X., Jansson V., Schmidutz F. Periprosthetic bone remodelling of short-stem total hip arthroplasty: a systematic review. Int Orthop. 2018;42:2077–2086. doi: 10.1007/s00264-017-3691-z. [DOI] [PubMed] [Google Scholar]
- 4.Jerosch J. [Differences between short stem prostheses] Orthopä. 2014;43:783–795. doi: 10.1007/s00132-014-2308-0. quiz 796. [DOI] [PubMed] [Google Scholar]
- 5.Budde S., Seehaus F., Schwarze M. Analysis of migration of the Nanos(R) short-stem hip implant within two years after surgery. Int Orthop. 2016;40:1607–1614. doi: 10.1007/s00264-015-2999-9. [DOI] [PubMed] [Google Scholar]
- 6.Ware J.E., Jr., Sherbourne C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 7.Harris W.H. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed] [Google Scholar]
- 8.Nilsdotter A.K., Lohmander L.S., Klassbo M., Roos E.M. Hip disability and osteoarthritis outcome score (HOOS)--validity and responsiveness in total hip replacement. BMC Muscoskel Disord. 2003;4:10. doi: 10.1186/1471-2474-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Aubigne R.M., Postel M. Functional results of hip arthroplasty with acrylic prosthesis. J Bone Joint Surg Am. 1954;36-a:451–475. [PubMed] [Google Scholar]
- 10.Amstutz H.C., Thomas B.J., Jinnah R., Kim W., Grogan T., Yale C. Treatment of primary osteoarthritis of the hip. A comparison of total joint and surface replacement arthroplasty. J Bone Joint Surg Am. 1984;66:228–241. [PubMed] [Google Scholar]
- 11.Freyd M. The graphic rating scale. J Educ Psychol. 1923;14:83–102. [Google Scholar]
- 12.Brooker A.F., Bowerman J.W., Robinson R.A., Riley L.H., Jr. Ectopic ossification following total hip replacement. Incidence and a method of classification. J Bone Joint Surg Am. 1973;55:1629–1632. [PubMed] [Google Scholar]
- 13.Kaipel M., Grabowiecki P., Sinz K., Farr S., Sinz G. Migration characteristics and early clinical results of the NANOS(R) short-stem hip arthroplasty. Wien Klin Wochenschr. 2015;127:375–378. doi: 10.1007/s00508-015-0756-0. [DOI] [PubMed] [Google Scholar]
- 14.Ettinger M., Ettinger P., Lerch M. The NANOS short stem in total hip arthroplasty: a mid term follow-up. Hip Int. 2011;21:583–586. doi: 10.5301/HIP.2011.8658. [DOI] [PubMed] [Google Scholar]
- 15.Stadler N., Lehner J., Abbas R., Trieb K. Prospective mid-term results of a consecutive series of a short stem. Acta Orthop Belg. 2016;82:372–375. [PubMed] [Google Scholar]
- 16.Czech S., Hermanson J., Kasperczyk S. Cementless neck preserving arthroplasty should be the treatment of choice in cases of coxarthrosis in middle-aged men: very good results in a mid-term follow-up study with NANOS stem and Verilast articulation. Chir Narzadow Ruchu Ortop Pol. 2018;83:103–107. doi: 10.31139/chnriop.2018.83.3.21. [DOI] [Google Scholar]
- 17.Gotze C., Ehrenbrink J., Ehrenbrink H. [Is there a bone-preserving bone remodelling in short-stem prosthesis? DEXA analysis with the Nanos total hip arthroplasty] Z für Orthop Unfallchirurgie. 2010;148:398–405. doi: 10.1055/s-0030-1250151. [DOI] [PubMed] [Google Scholar]
- 18.Amenabar T., Marimuthu K., Hawdon G., Gildone A., McMahon S. Total hip arthroplasty using a short-stem prosthesis: restoration of hip anatomy. J Orthop Surg. 2015;23:90–94. doi: 10.1177/230949901502300121. [DOI] [PubMed] [Google Scholar]
- 19.Capone A., Bienati F., Torchia S., Podda D., Marongiu G. Short stem total hip arthroplasty for osteonecrosis of the femoral head in patients 60 years or younger: a 3- to 10-year follow-up study. BMC Muscoskel Disord. 2017;18:301. doi: 10.1186/s12891-017-1662-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cornell C.N., Salvati E.A., Pellicci P.M. Long-term follow-up of total hip replacement in patients with osteonecrosis. Orthop Clin N Am. 1985;16:757–769. [PubMed] [Google Scholar]
- 21.Lidder S., Epstein D.J., Scott G. A systematic review of short metaphyseal loading cementless stems in hip arthroplasty. Bone Joint Lett J. 2019;101-b:502–511. doi: 10.1302/0301-620X.101B5.BJJ-2018-1199.R1. [DOI] [PubMed] [Google Scholar]
- 22.Thorey F., Hoefer C., Abdi-Tabari N., Lerch M., Budde S., Windhagen H. Clinical results of the metha short hip stem: a perspective for younger patients? Orthop Rev. 2013;5:e34. doi: 10.4081/or.2013.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wittenberg R.H., Steffen R., Windhagen H., Bucking P., Wilcke A. Five-year results of a cementless short-hip-stem prosthesis. Orthop Rev. 2013;5:e4. doi: 10.4081/or.2013.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidutz F., Grote S., Pietschmann M. Sports activity after short-stem hip arthroplasty. Am J Sports Med. 2012;40:425–432. doi: 10.1177/0363546511424386. [DOI] [PubMed] [Google Scholar]
- 25.Reimeringer M., Nuno N., Desmarais-Trepanier C., Lavigne M., Vendittoli P.A. The influence of uncemented femoral stem length and design on its primary stability: a finite element analysis. Comput Methods Biomech Biomed Eng. 2013;16:1221–1231. doi: 10.1080/10255842.2012.662677. [DOI] [PubMed] [Google Scholar]
- 26.Brinkmann V., Radetzki F., Gutteck N., Delank S., Zeh A. Influence of varus/valgus positioning of the Nanos(R) and Metha(R) short-stemmed prostheses on stress shielding of metaphyseal bone. Acta Orthop Belg. 2017;83:57–66. [PubMed] [Google Scholar]
- 27.Kobayashi S., Saito N., Horiuchi H., Iorio R., Takaoka K. Poor bone quality or hip structure as risk factors affecting survival of total-hip arthroplasty. Lancet. 2000;355:1499–1504. doi: 10.1016/S0140-6736(00)02164-4. [DOI] [PubMed] [Google Scholar]
- 28.Boese C.K., Bredow J., Ettinger M. The influence of hip rotation on femoral offset following short stem total hip arthroplasty. J Arthroplasty. 2016;31:312–316. doi: 10.1016/j.arth.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 29.Santaguida P.L., Hawker G.A., Hudak P.L. Patient characteristics affecting the prognosis of total hip and knee joint arthroplasty: a systematic review. Can J Surg. 2008;51:428–436. [PMC free article] [PubMed] [Google Scholar]
- 30.Thien T.M., Chatziagorou G., Garellick G. Periprosthetic femoral fracture within two years after total hip replacement: analysis of 437,629 operations in the nordic arthroplasty register association database. J Bone Joint Surg Am. 2014;96:e167. doi: 10.2106/JBJS.M.00643. [DOI] [PubMed] [Google Scholar]
- 31.Lindgren V., Gordon M., Wretenberg P., Karrholm J., Garellick G. Deep infection after total hip replacement: a method for national incidence surveillance. Infect Control Hosp Epidemiol. 2014;35:1491–1496. doi: 10.1086/678600. [DOI] [PubMed] [Google Scholar]