Abstract
Background
Milia are superficial keratinous cysts seen as pearly white, dome-shaped lesions 1–2 mm in diameter. Milia are associated with diseases that cause subepidermal blistering, such as hereditary forms of epidermolysis bullosa, epidermolysis bullosa acquisita, bullous pemphigoid, bullous lichen planus, and porphyria cutanea tarda. Multiple eruptive milia are rare and more extensive in number than primary milia.
Objective
The aim of this study was to search the literature for cases of blistering diseases with multiple milia formation, especially in areas of the skin where there was no evidence of blistering or trauma, and review the interpretations of their pathogenesis.
Methods
We performed a literature search with the terms multiple milia and bullous diseases, pemphigoid, and pemphigus.
Results
Very few studies have investigated the origin of milia. Primary milia are thought to originate from the sebaceous collar of vellus hairs, and secondary milia are believed to derive from eccrine ducts more commonly than from overlying epidermis, hair follicles, or sebaceous ducts. Milia secondary to blisters or trauma are speculated to be produced through the regeneration process of disrupted sweat glands or hair follicles. Immunological predisposition, aberrant interaction between the hemidesmosomes, and the extracellular matrix components beneath the hemidesmosomes have been described with regard to the formation of numerous milia during recovery. Multiple milia could be a primary manifestation of dystrophic epidermolysis bullosa in skin areas without evidence of blistering.
Conclusion
The exact etiology of multiple milia remains unknown. Immunological predisposition and improper interaction between hemidesmosomes and extracellular matrix components are speculated to play a role in the formation of milia during recovery of bullous lesions in blistering diseases. Still, further studies on the triggering mechanisms of keratinocyte dysfunction in cases of multiple milia formation without evidence of prior blistering are needed.
Keywords: Milia, Autoimmune blistering diseases, Blistering diseases, Pemphigoid, Pemphigus
Introduction
Milia are superficial keratinous cysts, clinically seen as pearly white, dome-shaped lesions 1–2 mm in diameter. Histologically, they are seen as miniature infundibular cysts with walls of stratified squamous epithelium containing a granular cell layer. Milia may be classified as primary or secondary, depending on how the lesions originated. Primary milia are thought to form spontaneously from the sebaceous collar of vellus hairs; secondary milia are derived from eccrine ducts and may be disease-associated, medication-associated, or caused by trauma (Berk and Bayliss, 2008).
Subepidermal blistering diseases, such as the hereditary forms of epidermolysis bullosa, epidermolysis bullosa acquisita (EBA), bullous pemphigoid (BP), bullous lichen planus, porphyria cutanea tarda, variegate porphyria, and pseudoporphyria, are associated with the formation of secondary milia in areas of previous blistering. In porphyria, bullae are known to be fragile and heal with scarring and milia. In other blistering disorders, however, multiple milia are less known to occur. In addition, multiple milia may also be observed in skin areas without evidence of blistering (Akaksa et al., 2017). Whether the formation of milia in seemingly unaffected skin is a criterion in diagnosing subepidermal blistering diseases remains questionable.
We conducted this study to search the literature for cases of blistering diseases with multiple eruptive milia formation, especially in areas of the skin where there is no evidence of blistering or trauma, and review the interpretations of their pathogenesis. We focused our search on blistering disorders, excluding porphyria to highlight the occurrence of extensive multiple milia formation in blistering disorders of autoimmune, inflammatory, or genetic origin (see Fig. 1, Fig. 2, Fig. 3, Fig. 4).
Fig. 1.
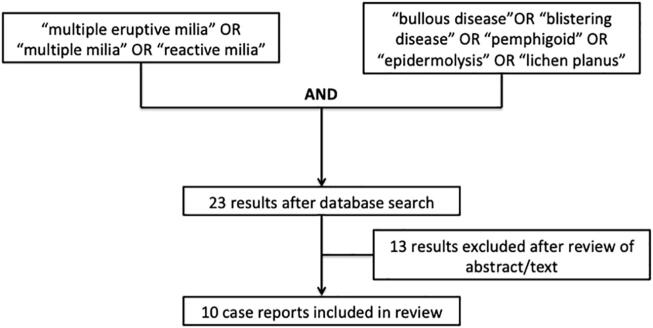
Methodology.
Fig. 2.
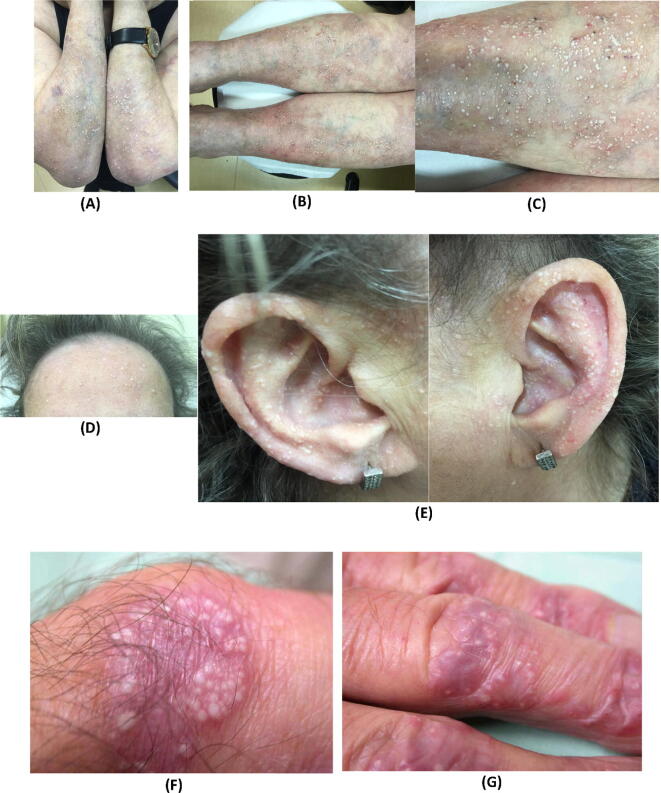
Multiple milia in two female patients with epidermolysis bullosa acquisita.
Fig. 3.
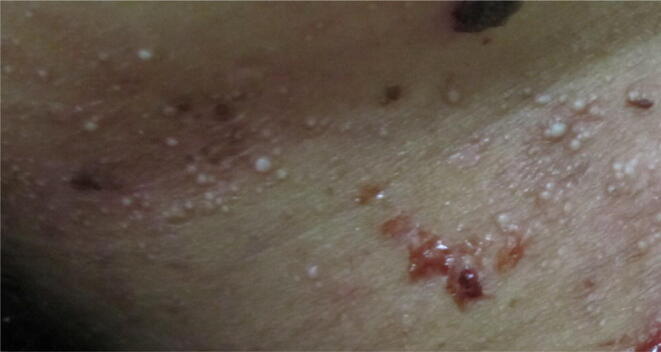
Multiple milia in a female patient with bullous pemphigoid.
Fig. 4.
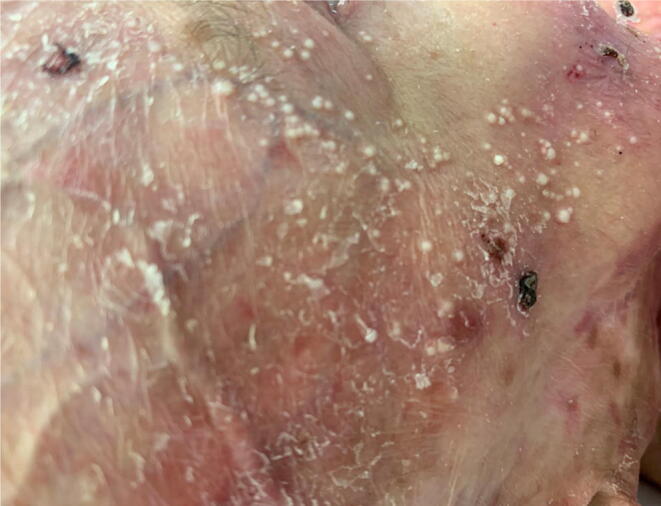
Multiple milia in a female patient with recessive dystrophic epidermolysis bullosa.
Methods
We performed a literature search on PubMed for the terms “multiple eruptive milia”, “multiple milia”, and “reactive milia”. Another search for “bullous disease”, “blistering disease”, “pemphigoid”, “epidermolysis”, and “lichen planus” was also conducted. The conjunction of these two queries was then searched, yielding 23 results. Only reports published in English and reports with autoimmune, inflammatory, or genetic blistering disorders were included.
Results and discussion
Few studies have investigated the origin of milia, especially the differences between primary and secondary milia. Milia may be a feature of many different dermatological diseases, as previously described (Tsuruta et al., 2013).
We found several cases reporting the formation of multiple milia in blistering diseases (Table 1). The underlying blistering disease present in these cases varies, ranging from hereditary forms of EB to autoimmune blistering diseases. In all cases but one, multiple milia appeared on sites of previous blistering. Akaksa et al. (2017) reported a case of dominant dystrophic EB in a 3-month-old girl where milia were observed before any report of blistering. Although blisters could have appeared in the prenatal or neonatal period, multiple milia appeared to be the only initial sign of dominant dystrophic epidermolysis bullosa in this patient. This presents an important opportunity for dermatologists and geneticists in the future who are presented with a similar case, as proper genetic counseling and advise should be given to expectant parents who have a family history of the disease.
Table 1.
Cases of multiple secondary milia formation in subepidermal blistering diseases.
| Authors | Year | Age/Sex | Blistering disorder present | Autoantibodies present | Milia on sites of previous blisters | Milia on unaffected skin |
|---|---|---|---|---|---|---|
| Mayuzumi et al. | 2006 | 4/M | Childhood EBA | NC1 and 2 of type VII collagen | + | – |
| Tsuruta et al. | 2013 | 55/F | BP | Anti-BP180 | + | – |
| Uchida et al. | 2014 | 62/M | BP | Anti-BP180 | + | – |
| Vink and Starink | 2014 | 79/F | Bullous acral lichen sclerosus | – | + | – |
| Sonthalia et al. | 2016 | 5/M | Linear lichen planus pigmentosus | – | + | – |
| Akasaka et al. | 2017 | 3 months/F | Dominant dystrophic epidermolysis bullosa | – | – | + (before visible blistering) |
| Ding et al. | 2017 | 25/M | BP | Anti-Dsg1Anti-Dsg3Anti-BP180 | + | – |
| Kumudhini et al. | 2018 | 35/F | BP | Anti-BP180Anti-BP230 | + | – |
| Amin et al. | 2019 | 80/M | BP | Anti-BP180 | + | – |
| Beiu et al. | 2019 | 54/M | EBA | – | + | – |
BP, bullous pemphigoid; EBA, epidermolysis bullosa acquisita; Dsg, desmoglein; F, female; M, male.
Milia are also known to develop in EBA (Mayuzumi et al., 2006) and mucous membrane pemphigoid, but are uncommon in BP (Amin et al., 2019). Because of this, many experts in EBA consider milia formation a possible diagnostic criterion and a differentiating factor from other blistering diseases. Because the diagnosis of blistering diseases may be challenging, especially in resource-limited settings, clear criteria that will help in diagnosis are important. Thus, despite the common occurrence of milia in EB and EBA, reports of milia in mucous membrane pemphigoid and BP could make milia formation a weaker differential criterion for the entire pemphigoid group (Tsuruta et al., 2013).
In the reported cases of BP with multiple milia, different autoantibodies were detected, including those seen in pemphigus, and Ding et al. (2017) reported a case of BP with high levels of anti-desmoglein 1 and anti-desmoglein 3, along with high anti-BP180 titers. In Kumudhini et al. (2018), milia developed in a patient with high autoantibody titers of anti-BP180; this patient required adjuvant treatment with cyclophosphamide. Similarly, Uchida et al. (2014) reported patients with BP who had high autoantibody titers that seemed to parallel high disease activity and that the resulting milia may be related to the intensity of inflammation along the dermo-epidermal junction. Thus it might be valuable to prospectively study high titer and/or refractory pemphigoid diseases to determine whether there is a relationship to milia formation.
Immunological predisposition is also thought to play a role in milia formation in patients with BP. Banfield et al. (1998) studied human leukocyte antigen associations in 74 patients with BP and compared their profiles with those of unrelated control subjects. The presence of the human leukocyte antigen DQ6 was linked with milia formation in both men and women (Banfield et al., 1998). Milia formation in BP may have also resulted from the aberrant interaction between the hemidesmosomes and the extracellular matrix components beneath the hemidesmosomes with IgG autoantibodies to LAD-1 and/or the recombinant protein of the BP180 C-terminal domain (Uchida et al., 2014). The pathophysiology of milia formation in patients with BP remains unknown; thus, investigating this could present an ancillary approach to managing patients with BP after recovery from bullous lesions, thereby affecting their quality of life.
In blistering diseases related to lichenoid processes, the ongoing intense inflammation leading to blister formation could result in the occurrence of milia. Patients with linear lichen planus pigmentosus (Sonthalia et al., 2016) and bullous acral lichen sclerosus (Vink and Starink, 2014) were reported to have milia after healing of subepidermal blisters. Future studies could perhaps investigate whether early or aggressive intervention to control inflammation can also prevent milia formation later.
The treatment of milia from bullous disorders is not different from that of primary milia. Because milia are benign cysts, they present more of a cosmetic problem for the patient. Spontaneous regression within several months has been seen. However, for patients who insist on an intervention, options may include excision and curettage, electrodessication, dermabrasion, cryotherapy, topical tretinoin, photodynamic therapy, Er:YAG or CO2 laser, and oral minocycline (Sharma et al., 2011). As of this writing, no comparative studies have evaluated the efficacy or superiority of any of these methods in the treatment of multiple milia.
Although the exact incidence of milia in blistering diseases is unknown, it may be more common than previously thought. Patients with blistering diseases are faced with an overwhelming burden of the disease itself, further complicated by the associated sequelae of both the disease and the treatment. Dermatologists treating these patients must always aim to provide a comprehensive and thoughtful explanation of the disease and its complications. Although they may seem inconsequential and are more of a cosmetic concern, patients must be made aware of the possibility of developing multiple milia in any phase of their disease.
Conclusion
Milia are benign keratinous cysts that result from the regeneration process of sweat glands or hair follicles. The exact etiology of multiple milia formation in subepidermal blistering disorders is still unknown, but an interplay of immunologic predisposition and atypical interactions between hemidesmosomal components and the extracellular matrix is suggested. Multiple milia are not exclusively seen in just one blistering disease; thus, clinicians must remember that the presence of these lesions does not definitively diagnose any specific blistering disorder. Further studies into the pathophysiology of milia formation in blistering diseases are needed to understand its mechanism and to provide the best approaches in improving the quality of life of patients afflicted with these diseases.
Financial disclosures
None.
Funding
None.
Study Approval
The author(s) confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies.
References
- Akaksa E., Nakano H., Takagi Y., Toyomaki Y., Sawamura D. Multiple milia as an isolated skin manifestation of dominant dystrophic epidermolysis bullosa: Evidence of phenotypic variability. Pediatr Dermatol. 2017;34(2):106–108. doi: 10.1111/pde.13047. [DOI] [PubMed] [Google Scholar]
- Amin S., Fiore C., Paek S.Y. Milia within resolving bullous pemphigoid lesions. Baylor Univ Med Cent Proc. 2019;32(1):90–92. doi: 10.1080/08998280.2018.1528962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfield C., Wojnarowska F., Allen J., George S., Venning V., Welsh K. The association of HLA-DQ7 with bullous pemphigoid is restricted to men. Br J Dermatol. 1998;138(6):1085–1090. doi: 10.1046/j.1365-2133.1998.02350.x. [DOI] [PubMed] [Google Scholar]
- Berk D.R., Bayliss S.J. Milia: A review and classification. J Am Dermatol. 2008;59(6):1050–1063. doi: 10.1016/j.jaad.2008.07.034. [DOI] [PubMed] [Google Scholar]
- Ding S., Xiang Y., Huang J., Deng Q., Chen J., Lu J. Bullous pemphigoid associated with milia, increased serum IgE, autoantibodies against desmogleins, and refractory treatment in a young patient. An Bras Dermatol. 2017;92(5 Suppl 1):34–36. doi: 10.1590/abd1806-4841.20176124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumudhini S., Rao R., Pai K., Shetty S., Pai S. Extensive milia formation in a young woman with bullous pemphigoid. Indian J Dermatol Venereol Leprol. 2018;84(2):248. doi: 10.4103/ijdvl.IJDVL_402_16. [DOI] [PubMed] [Google Scholar]
- Mayuzumi M., Akiyama M., Nishie W., Ukae S., Abe M., Sawamura D. Childhood epidermolysis bullosa acquisita with autoantibodies against noncollagenous 1 and 2 domains of type VII collagen: Case report and review of the literature. Br J Dermatol. 2006;155(5):1048–1052. doi: 10.1111/j.1365-2133.2006.07443.x. [DOI] [PubMed] [Google Scholar]
- Sharma R., Singal A., Sonthalia S. Multiple eruptive milia over both external ears. Indian J Dermatol Venereol Leprol. 2011;77(4):519–520. doi: 10.4103/0378-6323.82392. [DOI] [PubMed] [Google Scholar]
- Sonthalia S., Das A., Sharma S. Co-localization of linear lichen planus pigmentosus and milia in a child. Indian J Dermatol. 2016;61(2):237. doi: 10.4103/0019-5154.177790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruta D., Brzezinski P., Koga H., Ohata C., Furumura M., Hashimoto T. Bullous pemphigoid with prominent milium formation. Acta Dermatovenerol Croat. 2013;21(1):35–38. [PubMed] [Google Scholar]
- Uchida S., Oiso N., Koga H., Ishii N., Okahashi K., Matsuda H. Refractory bullous pemphigoid leaving numerous milia during recovery. J Dermatol. 2014;41:1003–1005. doi: 10.1111/1346-8138.12650. (August) [DOI] [PubMed] [Google Scholar]
- Vink L., Starink T. Bullous acral lichen sclerosus with milia. Clin Exp Dermatol. 2014;39:400–401. doi: 10.1111/ced.12278. [DOI] [PubMed] [Google Scholar]