ABSTRACT
The human microbiota is a key contributor to many aspects of human health and its composition is largely influenced by diet. There is a growing body of scientific evidence to suggest that gut dysbiosis (microbial imbalance of the intestine) is associated with inflammatory and immune-mediated diseases (e.g., inflammatory bowel disease and asthma). Regular consumption of fermented foods (e.g., kimchi, kefir, etc.) may represent a potential avenue to counter the proinflammatory effects of gut dysbiosis. However, an assessment of the available literature in this research area is lacking. Here we provide a critical review of current human intervention studies that analyzed the effect of fermented foods on the composition and/or function of the human gut microbiota. A total of 19 human intervention studies were identified that met this search criteria. In this review, we discuss evidence that consumption of fermented foods may modify the gut microbiota in humans. Further, there is cursory evidence to suggest that gut microbiota compositional changes mediate associations between fermented food consumption and human health outcomes. Although promising, there remains considerable heterogeneity in the human populations targeted in the intervention studies we identified. Larger longitudinal feeding studies with longer follow-up are necessary to confirm and enhance the current data. Further, future studies should consider analyzing microbiota function as a means to elucidate the mechanism linking fermented food consumption with human health. This review highlights methodologic considerations for intervention trials, emphasizing an expanse of research opportunities related to fermented food consumption in humans.
Keywords: microbiota, fermented foods, lactic acid bacteria, yogurt, probiotics
Lay Summary
The human gut microbiota includes all the bacteria, viruses, yeasts, and other small organisms that live in the human gut to help digest food. These microbes have been identified as strong influencers of human health. Many of these bacteria and yeasts are also used to prepare and preserve common foods such as yogurt, kimchi, and wine through the process of fermentation. These fermented foods may enhance human health by changing the amounts and types of good bacteria that live in the gut. However, a comprehensive review of scientific studies assessing this research question is lacking. In this review, we discuss results from 19 scientific studies in which humans consumed fermented foods. Through our review, we determined that consumption of fermented foods may change the amounts and types of good bacteria that live in the human gut. Further, these changes to gut bacteria may be linked to other health benefits. However, this is entirely dependent on the type and amount of fermented food consumed. In the future, longer and larger studies should be conducted with fermented foods in humans to better determine how these foods may affect gut microbes and enhance human health.
Introduction
The human microbiota is comprised of trillions of microbial cells and viruses that together have significant influences on many aspects of human health and physiology (1). The term microbiota refers to the microbial population (composition), whereas microbiome refers to the genetic makeup of this microbial population (2). Enhanced genomic strategies have streamlined analysis of this microbial consortium, allowing for the characterization of un-culturable microbial taxa (1). Application of these -omics strategies in humans suggests the microbiota may be modified by many environmental factors (3). Dysbiosis is an imbalanced microbial state, often instigated by environmental factors such as broad-spectrum antibiotic usage, which kills both pathogenic and resident microbes, and poor dietary habits (3). For example, regular consumption of a diet high in fat contributes to microbial dysbiosis by increasing the ratio of Firmicutes to Bacteroidetes (4).
Probiotics are live microbes which, when ingested in adequate amounts, confer a health benefit to the host (5). In addition, in order for a microbe to be considered probiotic, it also must adhere to the intestinal mucosa, because this is crucial for microbial colonization of the gut (6). Proper adhesion to the intestinal lining contributes to some of the benefits of consuming probiotic microbes, namely, protection from pathogen invasion and modulation of intestinal immune cells (6). Consumption of probiotics or other beneficial microbial taxa via dietary vehicles may also modify the host gut microbiota composition toward a more balanced state and counteract the effects of dysbiosis-promoting factors (7). In addition, many fermented foods contain bioactive compounds as a result of microbial fermentation (8). Regardless of whether or not the microbes remain in the food, consumption of these compounds can confer health benefits (8).
There is also increasing evidence to support the role of specific genetic determinants as strong predictors of microbiota composition (9, 10). For example, expression of specific genes in early life is reported to influence gut microbiota composition into adulthood (9). Further, in a study of 1126 twin pairs, researchers identified heritable microbes and associations between specific gene loci and bacterial taxa (10). Thus, intervention studies aimed at assessing the influence of dietary choices on the gut microbiota in humans are particularly challenging, because each person's microbiota may be genetically primed to react differently to the same intervention.
Fermented foods such as yogurt, sauerkraut, kimchi, and kefir contain relatively stable microbial ecosystems comprised primarily of lactic acid bacteria (LAB) and LAB primary metabolites (e.g., lactic acid) (8). Some LAB are considered probiotic (e.g., Lactobacillus spp.), whereas others are currently being studied to better characterize their probiotic potential (11). Nevertheless, these foods contain a variety of microbes that exhibit health-promoting properties, which could be integrated into one's diet as a means to support the health of the gut microbiota.
Fermentation is a common form of food preservation, in which a resilient microbial ecosystem prevents colonization by invading microbes that may cause spoilage (Erwinia spp., Pseudomonas spp.), maintaining the integrity of the food over long periods of time (8, 11). The initial microbial inhabitants of these ecosystems are preferentially selected from the environment based on the particular nutrient components and pH conditions of the food (4, 5). Over time, these initial microbes contribute to changes in the pH of the food, resulting in colonization by a succession of microbial species (12). For example, although cabbage fermentations like kimchi and sauerkraut are typically colonized by LAB, the particular species and strains of LAB differ based on the acidity of the food product (Leuconostoc mesenteroides dominates early in the colonization of the food at a pH of 5.6–4.3, whereas Lactobacillus sakei dominates later at a pH of 4.1) (13).
Observational and interventional studies in humans suggest that consumption of fermented foods is associated with protection from metabolic and immune-mediated diseases (14–16). However, whether modification of the gut microbiota composition mediates this association has yet to be determined. To assess the relevant empirical evidence, we reviewed published human intervention studies of fermented foods in which the gut microbiota composition (via culture-dependent or culture-independent methods) and/or metabolic function (i.e., production of bioactive metabolites) was analyzed. We present the available data on the topic, discuss gaps in the scientific literature and challenges in study design and implementation, and make recommendations for future assessment of the gut microbiota through dietary interventions in humans.
Fermented Foods Discussed in This Review
In this review, we assess the influence of spontaneous or multispecies starter-culture fermented food consumption on the composition and/or metabolic function of the human gut microbiota. Fermented foods such as kimchi, sauerkraut, coffee, and pickled vegetables (pickles, olives, etc.) undergo a spontaneous fermentation process and are subject to microbial colonization that is influenced by the surrounding environment (13) (Table 1). Yogurt, cheese, soy products, kefir, kombucha/fermented teas, and wine are typically fermented with multispecies starter cultures comprised of yeasts and bacteria [e.g., Kombucha SCOBY (symbiotic culture of bacteria and yeasts)] (17–19) (Table 1). There are many human intervention studies aimed at analyzing the effects on the gut microbiota of probiotic fortification of fermented food products (e.g., a comparison of the microbiota or other health effects after consumption of yogurt fortified with Bifidobacteria spp. or yogurt without this taxon) (20, 21). The goal of this review is to assess the effect of spontaneously or multispecies starter-culture fermented foods on the microbiota. Although common probiotic taxa may be used in the fermentation of the foods discussed in this review, these taxa are primary fermenters of the food rather than secondarily added to compare the probiotic effectiveness of that specific microbe. Articles comparing effects of specific probiotic-fortified fermentations are not included in this review.
TABLE 1.
Microbial compositions of fermented foods addressed in this review1
| Citations | Type of fermented food | Microbial components | Mode of initial bacterial colonization |
|---|---|---|---|
| Unno et al. (22); Tillisch et al. (23); Veiga et al. (24); Yilmaz et al. (25) | Fermented milk | Lactobacillus brevis,Lactobacillus casei,Lactobacillus acidophilus,Bifidobacterium longum, and Streptococcus thermophilus (22); Bifidobacterium animalis,S. thermophilus, and Lactobacillus bulgaricus (23); B. animalis subsp. lactis,S. thermophilus,Lactobacillus delbrueckii subsp. bulgaricus, and Lactococcus lactis (24); Lactobacillus spp. (25) | Starter culture |
| Lisko et al. (19); Yang and Sheu (26) | Yogurt | L. acidophilus, Bifidobacterium lactis, L. bulgaricus, S. thermophilus, and L. casei (18 only) | Starter culture |
| Firmesse et al. (27); Lay et al. (28) | Camembert cheese | S. thermophilus, Lactobacillus spp., L. lactis, Leuconostoc, Hafnia alvei, and Geotrichum | Starter culture or spontaneous |
| Not specified in the study cited in this review. General fermenters of fermented soybean milk were identified from Marzano et al. (29). | Wine | Predominantly Saccharomyces yeasts | Starter culture |
| Han et al. (15) | Kimchi | Lactobacillus spp. | Spontaneous |
| Nielsen et al. (30) | Sauerkraut | Lactobacillus spp. (L. plantarumandL. brevis), Leuconostoc spp., Weissella confusa,L. lactis, and Enterobacteriaceae | Spontaneous |
| Not specified in the study cited in this review. General fermenters of coffee were identified from Lee et al. (31). | Coffee | Primarily Klebsiella,Leuconostoc, LAB, Erwinia spp., Enterobacter spp. And a variety of yeasts (e.g., Kloeckera apis apicualata,Candida guilliermondii,Saccharomyces cerevisiae) | Spontaneous |
| Yamamoto et al. (32) | Fermented tea | Aspergillus luchuensis var kawachii kitahara, Lactobacillus spp. (L. sakei,L. acidophilus,L. casei,L. plantarum), B. longum | Starter culture |
| Not specified in the study cited in this review. General fermenters of fermented soybean milk were identified from Sirilun et al. (33). | Fermented soybean milk | Lactobacillus paracasei,L. casei,Lactobacillus mali, and Bifidobacterium breve | Starter culture |
| Not specified in the study cited in this review. | Fermented plant extract | Not specified | Not specified |
LAB, lactic acid bacteria.
Each study included in this review meets the following criteria: 1) the study analyzed a spontaneously fermented food or a food fermented with a multispecies starter culture commonly used in the commercial production of these foods, 2) the study was a human intervention (feeding) study (no observational studies were included), and 3) the composition and/or metabolic function of the gut (fecal) microbiota was analyzed. Three researchers (LTS, REN, and JGN) independently reviewed the scientific literature and consistently identified a total of 19 studies meeting these criteria using the following search terms in the PubMed database: “microbiome” or “microbiota” in combination with “fermented food,” “fermented,” “kefir,” “yogurt,” “wine,” “kimchi,” “sauerkraut,” “pickled food,” “cheese,” “fermented cabbage,” “fermented milk,” “kombucha,” “coffee,” and “fermented tea” (Table 2).
TABLE 2.
Human dietary intervention studies assessing the effect of fermented food consumption on the composition and/or function of the gut microbiota1
| Citation | Study design | Food product tested in the intervention | Comparison made | Participants | Duration of the intervention | Frequency and dose of the intervention | Effect on the gut microbiota | Other health or physiological observations |
|---|---|---|---|---|---|---|---|---|
| Veiga et al. (24) | Parallel-group design | Fermented milk | Microbiota of participants who consumed fermented milk compared with the microbiota of participants who received placebo | 28 women patients with IBS aged 20–69 y; 13 consumed fermented milk, 15 consumed placebo | 4 wk | 125 g, twice daily | Decrease in Bilophila wadsworthia (a pathobiont, P < 0.05), and an increase in butyrate-producing bacteria (P < 0.05). There was also a significant increase in SCFA production (particularly butyrate, P < 0.001) by the gut microbiota. | No other health outcomes were assessed. |
| Unno et al. (22) | Crossover intervention | Fermented milk | Microbiota after consumption of fermented milk compared with the microbiota after weeks of no intervention (washout) | 6 healthy adult women aged 20–24 y | 3 wk, followed by 3 wk of no intervention (washout) | 140 mL, twice daily | Decrease in members of the Bacteroidetes phylum (P < 0.05) and increase in members of the Firmicutes phylum (P < 0.05). | No other health outcomes were assessed. |
| Tillisch et al. (23) | Parallel-group design | Fermented milk | Microbiota of participants who consumed fermented milk compared with the microbiota of participants who consumed nonfermented milk, or who received no intervention | 36 healthy adult women aged 18–55 y; 12 consumed fermented milk, 11 consumed nonfermented milk, 13 received no intervention | 4 wk | 125 g, twice daily | There were no significant changes in the gut microbiota composition after the intervention. | After an emotional attention task, consumption of fermented milk resulted in reduced midbrain activity (P < 0.004). |
| Yilmaz et al. (25) | Parallel-group design | Fermented milk | Microbiota after consumption of fermented milk compared with the microbiota of subjects who received no intervention | 45 adult patients (18 y or older) with inflammatory bowel disease; 25 treated with fermented milk, 20 untreated | 4 wk | 200 mL, twice daily | Lactobacillus counts significantly increased in the feces of the treatment group. | Specifically, in patients with Crohn disease (compared with those with ulcerative colitis in the treatment group), there was a decrease in C-reactive protein and an increase in hemoglobin. In addition, in the last 2 wk, bloating scores were reduced and “feeling good” scores increased (P < 0.05). |
| Lisko et al. (19) | Parallel-group design | Yogurt | Microbiota after consumption of yogurt compared with the microbiota before consumption of yogurt | 6 healthy adults aged 18–54 y | 6 wk | 250 g, once daily | Nonsignificant shifts in Bifidobacteria spp. were observed. | No other health outcomes were assessed. |
| Yang and Sheu (26) | Parallel-group design | Yogurt | Microbiota after consumption of yogurt compared with the microbiota before consumption of yogurt | 38 Helicobacter pylori–infected and 38 healthy children aged 4–12 y | 4 wk | 200 mL, twice daily | Intervention reduced the Escherichia coli:Bifidobacterium ratio (P < 0.03) in H. pylori–infected children. | Intervention reduced H. pylori loads (P < 0.05) and elevated serum IgA and pepsinogen II concentrations (P < 0.001). |
| Firmesse et al. (43) | Before and after design | Camembert cheese | Microbiota after consumption of cheese compared with the microbiota before consumption of cheese | 12 healthy volunteers (no age specified) | 4 wk | 40 g, twice daily | Enterococcus faecalis increased in abundance after the intervention period (P < 0.05). | No other health outcomes were assessed. |
| Firmesse et al. (27) | Before and after design | Camembert cheese | Microbiota after consumption of cheese compared with the microbiota before consumption of cheese | 12 healthy volunteers (no age specified) | 4 wk | 40 g, twice daily | High concentrations of Lactococcus lactis and Leuconostoc mesenteroides measured in fecal samples during the intervention. L. mesenteroides persisted 15 d after the intervention ended. | Nitrate reductase activity decreased during the intervention. |
| Clemente-Postigo et al. (44) | Crossover intervention | Red wine | Microbiota of participants who consumed red wine compared with the microbiota of participants who consumed dealcoholized red wine, and gin | 10 healthy adult males aged 45–50 y | 20 d each, no washouts | 272 mL, once daily (red wine and dealcoholized red wine), 100 mL gin once daily | Red wine increased Prevotella abundance (P < 0.01), red wine polyphenols (alcoholized and dealcoholized) increased Bifidobacterium abundance (P < 0.01). | No significant differences were observed for LPS or LBP concentrations between interventions. Reductions in Prevotella and Bifidobacterium correlated with LPS concentrations (P < 0.05 and P < 0.01, respectively). |
| Queipo-Ortuño et al. (45) | Crossover intervention | Red wine | Microbiota of participants who consumed red wine compared with the microbiotas of participants who consumed dealcoholized red wine, and gin | 10 healthy adult males aged 45–50 y | 20 d each, no washouts | 272 mL, once daily (red wine and dealcoholized red wine), 100 mL gin once daily | Consumption of red wine polyphenols increased specific bacterial genera (e.g., Enterococcus, Prevotella, Bacteroides, and Bifidobacterium, P < 0.05). | Daily intake of red wine polyphenols was observed to decrease systolic and diastolic blood pressures, and triglyceride, total cholesterol, HDL cholesterol, C-reactive protein, and transaminase concentrations (P < 0.05). |
| Moreno-Indias et al. (46) | Crossover intervention | Red wine | Microbiota of participants who consumed red wine compared with the microbiotas of participants who consumed dealcoholized red wine | 10 healthy and 10 obese adult males aged 45–50 y | 4 wk, 15-d washout in between | 272 mL, once daily (red wine and dealcoholized red wine) | Red wine and dealcoholized red wine (polyphenols) decreased the abundances of Bifidobacteria, Lactobacillus, and butyrate-producing bacteria (P < 0.05) in obese adults. Further, red wine polyphenols reduced LPS-producing bacteria in obese patients (P < 0.05). | Red wine polyphenols were associated with reduction in BMI, weight, and LDL:HDL cholesterol (P < 0.05), among other markers of metabolic syndrome. |
| Barroso et al. (47) | Parallel-group design | Red wine | Microbiotas of participants in different polyphenol metabolizing groups after consumption of red wine | 20 healthy adults (age not specified) grouped according to their polyphenol metabolizing capacity | 4 wk | 250 mL, once daily | Consumption of red wine increased total diversity of the gut microbiota (P < 0.01). This was driven by specific low-abundant taxa; Slackia (P < 0.001), Gordonibacter, Oscillatoria, and Veillonella (P < 0.05). | No other health outcomes were assessed. |
| Inoguchi et al. (48) | Crossover intervention | Fermented soybean milk | Microbiota of participants who consumed fermented soybean milk compared with the microbiota of participants who consumed nonfermented soybean milk | 10 healthy adults aged 21–25 y; 5 consumed fermented soybean milk, 5 consumed nonfermented soybean milk | 2 wk | 100 g, once daily | Increase in Lactobacillus and decreased Clostridia after fermented soybean milk consumption (P < 0.05). | Fecal sulfide decreased after fermented soybean milk consumption (P < 0.01). |
| Cheng et al. (49) | Crossover intervention | Fermented soybean milk | Microbiota of participants who consumed fermented soybean milk compared with the microbiota of participants who consumed nonfermented soybean milk | 28 healthy adults aged 20–25 y; 14 consumed fermented soybean milk, 14 consumed nonfermented soybean milk | 2 wk | 250 mL, twice daily | Decrease in coliform organisms and Clostridium perfringens (P < 0.05), increase in Bifidobacterium and Lactobacillus spp. (P < 0.05). | No other health outcomes were assessed. |
| Nielsen et al. (30) | Parallel-group design | Lacto-fermented sauerkraut | Microbiota of participants who consumed unpasteurized sauerkraut compared with the microbiota of participants who consumed pasteurized sauerkraut | 34 patients with IBS aged 16–65 y; 15 consumed pasteurized sauerkraut, 19 consumed unpasteurized sauerkraut | 6 wk, followed by a 2-wk follow-up | 75 g, once daily | Both pasteurized and unpasteurized sauerkraut led to significant gut microbiota compositional changes (P < 0.001). Sauerkraut-related LAB were significantly increased in guts of patients who consumed unpasteurized sauerkraut. | Both pasteurized and unpasteurized sauerkraut resulted in decreased IBS severity scores. |
| Chiu et al. (50) | Parallel-group design | Fermented plant extract | Microbiota of participants who consumed fermented plant extract compared with the microbiota of participants who received placebo | 44 patients with hypercholesterolemia aged 30–60 y; 22 received fermented plant extract, 22 received placebo | 8 wk, followed by a 2-wk follow-up | 30 mL, twice daily | Individuals who consumed the fermented plant extract displayed increased gut Bifidobacteria (P < 0.05) and Lactobacillus spp. (P < 0.01) and decreased E. coli and C. perfringens (P < 0.05). | Consumption of fermented plant extract increased total antioxidant capacity (P < 0.05) and decreased the lipid profile (P < 0.05). |
| Han et al. (15) | Parallel-group design | Kimchi | Microbiota of participants who consumed fresh kimchi compared with the microbiota of participants who consumed fermented kimchi | 23 obese women aged 30–60 y; 12 consumed fresh kimchi, 11 consumed fermented kimchi | 8 wk | 60 g, thrice daily | Significant increase in Bifidobacterium abundance after kimchi consumption for 8 wk. Nonsignificant shifts in other bacterial taxa. | Negative correlation between Bifidobacterium and waist circumference (P < 0.05). This group also observed upregulation of genes associated with metabolism, immunity, and digestion (P < 0.05), which also correlated with bacterial taxa (P < 0.05). |
| Yamamoto et al. (32) | Before and after design | Fermented green tea (Cha-Koji) | Microbiota after consumption of Cha-Koji compared with the microbiota before consumption of Cha-Koji | 9 healthy adults aged 25–47 y | 4 wk | 2.14 g, once daily | Increase in Clostridium cluster XIVa and decrease in cluster IX (P < 0.05). | Increase in T regulatory cells after consumption of fermented tea (P < 0.01). |
| Jaquet et al. (51) | Before and after design | Coffee | Microbiota after consumption of coffee compared with the microbiota before consumption of coffee | 16 healthy adults aged 21–57 y | 3 wk | 3 cups/d | Increase in Bifidobacterium spp. (P < 0.05) | No other health outcomes were assessed. |
IBS, irritable bowel syndrome; LAB, lactic acid bacteria; LBP, LPS-binding protein.
The Micro- and Molecular Environment of Fermented Foods and Their Potential Role in Human Health
The fermented foods discussed in this review are fermented predominantly by LAB (e.g., Lactobacillus spp., Streptococcus thermophilus), Bifidobacteria spp., and, in the case of red wine and coffee, Saccharomyces yeasts (Table 1). Introduction of specific LAB and Bifidobacteria species and strains into both humans and animal models suggests these bacterial taxa are capable of modifying host metabolism and immunity (34–36). In addition, Saccharomyces yeasts are reported to exhibit anti-inflammatory properties, which may contribute to immune-supporting characteristics of modest wine consumption (37, 38).
Microbial fermentation can also modify the bioactive properties of the food itself. Through the process of fermentation, many microbes participate in the conversion of complex carbohydrates and phenolic compounds to bioactive metabolites (8, 39). Bioactive metabolites such as SCFAs are essential energy sources for host cells and are reported to have significant impact on host immune, neuronal, and metabolic functions (39–41). These particular metabolites also modulate gut immune function and energy production by colonic cells, affecting host–microbiota interactions (40, 42). Thus, consumption of fermented foods may introduce both probiotic microbes and their beneficial by-products, alluding to a mechanism by which these foods support aspects of human health.
Review of Human Intervention Studies
Of the 19 studies identified in our literature search, 8 analyzed fermented milk, yogurt, or cheese; 4 analyzed red wine; and 7 analyzed other varieties of fermented foods, including fermented tea, coffee, kimchi, fermented soybean milk, sauerkraut, and fermented plant extract (Table 2). We did not identify feeding studies that assessed the effect of pickled foods (olives, pickles, etc.) on the gut microbiota.
Studies testing fermented dairy products
Eight studies analyzed the gut microbiota after consumption of fermented dairy products, including fermented milk, yogurt, and cheese. Fermented dairy products are typically fermented by LAB (namely, Streptococcus thermophilus and Lactobacillus spp.) in addition to Bifidobacterium spp. (Table 1) (19, 22–28). Camembert cheese is also fermented with Leuconostoc spp., Hafnia alvei, and Geotrichum fungi (Table 1) (27, 28). These additional fermenters of the cheese could account for the varied effects of its consumption on the gut microbiota. However, it is important to note that although the fermented milks and yogurts tested were fermented with similar bacteria, the specific LAB and amounts of LAB in each product are not identical. These differences may account for the variability in results between the fermented milk and yogurt intervention studies.
Fermented milk
According to a crossover intervention study of 6 healthy women, consumption of fermented milk may be associated with changes in gut microbiota composition (22). Specifically, the authors report increases in the Firmicutes:Bacteroidetes ratio after consumption of fermented milk for 3 wk (22). Tillisch et al. (23) reported in a double-blind parallel-group intervention study comprised of 36 healthy adult women (12 received fermented milk, 11 received nonfermented milk, and 13 received no intervention) that changes in midbrain connectivity were associated with consumption of the fermented milk product for 4 wk. However, in a post hoc analysis of the gut microbiota, the authors report that these changes in neuronal activity were not mediated by shifts in the bacterial diversity of the gut (23). Thus, whether fermented milk products modify the gut microbiota of healthy participants remains inconclusive. The study by Unno et al. (22) was comprised of only 6 participants, limiting the researchers’ ability to detect small to medium shifts in microbial abundance. These studies also do not provide evidence of the gut microbiota mediating associations between fermented milk products and other physiological aspects in healthy participants.
Two studies analyzed the gut microbiota after consumption of fermented milk among immune-compromised patients. Veiga et al. (24) analyzed the gut microbiota before and after fermented milk consumption for 4 wk in 28 women with irritable bowel syndrome (IBS). After the intervention, the researchers observed a decrease in Bilophila wadsworthia (a pathobiont) and an increase in butyrate-producing bacteria (24). They also observed an increase in SCFA production, specifically butyrate, in the gut after consumption of fermented milk (24). Among 45 adult patients with inflammatory bowel disease, researchers reported increased Lactobacillus spp. counts in the feces of the patients who consumed fermented milk for 4 wk (n = 25) compared with untreated patients (n = 20) (25). This increase in abundance of Lactobacillus spp. was accompanied by decreased bloating scores and increased “feeling good” scores among the patients (25). These 2 studies suggest the gut microbiota as a mediator between fermented milk consumption and various health outcomes among immune-compromised individuals. Compared with studies in healthy participants, these works suggest that fermented food consumption may be more effective at modulating the gut microbiota among individuals with compromised gut health.
Yogurt
We identified 2 studies that tested the effect of yogurt consumption on the gut microbiota. Consumption of yogurt for 6 wk by 6 healthy participants was not linked to any statistically significant shifts in bacterial abundance in the gut (19). Conversely, a study by Yang and Sheu (26) reported that a 4-wk intervention with yogurt containing probiotic microbes reduced the intestinal Escherichia coli:Bifidobacterium spp. ratio in children infected with Helicobacter pylori compared with controls (antibiotic consumption within 1 mo of study start was an exclusion criterion). Notably, this intervention also reduced H. pylori loads and elevated serum IgA concentrations in infected children, highlighting an avenue for treatment of this infection with yogurt (26). Larger studies and possibly studies of longer intervention duration testing yogurt on the gut microbiota are necessary to shed more light on the potential beneficial effects of consuming yogurt products.
Camembert cheese
We identified 2 studies that analyzed the gut microbiota compositional effects of Camembert cheese consumption in healthy volunteers (27, 43). Both studies reported increases in specific microbial taxa in the guts of 12 participants consuming Camembert cheese for 4 wk, some of which (Leuconostoc spp.) persisted in the gut 15 d after completing the intervention (27, 43). Of note, the bacteria Firmesse et al. (27, 43) identified in the feces of these participants are colonizers of Camembert cheese, suggesting that the gut microbiota was effectively seeded by microbes found in this cheese. However, aside from the identification of cheese-colonizers in the guts of these individuals, no additional microbiota compositional alterations were observed.
Collectively, there are too few studies in each of these fermented dairy categories to suggest a particular pattern of gut microbiota modulation after consumption of these food products. Focusing specifically on fermented milk and yogurt, there is some evidence to suggest that consumption of these dairy products alters the gut microbiota preferentially in individuals with compromised gut health (24–26).
Studies testing red wine
Four studies analyzed the gut microbiota's response to red wine consumption (44–47). We did not identify any studies that focused on other fermented alcoholic beverages, although gin was used as a control in the studies by Queipo-Ortuño et al. (45) and Clemente-Postigo et al. (44). The specific fermenters of the red wine tested in the following studies were not disclosed in these publications (44–47). However, the goal of these studies was to compare the effects of red wine polyphenols on the gut microbiota, rather than the ethanol content. Red wine is predominately fermented with Saccharomyces yeast (Table 1), which, in addition to the production of ethanol, has the ability to modify the polyphenolic content of red wine (52).
A 2017 study (47) analyzed the effect of red wine consumption on the gut microbiota composition in 20 healthy individuals grouped according to their polyphenol metabolizing capacity (metabotypes). After 1 mo red wine consumption, researchers observed increased total gut microbial diversity driven primarily by shifts in abundances of specific bacterial genera (e.g., Enterococcus,Prevotella,Bacteroides, and Bifidobacterium spp.) (47). However, there was no association between red wine consumption and the metabotype classifications, suggesting significant gut microbiota interindividual variability (47).
Moreno-Indias et al. (46) assessed the effect of red wine polyphenols on the gut microbiota of healthy and obese males. The authors reported that consumption of both red wine and dealcoholized red wine for 4 wk increased Bifidobacteria, Lactobacillus, and butyrate-producing bacteria (e.g., Faecalibacterium prausnitzii) and reduced LPS-producing bacteria (e.g., E. coli) in obese males only (46). Notably, there were no significant gut microbiota compositional differences between the red wine and dealcoholized red wine interventions. This suggests that polyphenol conversion by microbial fermentation, rather than alcohol content, drove these gut microbiota changes in the participants.
The same research group also conducted 2 crossover intervention studies in 10 healthy male volunteers ( 44, 45). Participants consumed 272 mL red wine, dealcoholized red wine, or gin once daily for 20 d each ( 44, 45). Fecal samples were collected for gut microbiota analysis after each intervention period, but each study focused on different metabolic and immune outcomes ( 44, 45). Both studies reported similar changes in the gut microbiota composition (i.e., increases in Prevotella and Bifidobacteria spp.) after consumption of red wine and dealcoholized red wine, in comparison with gin ( 44, 45). The 2012 study also reported reduction in systolic and diastolic blood pressures, triglyceride concentrations, and total cholesterol and HDL cholesterol concentrations after red wine polyphenol (red wine or dealcoholized red wine) consumption (45). According to this group's 2013 study, red wine polyphenols had no effect on serum LPS or LPS-binding protein (LBP) concentrations (44).
These works provide preliminary evidence that red wine consumption modifies the composition of the gut microbiota. Interestingly however, the effect of red wine consumption on the gut microbiota may be driven primarily by the polyphenolic content of red wine, rather than the ethanol content. Two studies (45, 46) reported the polyphenol content of the red wine and dealcoholized red wine tested, indicating that other than the ethanol content, these 2 beverages were very similar in their chemical compositions. Because similar effects on the gut microbiota were observed with dealcoholized red wine, any positive effect of alcoholized red wine should be weighed against the carcinogenic effects of alcohol consumption.
Studies testing other fermented food products
We identified 7 human intervention studies that assessed the effects of other fermented food products (fermented tea, sauerkraut, fermented plant extract, coffee, kimchi, and fermented soybean milk) on the gut microbiota (Table 2).
All 7 studies reported that consumption of the fermented food tested modified the composition of the gut microbiota (Table 2). Four studies analyzed the gut microbiota of healthy individuals, all of which reported increased abundances of specific bacteria in the gut, which may exhibit health-promoting properties (32, 48, 49, 51). Consumption of 250 mL soybean milk twice daily for 2 wk was associated with increased abundance of Lactobacillus and Bifidobacteria spp. and decreased coliform bacteria and Clostridium perfringens (49). Some of these findings were corroborated in a 2012 study (48); consumption of 100 g fermented soybean milk once daily for 2 wk was associated with increased abundance of Lactobacillus spp. and decreased abundance of Clostridia spp. in 10 healthy adults. A 4-wk intervention study with Cha-Koji (fermented tea) increased the anti-inflammatory Clostridium cluster XIVa in the gut microbiota of 9 healthy adults (32). This shift in gut bacterial abundance was accompanied by an increase in systemic T-regulatory cells, suggesting the gut microbiota as a mediator between Cha-Koji consumption and these anti-inflammatory effects (32). Finally, coffee consumption (3 cups/d) for 3 wk was linked to increased Bifidobacteria spp. in the guts of 16 healthy adults (51). Notably, coffee beans are traditionally roasted before consumption. This study did not compare roasted with unroasted coffee beans or caffeinated with decaffeinated coffee products, highlighting opportunities for future investigation.
Three studies analyzed the gut microbiotas of immune-compromised or metabolic syndrome patients (15, 30, 50). Consumption of fermented plant extract for 8 wk by 22 hypercholesterolemic patients was reported to increase beneficial microbes, Bifidobacteria and Lactobacillus spp., and decrease potential pathogens, E. coli and C. perfringens (50). This shift in gut microbiota composition was accompanied by an increased antioxidant capacity and decreased lipid profile of these patients (50). In an 8-wk controlled clinical trial, kimchi consumption was linked to an increase in Bifidobacteria spp. abundance (15). Further, the abundance of Bifidobacterium was inversely correlated with waist circumference (15). Lastly, a 2018 intervention study by Nielsen et al. (30) compared the effects of pasteurized and unpasteurized sauerkraut on the gut microbiota composition. Fifteen IBS patients consumed 75 g pasteurized sauerkraut, whereas 19 IBS patients consumed unpasteurized sauerkraut for 6 wk (30). When the 2 groups were compared, there was a higher abundance of sauerkraut LAB in the fecal samples of patients in the unpasteurized group (30). However, both pasteurized and unpasteurized sauerkraut were linked to decreased IBS severity scores (30). This suggests that the beneficial effects of consuming sauerkraut may be attributed more to the enhanced bioactive properties (e.g., microbial metabolic content of the food after fermentation) of the fermented cabbage rather than the microbial content. An avenue for future studies testing sauerkraut would be to include unfermented cabbage as a control, because this would allow researchers to determine whether fermentation of cabbage is truly linked to health benefits and/or changes in gut microbiota composition.
The studies discussed in this review provide evidence that fermented food products induce compositional changes in the gut microbiota. However, there are too few studies within each fermented food category to identify a pattern of microbiota modulation that can be attributed to consumption of any specific fermented food. Further, the bacterial and yeast species used to ferment these foods and the amount of each of these fermenters within the food may also vary significantly between studies, increasing the variability of gut microbiota alterations observed after fermented food consumption (Table 1). Finally, many studies in which additional health outcomes were assessed suggest the microbiota as a mediator of the immune and metabolic benefits linked with fermented food consumption. However, sample size calculations for the analyses of microbiota composition were not reported for these studies and the majority are insufficiently powered to conduct mediation analyses to confirm this.
Challenges and Areas for Future Research
There are very few published intervention studies for the majority of the foods discussed in this review (some foods are represented by only 1 human intervention study). Consequently, the study designs used to conduct these dietary interventions are rather heterogeneous. Other than the duration of the intervention, which averaged ∼4 wk for most studies, the amount of fermented food consumed, the number of participants included, and the comparisons made vary significantly across studies (Table 2). The duration of intervention was generally not well justified. Therefore, a lack of change in the microbiota composition may be simply due to insufficient intervention duration. A better understanding of the minimum number of weeks required for a specific fermented food to be consumed to instigate changes in the microbiota will be essential to inform future research. Moreover, the choice of the study population will likely impact the influence of the fermented food on the microbiota. While some feeding studies included only healthy participants, other interventions focussed on populations with particular disorders. It is likely that fermented foods affect balanced microbiota differently than microbial dysbiosis. Future studies need to account for the baseline microbiota as an important predictor.
Suggestions for human intervention study design
Two study designs are most commonly used in dietary interventions: the parallel-group design and the crossover study design.
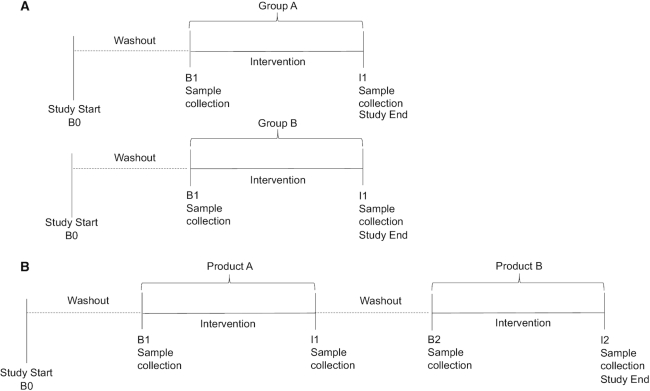
In the parallel-group design, participants are randomly assigned to ≥2 interventions (Figure 1A). Evaluations may be made by comparing 1 (or more) interventions with a no-intervention arm. This design is often employed when studying the effect of an intervention on 1 group of participants (e.g., healthy) and comparing the effects of the intervention to those of participants in another group (e.g., diseased). However, the parallel-group design presents many challenges with regard to individual variability, particularly in studies aimed at analyzing the human microbiota. Alternatively, in the crossover design, individual variability is reduced because comparisons are made within each participant. In this design, individuals are randomly assigned into intervention sequences (Figure 1B) and samples are collected before and after each intervention in the sequence. Both designs are represented in studies discussed in this review; additional benefits and pitfalls of each are discussed below.
FIGURE 1.
Study design strategies for human dietary intervention studies. (A) Parallel-group study design. Participants are randomly assigned to 1 intervention only. Samples are collected at B1 and I1. (B) Randomized crossover dietary intervention study design. Participants are randomly assigned to an intervention sequence. For example, group A consumes product A first and product B second. Samples are collected at B1, I1, B2, and I2. B0, beginning of study; B1, baseline 1; B2, baseline 2; I1, intervention 1; I2, intervention 2.
Currently no benchmark for the “normal” human microbiota exists against which to measure “change” of the microbiota composition. There is significant baseline (noninformative) variability in microbiota composition between individuals (53). The main bacterial colonizers of the gut microbiota can be grouped into 2 phyla: Bacteroidetes and Firmicutes (53). Even at this high taxonomic rank, the Bacteroidetes:Firmicutes ratio has been reported to differ by an order of magnitude between healthy individuals (53). The genera and species that comprise these phyla are highly variable and the presence of specific species depends strongly on the individual's genetic background and environmental exposome (e.g., diet, medications) (53). Further, there is differential responsiveness among people, with one individual's microbiota being responsive and another's being resilient to dietary modifications (54). For example, 1 study used a parallel-group design to analyze the effect of inulin consumption on the gut microbiota compared with placebo (maltodextrin) (55). The authors reported that the increase in Bifidobacteria after the intervention was largely driven by the baseline Bifidobacteria abundances (55). Thus, a key flaw in the parallel-group design is the inability to distinguish between microbiota compositional changes induced by the food product and those induced by interindividual differences at baseline.
In addition, when using the parallel-group design, the study size needs to be generally double that of a crossover design. In the crossover design, all participants complete both interventions, effectively doubling the total number of participants in each group. Although it may be necessary to use the parallel-group design when testing a product among 2 different groups of participants (e.g., healthy compared with diseased), this design should be avoided when analyzing the effects of multiple interventions (e.g., fermented food compared with nonfermented food).
The crossover design is often preferable for dietary interventions for the aforementioned reasons. However, this design has its own set of limitations. If the interventions tested are of significant duration (e.g., several months), it can be difficult to enroll and maintain participants in the study because the sequence of intervention doubles or triples the length of the study. Secondly, gut microbiota composition can shift over time, regardless of whether an individual has introduced a major change to their lifestyle (53). Moreover, if the dynamics of the exposure–microbiota association are not well understood, carryover effects may dilute comparisons between intervention sequences. However, these challenges may be mitigated by collecting new baseline samples after a washout period and before beginning the next intervention in the sequence.
Each study type presents its own challenges and advantages. However, if the research question is aimed at analyzing the effect of fermented foods compared with nonfermented foods on the gut microbiota, rather than comparing the effect of fermented food consumption between 2 groups of people, the crossover design is superior. This design reduces the impacts of genetic and environmental variability and allows for maintaining the same statistical power as a parallel-group study with a smaller sample size. Ultimately, selecting an unbiased study design is of the utmost importance because it can significantly affect the interpretation of the results collected.
Functional analyses and the gut mycobiome
Two areas which future intervention studies might consider expanding on include 1) analysis of the gut mycobiota (the fungal complement to the bacterial microbiota) and 2) analysis of microbiota function via metabolomics and metagenomics. All of the studies discussed in this review focused solely on characterization of the bacterial microbiota composition after fermented food consumption. By focusing solely on bacteria, researchers are currently excluding the potential influence of a large microbial population in the gut. There is growing evidence that the mycobiota plays an equally significant role in human health (56). Further, because many fermented foods are colonized by both yeasts and bacteria, analysis of the fungal populations would provide a more holistic view of the gut microbial populations affected by fermented food consumption.
In addition, changes in bacterial composition after a dietary intervention do not necessarily translate to functional changes in the gut microbiota. Given that sample size is a limitation in human intervention studies, many studies lack statistical power to conduct mediation analyses that might address whether the microbiota mediates the association between fermented food consumption and other health outcomes. However, it may be possible to address this mechanistic gap by conducting functional analyses on the gut microbiota in addition to the compositional assessment. Microbial metabolomics or meta-transcriptomics (analysis of the microbiota's gene expression) could be applied to intervention studies in the future as a means to elucidate the mechanisms by which diet-induced variation in microbiota composition can lead to changes in health outcomes.
Conclusions
In this review, we identified 19 human intervention studies that analyzed the effect of naturally fermented food consumption on the gut microbiota. Currently, the data are too sparse to suggest that 1 particular fermented food modifies the gut microbiota in a specific pattern. However, because the majority of these foods are colonized by LAB, Saccharomyces yeasts, and Bifidobacteria spp., we conclude that consumption of foods comprised of these particular fermenters may be potential dietary targets to prevent or overcome gut dysbiosis in humans. Further, a number of studies identified compositional changes to the gut microbiota along with changes to immune or metabolic factors in the human host. This suggests the microbiota as a mediator between fermented food consumption and these health outcomes. However, future studies should consider functional analyses (microbial metabolomics, meta-transcriptomics) to better characterize the mechanisms linking fermented food consumption to changes in human health. Researchers should also carefully consider the design of their intervention study. Crossover designs would enhance the statistical power owing to paired comparisons. Further, the mycobiota is extremely understudied in this research area, highlighting a novel opportunity for future intervention studies.
Acknowledgments
The authors’ responsibilities were as follows—LTS and KBM: conceptualized the study and have primary responsibility for the final content; LTS, REN, and JGN: conducted the literature review; LTS: wrote the manuscript; REN and JGN: contributed to the construction of Table 2 and reviewed and edited the manuscript; KBM: critically reviewed and provided significant revisions to the manuscript; and all authors: read and approved the final manuscript.
Notes
Supported by National Cancer Institute, NIH T-32 training grant 5T32CA009142-37 (to LTS).
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: IBS, irritable bowel syndrome; LAB, lactic acid bacteria; LBP, LPS-binding protein.
Contributor Information
Leah T Stiemsma, Department of Epidemiology, Fielding School of Public Health, University of California, Los Angeles, CA, USA.
Reine E Nakamura, Department of Integrative Biology and Physiology, University of California, Los Angeles, CA, USA.
Jennifer G Nguyen, Department of Biology, University of California, Los Angeles, CA, USA.
Karin B Michels, Department of Epidemiology, Fielding School of Public Health, University of California, Los Angeles, CA, USA; Institute for Prevention and Cancer Epidemiology, Faculty of Medicine and Medical Center, University of Freiburg, Freiburg, Germany.
References
- 1. Sekirov I, Russell SL, Antunes LCM, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. [DOI] [PubMed] [Google Scholar]
- 2. Mohajeri MH, Brummer RJM, Rastall RA, Weersma RK, Harmsen HJM, Faas M, Eggersdorfer M. The role of the microbiome for human health: from basic science to clinical applications. Eur J Nutr. 2018;57:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stiemsma LT, Michels KB. The role of the microbiome in the developmental origins of health and disease. Pediatrics. 2018;141:e20172437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Castaner O, Goday A, Park Y-M, Lee S-H, Magkos F, Shiow S-ATE, Schröder H. The gut microbiome profile in obesity: a systematic review. Int J Endocrinol. 2018:4095789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S et al.. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–14. [DOI] [PubMed] [Google Scholar]
- 6. Bermudez-Brito M, Plaza-Díaz J, Muñoz-Quezada S, Gómez-Llorente C, Gil A. Probiotic mechanisms of action. Ann Nutr Metab. 2012;61:160–74. [DOI] [PubMed] [Google Scholar]
- 7. Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Ther Adv Gastroenterol. 2013;6:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marco ML, Heeney D, Binda S, Cifelli CJ, Cotter PD, Foligné B, Gänzle M, Kort R, Pasin G, Pihlanto A et al.. Health benefits of fermented foods: microbiota and beyond. Curr Opin Biotechnol. 2017;44:94–102. [DOI] [PubMed] [Google Scholar]
- 9. Fulde M, Sommer F, Chassaing B, van Vorst K, Dupont A, Hensel M, Basic M, Klopfleisch R, Rosenstiel P, Bleich A et al.. Neonatal selection by Toll-like receptor 5 influences long-term gut microbiota composition. Nature. 2018;560:489–93. [DOI] [PubMed] [Google Scholar]
- 10. Goodrich JK, Davenport ER, Beaumont M, Jackson MA, Knight R, Ober C, Spector TD, Bell JT, Clark AG, Ley RE. Genetic determinants of the gut microbiome in UK twins. Cell Host Microbe. 2016;19:731–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Colombo M, Castilho NPA, Todorov SD, Nero LA. Beneficial properties of lactic acid bacteria naturally present in dairy production. BMC Microbiol. 2018;18:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patra JK, Das G, Paramithiotis S, Shin H-S. Kimchi and other widely consumed traditional fermented foods of Korea: a review. Front Microbiol. 2016;7:1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park K-Y, Jeong J-K, Lee Y-E, Daily JW. Health benefits of kimchi (Korean fermented vegetables) as a probiotic food. J Med Food. 2014;17:6–20. [DOI] [PubMed] [Google Scholar]
- 14. Gaskins AJ, Pereira A, Quintiliano D, Shepherd JA, Uauy R, Corvalán C, Michels KB. Dairy intake in relation to breast and pubertal development in Chilean girls. Am J Clin Nutr. 2017;105:1166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Han K, Bose S, Wang J, Kim B-S, Kim MJ, Kim E-J, Kim H. Contrasting effects of fresh and fermented kimchi consumption on gut microbiota composition and gene expression related to metabolic syndrome in obese Korean women. Mol Nutr Food Res. 2015;59:1004–8. [DOI] [PubMed] [Google Scholar]
- 16. An S-Y, Lee MS, Jeon JY, Ha ES, Kim TH, Yoon JY, Ok C-O, Lee H-K, Hwang W-S, Choe SJ et al.. Beneficial effects of fresh and fermented kimchi in prediabetic individuals. Ann Nutr Metab. 2013;63:111–9. [DOI] [PubMed] [Google Scholar]
- 17. Bourrie BCT, Willing BP, Cotter PD. The microbiota and health promoting characteristics of the fermented beverage kefir. Front Microbiol. 2016;7:647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chakravorty S, Bhattacharya S, Chatzinotas A, Chakraborty W, Bhattacharya D, Gachhui R. Kombucha tea fermentation: microbial and biochemical dynamics. Int J Food Microbiol. 2016;220:63–72. [DOI] [PubMed] [Google Scholar]
- 19. Lisko D, Johnston G, Johnston C. Effects of dietary yogurt on the healthy human gastrointestinal (GI) microbiome. Microorganisms. 2017;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shadnoush M, Hosseini RS, Khalilnezhad A, Navai L, Goudarzi H, Vaezjalali M. Effects of probiotics on gut microbiota in patients with inflammatory bowel disease: a double-blind, placebo-controlled clinical trial. Korean J Gastroenterol. 2015;65:215–21. [DOI] [PubMed] [Google Scholar]
- 21. Odamaki T, Sugahara H, Yonezawa S, Yaeshima T, Iwatsuki K, Tanabe S, Tominaga T, Togashi H, Benno Y, Xiao J. Effect of the oral intake of yogurt containing Bifidobacterium longum BB536 on the cell numbers of enterotoxigenic Bacteroides fragilis in microbiota. Anaerobe. 2012;18:14–18. [DOI] [PubMed] [Google Scholar]
- 22. Unno T, Choi J-H, Hur H-G, Sadowsky MJ, Ahn Y-T, Huh C-S, Kim G-B, Cha C-J. Changes in human gut microbiota influenced by probiotic fermented milk ingestion. J Dairy Sci. 2015;98:3568–76. [DOI] [PubMed] [Google Scholar]
- 23. Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, Guyonnet D, Legrain–Raspaud S, Trotin B, Naliboff B et al.. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394–401.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Veiga P, Pons N, Agrawal A, Oozeer R, Guyonnet D, Brazeilles R, Faurie J-M, van Hylckama Vlieg JET, Houghton LA, Whorwell PJ et al.. Changes of the human gut microbiome induced by a fermented milk product. Sci Rep. 2014;4:6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yilmaz I, Dolar ME, Ozpinar H. Effect of administering kefir on the changes in fecal microbiota and symptoms of inflammatory bowel disease: a randomized controlled trial. Turk J Gastroenterol. 2019;30:242–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang Y-J, Sheu B-S. Probiotics-containing yogurts suppress Helicobacter pylori load and modify immune response and intestinal microbiota in the Helicobacter pylori-infected children: yogurt benefits to gut microbiota and immunity. Helicobacter. 2012;17:297–304. [DOI] [PubMed] [Google Scholar]
- 27. Firmesse O, Alvaro E, Mogenet A, Bresson J-L, Lemée R, Le Ruyet P, Bonhomme C, Lambert D, Andrieux C, Doré J et al.. Fate and effects of Camembert cheese micro-organisms in the human colonic microbiota of healthy volunteers after regular Camembert consumption. Int J Food Microbiol. 2008;125:176–81. [DOI] [PubMed] [Google Scholar]
- 28. Lay C, Sutren M, Lepercq P, Juste C, Rigottier-Gois L, Lhoste E, Lemée R, Ruyet PL, Doré J, Andrieux C. Influence of Camembert consumption on the composition and metabolism of intestinal microbiota: a study in human microbiota-associated rats. Br J Nutr. 2004;92:429–38. [DOI] [PubMed] [Google Scholar]
- 29. Marzano M, Fosso B, Manzari C, Grieco F, Intranuovo M, Cozzi G, Mulè G, Scioscia G, Valiente G, Tullo A et al.. Complexity and dynamics of the winemaking bacterial communities in berries, musts, and wines from Apulian grape cultivars through time and space. PLoS One. 2016;11:e0157383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nielsen ES, Garnås E, Jensen KJ, Hansen LH, Olsen PS, Ritz C, Krych L, Nielsen DS. Lacto-fermented sauerkraut improves symptoms in IBS patients independent of product pasteurisation – a pilot study. Food Funct. 2018;9:5323–35. [DOI] [PubMed] [Google Scholar]
- 31. Lee LW, Cheong MW, Curran P, Yu B, Liu SQ. Coffee fermentation and flavor – an intricate and delicate relationship. Food Chem. 2015;185:182–91. [DOI] [PubMed] [Google Scholar]
- 32. Yamamoto B, Suzuki Y, Yonezu T, Mizushima N, Watanabe N, Sato T, Inoue S, Inokuchi S. Cha-Koji, comprising green tea leaves fermented with Aspergillus luchuensis var kawachii kitahara, increases regulatory T cell production in mice and humans. Biosci Biotechnol Biochem. 2018;82:885–92. [DOI] [PubMed] [Google Scholar]
- 33. Sirilun S, Sivamaruthi BS, Kesika P, Peerajan S, Chaiyasut C. Lactic acid bacteria mediated fermented soybean as a potent nutraceutical candidate. Asian Pac J Trop Biomed. 2017;7:930–6. [Google Scholar]
- 34. Linares DM, Gómez C, Renes E, Fresno JM, Tornadijo ME, Ross RP, Stanton C. Lactic acid bacteria and Bifidobacteria with potential to design natural biofunctional health-promoting dairy foods. Front Microbiol. 2017;8:846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ai C, Ma N, Zhang Q, Wang G, Liu X, Tian F, Chen P, Chen W. Immunomodulatory effects of different lactic acid bacteria on allergic response and its relationship with in vitro properties. PLoS One. 2016;11:e0164697.27764153 [Google Scholar]
- 36. Martín R, Miquel S, Ulmer J, Kechaou N, Langella P, Bermúdez-Humarán LG. Role of commensal and probiotic bacteria in human health: a focus on inflammatory bowel disease. Microb Cell Factories. 2013;12:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guerrini S, Mangani S, Romboli Y, Luti S, Pazzagli L, Granchi L. Impact of Saccharomyces cerevisiae strains on health-promoting compounds in wine. Fermentation. 2018;4:26. [Google Scholar]
- 38. Foligné B, Dewulf J, Vandekerckove P, Pignède G, Pot B. Probiotic yeasts: anti-inflammatory potential of various non-pathogenic strains in experimental colitis in mice. World J Gastroenterol. 2010;16:2134–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. LeBlanc JG, Chain F, Martín R, Bermúdez-Humarán LG, Courau S, Langella P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb Cell Factories. 2017;16:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wong JMW, de Souza R, Kendall CWC, Emam A, Jenkins DJA. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–43. [DOI] [PubMed] [Google Scholar]
- 41. den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud D-J, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Firmesse O, Rabot S, Bermúdez-Humarán LG, Corthier G, Furet J-P. Consumption of Camembert cheese stimulates commensal enterococci in healthy human intestinal microbiota. FEMS Microbiol Lett. 2007;276:189–92. [DOI] [PubMed] [Google Scholar]
- 44. Clemente-Postigo M, Queipo-Ortuño MI, Boto-Ordoñez M, Coin-Aragüez L, del Mar Roca-Rodriguez M, Delgado-Lista J, Cardona F, Andres-Lacueva C, Tinahones FJ. Effect of acute and chronic red wine consumption on lipopolysaccharide concentrations. Am J Clin Nutr. 2013;97:1053–61. [DOI] [PubMed] [Google Scholar]
- 45. Queipo-Ortuño MI, Boto-Ordóñez M, Murri M, Gomez-Zumaquero JM, Clemente-Postigo M, Estruch R, Cardona Diaz F, Andrés-Lacueva C, Tinahones FJ. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am J Clin Nutr. 2012;95:1323–34. [DOI] [PubMed] [Google Scholar]
- 46. Moreno-Indias I, Sánchez-Alcoholado L, Pérez-Martínez P, Andrés-Lacueva C, Cardona F, Tinahones F, Queipo-Ortuño MI. Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food Funct. 2016;7:1775–87. [DOI] [PubMed] [Google Scholar]
- 47. Barroso E, Muñoz-González I, Jiménez E, Bartolomé B, Moreno-Arribas MV, Peláez C, del Carmen Martínez-Cuesta M, Requena T. Phylogenetic profile of gut microbiota in healthy adults after moderate intake of red wine. Mol Nutr Food Res. 2017;61:1600620. [DOI] [PubMed] [Google Scholar]
- 48. Inoguchi S, Ohashi Y, Narai-Kanayama A, Aso K, Nakagaki T, Fujisawa T. Effects of non-fermented and fermented soybean milk intake on faecal microbiota and faecal metabolites in humans. Int J Food Sci Nutr. 2012;63:402–10. [DOI] [PubMed] [Google Scholar]
- 49. Cheng I-C, Shang H-F, Lin T-F, Wang T-H, Lin H-S, Lin S-H. Effect of fermented soy milk on the intestinal bacterial ecosystem. World J Gastroenterol. 2005;11:1225–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chiu H-F, Chen Y-J, Lu Y-Y, Han Y-C, Shen Y-C, Venkatakrishnan K, Wang C-K. Regulatory efficacy of fermented plant extract on the intestinal microflora and lipid profile in mildly hypercholesterolemic individuals. J Food Drug Anal. 2017;25:819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jaquet M, Rochat I, Moulin J, Cavin C, Bibiloni R. Impact of coffee consumption on the gut microbiota: a human volunteer study. Int J Food Microbiol. 2009;130:117–21. [DOI] [PubMed] [Google Scholar]
- 52. Grieco F, Carluccio MA, Giovinazzo G. Autochthonous Saccharomyces cerevisiae starter cultures enhance polyphenols content, antioxidant activity, and anti-inflammatory response of Apulian red wines. Foods. 2019;8:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Healey GR, Murphy R, Brough L, Butts CA, Coad J. Interindividual variability in gut microbiota and host response to dietary interventions. Nutr Rev. 2017;75:1059–80. [DOI] [PubMed] [Google Scholar]
- 55. Tuohy KM, Finlay RK, Wynne AG, Gibson GR. A human volunteer study on the prebiotic effects of HP-inulin—faecal bacteria enumerated using fluorescent in situ hybridisation (FISH). Anaerobe. 2001;7:113–8. [Google Scholar]
- 56. Arrieta M-C, Arévalo A, Stiemsma L, Dimitriu P, Chico ME, Loor S, Vaca M, Boutin RCT, Morien E, Jin M et al.. Associations between infant fungal and bacterial dysbiosis and childhood atopic wheeze in a nonindustrialized setting. J Allergy Clin Immunol. 2018;142:424–34.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]