ABSTRACT
Background
Observational evidence suggests that red meat intake is associated with type 2 diabetes (T2D) and cardiovascular disease incidence, but few randomized controlled trials have assessed effects of lean, unprocessed red meat intake on insulin sensitivity and other cardiometabolic risk factors.
Objective
This study compared the USDA Healthy US-Style Eating Pattern, low in saturated fat and red meat (<40 g/d red meat; USDA-CON), with a modified version with an additional 150 g/d lean beef as an isocaloric replacement for carbohydrate (USDA-LB) on insulin sensitivity and cardiometabolic risk markers.
Methods
Participants (7 men, 26 women; 44.4 y old) with overweight/obesity [BMI (kg/m2) = 31.3] and prediabetes and/or metabolic syndrome completed this randomized, crossover, controlled-feeding trial consisting of two 28-d treatments (USDA-CON and USDA-LB) separated by a ≥14-day washout. Insulin sensitivity (primary outcome variable), lipoprotein lipids, apolipoproteins (apoA-I and apoB), and high-sensitivity C-reactive protein (hs-CRP) (secondary outcome variables), in plasma or serum, and blood pressures were assessed at baseline and the end of each diet period.
Results
USDA-LB and USDA-CON did not differ significantly in effects on whole-body insulin sensitivity and other indicators of carbohydrate metabolism, lipoprotein lipids, apoA-I and apoB, hs-CRP, and blood pressures. USDA-LB produced a shift toward less cholesterol carried by smaller LDL subfractions compared with USDA-CON [least-squares geometric mean ratios for LDL1+2 cholesterol of 1.20 (P = 0.016) and LDL3+4 cholesterol of 0.89 (P = 0.044)] and increased peak LDL time versus USDA-CON (1.01; P = 0.008).
Conclusions
Substituting lean, unprocessed beef for carbohydrate in a Healthy US-Style Eating Pattern resulted in a shift toward larger, more buoyant LDL subfractions, but otherwise had no significant effects on the cardiometabolic risk factor profile in men and women with prediabetes and/or metabolic syndrome.
This trial was registered at clinicaltrials.gov as NCT03202680.
Keywords: insulin sensitivity, carbohydrate metabolism, lipoproteins, meat, beef, USDA, diet patterns, metabolic syndrome, prediabetes
Introduction
A large body of evidence indicates that insulin resistance (impaired insulin sensitivity) is a risk factor for the development of type 2 diabetes (T2D) and atherosclerotic cardiovascular disease (1, 2). Lifestyle changes, including weight loss and physical activity, which increase whole-body insulin sensitivity, have been shown to prevent or delay the onset of T2D in those at increased risk for its development (3). Relatively few intervention studies have been completed to assess the influences of foods or dietary patterns on insulin sensitivity while maintaining constant body weight and physical activity levels.
The 2015 Dietary Guidelines Advisory Committee reviewed 29 observational studies and 8 randomized trials and judged that there was “moderate” scientific evidence indicating that dietary patterns associated with a lower risk of T2D emphasize intakes of fruits, vegetables, low-fat dairy, and whole grains and limit intakes of red meat, sugar-sweetened foods/drinks, refined grains, French fries, and high-fat dairy (4). The 2015 Dietary Guidelines for Americans recommends that the US population move toward dietary patterns that increase consumption of plant foods and reduce consumption of animal-based foods, including red meat (5). The authors of a review of 9 prospective cohort studies (450,000 total subjects) concluded that each 100-g serving of unprocessed red meat/d is associated with an increased risk of T2D (relative risk: 1.19; 95% CI: 1.04, 1.37) (6). However, it is unclear whether the association of red meat consumption with increased T2D incidence is due to adverse physiologic effects of red meat consumption per se, or confounding due to the correlation of red meat intake with other dietary and lifestyle factors that increase T2D risk (i.e., residual confounding) (7–9). Authors of a systematic review of prospective cohort studies with ≥1000 participants followed for 2–34 y concluded that dietary patterns lower in red and processed meat intake “may result in very small reductions in adverse cardiometabolic outcomes,” but acknowledged that observational studies are prone to residual confounding (10).
Results from a limited number of intervention studies have shown that increasing unprocessed red meat intake does not adversely affect insulin sensitivity and other determinants of glucose tolerance, consistent with the possibility that the association in observational studies could be the result of confounding and not indicative of a causal relation. For example, a 4-wk diet containing ≥200 g/d red meat had no adverse effect on insulin sensitivity compared with a control diet that contained poultry and fish as the main protein sources (11). These results are similar to those from the Beef in an Optimal Lean Diet (BOLD) controlled-feeding trial (all food provided), which showed no differences in fasting concentrations of insulin and glucose after 5 wk of consuming a healthy American diet (20 g/d lean beef), a Dietary Approaches to Stop Hypertension (DASH) diet (28 g/d lean beef), and 2 diets with higher intakes of lean beef: 113 g/d (BOLD) and 153 g/d (BOLD+) (12).
Authors of a meta-analysis of 36 randomized controlled trials comparing red meat diets with diets that replaced red meat with a variety of other foods (i.e., plant protein, fish only, poultry only, mixed animal protein, carbohydrate, or usual diet) reported no significant differences in lipoprotein cholesterol, apolipoprotein (apo), or blood pressure responses between the red meat diets and all other diets combined (13). Red meat diets in which only lean meat was consumed resulted in larger reductions in total cholesterol (TC) and LDL cholesterol compared with other diets (13).
Moderation of carbohydrate intake is a dietary strategy that has been increasingly advocated for modification of the cardiometabolic risk factor profile (14). The OmniHeart trial that assessed the metabolic effects of a diet rich in carbohydrate, protein from mixed sources, or unsaturated fatty acids (UFAs) demonstrated that partial substitution of dietary carbohydrate with UFAs or mixed-source proteins produced favorable changes in triglycerides (TGs), non–HDL cholesterol, and blood pressure compared with the carbohydrate-rich diet (15). In addition, an index of insulin sensitivity (1/HOMA-IR) was increased by ∼15% (P < 0.05) with the UFA diet and by ∼5% (P > 0.05) with the protein diet, compared with the carbohydrate diet (16).
The objective of the present trial was to compare the effects of the USDA Healthy US-Style Eating Pattern, as outlined in the 2015 Dietary Guidelines for Americans (5), containing <40 g/d of red meat (USDA-CON), or modified to include an additional 150 g/d of lean, unprocessed beef as an isocaloric replacement for carbohydrate, primarily refined starches (USDA-LB), on insulin sensitivity and other indices of carbohydrate metabolism, as well as other cardiometabolic risk markers in men and women with risk factors for diabetes mellitus (prediabetes and/or metabolic syndrome).
Methods
Study design
This randomized, controlled-feeding, 2-period, crossover study was conducted in accordance with Good Clinical Practice Guidelines, the Declaration of Helsinki, and the US 21 Code of Federal Regulations (17). The trial was registered at clinicaltrials.gov as NCT03202680. The study statistician created the randomization scheme, and sealed envelopes containing diet sequences were generated. An envelope was opened at the time of randomization of a subject to a diet sequence. Due to the nature of the study treatments, neither subjects nor study staff were blinded, but the statistician was blinded to treatment during the initial analyses by identifying the diet conditions as A and B when the analyses of differences by diet conditions were completed. An institutional review board (Aspire IRB, Santee, CA) approved the protocol before the initiation of the study, and subjects provided written informed consent before any study procedures were performed. The trial included 2 screening visits, 1 baseline visit, two 28-d test periods with visits at weeks 3 and 4 and a visit at the conclusion of a ≥2-wk washout period between the test periods. The first screening visit occurred in July 2017 and the last subject visit occurred in July 2018.
Subjects
Men and women, 18–74 y of age, inclusive, each with a BMI (kg/m2) 25.0 to 39.9, and who met the criteria for having metabolic syndrome and/or prediabetes were eligible for the study (18, 19). Prediabetes was defined as fasting plasma glucose ≥100 and ≤125 mg/dL or glycated hemoglobin of 5.7% to 6.4%. Metabolic syndrome was defined as having ≥3 of the following 5 criteria (19): waist circumference ≥102 cm in men or ≥88 cm in women (≥90 cm and ≥80 cm, respectively, in Asian-American subjects); blood pressure ≥130 (systolic) or ≥85 (diastolic) mm Hg or use of antihypertensive medication; fasting plasma TG concentration ≥150 mg/dL; HDL cholesterol <40 mg/dL (men) or <50 mg/dL (women); and fasting plasma glucose ≥100 mg/dL. Subjects had to be otherwise generally healthy and have fasting TGs <400 mg/dL and fasting LDL cholesterol <200 mg/dL. Subjects also had to be willing to consume only study-related foods and beverages during each test period and had to make every effort to consume all study foods and beverages in their entirety each day. Subjects had to be willing to maintain their usual physical activity levels throughout the trial and not to change smoking or other nicotine-use habits during the study period. Potential subjects were excluded if they had atherosclerotic cardiovascular disease, pulmonary (including uncontrolled asthma), endocrine (including type 1 diabetes and T2D), chronic inflammatory (including irritable bowel disease, lupus, rheumatoid arthritis), hepatic, renal, hematologic, immunologic, dermatologic, neurologic, psychiatric, or biliary disorders, or if they had a recent history (prior 5 y) or presence of cancer other than nonmelanoma skin cancer. Those who had experienced significant weight change (±4.5 kg in past 3 mo), had extreme dietary habits, or who had a recent history or strong potential for drug or alcohol abuse were excluded. Subjects with uncontrolled hypertension (systolic blood pressure ≥160 mm Hg and/or diastolic blood pressure ≥100 mm Hg) or a known allergy, sensitivity, or intolerance to any study foods or their ingredients were also excluded. Eligible individuals were not permitted to have unstable use of antihypertensive medication, to be actively using antibiotics, or to use supplements or medications known to alter lipid metabolism (except for stable use of statins), carbohydrate metabolism, or weight-loss drugs/programs. Those who were pregnant, planning to be pregnant during the study period, lactating, or of childbearing potential and unwilling to commit to the use of a medically approved form of contraception throughout the study period were also excluded. Also, individuals who had a condition the investigator believed would interfere with his or her ability to provide informed consent, comply with the study protocol, or put the person at undue risk were excluded.
Eating patterns and procedures
Subjects were randomly assigned to a 7-day rotating menu for the first 28-d dietary intervention period which was composed of either USDA-CON or USDA-LB at the appropriate calorie level (1800–3600 kcal/d), based on each subject's calculated energy needs for weight maintenance (20). Both eating patterns were designed to provide ∼25–30% of fat energy (6% SFAs, 7% PUFAs, and 12% MUFAs). USDA-CON was designed to provide 16–18% of protein energy and 52–58% of carbohydrate energy, whereas USDA-LB was designed to provide 25–30% of protein energy and 40–45% of carbohydrate energy (5). The USDA-CON diet provided <40 g/d of red meat and the USDA-LB diet was modified to include an additional 150 g/d of lean, unprocessed beef. Specifically, the amount of lean red meat (including, but not limited to, beef) recommended in the 2015 Dietary Guidelines for Americans is ≤51 g/d (12.5 ounces/wk), which is equivalent to approximately one 3.5-oz serving every other day (5). Thus, the USDA-CON diet was designed to fall within that recommendation, with a limitation of lean beef consumption to <40 g/d (<10 ounces/wk), while the additional amount of lean beef in the USDA-LB condition was designed to be at the high end of actual red meat intake for some subgroups in the United States (∼2 servings/d) (5). Since this was a fully controlled feeding trial, all the foods consumed by the subjects during each 28-d intervention period were provided (Supplemental Table 1). After the last day of the first intervention period, subjects were instructed to return to their habitual eating patterns for the duration of the washout period of ≥2 wk, after which subjects crossed over to the other eating pattern.
The nutrient profiles of baseline dietary intake, calculated from baseline 3-d diet records, and both study eating patterns were analyzed using Food Processor® Nutrition Analysis software (version 11.4.548; ESHA Research). Dietary compliance with lean beef and control entrées was assessed by calculating the servings of uneaten study-food products returned by the subject and dividing this by the total number of servings of study-food products provided. Compliance with the consumption of non–entrée study foods was assessed semi-quantitatively based on deviations from consuming the food provided (uneaten food or non–study food consumed). Each day was rated as no deviations, minor deviations, or major deviations by the study coordinator.
Clinic assessments
Clinic visit procedures, including body weight and systolic and diastolic blood pressure measurements, were conducted at each clinic visit. Blood pressures were measured after the subject had been seated for a minimum of 5 min. An automated device (Spot Vital Signs; Welch Allyn) took 3 measurements, 1 min apart. Fasting (12 ± 2 h, water only) blood draws were completed at baseline and during each treatment period for measurement of plasma glucose, plasma insulin, serum high-sensitivity C-reactive protein (hs-CRP), plasma apoB, plasma apoA-I, serum lipoprotein lipids, and serum lipoprotein particle and subfraction concentrations. For fasting serum lipoprotein lipids and hs-CRP, measurements were obtained twice on separate days prior to the dietary intervention commencement and on 2 separate days during the last 3 days of each 28-d intervention period. At baseline and on the final day of each 28-d intervention period, a short intravenous-glucose-tolerance test (IVGTT) was performed. The Stanford 7-d Physical Activity Questionnaire was completed at baseline and the end of each intervention period (21). Adverse experiences were evaluated by asking the subjects a nonleading question at each clinic visit.
Fasting lipid profile, lipoprotein subfractions and particles, and apolipoproteins
Fasting blood samples were collected for assessment of fasting serum lipids: TC, HDL cholesterol, non–HDL cholesterol, calculated LDL cholesterol, and TGs. Fasting lipid concentrations were analyzed according to the Standardization Program of the Centers for Disease Control and Prevention and the National Heart, Lung, and Blood Institute using enzymatic colorimetric methodology. LDL-cholesterol concentrations (milligrams per deciliter) were calculated according to the Friedewald equation as follows (in mg/dL): LDL cholesterol = TC – HDL cholesterol − TGs/5. Serum lipoprotein particle and subfraction cholesterol concentrations were analyzed using a density gradient ultracentrifugation technique, termed Vertical Auto Profile (VAP), from fasting blood samples (VAP Diagnostics Laboratory, Inc.) (22). Additionally, plasma apoB and apoA-I were both assessed using immunoturbidimetric assays (Cleveland HeartLab).
Fasting hs-CRP
Fasting serum hs-CRP analysis was assessed using an immunoturbidimetric assay carried out by Cleveland HeartLab.
Insulin sensitivity and carbohydrate metabolism
Fasting plasma glucose was assessed using an enzymatic colorimetric assay (Cleveland HeartLab), and fasting plasma insulin was assessed using an electrochemiluminescence immunoassay (Cleveland HeartLab). For the short IVGTT, after an overnight fast, an indwelling catheter was inserted to deliver the glucose load and obtain blood samples. To maintain patency of the intravenous catheter, normal saline solution was used to flush the catheter and/or infused as a slow, continuous drip. Samples were collected at t = −5, 5, 10, 20, 30, 40, and 50 ± 1 min, where an intravenous glucose bolus [t = 0 min; 0.3 g/kg body weight, 50% dextrose solution (with a maximum of 25 g of glucose)] was provided over ∼1.5 min. The glucose loads were administered within ±30 min of the t = 0 min time established at the baseline visit. An insulin sensitivity index (ISI) was calculated as the fractional disappearance rate of glucose (Kg) from 10–50 min divided by the average plasma insulin concentration from 10–50 min [i.e., Kg/(total AUC for insulin from 10–50 min/40 min)]. The Kg value for plasma glucose was calculated as the absolute value of the linear slope of the relation between the natural logarithm of glucose regressed on time from 10 to 50 min. Fasting homeostasis model assessments of insulin sensitivity (HOMA2-%S) and β-cell function (HOMA2-%B) indices were estimated using average fasting plasma glucose and insulin values (www.dtu.ox.ac.uk/homacalculator/index.php).
Statistical analysis
Sample size calculations indicated that an evaluable sample of 30 subjects was expected to provide 80% power to detect a difference of 0.53 SDs for the difference between diet conditions in ISI (the primary outcome variable), based on an ɑ of 0.05 for a 2-sided paired t test. Tests of significance were performed at an ɑ = 0.05, 2-sided, unless otherwise specified. All outcome variables other than ISI were considered secondary. The primary analysis utilized data from all subjects who provided data during both intervention periods (evaluable analysis sample). For continuous variables, means and SEMs are presented, or medians and interquartile (25th and 75th percentile) limits if not normally distributed; for outcome variables, geometric mean (log-mean back-transformed into the original units) and geometric SD limits are reported (i.e., log-mean plus 1 SD and log-mean minus 1 SD, back-transformed to the original units, for which values are not symmetrical around the back-transformed geometric mean value). Numbers of subjects and percentages are presented for categorical variables.
Statistical modeling was completed using SPSS Statistics (version 24.0 or higher; IBM Corporation). The initial models contained terms for diet condition, sequence, period, and baseline value as a covariate, with subject as a random effect. Models were reduced in a stepwise manner until only significant (P < 0.05) terms or diet condition and baseline value remained in the model. Separate models were run to evaluate possible treatment by sequence interactions (e.g., carryover effects). Repeated-measures linear mixed models were generated in SPSS Statistics using the MIXED procedure. Subject nested within treatment sequence was included as a random effect to account for the fact that 1) the values from the same subject are not independent and 2) values from subjects in the same treatment sequence may be more similar to one another than to subjects in the opposing sequence.
Because the residuals from the statistical models for several outcome variables were not normally distributed (Shapiro-Wilk test, P < 0.01), natural logarithm (log) transformations were applied for all continuous outcome variables, and least-squares geometric mean ratios for the 2 diet conditions (USDA-LB and USDA-CON) and 95% CIs from the final statistical models are presented.
A post hoc sensitivity analysis was completed that included missing values for the second period for the 4 subjects who completed the first diet condition but discontinued the study and provided no data for the second diet condition. Missing values for the second period were addressed with multiple imputation in SPSS, using the automatic method. This method selected a monotone linear regression approach for all variables except for LDL peak time, for which the Markov chain Monte Carlo method was used. Additional sensitivity analyses were conducted to examine the effects of each period separately, to explore the possibility of a carryover. Since no statistically significant or clinically relevant evidence was present to suggest there were differential responses by treatment sequence, pooled data are presented for both sequences. Subgroup analyses were also completed to assess whether evidence for differences in responses was present according to whether stable-regimen statin therapy was being used.
Results
A total of 152 subjects were screened, of whom 49 were randomized. The causes for screen failure included 79 subjects not meeting laboratory criteria, 5 subjects lost to follow-up, and 19 subjects not meeting other entry criteria (e.g., exclusionary medical history, BMI outside of the allowable range, inadequate vein access, and food allergy) (Figure 1). The subject attrition rate was higher than anticipated, which was largely attributable to subject dropout in the weeks following a hurricanethat left some participants without power for periods of ≤3 wk. Originally, the plan was to randomize 40 subjects; however, an additional 9 subjects were randomized to replace individuals who discontinued participation shortly after the hurricane.
FIGURE 1.
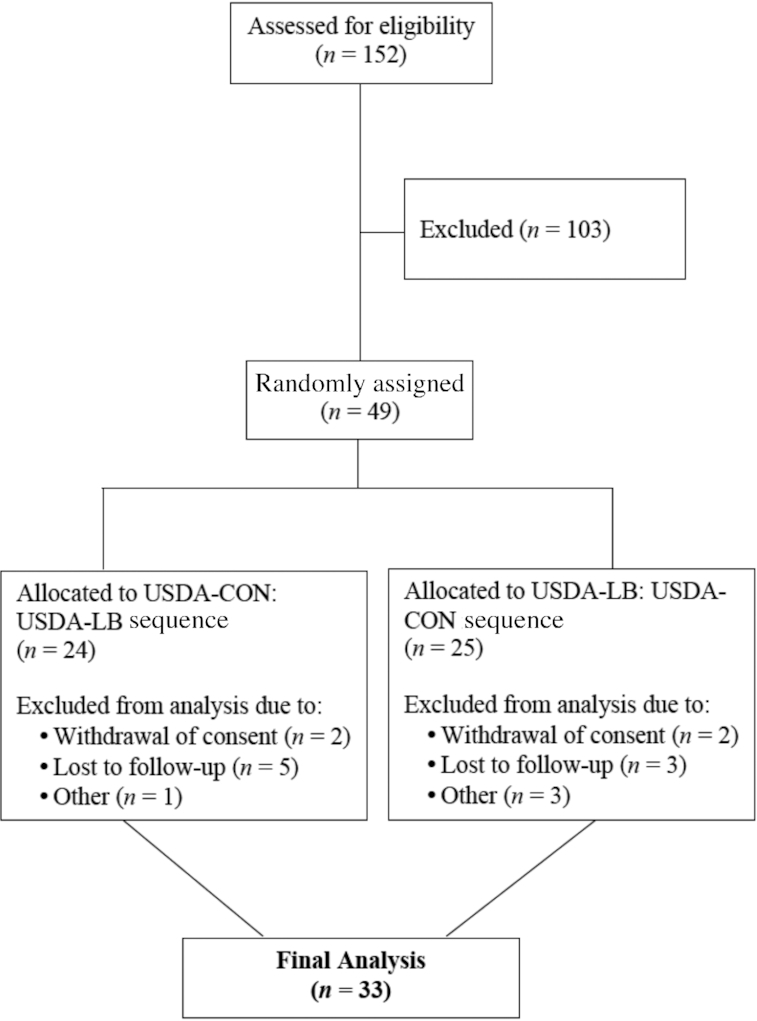
Flow diagram of subjects assessed for eligibility, excluded, randomized, and analyzed for the study. See Results section for a description of the subjects who terminated the study early. USDA-CON, a USDA Healthy US-Style Eating Pattern, as outlined by the 2015 Dietary Guidelines for Americans (5), containing <40 g/d red meat; USDA-LB, a USDA Healthy US-Style Eating Pattern, as outlined by the 2015 Dietary Guidelines for Americans (5), modified to incorporate an additional 150 g/d of fresh/unprocessed lean beef to that of the USDA-CON diet, in place of carbohydrate, primarily refined starches.
Thirty-three subjects (7 males and 26 females) completed the trial and provided evaluable data. Of the 16 subjects who terminated early, 8 were in the USDA-LB:USDA-CON sequence and 8 were in the USDA-CON:USDA-LB sequence; 7 were male and 9 were female. Four of the early terminators completed the first treatment condition and 12 did not. Two of the early terminators returned after the washout, and none provided outcome data during the second diet condition. Baseline demographic and anthropometric characteristics are presented in Table 1, and baseline metabolic syndrome component and prediabetes characteristics are presented in Table 2. Mean energy and nutrient intakes at baseline, and average energy and nutrient contents of the eating patterns for each condition, are provided in Tables 3 and 4, respectively. Notably, diet record data show that subjects underreported energy intake by ∼22%, which is consistent with results that our group and others have reported previously (23, 24). Participants had a median compliance of 100% (interquartile limits: 96.0–100% for the USDA-CON condition and 96.1–100% for the USDA-LB condition) with the assigned eating pattern conditions during both intervention periods based on consumption of the beef and nonbeef entrées, and there was a median of 0% of days (interquartile limits: 0%, 12%) with major dietary violations for each eating pattern. Median (interquartile limits) baseline body weight was 83.0 kg (79.0, 93.4 kg), and decreased by 0.9 kg (−2.4, 0.0 kg) following the USDA-CON and 0.7 kg (−2.1, 0.4 kg) following the USDA-LB (not significantly different between conditions). Median baseline physical activity was 255 (interquartile limits: 243, 292) metabolic-equivalent hours (i.e., 87 metabolic-equivalent hours above resting). During both eating patterns the median physical activity levels decreased significantly (P < 0.05) from baseline [−10.5 metabolic-equivalent hours (interquartile limits: −35.8, −0.5) for USDA-CON and −6.0 (interquartile limits: −22.8, 3.0) for USDA-LB], but the difference between eating patterns was not statistically significant. There were no adverse events reported by the subjects that the study physicians judged to be related to the study diets.
TABLE 1.
Demographic and anthropometric baseline characteristics of subjects in the evaluable sample according to eating pattern sequence and overall1
| Characteristic | USDA-CON:USDA-LB Sequence | USDA-LB:USDA-CON Sequence | Evaluable sample |
|---|---|---|---|
| Sex | |||
| Male | 3 (18.8) | 4 (23.5) | 7 (21.2) |
| Female | 13 (81.3) | 13 (76.5) | 26 (78.8) |
| Race | |||
| White/Caucasian | 12 (75.0) | 12 (70.6) | 24 (72.7) |
| Black/African American | 2 (12.5) | 3 (17.6) | 5 (15.2) |
| Other | 2 (12.5) | 2 (11.8) | 4 (12.1) |
| Ethnicity | |||
| Not Hispanic/Latino | 10 (62.5) | 8 (47.1) | 18 (54.5) |
| Hispanic/Latino | 6 (37.5) | 5 (29.4) | 11 (33.3) |
| Current smoker | 2 (12.5) | 2 (11.8) | 4 (12.1) |
| Consumes alcohol | 10 (62.5) | 6 (35.3) | 16 (48.5) |
| Age, y | 48.4 ± 3.0 | 40.6 ± 3.4 | 44.4 ± 2.4 |
| Height, cm | 163 ± 2.4 | 167 ± 1.8 | 1651 ± 1.5 |
| Weight, kg | 81.8 ± 3.0 | 88.4 ± 2.9 | 85.2 ± 2.2 |
| BMI, kg/m2 | 30.7 ± 1.2 | 31.8 ± 1.3 | 31.3 ± 0.8 |
| BMI ≥25.0 to <30.0 | 8 (50.0) | 7 (41.2) | 15 (45.5) |
| BMI ≥30.0 | 8 (50.0) | 10 (58.8) | 18 (54.5) |
| Waist circumference, cm | 98.1 ± 2.5 | 97.0 ± 3.1 | 97.5 ± 2.0 |
| Men | 100 ± 8.4 | 90.3 ± 7.9 | 94.5 ± 5.7 |
| Women | 97.6 ± 2.5 | 99.0 ± 3.2 | 98.3 ± 2.0 |
Values are means ± SEMs or frequencies (%); n = 16 for USDA-CON:USDA-LB; n = 17 for USDA-LB:USDA-CON; n = 33 for the evaluable sample. USDA-CON, a USDA Healthy US-Style Eating Pattern, as outlined by the 2015 Dietary Guidelines for Americans (5), containing <40 g/d red meat; USDA-LB, a USDA Healthy US-Style Eating Pattern, as outlined by the 2015 Dietary Guidelines for Americans (5), modified to incorporate an additional 150 g/d of fresh/unprocessed lean beef to that of the USDA-CON diet, in place of carbohydrate, primarily refined starches.
TABLE 2.
Metabolic syndrome component characteristics and/or presence of prediabetes at baseline among subjects in the evaluable sample1
| Characteristic | Evaluable sample |
|---|---|
| Triglycerides ≥150 mg/dL | 19 (57.6) |
| HDL cholesterol | |
| <40 mg/dL, Men | 6 (18.2) |
| <50 mg/dL, Women | 14 (42.4) |
| SBP ≥130 and/or DBP ≥85 mm Hg and/or BP medication | 10 (30.3) |
| Men, waist circumference, ≥102 cm | 2 (6.1) |
| Women, waist circumference, ≥88 cm | 21 (63.6) |
| Presence of prediabetes | 31 (93.9) |
| Fasting plasma glucose 100–125 mg/dL | 29 (87.9) |
| HbA1c 5.7–6.4% | 8 (24.2) |
| Number of metabolic syndrome components present | |
| 0 | 2 (6.1) |
| 1 | 2 (6.1) |
| 2 | 8 (24.2) |
| 3 | 8 (24.2) |
| 4 | 7 (21.2) |
| 5 | 6 (18.2) |
| ≥3 | 21 (63.6) |
Values are n (%); n = 33. Metabolic syndrome component criteria differ by sex for HDL cholesterol and waist circumference; thus, the n values presented are derived from an eligible total of n = 7 for men and n = 26 for women. The adjacent percentages present the percentage of the total subject population. BP, blood pressure; DBP, diastolic blood pressure; HbA1c, glycated hemoglobin; SBP, systolic blood pressure.
TABLE 3.
Energy, macronutrient, and select nutrient intakes at baseline from 3-d diet record analysis1
| Parameter | Baseline |
|---|---|
| Energy, kcal/d | 1880 (1620, 2440) |
| Carbohydrate, % of energy | 46.8 (42.0, 52.9) |
| Protein, % of energy | 16.5 (13.0, 19.0) |
| Total fat, % of energy | 36.4 (31.4, 41.7) |
| SFAs | 11.8 (10.1, 13.8) |
| UFAs | 24.0 (20.5, 28.1) |
| Dietary fiber, g/d | 16.8 (11.9, 21.9) |
| Cholesterol, mg/d | 342 (182, 478) |
| Sodium, mg/d | 3000 (2300, 3910) |
| Calcium, mg/d | 648 (490, 844) |
Values are medians (interquartile limits); n = 33. SFA, saturated fatty acid; UFA, unsaturated fatty acid.
TABLE 4.
Average nutrient content from USDA-CON and USDA-LB daily menus1
| Parameter | USDA-CON | USDA-LB |
|---|---|---|
| Energy, kcal/d | 2400 | 2400 |
| Carbohydrate, % of energy | 50.6 | 44.1 |
| Sugar, % of energy | 11.4 | 10.0 |
| Protein, % of energy | 20.0 | 26.1 |
| Total fat, % of energy | 32.1 | 32.4 |
| SFAs | 7.9 | 8.0 |
| UFAs | 24.2 | 24.4 |
| Dietary fiber, g/d | 32.0 | 33.9 |
| Cholesterol, mg/d | 235 | 360 |
| Sodium, mg/d | 3270 | 3330 |
| Calcium, mg/d | 760 | 746 |
Values for USDA-CON and USDA-LB are from an average of selected daily menus at the average kilocalorie level; n = 33. SFA, saturated fatty acid; UFA, unsaturated fatty acid; USDA-CON, a USDA Healthy US-Style Eating Pattern, as outlined by the 2015 Dietary Guidelines for Americans (5), containing <40 g/d red meat; USDA-LB, a USDA Healthy US-Style Eating Pattern, as outlined by the 2015 Dietary Guidelines for Americans (5), modified to incorporate an additional 150 g/d of fresh/unprocessed lean beef to that of the USDA-CON diet, in place of carbohydrate, primarily refined starches.
No significant differences were observed between the USDA-CON or USDA-LB diet conditions for carbohydrate metabolism parameters, including the primary outcome variable, ISI (Table 5). No significant differences between diet conditions were observed for Kg after intravenous glucose and total AUCs for plasma insulin from 0–10 min and 10–50 min. Fasting plasma glucose and insulin and the HOMA2-%S and HOMA2-%B indices were also not significantly different between eating patterns.
TABLE 5.
Plasma outcomes related to carbohydrate metabolism at baseline and following USDA-CON and USDA-LB diet patterns among subjects in the evaluable sample1
| Parameter | Baseline | USDA-CON | USDA-LB | USDA-LB:USDA-CON | P |
|---|---|---|---|---|---|
| ISI, (%/min)/(mU/L) | 391 (187, 814) | 379 (200, 717) | 340 (180, 643) | 0.90 (0.68, 1.19) | 0.459 |
| Kg, % | 1.68 (1.16, 2.43) | 1.51 (0.88, 2.58) | 1.35 (0.79, 2.31) | 0.89 (0.69, 1.16) | 0.408 |
| Insulin AUC0–10 min | 605 (59.7, 1411) | 511 (321, 814) | 514 (323, 818) | 1.00 (0.81, 1.25) | 0.966 |
| Insulin AUC10–50 min | 1720 (93.3, 3310) | 1580 (940, 2640) | 1570 (937, 2640) | 1.00 (0.82, 1.21) | 0.981 |
| Fasting glucose, mg/dL | 93.1 (82.1, 106) | 90.2 (71.3, 114) | 97.1 (76.7, 123) | 1.08 (0.96, 1.21) | 0.206 |
| Fasting insulin, mU/L | 12.4 (6.53, 23.4) | 11.5 (5.99, 22.2) | 12.3 (6.39, 23.7) | 1.07 (0.84, 1.35) | 0.602 |
| HOMA2-%S | 62.7 (33.4, 118) | 67.6 (34.6, 133) | 62.7 (32.0, 123) | 0.93 (0.72, 1.19) | 0.557 |
| HOMA2-%B | 123 (82.5, 184) | 126 (83.2, 192) | 116 (76.1, 176) | 0.91 (0.80, 1.05) | 0.209 |
Values for baseline are geometric means (−1 SD, +1 SD); values for USDA-CON and USDA-LB are least-squares geometric means (−1 SD, +1 SD); values for USDA-LB:USDA-CON are least-squares geometric mean ratios (95% CIs); n = 33. P values are for USDA-LB vs. USDA-CON. Units for insulin total AUC0–10 min and insulin AUC10–50 min are (mU/L) × min (calculated using the linear trapezoidal method). HOMA2-%B, homeostasis model assessment 2-β-cell function; HOMA2-%S, homeostasis model assessment 2-insulin sensitivity; ISI, insulin sensitivity index; Kg, fractional disappearance of glucose constant; USDA-CON, a USDA Healthy US-Style Eating Pattern, as outlined by the 2015 Dietary Guidelines for Americans (5), containing <40 g/d red meat; USDA-LB, a USDA Healthy US-Style Eating Pattern, as outlined by the 2015 Dietary Guidelines for Americans (5), modified to incorporate an additional 150 g/d of fresh/unprocessed lean beef to that of the USDA-CON diet, in place of carbohydrate, primarily refined starches.
Results for serum lipoprotein lipids, plasma apoA-I and apoB, serum hs-CRP, and blood pressures are shown Table 6. No significant differences were observed between diet conditions for any of the lipoprotein lipids, apoA-I and apoB, hs-CRP, or blood pressures.
TABLE 6.
Fasting serum lipoprotein lipids, plasma apolipoproteins, serum hs-CRP, and blood pressures at baseline and following USDA-CON and USDA-LB diet patterns among subjects in the evaluable sample1
| Parameter | Baseline | USDA-CON | USDA-LB | USDA-LB:USDA-CON | P |
|---|---|---|---|---|---|
| LDL cholesterol, mg/dL | 112 (85.8, 145) | 105 (85.4, 130) | 109 (88.3, 134) | 1.03 (0.97, 1.10) | 0.318 |
| HDL cholesterol, mg/dL | 47.6 (38.7, 58.7) | 44.6 (39.1, 50.9) | 45.5 (39.9, 51.9) | 1.02 (0.97, 1.07) | 0.447 |
| Non–HDL cholesterol, mg/dL | 137 (104, 181) | 130 (106, 159) | 133 (109, 163) | 1.03 (0.97, 1.08) | 0.348 |
| TC, mg/dL | 188 (157, 226) | 178 (153, 206) | 181 (156, 211) | 1.02 (0.97, 1.07) | 0.378 |
| Triglycerides, mg/dL | 115 (72.2, 183) | 111 (75.4, 163) | 113 (76.7, 166) | 1.02 (0.93, 1.11) | 0.697 |
| TC:HDL cholesterol | 3.96 (2.93, 5.34) | 3.98 (3.26, 4.87) | 3.99 (3.26, 4.87) | 1.00 (0.97, 1.04) | 0.947 |
| ApoB, mg/dL | 88.4 (66.5, 118) | 85.2 (68.6, 106) | 87.0 (70.0, 108) | 1.02 (0.95, 1.10) | 0.596 |
| ApoA-I, mg/dL | 131 (113, 151) | 124 (104, 147) | 127 (107, 151) | 1.03 (0.96, 1.10) | 0.451 |
| ApoB:apoA-I | 0.68 (0.47, 0.97) | 0.69 (0.54, 0.89) | 0.68 (0.53, 0.87) | 0.98 (0.94, 1.04) | 0.561 |
| hs-CRP, mg/dL | 2.55 (0.79, 8.27) | 2.08 (1.06, 4.08) | 1.91 (0.98, 3.74) | 0.92 (0.75, 1.12) | 0.399 |
| Systolic BP, mm Hg | 121 (108, 136) | 120 (111, 129) | 120 (112, 130) | 1.00 (0.98, 1.03) | 0.808 |
| Diastolic BP, mm Hg | 80.4 (72.0, 89.7) | 79.4 (74.5, 84.5) | 79.4 (74.6, 84.6) | 1.00 (0.98, 1.03) | 0.914 |
Values for baseline are geometric means (−1 SD, +1 SD); values for USDA-CON and USDA-LB are least-squares geometric means (−1 SD, +1 SD); values for USDA-LB:USDA-CON are least-squares geometric mean ratios (95% CI); n = 31–33. P values are for USDA-LB vs. USDA-CON. BP, blood pressure; hs-CRP, high sensitivity C-reactive protein; TC, total cholesterol; USDA-CON, a USDA Healthy US-Style Eating Pattern, as outlined by the 2015 Dietary Guidelines for Americans (5), containing <40 g/d red meat; USDA-LB, a USDA Healthy US-Style Eating Pattern, as outlined by the 2015 Dietary Guidelines for Americans (5), modified to incorporate an additional 150 g/d of fresh/unprocessed lean beef to that of the USDA-CON diet, in place of carbohydrate, primarily refined starches.
VAP assessment of serum lipoprotein subfraction cholesterol and LDL-particle concentration indicated that the LDL subclass distribution shifted toward a greater proportion of LDL cholesterol being carried by larger, more buoyant particles in the USDA-LB diet condition compared with the USDA-CON diet condition (Table 7). USDA-LB resulted in a significant (P < 0.05) increase in peak LDL time (a measure of LDL particle size) (Figure 2) compared with the USDA-CON condition, consistent with the differences in cholesterol carried by larger LDL1+2 subfractions and smaller, higher-density LDL3+4 (Table 7).
TABLE 7.
Fasting serum lipoprotein subfractions and particles concentrations at baseline and following USDA-CON and USDA-LB diet patterns among subjects in the evaluable sample1
| Parameter | Baseline | USDA-CON | USDA-LB | USDA-LB:USDA-CON | P |
|---|---|---|---|---|---|
| LDL particle, nmol/L | 1420 (957, 2110) | 1440 (1100, 1870) | 1480 (1140, 1930) | 1.03 (0.95, 1.12) | 0.443 |
| LDL-real, mg/dL | 82.2 (58.3, 116) | 82.1 (66.2, 102) | 81.2 (65.5, 101) | 0.99 (0.92, 1.07) | 0.782 |
| LDL1+2 cholesterol, mg/dL | 29.6 (18.7, 46.6) | 28.1 (19.7, 40.1) | 33.6 (23.5, 48.0) | 1.20 (1.04, 1.37) | 0.016 |
| LDL3+4 cholesterol, mg/dL | 49.7 (31.8, 77.6) | 50.8 (38.1, 67.8) | 45.1 (33.8, 60.1) | 0.89 (0.79, 0.99) | 0.044 |
| HDL cholesterol, mg/dL | 45.6 (35.9, 58.0) | 45.8 (41.2, 51.0) | 46.4 (41.7, 51.7) | 1.01 (0.96, 1.07) | 0.641 |
| HDL2 cholesterol, mg/dL | 10.7 (6.96, 16.4) | 10.6 (8.87, 12.7) | 11.2 (9.30, 13.4) | 1.05 (0.96, 1.14) | 0.302 |
| HDL3 cholesterol, mg/dL | 34.8 (28.6, 42.4) | 34.9 (31.4, 38.9) | 35.1 (31.5, 39.1) | 1.00 (0.95, 1.06) | 0.877 |
Values for baseline are geometric means (−1 SD, +1 SD); values for USDA-CON and USDA-LB are least-squares geometric means (−1 SD, +1 SD); values for USDA-LB:USDA-CON are least-squares geometric mean ratios (95% CI); n = 32. P values are for USDA-LB vs. USDA-CON. USDA-CON, a USDA Healthy US-Style Eating Pattern, as outlined by the 2015 Dietary Guidelines for Americans (5), containing <40 g/d red meat; USDA-LB, a USDA Healthy US-Style Eating Pattern, as outlined by the 2015 Dietary Guidelines for Americans (5), modified to incorporate an additional 150 g/d of fresh/unprocessed lean beef to that of the USDA-CON diet, in place of carbohydrate, primarily refined starches.
FIGURE 2.
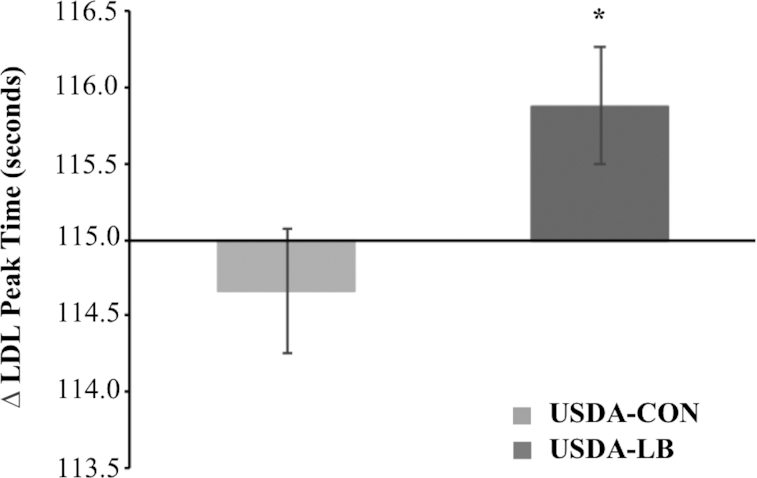
VAP assessment of change from baseline of LDL peak time after 28-d intake of either the USDA-CON or the USDA-LB among subjects in the evaluable sample. n = 32. The line at 115.0 s represents the baseline value. Values are medians (interquartile limits). *Difference from USDA-CON from analysis of geometric mean ratios, P = 0.008. Least-squares geometric means (−1 SD, +1 SD) are 115 (113, 117) for USDA-CON and 116 (114, 118) for USDA-LB. USDA-CON, a USDA Healthy US-Style Eating Pattern, as outlined by the 2015 Dietary Guidelines for Americans (5), containing <40 g/d red meat; USDA-LB, a USDA Healthy US-Style Eating Pattern, as outlined by the 2015 Dietary Guidelines for Americans (5), modified to incorporate an additional 150 g/d of fresh/unprocessed lean beef to that of the USDA-CON diet, in place of carbohydrate, primarily refined starches; VAP, vertical auto profile.
Post hoc sensitivity analyses showed no evidence of differential effects according to the use of statin therapy. A sensitivity analysis was also completed that included data for the 4 subjects who completed the first treatment period with imputation of results for the second treatment period for those subjects. The results were not materially altered, except that the difference between conditions for LDL1+2 cholesterol lost statistical significance, although differences for LDL3+4 cholesterol and LDL peak time retained statistical significance (data not shown). In addition, a separate sensitivity analysis was completed for the first treatment condition only (i.e., treating the study as if it were a parallel-arm trial). The same general patterns were apparent during the first treatment period as observed in the pooled analysis.
Discussion
The results of this trial indicate that following the USDA Healthy US-Style Eating Pattern, as outlined in the 2015 Dietary Guidelines for Americans (5), modified to incorporate an additional 150 g/d of lean, unprocessed beef as an isocaloric replacement for carbohydrate, primarily refined starches (USDA-LB), did not have significant effects on whole-body insulin sensitivity or other indicators of carbohydrate metabolism compared with USDA-CON (which provided <40 g/d red meat).
Consistent with the results from the present study, Gadgil et al. (16) reported that partial replacement of 10% of energy from carbohydrate in a low-SFA diet with protein (mixed source) did not significantly alter fasting indicators of insulin sensitivity, although replacement with UFAs did produce a net increase of ∼15%. Our group previously found that partial replacement of refined starches and added sugars with a combination of UFAs and egg protein resulted in a 24% net increase in insulin sensitivity (23). In the present study, the main macronutrient difference between diet conditions was the substitution of beef protein for starches, while sugar intakes were similar between the diet conditions. Thus, the present results are generally consistent with those from other studies showing that replacing dietary carbohydrate (mainly as starches) with protein has a neutral effect on insulin sensitivity (16, 25). Additional research is needed to further define the effects of different protein types on insulin sensitivity (e.g., animal vs. plant, and different types of animal proteins such as those from dairy, red meat, poultry, eggs, and seafood), as the present investigation was not designed to address that question.
Geometric mean values for serum LDL cholesterol, TC, non–HDL cholesterol, and TGs did not differ significantly between diet conditions. Dietary cholesterol intake was 125 mg/d higher in the USDA-LB diet than in the USDA-CON diet. The observed, non–statistically significant (P = 0.318) difference of 4 mg/dL in geometric mean LDL cholesterol between USDA-CON and USDA-LB aligns well with the predicted effect of a difference of this magnitude in dietary cholesterol based on a meta-regression analysis of the relation between changes in dietary cholesterol and changes in LDL cholesterol conducted by our group (26).
The BOLD study was also a crossover, controlled-feeding trial that compared the effects of lean beef intake, incorporated into a healthy dietary pattern, on cardiometabolic disease risk markers (12). That study compared 4 dietary conditions: an American diet, a DASH diet, a BOLD Diet, and a BOLD+ diet. Macronutrient intakes were similar in the BOLD+ diet (27%, 45%, and 28% of energy from protein, carbohydrate, and fat, respectively) and the USDA-LB diet (26%, 44%, and 32% of energy from protein, carbohydrate, and fat, respectively) used in the present trial. The DASH diet pattern (18%, 55%, and 20% of energy from protein, carbohydrate, and fat, respectively) was similar to the USDA-CON (20%, 51%, and 32% of energy from protein, carbohydrate, and fat, respectively). SFA intake was low in DASH and BOLD+ (6% of energy), as well as in the USDA-CON and USDA-LB diets (8% of energy). Subjects were selected for the BOLD trial on the basis of having elevated concentrations of LDL cholesterol, whereas the present trial selected subjects at increased risk for developing T2D.
No significant differences were observed in TC, LDL cholesterol, or non–HDL cholesterol between the DASH and BOLD+ diets, which was also the case in the present study for the USDA-CON and USDA-LB diets. Similarly, fasting glucose and insulin concentrations did not differ significantly between the DASH and BOLD+ diet conditions, or the USDA-CON and USDA-LB diet conditions.
O'Connor and colleagues (27) completed a randomized, crossover, controlled-feeding trial that assessed the effects of 4-wk intake of a Mediterranean dietary pattern containing either ∼500 g/wk (typical US intake) of lean red meat (including pork) or ∼200 g/wk of lean red meat. Both eating patterns in that study contained a mix of other proteins (poultry, seafood, whole eggs, nuts, seeds, and soy) to provide 18–19% of total dietary protein energy in both conditions. Results indicated that both Mediterranean-style eating patterns produced reductions in TC and blood pressure parameters, but only the Mediterranean-style eating pattern containing ∼500 g/wk lean red meat resulted in significant reductions in LDL cholesterol from baseline and compared with the Mediterranean-style eating pattern containing ∼200 g/wk lean red meat (27). Thus, the results suggested a favorable effect on LDL cholesterol of a higher intake of red meat protein, compared with a mixture of proteins from other sources.
In the present study, the LDL subclass distribution shifted toward a greater proportion of LDL cholesterol being carried by larger, more buoyant particles in USDA-LB compared with USDA-CON. Specifically, the USDA-LB had 5.5 mg/dL greater geometric mean LDL1+2-cholesterol subfraction concentrations and 5.8 mg/dL lower geometric mean LDL3+4-cholesterol subfraction concentrations versus the USDA-CON. A shift toward larger particles is potentially favorable, since smaller, less buoyant LDL particles may be more atherogenic (28). It is unknown whether this shift was due to the decrease in carbohydrate intake alone or due, in part, to the increase in lean beef intake. The LDL subfraction results from a recent trial that assigned subjects to high-SFA and low-SFA treatment arms within red meat, white meat, and nonmeat protein diets align with those from the present study (29). Bergeron and colleagues (29) provided diets in which the predominant protein sources tested were unprocessed lean red meat, unprocessed lean white meat (poultry), or nonmeat sources (legumes, nuts, grains, and isoflavone-free soy products). The 3 diet conditions (red meat, white meat, and nonmeat) were matched for macronutrient intakes. The 2 meat diets resulted in higher LDL cholesterol than the nonmeat diet, independent of SFA content, and the LDL-cholesterol concentration did not differ significantly between the red and white meat diets (29). This lack of difference in LDL-cholesterol concentration between the diets containing lean, unprocessed red, and white meats is in agreement with results from our prior research (30, 31). Under low-SFA conditions (7–8% of energy), the statistically significant difference in LDL cholesterol between red meat and nonmeat conditions was 5 mg/dL, which is similar in magnitude to the nonsignificant (P = 0.318) difference of ∼4 mg/dL in the present investigation. The difference between these conditions for mean dietary cholesterol intake was 78 mg/d. In a meta-regression analysis of the effects of changes in dietary cholesterol intake on LDL cholesterol completed by our research group, each 100-mg/d increase in dietary cholesterol was associated with an increase of ∼4.5 mg/dL LDL cholesterol based on the best-fitting nonlinear models (26). Thus, the difference in LDL cholesterol in the present trial, and in that of Bergeron and colleagues, may be explained, at least in part, by higher dietary cholesterol intakes (26). Small + medium LDL particle concentrations did not differ between the meat and nonmeat conditions in the Bergeron trial, but the large LDL-particle concentration was higher during both meat conditions compared with the nonmeat condition (29). This is consistent with the finding in the present study that higher beef intake in the USDA-LB condition produced a shift in the proportion of LDL cholesterol carried by larger, more buoyant LDL particles, although the results are difficult to compare directly because of differences in methodology (i.e., measurement of cholesterol carried by LDL particles in different density bands vs. measurement of LDL particles of different sizes).
In a prior study, our group showed that partially replacing dietary carbohydrate with UFAs and egg protein also resulted in a shift toward larger LDL-particle size, which aligns with the results of the present study (23). Thus, partial replacement of dietary carbohydrate with protein may contribute to a shift in LDL-subfraction distribution, although results reported by Bergeron et al. (29), described above, suggest that meat and plant protein sources may differ in their effects on LDL-subclass distribution. Additional research will be needed to investigate this issue further and to assess whether such a shift affects cardiovascular disease risk.
Several limitations of the present study should be considered. Because of the nature of the study treatments, neither subjects nor study staff were blinded, which leads to the potential for bias associated with lack of blinding. The trial did not have sufficient statistical power to detect small differences. Differences observed between diet conditions for some variables measured were in the range of 8–11% (least-square geometric mean ratios of 0.89 to 1.08). Although none of the P values for these differences approached statistical significance (all P > 0.20), it is possible that differences of this magnitude could have clinical importance if maintained over extended periods. Also, the finding that the distribution of cholesterol across LDL subclasses differed between diet conditions should be interpreted with caution because several secondary outcomes were tested; thus, there is an increased risk for type I statistical errors. The short duration of the intervention, 28 days, may also be considered a limitation. However, previous research conducted by the authors and others has shown that changes in the cardiometabolic risk factor profile, including insulin sensitivity and changes in the lipoprotein lipid profile, are evident within ≤3 wk of making dietary changes (15, 16, 23, 32). Additional research will be needed to assess the potential durability of these findings over longer periods. Another limitation is that participants were predominantly female and, although there was no indication of differences in responses by sex, the subset of male subjects is too small to draw conclusions about potential differences in responses between men and women. Finally, the dropout rate was higher for this trial than other similar studies completed by the investigators, largely attributable to a hurricane that produced extended power outages. Thus, the possibility cannot be ruled out that this produced bias in the findings.
Sodium intake amounts were somewhat high at ∼3300 mg/d, because some of the study foods contained higher sodium to ensure adequate viability under frozen conditions. While the latest DRIs no longer provide a Tolerable Upper Intake Level, a chronic disease risk-reduction intake recommendation has been introduced and advises to reduce sodium intakes if >2300 mg/d, largely aimed at lowering blood pressure (33). Systolic and diastolic blood pressures did not differ across diet conditions, but it is uncertain whether this would also have been the case with lower sodium intakes.
In conclusion, with the exception of a shift toward a greater fraction of LDL cholesterol being carried by larger, more buoyant subspecies of LDL particles, substituting a portion of carbohydrate, primarily refined starches, with lean unprocessed beef, in a USDA Healthy US-Style Eating Pattern did not significantly impact insulin sensitivity, other indices of carbohydrate metabolism, or markers of cardiometabolic health in men and women at risk for T2D.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—KCM, MRD, and OMP: designed the research; MK and MB: provided trial medical oversight; MLW and CEM: managed and coordinated the clinical trial; KCM and MLW: performed statistical analysis; KCM, MRD, and OMP: wrote the manuscript; KCM and MRD: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
This research was funded by The Beef Checkoff. The Beef Checkoff provided comments on early aspects of the study design. Interim analyses and the final data were shared with the sponsor prior to publication, but the substance and conclusions are those of the authors alone.
Author disclosures: KCM has received research funding and/or consulting fees from the Almond Board of California, Egg Nutrition Center, General Mills, Inc., Hass Avocado Board, Kellogg Company, National Cattlemen's Beef Association, and the National Dairy Council. KCM and OMP have also received research funding from the National Pork Board. This funding was not used to support this analysis. Likewise, the other authors are employees of Midwest Biomedical Research Center for Metabolic and Cardiovascular Health, which has received funding from the aforementioned organizations.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: BOLD, Beef in an Optimal Lean Diet; DASH, Dietary Approaches to Stop Hypertension; HOMA2-%B, homeostasis model assessment 2-B-cell function; HOMA2-%S, homeostasis model assessment 2-insulin sensitivity; hs-CRP, high sensitivity C-reactive protein; ISI, insulin sensitivity index; IVGTT, intravenous-glucose-tolerance test; Kg, fractional disappearance of glucose constant; TC, total cholesterol; TG, triglyceride; T2D, type 2 diabetes; UFA, unsaturated fatty acid; USDA-CON, a USDA Healthy US-Style Eating Pattern, as outlined by the 2015 Dietary Guidelines for Americans, containing <40 g/d red meat; USDA-LB, a USDA Healthy US-Style Eating Pattern, as outlined by the 2015 Dietary Guidelines for Americans, modified to incorporate an additional 150 g/d of fresh/unprocessed lean beef to that of the USDA-CON diet, in place of carbohydrate, primarily refined starches; VAP, Vertical Auto Profile.
Contributor Information
Kevin C Maki, Midwest Biomedical Research Center for Metabolic and Cardiovascular Health, Addison, IL, USA; Department of Applied Health Science, School of Public Health, Indiana University, Bloomington, IN, USA.
Meredith L Wilcox, Midwest Biomedical Research Center for Metabolic and Cardiovascular Health, Addison, IL, USA.
Mary R Dicklin, Midwest Biomedical Research Center for Metabolic and Cardiovascular Health, Addison, IL, USA.
Mary Buggia, Midwest Biomedical Research Center for Metabolic and Cardiovascular Health, Addison, IL, USA.
Orsolya M Palacios, Midwest Biomedical Research Center for Metabolic and Cardiovascular Health, Addison, IL, USA.
Cathleen E Maki, Midwest Biomedical Research Center for Metabolic and Cardiovascular Health, Addison, IL, USA.
Melvyn Kramer, Midwest Biomedical Research Center for Metabolic and Cardiovascular Health, Addison, IL, USA.
References
- 1. Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. 2012;32(8):1754–9. [DOI] [PubMed] [Google Scholar]
- 2. Rader DJ. Effect of insulin resistance, dyslipidemia, and intra-abdominal adiposity on the deveopment of cardiovascular disease and diabetes mellitus. Am J Med. 2007;120(3 Suppl 1):S12–8. [DOI] [PubMed] [Google Scholar]
- 3. American Diabetes Association. 5. Prevention or delay of type 2 diabetes: standards of medical care in diabetes–2018. Diabetes Care. 2018;41(Suppl 1):S51–4. [DOI] [PubMed] [Google Scholar]
- 4. Dietary Guidelines Advisory Committee. Scientific report of the 2015 Dietary Guidelines Advisory Committee: advisory report to the Secretary of Health and Human Services and the Secretary of Agriculture. U.S. Department of Agriculture, Agricultural Research Service, Washington (DC): Office of Disease Prevention and He; alth Promotion; 2015; [Internet]. [Accessed 2019 Dec 12]. Available from: https://health.gov/sites/default/files/2019-09/Scientific-Report-of-the-2015-Dietary-Guidelines-Advisory-Committee.pdf. [Google Scholar]
- 5. US Department of Health and Human Services; US Department of Agriculture. 2015–2020 Dietary guidelines for Americans. 8th ed. December 2015; [Internet]. [Accessed 2019 Dec 12]. Available from: https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf. [Google Scholar]
- 6. Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Willett WC, Hu FB. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr. 2011;94(4):1088–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cross AJ, Leitzmann MF, Gail MH, Hollenbeck AR, Schatzkin A, Sinha R. A prospective study of red and processed meat intake in relation to cancer risk. PLoS Med. 2007;4(12):e325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bradbury KE, Tong TYN, Key TJ. Dietary intake of high-protein foods and other major foods in meat-eaters, poultry-eaters, fish-eaters, vegetarians, and vegans in UK Bio. bank. Nutrients. 2017;9(12):1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Papier K, Tong TY, Appleby PN, Bradbury KE, Fensom GK, Knuppel A, Perez-Cornago A, Schmidt JA, Travic RC, Key TJ. Comparison of major protein-source foods and other food groups in meat-eaters and non-meat-eaters in the EPIC-Oxford Cohort. Nutrients. 2019;11(4):824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vernooij RWM, Zeraatkar D, Han MA, El Dib R, Zworth M, Milio K, Sit D, Lee Y, Gomaa H, Valli C et al.. Patterns of red meat and processed meat consumption and risk for cardiometabolic and cancer outcomes: a syste. matic review and meta-analysis of cohort studies. Ann Intern Med. 2019[Epub ahead of print2019 Oct 1. doi:10.7326/M19-1583]. [DOI] [PubMed] [Google Scholar]
- 11. Turner KM, Keogh JB, Clifton PM. Red meat, dairy, and insulin sensitivity: a randomized crossover intervention study. Am J Clin Nutr. 2015;101(6):1173–9. [DOI] [PubMed] [Google Scholar]
- 12. Roussell MA, Hill AM, Gaugler TL, West SG, Heuvel JP, Alaupovic P, Gillies PJ, Kris-Etherton PM. Beef in an Optimal Lean Diet study: effects on lipids, lipoproteins, and apolipoproteins. Am J Clin Nutr. 2012;95(1):9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guasch-Ferr M, Satija A, Blondin SA, Janiszewski M, Emlen E, O'Connor LE, Campbell WW, Hu FB, Willett WC, Stampfer MJ. Meta-analysis of randomized controlled trials of red meat consumption in comparison with various comparison diets on cardiovascular risk factors. Circulation. 2019;139(15):1828–45. [DOI] [PubMed] [Google Scholar]
- 14. Kirkpatrick CF, Bolick JP, Kris-Etherton PM, Sikand G, Aspry KE, Soffer DE, Willard KE, Maki KC. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: a scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. J Clin Lipidol. 2019;13(5):689–711. [DOI] [PubMed] [Google Scholar]
- 15. Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER3rd, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM et al.. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294(19):2455–64. [DOI] [PubMed] [Google Scholar]
- 16. Gadgil MD, Appel LJ, Yeung E, Anderson CAM, Sacks FM, Miller ER III. The effects of carbohydrate, unsaturated fat, and protein intake on measures of insulin sensitivity: results from the OmniHeart trial. Diabetes Care. 2013;36(5):1132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Medical Association. Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2000;284(23):3043–5. [PubMed] [Google Scholar]
- 18. American Diabetes Association. 2. Classification and diagnosis of diabetes. Standard of medical care in diabetes–2016. Diabetes Care. 2016;39(Suppl 1):S13–22. [DOI] [PubMed] [Google Scholar]
- 19. Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WPT, Loria CM, Smith SC Jr et al.. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(6):1640–5. [DOI] [PubMed] [Google Scholar]
- 20. Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51(2):241–7. [DOI] [PubMed] [Google Scholar]
- 21. Blair SN, Haskell WL, Ho P, Paffenbarger RS Jr, Vranizan KM, Farquhar JW, Wood PD. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122(5):794–804. [DOI] [PubMed] [Google Scholar]
- 22. Kulkarni KR, Garber DW, Marcovina SM, Segrest JP. Quantification of cholesterol in all lipoprotein classes by the VAP-II method. J Lipid Res. 1994;35(1):159–68. [PubMed] [Google Scholar]
- 23. Maki KC, Palacios OM, Lindner E, Nieman KM, Bell M, Sorce J. Replacement of refined starches and added sugars with egg protein and unsaturated fats increases insulin sensitivity and lowers triglycerides in overweight or obese adults with elevated triglycerides. J Nutr. 2017;147(7):1267–74. [DOI] [PubMed] [Google Scholar]
- 24. Trabulsi J, Schoeller DA. Evaluation of dietary assessment instruments against doubly labeled water, a biomarker of habitual energy intake. Am J Physiol Endocrinol Metab. 2001;281(5):E891–9. [DOI] [PubMed] [Google Scholar]
- 25. Chiu S, Williams PT, Dawson T, Bergman RN, Stefanovski D, Watkins SM, Krauss RM. Diets high in protein or saturated fat do not affect insulin sensitivity or plasma concentrations of lipids and lipoproteins in overweight and obese adults. J Nutr. 2014;144(11):1753–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vincent MJ, Allen B, Palacios OM, Haber LT, Maki KC. Meta-regression analysis of the effects of dietary cholesterol intake on LDL and HDL cholesterol. Am J Clin Nutr. 2019;109(1):7–16. [DOI] [PubMed] [Google Scholar]
- 27. O'Connor LE, Paddon-Jones D, Wright AJ, Campbell WW. A Mediterranean-style eating pattern with lean, unprocessed red meat has cardiometabolic benefits for adults who are overweight or obese in a randomized, crossover, controlled feeding trial. Am J Clin Nutr. 2018;108(1):33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krauss RM, Siri PW. Metabolic abnormalities: triglyceride and low-density lipoprotein. Endocrinol Metab Clin North Am. 2004;33(2):405–15. [DOI] [PubMed] [Google Scholar]
- 29. Bergeron N, Chiu S, Williams PT, King SM, Krauss RM. Effects of red meat, white meat, and nonmeat protein sources on atherogenic lipoprotein measures in the context of low compared with high saturated fat intake: a randomized controlled trial. Am J Clin Nutr. 2019;110(1):24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Davidson MH, Hunninghake D, Maki KC, Kwiterovich PO Jr, Kafonek S. Comparison of the effects of lean red meat vs. lean white meat on serum lipid levels among free-living persons with hypercholesterolemia: a long-term, randomized clinical trial. Arch Intern Med. 1999;159(12):1331–8. [DOI] [PubMed] [Google Scholar]
- 31. Hunninghake DB, Maki KC, Kwiterovich PO Jr, Davidson MH, Dicklin MR, Kafonek SD. Incorporation of lean red meat into a National Cholesterol Education Program Step I diet: a long-term randomized clinical trial in free-living persons with hypercholesterolemia. J Am Coll Nutr. 2000;19(3):351–60. [DOI] [PubMed] [Google Scholar]
- 32. Anderson JW, Story L, Sieling B, Chen WJ, Petro MS, Story J. Hypocholesterolemic effects of oat-bran or bean intake for hypercholesterolemic men. Am J Clin Nutr. 1984;40(6):1146–55. [DOI] [PubMed] [Google Scholar]
- 33. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board; Committee to Review the Dietary Reference Intakes for Sodium and Potassium;. Oria M, Harrison M, Stallings VA, Dietary Reference Intakes for sodium and potassium. Washington (DC): National Academies Press; 2019. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


