Abstract
Sulfasalazine is an aminosalicylate primarily used in the treatment of rheumatoid arthritis and ulcerative colitis. Its immunomodulatory, anti-inflammatory, and antiproliferative properties make it a potential therapeutic option for various dermatological disorders. Owing to its wide range of effects, it is often used off-label in dermatological diseases, such as alopecia areata, psoriasis and psoriatic arthritis, lichen planus, and pemphigus. However, the level of evidence supporting its efficacy and safety in dermatology is limited. More research is needed to uncover the full potential of sulfasalazine in dermatology. The present article is a detailed review of the pharmacology, modes of action, side effects, and contraindications of sulfasalazine, along with an up-to-date review of the evidence underlying its use in various dermatological conditions.
Keywords: Sulfasalazine, Dermatology, Immunomodulator, DMARD, Psoriasis
Introduction
Sulfasalazine (SSZ; salazopyrin, sulfasalazopyridine, salicylazosulfapyridine) is a U.S. Food and Drug Administration–approved disease-modifying antirheumatic drug (DMARD) commonly used in the treatment of rheumatoid arthritis and ulcerative colitis (Rains et al., 1995). Owing to its anti-inflammatory action, SSZ is used off-label in a wide spectrum of dermatological diseases; however, there is dearth of literature on its use in dermatology.
History
SSZ was devised by Dr. Nana Svartz, a Scandinavian rheumatologist and professor of medicine at the Karolinska Institute in Stockholm, in cooperation with Swedish pharmaceutical company Pharmacia in 1941. It was an attempt to treat rheumatoid arthritis (then known as rheumatic polyarthritis), which was believed to be a disease of bacterial origin. It was observed that when given concomitantly, sulfonamides and aspirin did not produce concrete results, which shifted the research focus to combining the two agents chemically to produce an antibiotic that would have an affinity for connective tissue. The result was the drug SSZ, which was first tried in rheumatic polyarthritis with impressive results and later in ulcerative colitis (Dover, 1971, Svartz, 1948, Watkinson, 1986). The use of SSZ in dermatology can be traced back to 1971 when it was first used in scleroderma (Dover, 1971).
Pharmacokinetics
Absorption
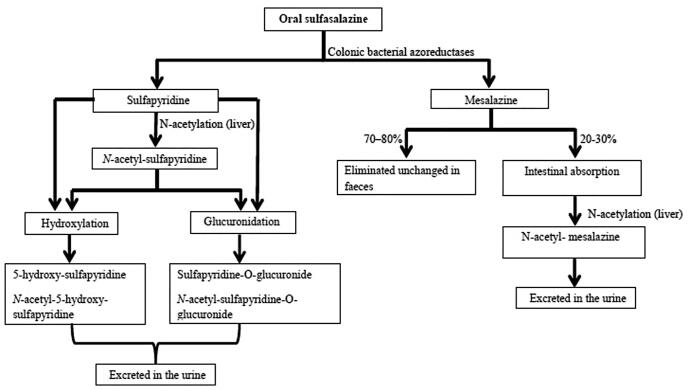
SSZ is the combination of a sulfonamide (sulfapyridine) and mesalazine (mesalamine, 5-aminosalicylic acid) covalently linked by an azo bond. It is absorbed in the small intestine with an oral bioavailability of approximately 10% to 30%. SSZ that reaches the large bowel undergoes azoreduction by bacterial azoreductases to release sulfapyridine and mesalazine. Sulfapyridine is almost fully (>90%) absorbed, whereas mesalazine undergoes only 20% to 30% absorption, and the remainder is eliminated in the feces (Fig. 1; Plosker and Croom, 2005, Rains et al., 1995).
Fig. 1.
Flow diagram of the pharmacokinetics of sulfasalazine on oral administration.
Distribution
Absorbed sulfasalazine is distributed throughout the body but does not cross the blood–brain barrier. Both SSZ and sulfapyridine cross the placenta and are found in breast milk. Breast milk concentrations of sulfapyridine are approximately 40% of those seen in plasma, but SSZ concentrations are negligible. Plasma concentrations of mesalazine are very low, and it is therefore unlikely to be found in breast milk. Both sulfapyridine and SSZ distribute to synovial fluid, with concentrations generally lower than those found in plasma. The peak plasma level of sulfapyridine is attained later than SSZ. The plasma protein binding is up to 99% for SSZ and 50% for sulfapyridine (Rains et al., 1995).
Metabolism and excretion
Systemically absorbed sulfasalazine is metabolized to some extent in the liver to sulfapyridine and mesalazine (U.S. National Library of Medicine, 2001); a small proportion of an administered dose of SSZ is eliminated unchanged in the urine (Rains et al., 1995, Tett, 1993). Sulfapyridine is primarily metabolized by acetylation in the liver to form N-acetyl-sulfapyridine (inactive), some of which is eliminated in the urine. Sulfapyridine and its N-acetyl metabolite also undergo hydroxylation and glucuronidation; the glucuronide conjugates and the hydroxylated metabolites (5-hydroxy-sulfapyridine and N-acetyl-5-hydroxy-sulfapyridine) are eliminated in the urine (Rains et al., 1995, Tett, 1993, U.S. National Library of Medicine, 2001).
Mesalazine is mainly (70%–80%) eliminated unchanged in the feces, but it also undergoes acetylation to form N-acetyl- mesalazine (inactive), which is eliminated in the urine (Fig. 1). The metabolism of sulfapyridine is affected by the acetylator phenotype. In the Caucasian population, there is an approximately equal distribution in fast and slow acetylators (Plosker and Croom, 2005, Tett, 1993). The elimination half-life of sulfapyridine ranges between 5 and 18 hours and is approximately 50% to 100% longer in slow acetylators than in fast acetylators. Slow acetylators have higher plasma concentrations of sulfapyridine, which is thought to be related to the higher prevalence of minor adverse effects (Plosker and Croom, 2005, Taggart et al., 1992, Tett, 1993).
Mechanism of action
The sulfa moiety is known to have antimicrobial properties, whereas the salicylate component acts as an anti-inflammatory agent. The mechanism of action of SSZ is summarized in Table 1 (Akahoshi et al., 1997, Bissonnette et al., 1996, Camp, 1992, Cannella and O’Dell, 2017, Feeley et al., 1999, Fujiwara et al., 1990, Gadangi et al., 1996, Gupta, 1990, Halasz, 1990, Hashimoto et al., 1991, Hirohata et al., 2002, Kang et al., 1999, Plosker and Croom, 2005, Pruzanski et al., 1997, Rodenburg et al., 2000, Smedegård and Björk, 1995, Yamazaki et al., 1991).
Table 1.
Mechanism of action of sulfasalazine.
| Action of sulfasalazine | Mechanism |
|---|---|
| Immunomodulatory/anti-inflammatory action | Inhibition of cytokine release
|
| Antiproliferative action | Competitive inhibitor of brush border folate conjugase enzyme
|
| Antibacterial activity | Competitive inhibition of the bacterial enzyme dihydropteroate synthetase, necessary for the synthesis of folic acid |
ELAM, endothelial cell adhesion molecule; ICAM, intercellular adhesion molecule; IL, interleukin; TNF, tumor necrosis factor; VCAM, vascular cell adhesion molecule.
Dermatological uses
The use of SSZ in rheumatoid arthritis and inflammatory bowel disease is supported by robust evidence (Plosker and Croom, 2005, Watkinson, 1986). The uses of SSZ in the field of dermatology are summarized in Table 2.
Table 2.
Review of literature on SSZ use in various dermatological diseases.
| S. No. | Dermatological disease | Study (year) | Sample size | Maximum daily dose | Maximum duration of treatment | Outcome | Adverse effects reported |
|---|---|---|---|---|---|---|---|
| 1. | Psoriasis | Uncontrolled open-label study (Gupta et al., 1989) | 32 | 3 g | 8 weeks | Twenty-four patients completed the treatment, and 17 had modest to marked improvement or clearing. | Nausea, indigestion, diarrhea, fatigue, cutaneous reaction |
| Double-blind placebo-controlled randomized study (Gupta et al, 1990) | 50 | 4 g | 8 weeks | In the SSZ group, 7 of 17 patients had marked, 7 of 17 had moderate, and 3 of 17 had minimal improvement. In the placebo group, only 1 of 27 patients demonstrated moderate improvement. |
Cutaneous reactions, nausea | ||
| Comparative randomized study (Bharti and Girgla, 1996) | 30 | 1.5 g | 12 weeks | Efficacy was comparable for the 2 drugs: Decrease in mean EST in patients on methotrexate and SSZ therapy was 86.55% and 83.64% at 4 weeks and 92.86% and 92.13% at 12 weeks, respectively. | Transient rise in transaminases | ||
| Randomized controlled trial (El-Mofty et al., 2011) | 32 | 2 g | 8 weeks | Twenty-one of 32 patients completed treatment. The percentage reduction in PASI score achieved with methotrexate was significantly higher than that with SSZ alone, PTX alone, or the combined use of SSZ and PTX | Nausea | ||
| Case report (Li et al., 2018a) | 1 | 1 g | 6 weeks | Skin lesions disappeared after 6 weeks of treatment | NR | ||
| 2. | Alopecia areata | Retrospective record based (Ellis et al., 2002) | 249 | 4 g | Variable | Of the 19 patients who took SSZ for ≥ 3 months, 7 patients had excellent, 3 had slight, and 9 had no improvement | GI distress, rash, laboratory abnormalities, headache |
| Uncontrolled open-label study (Aghaei, 2008) | 26 | 3 g | 6 months | Twenty-two patients completed treatment. Overall, 15 of 22 patients (68.2%) responded to therapy. Six of 22 (27.3%) achieved complete hair regrowth, and 40.9% had partial hair regrowth. Seven (31.8%) had no hair regrowth. Of the 22 patients with complete and partial remission, 10 (45.5%) had partial or complete relapse | GI distress, rash, laboratory abnormalities, headache | ||
| 3. | Lichen planus | Uncontrolled open-label study (Bauza et al., 2005) | 20 | 3 g | 12 months | Complete responses were observed in 13 patients and partial responses in 7 patients. All patients reported an early resolution of the pruritus. No changes were detected in mucosal lichen planus |
GI distress, cutaneous rash, leukopenia, poor glycemic control |
| Double-blind, randomized, placebo-controlled study (Omidian et al., 2010) | 52 | 2.5 g | 6 weeks | Twenty-three patients in the SSZ group and 21 patients in the placebo group evaluated for efficacy. After 6 weeks of treatment, the rate of improvement was 9.6% (two patients) in the placebo group and 82.6% (19 patients) in the sulfasalazine group. The improvement rate of pruritus was 14.3% in the placebo group and 91.3% in the sulfasalazine group |
Skin rash, headache, GI distress, mild leucopenia | ||
| Uncontrolled open-label study (Jeong et al., 2016) | 21 | Topical SSZ 3 times/day | 4 weeks | Seventeen patients (81%) reported improvement of discomfort and 12 patients (57%) had lesions decrease in size > 50% | NR | ||
| 4. | Pemphigus vulgaris | Comparative, double-blind study (El-Darouti et al., 2009) | 64 | SSZ 1.5 g (+PTX 1.2 g)* | Variable | Forty-two patients received SSZ+PTX and 22 patients received placebo. A statistically significant decrease in serum levels of TNF-alpha in the SSZ group compared with those in placebo group at 6 and 8 weeks. Significant clinical improvement was observed in patients in the SSZ group compared with those in the placebo group. | Gastric pain, nausea, and headache |
| Uncontrolled, open-label study (Dogra et al., 2015) | 15 | SSZ 1.5 g (+PTX 1.2 g)* | Variable | Patients achieved remission period ranging from 6 months to 3 years | Nausea, vomiting, headache, and fatigue | ||
| 5. | Dermatitis herpetiformis | Case report (Willsteed et al., 2005) | 2 | 4 g | Variable | – | NR |
| 6. | Mucus membrane pemphigoid | Retrospective study (Doan et al., 2001) | 9 | 4 g | NR | Disease remained controlled with SSZ alone in four patients (45%). Two patients (22%) required adjunctive oral cyclophosphamide | GI discomfort, hemolysis |
| 7. | Urticaria | Retrospective study (McGirt et al., 2006) | 19 | 4 g | 24 months | Fourteen patients (74%) reported significant improvement, four patients (21%) reported minimal improvement but were not satisfied with their symptom relief, and one patient (5%) reported worsening of symptoms | Headache, GI discomfort, leukopenia, elevated liver enzymes |
| Retrospective study (Orden et al., 2014) | 39 | 3 g | 74 weeks (mean) | Eight patients were excluded from the final analysis. Twenty-six patients (83.9%) showed an improvement in symptoms within the first 3 months, with 51.6% of patients becoming asymptomatic within the first 6 months of starting SSZ. Eleven patients (35.4%) achieved complete relief of symptoms after tapering off SSZ therapy. Five of the 31 patients (16.1%) failed treatment | Leucopenia, anemia, raised transaminases, rhabdomyolysis | ||
| Case report (Engler et al., 1995) | 2 | 4 g | NR | - | NR | ||
| 8. | DLE | Pilot study (Artüz et al., 1996) | 13 | 4.5 g | 3 months | - | Headache, nausea, epigastric pain |
| Case series (Delaporte et al., 1997) | 11 | 2 g | NR | Seven patients had complete response, one had partial response, and 3 were nonresponders. Six of eight responders were given SSZ exclusively | Light sensitization, neutropenia, transient rise in liver enzymes | ||
| 9. | Atrophie blanche | Case report (Gupta et al., 1990) | 2 | 3 g | Variable | - | NR |
| Case series (Bisalbutra and Kullavanijaya, 1993) | 8 | 3 g | 2 months | - | Facial edema, lip paresthesia | ||
| 10. | Generalized morphea | Case report (Taveira et al., 1999) | 1 | 2 g | 7 months | Marked decrease in the infiltration of the plaques and restoration of the mobility of the affected areas after 1 month of treatment with SSZ | NR |
| Case report (Micalizzi et al., 1996) | 1 | 2 g | 8 months | – | NR | ||
| 11. | PD-PSV | Case report (Li et al., 2018b) | 1 | 0.5 g | NR | – | NR |
| 12. | EED | Case report (Chen et al., 2017) | 1 | NR | NR | Patient reported full remission of EED symptoms soon after initiation of SSZ | NR |
DLE, discoid lupus erythematosus; EED; erythema elevatum diutinum; EST, erythema, scaling, and thickness; GI, gastrointestinal; NR, not reported; PASI, Psoriasis Area and Severity Index; PD-PSV, pyodermatitis-pyostomatitis vegetans; PTX, pentoxifylline; SSZ, sulfasalazine; TNF, tumor necrosis factor.
Combined therapy with IV pulsed steroid.
Psoriasis and psoriatic arthritis
SSZ has been tried successfully alone and in combination with pentoxifylline in patients with psoriasis (Bharti and Girgla, 1996, El-Mofty et al., 2011, Gupta, 1990, Gupta et al., 1989) and psoriatic arthritis (Jones et al., 2000, Marguerie et al., 2002) owing to its anti-inflammatory and antiproliferative action. The prescribed dose ranges from 1500 mg/day to 3 g/day.
Reductions have been observed in ICAM-1 expression, leukotriene synthesis, and in the number of intraepidermal and dermal T lymphocytes, as well as T-helper CD4 cells in the skin biopsy specimens of patients affected by psoriasis and treated with SSZ (Gupta, 1990). SSZ was also found to be effective in a case of acrodermatitis continua of Hallopeau (Li et al., 2018a).
Alopecia areata
SSZ in a dose of 500 mg twice daily for 1 month, 1 g twice daily for 1 month, and then 1.5 g twice daily for a maximum of 3 months was used in recalcitrant cases of alopecia areata with good response in 27% of cases (Aghaei, 2008). Ellis et al. (2002) reported a 23% response in severe cases of alopecia areata (Ellis et al. 2002).
Lichen planus
In a prospective uncontrolled study of 20 patients with lichen planus (LP), SSZ at initial doses of 1.5 g/day and increased by 0.5 g/week to 3 g/day for 4 to 16 weeks yielded a complete response in 13 patients and a partial response in 7 patients. Patients with mucosal LP showed a poor response (Bauza et al., 2005).
A randomized, double-blind, placebo-controlled, prospective study of 52 patients with LP concluded that SSZ is a relatively safe and effective alternative treatment option for generalized LP (Omidian et al., 2010). SSZ has also been tried in a topical formulation as a mouthwash in oral LP with good results (Jeong et al., 2016).
Immunobullous disorders
In a comparative double-blind study, a combination of SSZ and pentoxifylline used as adjuvant therapy in the treatment of PV induced a faster and more significant decrease in the serum level of tumor necrosis factor alpha (TNF-α), and this decrease was associated with rapid clinical improvement. These adjuvant therapies can replace cyclophosphamide, especially in younger patients, owing to their relatively fewer side effects (Dogra et al., 2015, El-Darouti et al., 2009).
SSZ is one of the first-line drugs for the management of ocular mucus membrane pemphigoid, alongside dapsone. Incidence of complications is also less frequently reported with SSZ than with dapsone (Doan et al., 2001, Sobolewska et al., 2013). However, limited data exist on the use of SSZ in other bullous disorders, such as dermatitis herpetiformis (Willsteed et al., 2005).
Urticaria and angioedema
Sulfasalzine has been effectively tried in antihistamine-resistant cases of chronic idiopathic urticaria and angioedema (McGirt et al., 2006, Orden et al., 2014). An isolated case has been reported of the usefulness of sulfasalzine in delayed pressure urticaria (Engler et al., 1995).
Pyoderma gangrenosum, erythema elevatum diutinum, and Behcet’s disease
Anecdotal cases have been reported on the successful use of SSZ in pyoderma gangrenosum and erythema elevatum diutinum (Bhat, 2012, Chen et al., 2017, Miranda, 2002). Low-dose SSZ has also been successfully used in a case of pyodermatitis-pyostomatitis vegetans (Li et al., 2018b). SSZ along with steroids is the first-line treatment for gastrointestinal Behcet’s disease (Alpsoy, 2012).
Atrophie blanche
Anecdotal reports exist on the use of SSZ in atrophie blanche (Bisalbutra and Kullavanijaya, 1993, Gupta et al., 1990).
Discoid lupus erythematosus
SSZ has been tried in the treatment of discoid lupus erythematosus. In one study comprising 11 cases, SSZ was found to be more effective in rapid acetylators (Artüz et al., 1996, Delaporte et al., 1997).
Morphea and lichen sclerosus et atrophicus
Generalized bullous morphea and generalized morphea with Lichen sclerosus et atrophicus was successfully treated with SSZ in occasional case reports (Micalizzi et al., 1996, Taveira et al., 1999).
Miscellaneous
Anecdotal reports exist on the use of SSZ in combination and sequential therapy with other DMARDs in the management of SAPHO syndrome with good response (Huber et al., 2009, Özen and Kalyoncu, 2011). The use of SSZ has also been reported in adult-onset still disease, but it should be used with caution owing to the high incidence of adverse effects (Jung et al., 2000).
Dosage and administration
SSZ is available in regular and enteric coated tablets of 500 mg and a suspension of 500 mg/ml. The dose is gradually titrated to minimize gastrointestinal adverse effects. The initial recommended dosage is 500 mg daily, escalated by 500 mg/day every week to the standard dose of 1500 to 3000 mg daily in divided doses. If a patient is intolerant of a new dose level, the dose should be reduced to a previously tolerated level for a further week and then increased again as appropriate. In pediatric patients, the initial dose is 10 to 12.5 mg/kg/day, which is increased weekly at 50 mg/kg/day in two divided doses until a maintenance of 2 g/day is achieved (Akil and Amos, 1995, Cannella and O’Dell, 2017).
Concomitant folic acid supplementation is advised because SSZ reduces folate absorption and is an inhibitor of the folate-dependent pathway. The dose should be reduced for patients with renal insufficiency (Cannella and O’Dell, 2017).
Adverse effects
Most adverse events due to SSZ occur during the first few months of treatment, and occurrences decrease with time. The most common adverse effects are gastrointestinal effects, headache, dizziness, and rash (Rains et al., 1995).
Gastrointestinal and hepatic
The most frequently reported adverse effects with SSZ therapy are gastrointestinal effects (nausea, vomiting, dyspepsia, anorexia, abdominal pain, and diarrhea). SSZ is therefore prescribed as enteric coated tablets for most patients to minimize gastrointestinal effects. Elevated levels of liver enzymes and hepatic dysfunction are reported less frequently and are mostly transient (Cannella and O’Dell, 2017).
Hematologic
Hematologic disturbances are reported in ≤3% of SSZ recipients and usually occur within the first 3 months. Leukopenia is the most common abnormality and usually reverses after discontinuing SSZ. The incidence of hematologic disturbances is higher in patients with rheumatoid arthritis than in patients receiving the drug for other diagnoses.
Macrocytosis due to folate deficiency and hemolysis have been reported with SSZ; its use should be avoided in patients with G6PD deficiency. Thrombocytopenia is rare (Cannella and O’Dell, 2017, Remlinger, 2012).
Dermatologic
The sulfapyridine moiety in SSZ is believed to be responsible for most of the hypersensitivity reactions that occur. Cutaneous rashes are reported in <5% of patients and are usually maculopapular, pruritic, and generalized (Cannella and O’Dell, 2017). Anecdotal reports exist of occurrences of erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms, lichenoid eruption, lupus-like syndrome, and phototoxicity (Ghosh et al., 2013, Jullien et al., 1995, Santhanam and Singh, 2018, Tohyama et al., 1998, Tremblay et al., 2011).
Raynaud phenomenon, thinning of the hair, and cutaneous pigmentation have also been reported with SSZ use (Saigal et al., 2010, Brzezińska-Wcisło and Wcisło-Dziadecka, 2016, Gabazza et al., 1992).
Central nervous system
Headache and dizziness are the most common neurologic side effects (Plosker and Croom, 2005, Rains et al., 1995). Axonal neuropathy may occur after prolonged SSZ use but is very rare (Liedorp et al., 2008).
Fertility
Prolonged treatment with SSZ may universally depress semen quality (i.e., increase the number of abnormal forms and impaired sperm motility) and cause oligospermia, leading to infertility. However, the condition appears to be reversible upon discontinuation of the drug, returning to normal 2 to 3 months after cessation. SSZ has no effect on fertility in women (Cannella and O’Dell, 2017, Plosker and Croom, 2005).
Pregnancy and lactation
SSZ and sulfapyridine cross the placenta, but they do not seem to cause or increase fetal anomalies, spontaneous abortions and therefore can be safely used in women of childbearing age and pregnant women. However, it should be used with caution owing to reports of neural tube defects and hematological complications, such as hemolytic anemia in the fetus. Administration of folic acid (5 mg/day) with SSZ is recommended during pregnancy to decrease the risk of neural tube defects.
Sulfapyridine is secreted in breast milk. The American Academy of Pediatrics has classified SSZ as a drug that must be given with caution to nursing women, especially in cases of premature infants and those with G6PD deficiency due to an increased risk of hemolytic anemia and jaundice (Bokstrӧm et al., 2006, Wu and Ying, 2019).
Pulmonary
Pulmonary toxicity due to SSZ is rare. Typical presentation of SSZ-induced lung disease is new-onset dyspnea and infiltrates on chest radiography. Pulmonary pathology is variable, the commonest being eosinophilic pneumonia with peripheral eosinophilia and interstitial inflammation with or without fibrosis (Parry et al., 2002).
Monitoring
Complete blood counts and liver and renal function tests should be performed prior to commencing therapy. These tests should be repeated every 2 to 4 weeks for the first 3 months and then every 8 to 12 weeks for the next 3 months of treatment. Beyond 6 months of therapy, longer intervals (12 weeks) of monitoring are suggested. Patients should be advised to report immediately in case of fever, sore throat, malaise, or nonspecific illness (Saag et al., 2008).
Drug interactions
SSZ inhibits thio-purine methyl transferase enzyme activity and thus may potentiate azathioprine toxicity (Anstey et al., 2004). SSZ reduces the absorption of folic acid and digoxin (U.S. National Library of Medicine, 2001). Rarely, SSZ can increase the effects of oral hypoglycemic drugs and the anticoagulant effect of warfarin (Cannella and O’Dell, 2017).
Contraindications
Contraindications to SSZ include Hypersensitivity to any component of SSZ, or with a sulfonamide or salicylate allergy (Saag et al., 2008); thrombocytopenia (platelet count <50,000/mm3; Saag et al., 2008); hepatic disease (e.g., liver transaminases >2-fold the upper limit of normal, acute hepatitis B or C, untreated chronic hepatitis B; Saag et al., 2008); significant renal impairment (Saag et al., 2008); porphyria (Cannella and O’Dell, 2017); and G6PD deficiency (Cannella and O’Dell, 2017).
Discussion
SSZ is approved by the U.S. Food and Drug administration for the management of ulcerative colitis and rheumatoid arthritis. It has been used for a variety of dermatological diseases, such as psoriasis, alopecia areata, pemphigus vulgaris, urticaria, and mucus membrane pemphigoid. However, its use in dermatology is limited by the lack of randomized trials.
We could find only a few randomized controlled trials supporting its use in psoriasis (Bharti and Girgla, 1996, Gupta et al., 1990), lichen planus (Omidian et al., 2010), and pemphigus vulgaris (El-Darouti et al., 2009). The use of SSZ in other dermatological diseases is largely supported by uncontrolled trials, case series, and case reports.
Conclusions
SSZ is an inexpensive drug that has immunomodulatory, anti-inflammatory, and antiproliferative properties with relatively few side effects. The wide range of applications of SSZ in varied dermatological diseases makes it an important drug in the therapeutic arsenal of a dermatologist. SSZ can be especially useful in patients who fail to respond to standard therapy or in those for whom standard therapy is contraindicated. However, the therapeutic potential of the drug in the field of dermatology needs to be further established by multicenter, large-scale randomized controlled trials.
Financial disclosures
none.
Funding
none.
Study approval
N/A.
Conflict of interest
none.
References
- Aghaei S. An uncontrolled, open label study of sulfasalazine in severe alopecia areata. Indian J Dermatol Venereol Leprol. 2008;74(6):611–613. doi: 10.4103/0378-6323.45103. [DOI] [PubMed] [Google Scholar]
- Akahoshi T., Namai R., Sekiyama N., Tanaka S., Hosaka S., Kondo H. Rapid induction of neutrophil apoptosis by sulfasalazine: implications of reactive oxygen species in the apoptotic process. J Leukoc Biol. 1997;62:817–826. doi: 10.1002/jlb.62.6.817. [DOI] [PubMed] [Google Scholar]
- Akil M., Amos R.S. ABC of rheumatology: rheumatoid arthritis –II: treatment. BMJ. 1995;310(6980):652–654. doi: 10.1136/bmj.310.6980.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpsoy E. New evidence-based treatment approach in Behçet’s disease. Patholog Res Int. 2012;2012 doi: 10.1155/2012/871019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey A.V., Wakelin S., Reynolds N.J. Guidelines for prescribing azathioprine in dermatology. Br J Dermatol. 2004;151:1123–1132. doi: 10.1111/j.1365-2133.2004.06323.x. [DOI] [PubMed] [Google Scholar]
- Artüz F., Lenk N., Deniz N., Alli N. Efficacy of sulfasalazine in discoid lupus erythematosus. Int J Dermol. 1996;35:746–748. doi: 10.1111/j.1365-4362.1996.tb00657.x. [DOI] [PubMed] [Google Scholar]
- Bauza A., Espana A., Gil P., Lloret P., Doval F.J.V. Successful treatment of lichen planus with sulfasalazine in 20 patients. Int J Dermatol. 2005;44(2):158–162. doi: 10.1111/j.1365-4632.2005.02070.x. [DOI] [PubMed] [Google Scholar]
- Bharti R., Girgla S. Sulfasalazine in treatment of psoriasis. Indian J Dermatol Venereol Leprol. 1996;62(2):87–88. [PubMed] [Google Scholar]
- Bhat R.M. Pyoderma gangrenosum: an update. Indian Dermatol Online J. 2012 Jan;3(1):7–13. doi: 10.4103/2229-5178.93482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisalbutra P., Kullavanijaya P. Sulfasalazine in atrophie blanche. J Am Acad Dermatol. 1993;28:275–276. doi: 10.1016/s0190-9622(08)81156-5. [DOI] [PubMed] [Google Scholar]
- Bissonnette E.Y., Enciso J.A., Befus A.D. Inhibitory effects of sulfasalazine and its metabolites on histamine release and TNF-alpha production by mast cells. J Immunol. 1996;156(1):218–223. [PubMed] [Google Scholar]
- Bokstrӧm H., Holst R.M., Hafstrom O., Swolin B., Johansson M.L., Brunlof G. Fetal hemolytic anemia associated with maternal sulfasalazine therapy during pregnancy. Acta Obstet Gynecol Scand. 2006;85(1):118–121. doi: 10.1080/00016340500334810. [DOI] [PubMed] [Google Scholar]
- Brzezińska-Wcisło L.A., Wcisło-Dziadecka D. Hair diseases: a big problem on a small surface. Adv Dermatol Allergol. 2016;33(5):317. doi: 10.5114/ada.2016.62834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp R.D.R. Psoriasis. In: Champian R.H., Burton J.L., Ebling F.J.G., editors. Textbook of dermatology. 5th ed. Blackwell Scientific Publications; Oxford: 1992. pp. 1391–1457. [Google Scholar]
- Cannella A.C., O’Dell J.R. Traditional DMARDs: methotrexate, sulfasalazine, leflunomide, hydroxychloroquine and combination therapies. In: Firestein G.S., Budd R., Gabriel S.E., McInnes I.B., editors. Kelley’s textbook of rheumatology. 10th ed. Elsevier; 2017. pp. 971–974. [Google Scholar]
- Chen M.L., Chlopik A., Hoang M.P., Smith G.P. Complete resolution of erythema elevatum diutinum using oral sulfasalazine. Dermatol Online J. 2017;23(10) [PubMed] [Google Scholar]
- Delaporte E., Catteau B., Sabbagh N., Gosselin P., Breuillard F., Doutre M.S. Treatment of discoid lupus erythematosus with sulfasalazine: 11 cases. Ann Dermatol Venereol. 1997;124(2):151–156. [PubMed] [Google Scholar]
- Doan S., Lerouic J.F., Robin H., Prost C., Savoldelli M., Hoang-Xuan T. Treatment of ocular cicatricial pemphigoid with sulfasalazine. Ophthalmology. 2001;108(9):1565–1568. doi: 10.1016/s0161-6420(01)00657-1. [DOI] [PubMed] [Google Scholar]
- Dogra D., Dogra N., Gupta N., Gupta V. Sulfasalazine and pentoxifylline, a new adjuvant in young pemphigus patients: a pilot study. Indian J Dermatol Venereol Leprol. 2015;22:31–37. doi: 10.4103/0378-6323.168341. [DOI] [PubMed] [Google Scholar]
- Dover N. Salazopyrin (azulfidine) treatment in scleroderma. Isr J Med Sci. 1971;7(11):1301–1303. [PubMed] [Google Scholar]
- El-Darouti M., Marzouk S., Abdel Hay R., El Tawdy A., Fawzy M., Leheta T. The use of sulfasalazine and pentoxifylline (low-cost antitumour necrosis factor drugs) as adjuvant therapy for the treatment of pemphigus vulgaris: a comparative study. Br J Dermatol. 2009;161(2):313–319. doi: 10.1111/j.1365-2133.2009.09208.x. [DOI] [PubMed] [Google Scholar]
- El-Mofty M., El-Darouti M., Rasheed H., Bassiouny D.A., Abdel-Halim M., Zaki N.S. Sulfasalazine and pentoxifylline in psoriasis: a possible safe alternative. J Dermatolog Treat. 2011;22:31–37. doi: 10.3109/09546630903460260. [DOI] [PubMed] [Google Scholar]
- Ellis C.N., Brown M.F., Voorhees J.J. Sulfasalazine for alopecia areata. J Am Acad Dermatol. 2002;46(4):541–544. doi: 10.1067/mjd.2002.119671. [DOI] [PubMed] [Google Scholar]
- Engler R.J., Squire E., Benson P. Chronic sulfasalazine therapy in the treatment of delayed pressure urticaria and angioedema. Ann Allergy Asthma Immunol. 1995;74(2):155–159. [PubMed] [Google Scholar]
- Feeley B.T., Park A.K., Hoyt E.G., Robbins R.C. Sulfasalazine inhibits reperfusion injury and prolongs allograft survival in rat cardiac transplants. J Hear Lung Transplant. 1999;18(11):1088–1095. doi: 10.1016/s1053-2498(99)00078-9. [DOI] [PubMed] [Google Scholar]
- Fujiwara M., Mitsui K., Yamamoto I. Inhibition of proliferative responses and interleukin 2 productions by salazosulfapyridine and its metabolites. Jpn J Pharmacol. 1990;54(2):121–131. doi: 10.1254/jjp.54.121. [DOI] [PubMed] [Google Scholar]
- Gabazza E.C., Taguchi O., Yamakami T., Machishi M., Ibata H., Suzuki S. Pulmonary infiltrates and skin pigmentation associated with sulfasalazine. Am J Gastroenterol. 1992;87(11):1654–1657. [PubMed] [Google Scholar]
- Gadangi P., Longaker M., Naime D., Levin R.I., Recht P.A., Montesinos M.C. The anti-inflammatory mechanism of sulfasalazine is related to adenosine release at inflamed sites. J Immunol. 1996;156(5):1937–1941. [PubMed] [Google Scholar]
- Ghosh S., Jain V., Chaudhuri S., Mathur S. Sulfasalazine induced lichen planus in a patient of rheumatoid arthritis. Indian J Dermatol Venereol Leprol. 2013;79(4):541. doi: 10.4103/0378-6323.113103. [DOI] [PubMed] [Google Scholar]
- Gupta A.K. Sulfasalazine improves psoriasis. Arch Dermatol. 1990;126(4):487. [PubMed] [Google Scholar]
- Gupta A.K., Ellis C.N., Siegel M.T., Voorhees J.J. Sulfasalazine: a potential psoriasis therapy? J Am Acad Dermatol. 1989;20(5 Pt 1):797–800. doi: 10.1016/s0190-9622(89)70092-x. [DOI] [PubMed] [Google Scholar]
- Gupta A.K., Goldfarb M.T., Voorhees J.J. The use of sulfasalazine in atrophie blanche. Int J Dermatol. 1990;29:663–665. doi: 10.1111/j.1365-4362.1990.tb02594.x. [DOI] [PubMed] [Google Scholar]
- Halasz C.L.G. Sulfasalazine as folic acid inhibitor in psoriasis. Arch Dermatol. 1990;126(11):1516. [PubMed] [Google Scholar]
- Hashimoto J., Sato K., Higaki M., Nishioka K., Kashiwazaki S., Miyasaka N. The effects of antirheumatic drugs on the production of, and the responsiveness to cytokines (IL-1 and IL-6) Ensho. 1991;11(3):279–286. [Google Scholar]
- Hirohata S., Ohshima N., Yanagida T., Aramaki K. Regulation of human B cell function by sulfasalazine and its metabolites. Int Immunopharmacol. 2002;2(5):631–640. doi: 10.1016/s1567-5769(01)00186-2. [DOI] [PubMed] [Google Scholar]
- Huber C.E., Judex A.G., Freyschmidt J., Feuerbach S., Schölmerich J., Müller-Ladner U. Sequential combination therapy leading to sustained remission in a patient with SAPHO syndrome. Open Rheumatol J. 2009;3:18. doi: 10.2174/1874312900903010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S.H., Na H.S., Park S.H., Ahn Y.W., Chung J. Topical sulfasalazine for unresponsive oral lichen planus. Quintessence Int. 2016;47(4):319–327. doi: 10.3290/j.qi.a34974. [DOI] [PubMed] [Google Scholar]
- Jones G., Crotty M., Brooks P. Interventions for psoriatic arthritis. Cochrane Database Syst Rev. 2000;3:CD000212. doi: 10.1002/14651858.CD000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jullien D., Wolkenstein P., Roupie E., Roujeau J.C., Revuz J. Toxic epidermal necrolysis after sulfasalazine treatment of mild psoriatic arthritis: warning on the use of sulfasalazine for a new indication. Arthritis Rheum. 1995;38:573. doi: 10.1002/art.1780380420. [DOI] [PubMed] [Google Scholar]
- Jung J.H., Jun J.B., Yoo D.H., Kim T.H., Jung S.S., Lee I.H. High toxicity of sulfasalazine in adult-onset Still’s. Clin Exp Rheumatol. 2000;18:245–248. [PubMed] [Google Scholar]
- Kang B.Y., Chung S.W., Im S.Y., Choe Y.K., Kim T.S. Sulfasalazine prevents T-helper 1 immune response by suppressing interleukin-12 production in macrophages. Immunology. 1999;98(1):98–103. doi: 10.1046/j.1365-2567.1999.00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remlinger KA. Hematologic toxicity of drug therapy. In: Wolverton, SE, editor. Comprehensive dermatologic drug therapy. 3rd ed. 2012. p. 696.
- Li M., Zhang Y., Xu H., Chen Z.Q., Li Y.M. A case of acrodermatitis continua of Hallopeau (ACH) successfully treated with sulfasalazine. Dermatol Ther. 2018;31 doi: 10.1111/dth.12596. [DOI] [PubMed] [Google Scholar]
- Li S., Li Z., Feng S. Low-dose sulfasalazine in a case of pyodermatitis-pyostomatitis vegetans. J Am Acad Dermatol. 2018 doi: 10.1016/j.jaad.2018.10.020. [DOI] [PubMed] [Google Scholar]
- Liedorp M., Voskuyl A.E., van Oosten B.W. Axonal neuropathy with prolonged sulphasalazine use. Clin Exp Rheumatol. 2008;26:671. [PubMed] [Google Scholar]
- Marguerie L., Flipo R.M., Grardel B., Beaurain D., Duquesnoy B., Delcambre B. Use of disease-modifying antirheumatic drugs in patients with psoriatic arthritis. Joint Bone Spine. 2002;69(3):275–281. doi: 10.1016/s1297-319x(02)00396-2. [DOI] [PubMed] [Google Scholar]
- McGirt L.Y., Vasagar K., Gober L.M., Saini S.S., Beck L.A. Successful treatment of recalcitrant chronic idiopathic urticaria with sulfasalazine. Arch Dermatol. 2006;142:1337–1342. doi: 10.1001/archderm.142.10.1337. [DOI] [PubMed] [Google Scholar]
- Micalizzi C., Parodi A., Rebora A. Generalized bullous morphoea. Efficacy of salazopyrin. Clin Exp Dermatol. 1996;21:246–247. doi: 10.1111/j.1365-2230.1996.tb00080.x. [DOI] [PubMed] [Google Scholar]
- Miranda M.F.M. Pyoderma gangrenosum treated with sulfasalazine and dapsone. Indian J Dermatol Venereol Leprol. 2002;68(3):160–161. [PubMed] [Google Scholar]
- Omidian M., Ayoobi A., Mapar M., Feily A., Cheraghian B. Efficacy of sulfasalazine in the treatment of generalized lichen planus: randomized double-blinded clinical trial on 52 patients. J Eur Acad Dermatology Venereol. 2010;24:1051–1054. doi: 10.1111/j.1468-3083.2010.03583.x. [DOI] [PubMed] [Google Scholar]
- Orden R.A., Timble H., Saini S.S. Efficacy and safety of sulfasalazine in patients with chronic idiopathic urticaria. Ann Allergy Asthma Immunol. 2014;112(1):64–70. doi: 10.1016/j.anai.2013.09.028. [DOI] [PubMed] [Google Scholar]
- Özen M., Kalyoncu U. SAPHO syndrome may be treated effectively with combined drug regimens-a case report. IJCRI. 2011;2:8–11. [Google Scholar]
- Parry S.D., Barbatzas C., Peel E.T., Barton J.R. Sulphasalazine and lung toxicity. Eur Respir J. 2002;19(4):756–764. doi: 10.1183/09031936.02.00267402. [DOI] [PubMed] [Google Scholar]
- Plosker G.L., Croom K.F. Sulfasalazine: a review of its use in the management of rheumatoid arthritis. Drugs. 2005;65(13):1825–1849. doi: 10.2165/00003495-200565130-00008. [DOI] [PubMed] [Google Scholar]
- Pruzanski W., Stefanski E., Vadas P., Ramamurthy N.S. Inhibition of extracellular release of proinflammatory secretory phospholipase A2 (sPLA2) by sulfasalazine. Biochem Pharmacol. 1997;53:1901–1907. doi: 10.1016/s0006-2952(97)00137-8. [DOI] [PubMed] [Google Scholar]
- Rains C.P., Noble S., Sulfasalazine Faulds D. A review of its pharmacological properties and therapeutic efficacy in the treatment of rheumatoid arthritis. Drugs. 1995;50:137–156. doi: 10.2165/00003495-199550010-00009. [DOI] [PubMed] [Google Scholar]
- Rodenburg R.J., Ganga A., Van Lent P.L., Van De Putte L.B., Van Venrooij W.J. The antiinflammatory drug sulfasalazine inhibits tumor necrosis factor α expression in macrophages by inducing apoptosis. Arthritis Rheum. 2000;43(9):1941–1950. doi: 10.1002/1529-0131(200009)43:9<1941::AID-ANR4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Saag K.G., Gim G.T., Patkar N.M., Anuntiyo J., Finney C., Curtis J.R. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Care Res. 2008;59:762–784. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- Saigal R., Kansal A., Mittal M., Singh Y., Ram H. Raynaud’s phenomenon. JAPI. 2010;58:309–313. [PubMed] [Google Scholar]
- Santhanam S., Singh N. Sulfasalazine induced photo toxicity. Indian J Rheumatol. 2018;13(1):64. [Google Scholar]
- Smedegård G., Björk J. Sulphasalazine: mechanism of action in rheumatoid arthritis. Br J Rheumatol. 1995;34(Suppl 2):7–15. [PubMed] [Google Scholar]
- Sobolewska B., Deuter C., Zierhut M. Current medical treatment of ocular mucous membrane pemphigoid. Ocul Surf. 2013;11(4):259–266. doi: 10.1016/j.jtos.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Svartz N. The treatment of rheumatic polyarthritis with acid azo compounds. Rheumatism. 1948;4(1):180–185. [PubMed] [Google Scholar]
- Taggart A.J., McDermott B.J., Roberts S.D. The effect of age and acetylator phenotype on the pharmacokinetics of sulfasalazine in patients with rheumatoid arthritis. Clin Pharmacokinet. 1992;23(4):311–320. doi: 10.2165/00003088-199223040-00006. [DOI] [PubMed] [Google Scholar]
- Taveira M., Selores M., Costa V., Massa A. Generalized morphea and lichen sclerosus et atrophicus successfully treated with sulphasalazine. J Eur Acad Dermatol Venereol. 1999;12:283–284. doi: 10.1111/j.1468-3083.1999.tb01053.x. [DOI] [PubMed] [Google Scholar]
- Tett S.E. Clinical pharmacokinetics of slow-acting antirheumatic drugs. Clin Pharmacokinet. 1993;25:392–407. doi: 10.2165/00003088-199325050-00005. [DOI] [PubMed] [Google Scholar]
- Tohyama M., Yahata Y., Yasukawa M., Inagi R., Urano Y., Yamanishi K. Severe hypersensitivity syndrome due to sulfasalazine associated with reactivation of human herpesvirus 6. Arch Dermatol. 1998;134:1113–1117. doi: 10.1001/archderm.134.9.1113. [DOI] [PubMed] [Google Scholar]
- Tremblay L., de Chambrun G.P., De Vroey B., Lavogiez C., Delaporte E., Colombel J.F. Stevens-Johnson syndrome with sulfasalazine treatment: report of two cases. J Crohn’s Colitis. 2011;5:457–460. doi: 10.1016/j.crohns.2011.03.014. [DOI] [PubMed] [Google Scholar]
- U.S. National Library of Medicine. Azulfidine EN-tabs (sulfasalazine delayed release tablets, USP) prescribing information [Internet]. 2001 [Last accessed on 19 May 2018]. Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b9ef541a-93c8-4428-ba45-398aa0b327d1.
- Watkinson G. Sulphasalazine: a review of 40 years’ experience. Drugs. 1986;32(1):1–11. doi: 10.2165/00003495-198600321-00003. [DOI] [PubMed] [Google Scholar]
- Willsteed E., Lee M., Wong L.C., Cooper A. Sulfasalazine and dermatitis herpetiformis. Australas J Dermatol. 2005;46:101–103. doi: 10.1111/j.1440-0960.2005.00152.x. [DOI] [PubMed] [Google Scholar]
- Wu TY, Ying KY. Disease modifying anti-rheumatic drugs: review of pregnancy and lactation. 2019;19(1):18–26.
- Yamazaki T., Miyai E., Shibata H., Yamamoto I. Pharmacological studies of salazosulfapyridine (SASP) evaluation of anti-rheumatic action. Pharmacometrics. 1991;41(6):563–574. [Google Scholar]