Abstract
Background
Bullous pemphigoid (BP) is an autoimmune subepidermal blistering disease associated with immune response against BP-180 and BP-230. Peripheral blood eosinophilia and dermal infiltration of eosinophils are common findings in BP.
Objective
The aim of our study was to demonstrate a statistical correlation between dermal and peripheral blood eosinophilia, anti BP-180, and anti BP-230 IgG and clinical severity of BP.
Methods
A total of 27 patients with newly diagnosed BP were included. Severity of disease was assessed according to the bullous pemphigoid disease activity index (BPDAI). Anti-BP-180 and anti-BP-230 titers, peripheral blood eosinophilia, and dermal eosinophil infiltration and tissue inflammation severity were assessed for each patient.
Results
A significant correlation was found between the serum levels of anti-BP-180 and anti-BP-230, and dermal eosinophilia and tissue inflammation severity with objective and subjective BPDAI scores. In addition, there was a significant correlation between the percentage of peripheral blood eosinophils and subjective BPDAI scores and urticarial/eczematous lesions. Moreover, the mucosal component did not show any correlation with autoantibody levels and inflammation severities.
Conclusion
Anti-BP-180 and anti-BP-230 levels, tissue inflammation severity, and dermal eosinophilia had a strong and significant correlation with BP severity. In addition, percentage of peripheral blood eosinophilia showed a correlation with subjective BPDAI scores.
Keywords: Bullous pemphigoid, Anti-BP-180, Anti-BP-230, Peripheral blood eosinophilia, Dermal eosinophils
Introduction
Bullous pemphigoid (BP) is the most common autoimmune subepidermal blistering disease and typically presents in the elderly. BP is characterized by a generalized pruritic and blistering eruption; however, the clinical presentation can be polymorphic, especially in the early pre-bullous phase of the disease (Van Beek et al., 2018).
BP is an immune-mediated disease associated with a humoral and cellular response directed against two hemidesmosomal proteins: BP-180 (BPAg2, type XVII collagen) and BP-230 (BPAg1). The binding of autoantibodies leads to an inflammatory reaction cascade resulting in complement activation, inflammatory cell infiltration, and the release of proteases and reactive oxygen species that induces subepidermal separation (Ishiura et al., 2008, Murrell et al., 2012). Enzyme-linked immunosorbent assay (ELISA) is a sensitive method for detection of anti-BP-180 and anti-BP-230. Almost all patients with BP develop circulating IgG autoantibodies that bind to BP-180, but they also exhibit significant autoreactivity to intracellular BP-230.
Studies have found that patients with BP develop an autoreactive T-cell response against BP-180 and BP-230, which is probably crucial for stimulating B-cells to produce pathogenic autoantibodies. Anti-BP-180 IgG titers were demonstrated to have parallel disease activity and control in several studies. In contrast to BP180‐specific antibodies, the pathogenicity of anti-BP-230 IgG remains undetermined (Esmaili et al., 2015, Feng et al., 2008, Izumi et al., 2004, Koga et al., 2018, Le Saché-de et al., 2012, Schmidt et al., 2000).
Cutaneous infiltration by eosinophils is considered an early and crucial event in the development of bullous lesions in BP. The role of eosinophils in the pathogenesis of BP is supported by the presence of extracellular eosinophil granules, degranulated eosinophils, and free granule proteins within the bullous lesions (Bernard et al., 1987, Kridin, 2018, Marzano et al., 2009). On the contrary, the clinical significance and relationship between tissue eosinophilia and disease severity has not been studied yet. Moreover, peripheral blood eosinophilia has been reported in 50% to 60% of BP cases with a positive correlation with disease severity (Bernard et al., 1987; Kridin et al., 2018; Zuo et al., 2012). Count of peripheral eosinophils have been shown to reflect disease activity more accurately than anti-BP-180 levels (Kenneth et al., 2014).
To the best of our knowledge, no study has focused on the correlation between disease severity in BP cases and severity of dermal eosinophils. The objective of our study was to demonstrate a statistical correlation between dermal and peripheral blood eosinophilia, anti-BP-180, and anti-BP-230 IgG with clinical severity of BP.
Methods
This prospective study was performed over a period of 2 years and analyzed 27 new cases of BP admitted to the outpatient and inpatient clinics of Razi hospital, from 2016 to 2017. The study was approved by the ethical committee of Tehran University of Medical Sciences (IR.TUMS.REC.1394.253).
The inclusion criteria were patients with a definite diagnosis of BP that were new cases and who consented to participate in the study. The diagnosis of BP was based on characteristic clinical features suggestive of BP, subepidermal blister formation with dermal eosinophilic infiltration based on skin biopsy test results, and linear IgG and/or C3 deposits along the dermoepidermal junction by direct immunofluorescence. The exclusion criteria were cases with an inconclusive diagnosis of BP or patients under treatment for their disease.
Severity of disease was assessed with the BP disease activity index (BPDAI), which measures separate scores of the skin (cutaneous blisters/erosions and cutaneous urticaria/erythema) and mucous membrane (mucosal blisters/erosions) disease activity based on the number and size of the lesions. Damage scores are separate as well and included the postinflammatory pigmentation in different skin locations. Total BPDAI score ranges from 0 to 360 (up to 240 for cutaneous activity and up to 120 for mucosal activity).
Severity of pruritus was also determined as a major symptom of BP by a subjective component of the BPDAI that uses a visual analogue scale from 0 to 10 for the past 24 hours, past week, and past month (score, 0–30). If a patient was incapable of completing a reliable visual analogue scale rating, the degree of pruritus was assessed based on the extent of excoriations, scored out of 30 (Murrell et al., 2012).
Routine histopathology (hematoxylin and eosin staining) and direct immunofluorescence studies were performed for all patients, and the same tissue samples were evaluated for assessment of tissue inflammation and dermal eosinophils. Tissue inflammation was scored as Grade 0 = 0%, Grade 1 < 25%, Grade 2 = 25% to 50%, Grade 3 = 50% to 75%, and Grade 4 > 75% based on the percentage of light microscope fields that included inflammatory cells. In this regard, four fields of each case were evaluated by a power of ×10, each field was divided into four quadrants (each 25%), and the severity of tissue inflammation was scored according to the existence of inflammation in each quadrant. Finally, the mean of percent in four fields was considered the final score of tissue inflammation for each case.
Dermal eosinophils were scored as Grade 0 = 0%, Grade 1 < 25%, Grade 2 = 25% to 50%, Grade 3 = 50% to 75%, and Grade 4 > 75% based on the mean of percentage of eosinophils among inflammatory cells in the same four fields by a power of ×40. Count of inflammatory cells and eosinophils was done as cell by cell and then converted to percentage.
To assess anti-BP-180 and anti-BP-230 antibody titers, commercial ELISA kits (Anti-BP180-NC16A-4X and Anti-BP230-CF, Euroimmun medizinische labordiagnostika AG, Germany) were used. Serum samples were diluted at 1:100 per the manufacturer’s instructions, and the proposed positive cutoff value was 20 RU/ml.
All statistical analyses were performed with SPSS software (version 21.0, IBM; Chicago, IL). Spearman correlation coefficient was used to investigate the relationship between number of peripheral blood eosinophils, percent of peripheral blood eosinophils, anti-BP-180 antibody titers, anti-BP-230 antibody titers, dermal eosinophil infiltration, and tissue inflammation severity with objective and subjective BPDAI (severity of itching). A p-value of <.05 was considered statistically significant.
Results
In total, 27 patients with a confirmed diagnosis of BP were enrolled in this study. Thirteen cases (48.1%) were male patients and 14 cases (51.9 %) were female. The mean patient age was 71.77 ± 13.48 years (range, 31–91 years). Twenty-five of 27 patients (92.6%) tested positive for anti-BP-180, and 16 (59.25%) tested positive for anti-BP-180 and anti-BP-230, but none of the patients tested positive for anti-BP-230 alone. Four patients had mucosal lesions.
Using ELISA, anti-BP-180 levels in patients ranged from 1.9 to >1600 u/ml with an average of 718.99 ± 681 u/ml, and anti BP-230 levels ranged from 0.5 to 688 u/ml with an average of 133.59 ± 209.33 u/ml. Patients’ clinical and histological characteristics are summarized in Table 1. The reported diseases in patients were hypertension (12 cases), diabetes mellitus (3 cases), cerebrovascular accident (2 cases), benign prostate hyperplasia (2 cases), and hyperlipidemia, Parkinson disease, Alzheimer disease, rheumatoid arthritis, epilepsy, and laryngeal cancer (one case each).
Table 1.
Clinical and histological characteristics of patients with bullous pemphigoid.
| Clinical and histological variables | Mean ± SD | Median | Range |
|---|---|---|---|
| Anti-BP180 | 718.99 ± 681.36 | 296 | 1.9–˃1600 |
| Anti-BP230 | 133.58 ± 209.33 | 29.6 | 0.5–688 |
| Peripheral blood eosinophilia (count) | 597.62 ± 827.76 | 210 | 0–3507 |
| Peripheral blood eosinophilia (%) | 5.38 ± 6 | 2 | 0–21 |
| Objective BPDAI | 109.44 ± 44.68 | 106 | 14–174 |
| Erosive and bullous lesions | 59.74 ± 26.39 | 2–95 | |
| Urticarial and eczematous lesions | 41.57 ± 22.82 | 1–82 | |
| Pigmented and scar lesions | 7.92 ± 2.5 | 1–11 | |
| Mucosal lesions | 1.07 ± 3.14 | 0–13 | |
| Subjective BPDAI, n (%) | |||
| Mild (0–10) | 4 (14.8%) | ||
| Moderate (11–20) | 7 (25.9%) | ||
| Severe (21–30) | 16 (59.3%) | ||
| Tissue inflammation, n(%) | |||
| Grade 0 (0%) | 0 | ||
| Grade 1 (<25%) | 2 (7.4%) | ||
| Grade 2 (25%–50%) | 8 (29.6%) | ||
| Grade 3 (50%–75%) | 11 (40.7%) | ||
| Grade 4 (˃75%) | 6 (22.2%) | ||
| Dermal eosinophils, n(%) | |||
| Grade 0 (0%) | 0 | ||
| Grade 1 (<25%) | 6 (22.2%) | ||
| Grade 2 (25%–50%) | 7 (25.9%) | ||
| Grade 3 (50%–75%) | 6 (22.2%) | ||
| Grade 4 (˃75%) | 8 (29.6%) | ||
BPDAI, bullous pemphigoid disease activity index; SD, standard deviation; n, number.
A significant correlation was found between objective BPDAI scores and serum levels of anti-BP-180 and anti-BP-230 antibodies (p < .0001), dermal eosinophils (p = .012), and tissue inflammation severity (p = .002), but not with peripheral blood eosinophils (Table 2).
Table 2.
Correlation between histopathologic and laboratory parameters with severity of bullous pemphigoid.
| Variables | Objective BPDAI | Subjective BPDAI | Urticarial/eczematous lesion | Erosive/bullous lesions | Mucosal lesions |
|---|---|---|---|---|---|
| Anti-BP180 |
r = .82 p < .0001 |
r = .56 p = .002 |
r = .787 p < .0001 |
r = .65 p < .0001 |
r = .009 p = .96 |
| Anti-BP230 |
r = .64 p < .0001 |
r = .43 p = .025 |
r = .71 p < .0001 |
r = .428 p = .026 |
r = .06 p = .76 |
| Eosinophil count |
r = .36 p = .062 |
r = .35 p = .07 |
r = .368 p = .059 |
r = .28 p = .156 |
r = -.19 p = .31 |
| Eosinophil percent |
r = .378 p = .052 |
r = .41 p = .03 |
r = .38 p = .049 |
r = .29 p = .139 |
r = -.21 p = .28 |
| Dermal eosinophils |
r = .47 p = .012 |
r = .44 p = .022 |
r = .34 p = .23 |
r = .53 p = .004 |
r = .13 p = .49 |
| Tissue inflammation |
r = .57 p = .002 |
r = .44 p = .022 |
r = .55 p = .003 |
r = .44 p = .02 |
r = -.08 p = .97 |
BPDAI, bullous pemphigoid disease activity index.
Aside from the total scores of objective BPDAI, the Spearmen correlation coefficient was used to analyze the relationship among the three components of the objective BPDAI (urticarial/eczematous lesions, erosive/bullous lesions, and mucosal lesions). There was a significant correlation between severity of erosive/bullous lesions and anti-BP-180 (p < .0001), anti-BP-230 (p = .026), dermal eosinophils (p = .004), and tissue inflammation (p = .02). Severity of urticarial/eczematous lesions was significantly correlated with anti-BP-180, anti-BP-230 (p < .0001), percentage of peripheral blood eosinophils (p = .049), and tissue inflammation severity (p = .003), but this correlation was not found for the number of peripheral blood and dermal eosinophils. Moreover, there was no significant relationship between the severity of mucosal lesions and any of the aforementioned variables (Fig. 1).
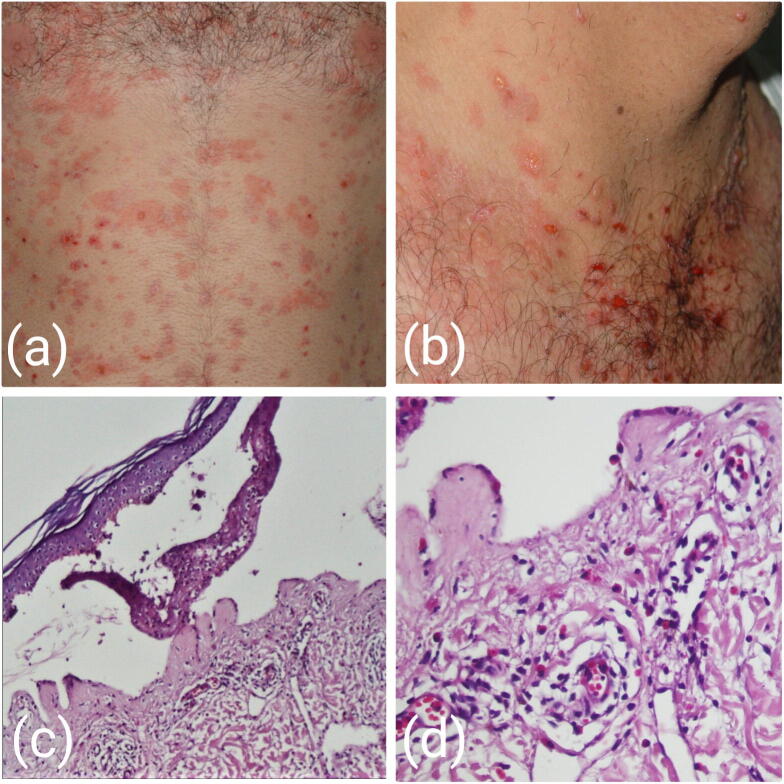
Fig. 1.
(A, B) Generalized urticarial and blistering lesions, (C) corresponding histological findings, including cell-rich subepidermal bulla, and (D) tissue inflammation and dermal eosinophilic infiltrate Grade 2 (hematoxylin and eosin staining, C×100, D×400).
Furthermore, like objective BPDAI, subjective BPDAI had a significant correlation with serum levels of anti-BP-180 (p = .002), anti-BP-230 antibodies (p = .025), dermal eosinophils (p = .022), tissue inflammation severity (p = .022), and percentage of peripheral blood eosinophilia (p = .03). Subjective BPDAI (severity of itching) was also significantly correlated with patient age (r = .45; p = .017), but there was no significant correlation between age and other variables (Table 2, Table 3).
Table 3.
Correlation between age and other parameters in bullous pemphigoid disease.
| BPDAI | Subjective BPDAI | Peripheral blood eosinophilia (count) | Peripheral blood eosinophilia (%) | Anti-BP180 | Anti-BP230 | Dermal eosinophilia | Tissue inflammation | |
|---|---|---|---|---|---|---|---|---|
| Age |
r = .37 p = .55 |
r = .45 p = .017 |
r = .15 p = .44 |
r = .18 p = .36 |
r = .22 p = .25 |
r = .13 p = .49 |
r = .33 p = .09 |
r = .25 p = .19 |
BPDAI, bullous pemphigoid disease activity index.
Discussion
This study illustrates the significant correlation between anti-BP-180, anti-BP-230, dermal eosinophilia and tissue inflammation severity and severity of BP (objective BPDAI). There was also a significant correlation between severity of itching (subjective BPDAI) and serum levels of anti-BP-180, anti-BP-230 antibodies, dermal eosinophilia, tissue inflammation severity, and percentage of peripheral blood eosinophilia. This was the first study to examine the association between BP severity and histopathological parameters (dermal eosinophilia and tissue inflammation severity).
Our findings revealed no relationship between peripheral blood eosinophil count and objective BPDAI, and this held for the three components of objective BPDAI. This is inconsistent with the results of previous studies, which reported a significant positive correlation between BP severity and serum eosinophilia. In a study by Kenneth et al. (2014) of patients with BP treated with omalizumab, the parameter that followed disease activity the most closely was eosinophil count. Another study by Van Beek et al. (2015) reported a significant correlation between circulating eosinophil counts and the classical phenotype of BP (blisters and erosions), but not with the extent of urticarial lesions and erythema.
Moreover, we found a significant correlation between percentage of peripheral blood eosinophils and urticarial/eczematous lesions (r = .38; p = .049), but this result was not obtained for erosive/bullous lesions (r = .29; p = .139). This difference could be explained by the paucity of reports in this field and the fact that in most studies a range of cut point of 400 to 600 eosinophils/µL was used as a definition for eosinophilia, which can cause different results.
In a recent case-control study by Kridin (2018), patients with BP and moderate or severe eosinophilia (>1500 cells/µL) were older at the time of presentation and had higher proportions of extensive disease, palmoplantar involvement, positive indirect immunofluorescence, and more frequently were in the urticarial stage of BP (albeit lacking a statistical significance) compared with patients with normal eosinophil counts and percentage. This is in line with our findings, where the percentage of peripheral blood eosinophils had a significant correlation with urticarial/eczematous lesions; however, there was no correlation between eosinophilia and age in our study.
Moreover, pruritus severity was significantly correlated with age, eosinophil percentage (r = .41; p = .03), dermal eosinophilia (r = .44; P = .022), and tissue inflammation (r = .44; p = .022), but not with eosinophil count. In other words, we can assume that percentage of peripheral blood eosinophils is more valuable than the absolute count of these cells in estimating BP severity.
In our study, the correlation between severity of tissue inflammation and severity of disease or BPDAI (r = .57; p = .002), including severity of erosive/bullous lesions (r = .44; p = .02), urticarial/eczematous lesions (r = .55; p = .003), and pruritus (r = .44; p = .022), was found to be significant. The same results were found for the severity of dermal eosinophilia, but interestingly this factor was not related with severity of urticarial/eczematous lesions (r = .368; p = .059). In a study by Izumi et al. (2016), a noninflammatory phenotype of BP, in which autoantibodies specifically target the midportion of collagen XVII but not NC16A, clinically showed significantly reduced erythema associated with scant lesional infiltration of eosinophils. Studies that consider the relationship between clinical features of BP and tissue inflammation are rare, and there is a need for more detailed analyses of the relevance of tissue eosinophils to erythematous lesions in further investigations. Of note, this parameter could be of high value in the determination of BP severity and associated pruritus.
In our study, there was a significant correlation between the level of anti-BP-180 and anti-BP-230 with BPDAI and the severity of itching. This finding is consistent with the results of a similar study in France in 2012, which showed that levels of both autoantibodies paralleled the clinical evolution, especially in cases of disease control, but no correlation was revealed by clinical severity (Le Saché-de Peufeilhoux et al., 2012). In contrast, the vast majority of studies found a significant correlation between BPDAI and pruritus severity with anti-BP-180 but not with anti-BP-230 (Charneux et al., 2011, Daneshpazhooh et al., 2018, Feng et al., 2008, Giusti et al., 2017, Ishiura et al., 2008, Schmidt et al., 2000).
There are contradictory results with regard to the role of anti-BP-230 in the pathogenesis of BP. It has shown that although major epitopes for IgG anti-BP-180 reside within the extracellular domain, IgG anti-BP-230 reacts with various domains on the entire BP-230 molecule (Hashimoto et al., 2017). In contrast with BP-180, which is a transmembrane protein with well-documented roles in BP pathogenicity, the pathogenic role of BP-230 as an intracytoplasmic protein is questionable. Anti-BP-230 autoantibodies may be helpful in diagnosis, with a diagnostic added value of 5% compared with BP-180 alone, and have been reported to be associated with localized types of BP (Giusti et al., 2017, Hayakawa et al., 2016). A study by Hayakawa et al. (2016) revealed a new disease entity as anti-BP-230 type BP, in which IgG anti-BP-230 is the sole autoantibody.
Additionally, we have not found any significant correlation between the severity of mucosal involvement and any of the studied variables, including anti-BP-180, anti-BP-230, and tissue and peripheral eosinophilia. This finding is similar to that of a previous study by Daneshpazhooh et al. (2018), which reported that neither anti-BP-180 nor anti-BP-230 levels had a significant relationship with the oral mucosa subcomponent of the BPDAI scoring systems. The researchers concluded that this finding reflects a need for modification in the scoring system with proportionate emphasis on BP mucosal involvement (Daneshpazhooh et al., 2018). Interestingly, in a study by Clapé et al. (2018), a shift toward higher blister/erosion BPDAI scores has been shown for patients with mucosal involvement. The researchers also found that mucosal lesions are related clinically to disease severity and immunologically to the absence of anti-BP-230 antibodies (Clapé et al., 2018).
A limitation of our study is the low patient number, which may affect the significance of the results.
Conclusion
We found that anti-BP-180 and anti-BP-230 levels, tissue inflammation severity, and dermal eosinophilia had a strong and significant correlation with BP severity. In addition, percentage of peripheral blood eosinophils showed a correlation with subjective BPDAI and urticarial/eczematous lesions. Moreover, the mucosal component did not show any correlation with autoantibody levels and inflammation severities.
These findings indicate the need for a modification of the existing BPDAI scale and the utilization of peripheral eosinophilia as a marker of severity of pruritus and the need for treatments that could alleviate symptoms. Moreover, the severity of tissue inflammation and eosinophil infiltration might be an indicator and/or prognostic factor of BP severity.
Conflict of Interest
None.
Acknowledgments
Acknowledgements
The authors gratefully acknowledge Prof. Maryam Daneshpazhooh for her great support in reviewing the article.
Financial disclosures
None.
Funding
None.
Study approval
The author(s) confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies.
References
- Bernard P., Venot J., Constant F., Bonnetblane J.M. Blood eosinophilia as a severity marker for bullous pemphigoid. J Am Acad Dermatol. 1987;16(4):879–881. doi: 10.1016/s0190-9622(87)80227-x. [DOI] [PubMed] [Google Scholar]
- Charneux J., Lorin J., Vitry F., Antonicelli F., Reguiai Z., Barbe C. Usefulness of BP230 and BP180-NC16a enzyme-linked immunosorbent assays in the initial diagnosis of bullous pemphigoid: a retrospective study of 138 patients. Arch Dermatol. 2011;147(3):286–291. doi: 10.1001/archdermatol.2011.23. [DOI] [PubMed] [Google Scholar]
- Clapé A., Muller C., Gatouillat G., Le Jan S., Barbe C., Pham B.N. Mucosal involvement in bullous pemphigoid is mostly associated with disease severity and to absence of anti-BP230 autoantibody. Front Immunol. 2018;9:479. doi: 10.3389/fimmu.2018.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneshpazhooh M., Ghiasi M., Lajevardi V., Nasiri N., Balighi K., Teimourpour A. BPDAI and ABSIS correlate with serum anti-BP180 NC16A IgG but not with anti-BP230 IgG in patients with bullous pemphigoid. Arch Dermatol Res. 2018;310(3):255–259. doi: 10.1007/s00403-018-1817-9. [DOI] [PubMed] [Google Scholar]
- Esmaili N., Mortazavi H., Kamyab-Hesari K., Aghazadeh N., Daneshpazhooh M. Diagnostic accuracy of BP180 NC16a and BP230-C3 ELISA in serum and saliva of patients with bullous pemphigoid. Clin Exp Dermatol. 2015;3:324–330. doi: 10.1111/ced.12510. [DOI] [PubMed] [Google Scholar]
- Feng S., Wu Q., Jin P., Lin L., Zhou W., Sang H. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Int J Dermatol. 2008;47:225–228. doi: 10.1111/j.1365-4632.2008.03473.x. [DOI] [PubMed] [Google Scholar]
- Giusti D., Le Jan S., Gatouillat G., Bernard P., Pham B.N., Antonicelli F. Biomarkers related to bullous pemphigoid activity and outcome. Exp Dermatol. 2017;26(12):1240–1247. doi: 10.1111/exd.13459. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Ohzono A., Teye K., Numata S., Hiroyasu S., Tsuruta D. Detection of IgE autoantibodies to BP 180 and BP 230 and their relationship to clinical features in bullous pemphigoid. Br J Dermatol. 2017;177(1):141–151. doi: 10.1111/bjd.15114. [DOI] [PubMed] [Google Scholar]
- Hayakawa T., Teye K., Hachiya T., Uehara R., Hashiguchi M., Kawakami T. Clinical and immunological profiles of anti-BP230-type bullous pemphigoid: Restriction of epitopes to the C-terminal domain of BP230, shown by novel ELISAs of BP230-domain specific recombinant proteins. Eur J Dermatol. 2016;26(2):155–163. doi: 10.1684/ejd.2015.2719. [DOI] [PubMed] [Google Scholar]
- Ishiura N., Fujimoto M., Watanabe R., Nakashima H., Kuwano Y., Yazawa N. Serum levels of IgE anti-BP180 and anti-BP230 autoantibodies in patients with bullous pemphigoid. J Dermatol Sci. 2008;49:153–161. doi: 10.1016/j.jdermsci.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Izumi T., Ichiki Y., Esaki C., Kitajima Y. Monitoring of ELISA for antiBP180 antibodies: clinical and therapeutic analysis of steroid-treated patients with bullous pemphigoid. J Dermatol. 2004;31:383–391. doi: 10.1111/j.1346-8138.2004.tb00689.x. [DOI] [PubMed] [Google Scholar]
- Izumi K., Nishie W., Mai Y., Wada M., Natsuga K., Ujiie H. Autoantibody profile differentiates between inflammatory and noninflammatory bullous pemphigoid. J Invest Dermatol. 2016;136(11):2201–2210. doi: 10.1016/j.jid.2016.06.622. [DOI] [PubMed] [Google Scholar]
- Kenneth K.Y., Crew A.B., Messingham K.A., Fairley J.A., Woodley D.T. Omalizumab therapy for bullous pemphigoid. J Am Acad Dermatol. 2014;71(3):468–474. doi: 10.1016/j.jaad.2014.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga H., Teye K., Ishii N., Ohata C., Nakama T. High index values of enzyme-linked immunosorbent assay for BP180 at baseline predict relapse in patients with bullous pemphigoid. Front Med. 2018;5:139. doi: 10.3389/fmed.2018.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kridin K. Peripheral eosinophilia in bullous pemphigoid: prevalence and influence on the clinical manifestation. Br J Dermatol. 2018;179(5):1141–1147. doi: 10.1111/bjd.16679. [DOI] [PubMed] [Google Scholar]
- Le Saché-de Peufeilhoux L., Ingen-Housz-Oro S., Hue S., Sbidian E., Valeyrie-Allanore L., Ortonne N. The value of BP230 enzyme-linked immunosorbent assay in the diagnosis and immunological follow-up of bullous pemphigoid. Dermatol. 2012;224:154–159. doi: 10.1159/000337545. [DOI] [PubMed] [Google Scholar]
- Marzano A.V., Tedeschi A., Fanoni D., Bonanni E., Venegoni L., Berti E. Activation of blood coagulation in bullous pemphigoid: role of eosinophils, and local and systemic implications. Br J Dermatol. 2009;160(2):266–272. doi: 10.1111/j.1365-2133.2008.08880.x. [DOI] [PubMed] [Google Scholar]
- Murrell D.F., Daniel B.S., Joly P., Borradori L., Amagai M., Hashimoto T. Definitions and outcome measures for bullous pemphigoid: recommendations by an international panel of experts. J Am Acad Dermatol. 2012;66:479–485. doi: 10.1016/j.jaad.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E., Obe K., Bröcker E.B., Zillikens D. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol. 2000;136:174–178. doi: 10.1001/archderm.136.2.174. [DOI] [PubMed] [Google Scholar]
- Van Beek N., Schwemm N., Schulze F., Recke A., Zillikens D., Schmidt E. Die Serumspiegel von IgE Antikörpern gegen die Immunodominante BP180-NC16A Domäne korrelieren mit der Krankheitsaktivität von Patienten mit bullösem Pemphigoid. J German Soc Dermatol. 2015;13:69–110. [Google Scholar]
- Van Beek N., Zillikens D., Schmidt E. Diagnosis of autoimmune bullous diseases. J German Soc Dermatol. 2018;16:1077–1091. doi: 10.1111/ddg.13637. [DOI] [PubMed] [Google Scholar]
- Zuo Y.G., Liu B., Li L., Jin H.Z., Sun Q.N. Correlation between blood eosinophil level and steroid doses in patients with bullous pemphigoid. Acta Academiae Medicinae Sinicae. 2012;34(2):130–133. doi: 10.3881/j.issn.1000-503X.2012.02.006. [DOI] [PubMed] [Google Scholar]