Abstract
Background
Neural trajectories of gait are not well established. We determined two distinct, clinically relevant neural trajectories, operationalized via functional near-infrared spectroscopy (fNIRS) HbO2 measures in the prefrontal cortex (PFC), under Single-Task-Walk (STW), and Dual-Task-Walk (DTW) conditions. Course trajectory assessed neural activity associated with attention during the course of a walking task; the second trajectory assessed neural activity associated with learning over repeated walking trials. Improved neural efficiency was defined as reduced PFC HbO2 after practice.
Methods
Walking was assessed under STW and DTW conditions. fNIRS was utilized to quantify HbO2 in the PFC while walking. Burst measurement included three repeated trials for each experimental condition. The course of each walking task consisted of six consecutive segments.
Results
Eighty-three nondemented participants (mean age = 78.05 ± 6.37 years; %female = 49.5) were included. Stride velocity (estimate = −0.5259 cm/s, p = <.0001) and the rate of correct letter generation (log estimate of rate ratio = −0.0377, p < .0001) declined during the course of DTW. In contrast, stride velocity (estimate = 1.4577 cm/s, p < .0001) and the rate of correct letter generation (log estimate of rate ratio = 0.0578, p < .0001) improved over repeated DTW trials. Course and trial effects were not significant in STW. HbO2 increased during the course of DTW (estimate = 0.0454 μM, p < .0001) but declined over repeated trials (estimate = −0.1786 μM, p < .0001). HbO2 declined during the course of STW (estimate = −.0542 μM, p < .0001) but did not change significantly over repeated trials.
Conclusion
We provided evidence for distinct attention (course) and learning (repeated trials) trajectories and their corresponding PFC activity. Findings suggest that learning and improved PFC efficiency were demonstrated in one experimental session involving repeated DTW trials.
Keywords: fNIRS, Burst measurement, Mobility, Brain
Research concerning brain activation during walking has been limited because locomotion cannot be assessed in MRI and PET scanners (1). The pivotal role of the prefrontal cortex (PFC) in executive control is well documented (2), but its influence on human locomotion has only been explored recently, though supervisory control of the PFC over motor acts has been reported in classic studies with rhesus monkeys (3).
Accumulating evidence provides strong support for using functional near-infrared spectroscopy (fNIRS) to probe brain activations under different experimental conditions (4). Notably, fNIRS has been recognized as a viable method to explore neural activity of locomotion in aging and disease populations (5). Human studies using fNIRS revealed that the PFC plays a critical role in walking in healthy individuals (6–8), older adults (9,10), as well as patients with stroke (11), Multiple Sclerosis (12), and Parkinson’s disease (13,14).
Dual-tasks that involve walking impose additional demands on attention resources relative to the single tasks (15). Studies using fNIRS revealed increased HbO2 in the PFC in Dual-Task-Walk (DTW) compared to Single-Task-Walk (STW) conditions in young and old participants (16,17). These findings have been extended to a large cohort supporting the key role of the PFC in cortical control of locomotion, notably under attention-demanding conditions (18–20). Furthermore, HbO2 in the PFC declined during the course of STW but increased during the course of DTW; the increase in HbO2 in the latter walking condition was attributed to the acute mental effort it requires (21).
Burst measurement, which involves repeated administration of the same task/measure, has been applied to the assessment of intraindividual changes in cognitive function (22) and stress response (23) in older adults. Burst measurement is also optimal for examining distinct trajectories of attention, assessed during the course of a task, and learning, which is defined as improved performance due to practice, and can be evaluated over repeated trials of the same task. Indeed, opposite trajectories demonstrating decline during the course and improvement over repeated trials of a cognitive task were observed in patients with multiple sclerosis and controls (24). Burst measurement has not been utilized to determine attention and learning trajectories and their corresponding neural activity under STW and DTW conditions.
Current Study
Using burst measurement, we determined two distinct, clinically relevant neural trajectories, operationalized via fNIRS HbO2 measures in the PFC, under STW and DTW conditions. Course trajectory assessed neural activity associated with attention during the course of a walking task; the second trajectory assessed neural activity associated with learning over repeated walking trials. We hypothesized that course performance will remain unchanged during STW but decline in DTW due to the acute attention demands inherent in the latter condition. We further hypothesized that learning, defined as improvement in performance over repeated trials, would manifest in DTW but not in STW. Establishing behavioral evidence for distinct trajectories during the course and over repeated trials of STW and DTW was necessary to characterize their corresponding neural activity. Consistent with our previous findings (21), we expected that HbO2 would increase during the course of DTW. In contrast, we expected that HbO2 would decline during the course of STW because this task is relatively simple. Moreover, we predicted that HbO2 in DTW but not in STW would decrease over repeated trials due to learning. Reduced brain activation after practice served as a marker for improved brain efficiency (25). Here, decline in PFC HbO2 after practice was used to operationalize improved PFC efficiency.
Methods
Participants
Participants were community-residing older adults (age ≥ 65 years) enrolled in “Central Control of Mobility in Aging” (CCMA) (15). Structured telephone interviews were administered to obtain verbal assent and determine initial eligibility including screens for dementia and assessments of medical history and current physical function. Individuals who passed the telephone interview were invited to two annual in-person visits during which trained research assistants administered comprehensive neuropsychological, psychological, and mobility assessments. Dementia diagnoses were assigned at consensus diagnostic case conferences (26). Exclusion criteria were: current or history of severe neurological or psychiatric disorders, inability to ambulate independently, significant loss of vision and/or hearing, and recent or anticipated medical procedures that may affect ambulation. Participants who completed the burst design protocol (n = 83) were included in the current study. Written informed consents were obtained in-person and approved by the IRB.
Measures
Walking task
We used a well-validated DTW procedure (15). There were two single tasks: (i) STW and (ii) Cognitive (Alpha). In STW participants were asked to walk around the electronic walkway at their “normal pace” for three consecutive loops. In Alpha, participants were required to stand still while reciting alternate letters of the alphabet (eg, A C E…) for 30-second out loud. The rate of correct letter generation (ie, letters produced in the correct order) was used to quantify performance on this task. In DTW, participants were instructed to walk around the walkway for three consecutive loops at their normal pace while reciting alternate letters of the alphabet. Participants were instructed to pay equal attention to both tasks to minimize task prioritization effects.
Quantitative gait assessment
Zenometrics
A 4 × 20 foot Zeno electronic walkway was utilized to assess quantitative measures of gait during STW and DTW (Zenometrics, LLC, Peekskill, NY). Walking distance was identical and fixed in both walking conditions but the time it took participants to complete STW (mean ± SD = 50.13 ± 12.72 seconds) and DTW (mean ± SD = 60.24 ± 21.49 seconds) was determined by their walking speed. Stride velocity (cm/s), was measured based on the location and mathematical parameters between footfalls on the instrumented walkway. Split-half intraclass correlations (ICC) for stride velocity (cm/s) in STW and DTW were greater than 0.95 revealing excellent internal consistency (18). Using ProtoKinetics Movement Analysis Software technology (PKMAS), a validated computer algorithm determined entry and exit points to and from turns during the three consecutive loops that each participant was required to walk under STW and DTW conditions (27). This procedure yielded six consecutive straight-walk course segments that were used to operationalize the time course for STW and DTW. Research assistants recorded the letters generated during each of the six trial segments. Inter-rater reliability between the algorithm and rater who determined entry and exit points to and from each trial segments was high (27).
Burst measurement
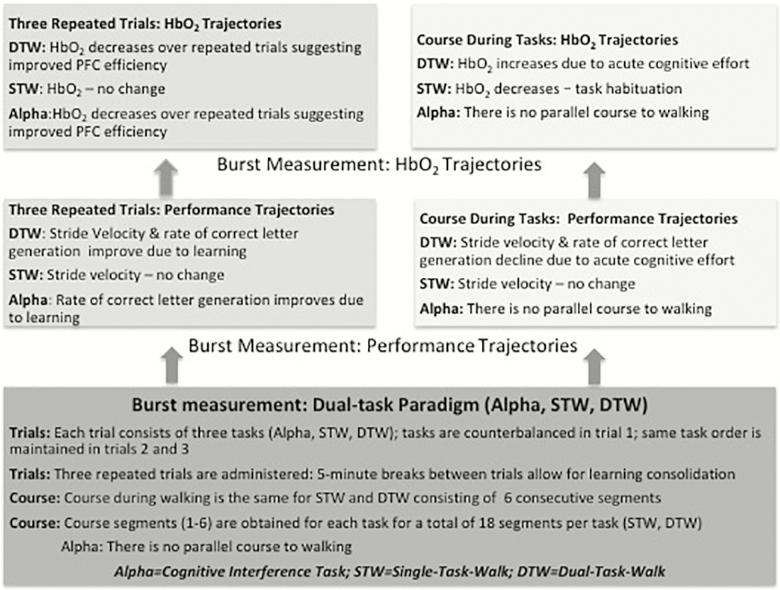
Three trials were administered separated by 5-minute time intervals allowing participants to rest. In the first trial, the three test conditions were presented in a counterbalanced order using a Latin-square design to minimize task order effects on the outcome measures. Then, the same test order was maintained for each participant for the second and third trials. Figure 1 depicts the burst measurement and predictions of the current study.
Figure 1.
Burst measurement and primary predictions.
fNIRS System
Validation of the fNIRS system has been reported including its advantages in handling motion artifacts compared to traditional neuroimaging methods (28). A recent MRI fNIRS coregistration study provided further evidence for the spatial resolution of the current fNIRS device in older adults (29). fNIRS measures changes in cortical oxygenated hemoglobin (HbO2) levels using light–tissue interaction properties of light within the near-infrared range. Changes in hemodynamic activity in the PFC were assessed using fNIRS Imager 1100 (fNIRS Devices, LLC, Potomac, MD). The system collects data at a sampling rate of 2 Hz. The fNIRS sensor consists of 4 LED light sources and 10 photodetectors, which cover the forehead using 16 voxels, with a source-detector separation of 2.5 cm. The light sources on the sensor (Epitex Inc. type L4X730/4X805/4X850-40Q96-I) contain three built-in LEDs having peak wavelengths at 730, 805, and 850 nm, with an overall outer diameter of 9.2 ± 0.2 mm. The photodetectors (Bur Brown, type OPT101) are monolithic photodiodes with a single supply transimpedance amplifier. We implemented a standard sensor placement procedure based on landmarks from the international 10–20 system (30).
Preprocessing and hemodynamic signal extraction
Raw data at 730 and 850 nm wavelengths were inspected for excessive noise, saturation, or dark current conditions. To eliminate possible respiration, heart rate signals and unwanted high frequency noise raw intensity measurements at 730 and 850 nm were low-pass filtered with a finite impulse response filter of cutoff frequency at 0.14 Hz (28). Saturation or dark current conditions were excluded. Oxygenated hemoglobin (HbO2), deoxygenated hemoglobin (Hb), oxygenation index (HbO2-Hb), and total hemoglobin (HbO2+Hb) signals can be calculated from the artifact-removed raw intensity measurements at 730 and 850 nm using modified Beer-Lambert law (DPF = 6). HbO2 is more reliable and sensitive to locomotion-related changes in cerebral oxygenation (7,9) and was thus used as the primary neuroimaging outcome in this study. Separate proximal 10-second baselines, administered prior to each experimental condition, were used to determine relative changes in HbO2 concentrations (18–20).
Epoch and feature extraction
Individual mean HbO2 data were extracted for each experimental condition using all available data from the 16 channels. A central “hub” computer with E-Prime 2.0 software sent synchronized triggers to both the fNIRS system and PKMAS. A second-level postprocessing time synchronization method was implemented using the first recorded foot contact with the walkway as a time stamp. A PKMAS algorithm terminated the fNIRS recording at the end of the sixth straight walk (27).
Reliability
Internal consistency of HbO2 measurements, determined by split-half intraclass correlations within each task, was excellent for STW (0.830), Alpha (0.864), and DTW (0.849) (18).
Covariates
The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) was used to assess overall level of cognitive function (31). The Geriatric Depression Scale (GDS) was used to assess depressive symptoms (32). A disease comorbidity index quantified illness burden based on the presence or absence of diabetes, chronic heart failure, arthritis, hypertension, depression, stroke, Parkinson’s disease, chronic obstructive lung disease, angina, and myocardial infarction (range 0–10) (26).
Statistical Analysis
Linear mixed effects models (LME) were used to assess the change in stride velocity during the six course segments and over the three trials of STW and DTW. In each model, separate linear slopes of the time course (six-level) and trial (three-level) quantified attention (course) and learning (trial) trajectories. Generalized Estimating Equation (GEE) Poisson model was used to assess the change in the rate of correct letter generation during the six course segments and over the three trials of DTW. Separate LMEs for STW and DTW were used to assess the change in HbO2 slopes during the six course segments and over the three trials. Individual channel data were treated as both fixed and random effect factor thus allowing it contribute to both the mean and variation. LME and GEE Poisson models were used to determine the change in HbO2 and the rate of correct letter generation in Alpha, respectively, over repeated trials. Analyses controlled for gender, age, education, depressive symptoms, disease comorbidity, and total RBANS index score. Statistical analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC).
Results
The sample consisted of 83 nondemented participants (mean age = 78.05 ± 6.37 years; mean education = 15.00 ± 2.99 years; %female = 49.5). The mean RBANS Index score (93.72 ± 12.37) was indicative of average cognitive function. Mean disease comorbidity index (0.75 ± 1.04) and GDS scores (4.38 ± 3.31) were low indicating the sample was relatively healthy and with minimal depressive symptoms, respectively. Demographic information, summarized in Table 1, also included trial-stratified descriptive statistics for behavioral performance and HbO2. Descriptive statistics for behavioral performance and HbO2 stratified by course and trial per task are summarized in Table 2. Stride velocity significantly decreased in DTW compared to STW (estimate = 12.522, 95% confidence interval [CI]: 11.868–13.176, p < .001). The rate of correct letter generation was not significantly different between Alpha and DTW (estimate = 0.047, 95% CI: −0.004–0.099, p = .074).
Table 1.
Sample Characteristics Behavioral Performance and HbO2 Levels per Trial
| Number (%) | |
|---|---|
| Participants | 83 |
| Women | 41 (49.4) |
| Mean (SD) | |
| Age, years | 78.05 (6.37) |
| Education, years | 15 (2.99) |
| Disease Comorbidity Index | 0.75 (1.04) |
| GDS (total score) | 4.38 (3.31) |
| Trial 1 | |
| STW stride velocity(cm/s) | 84.55 (18.33) |
| DTW stride velocity(cm/s) | 70.11 (19.30) |
| Alpha rate of letter generation (correct/min) | 35.09 (10.71) |
| DTW rate of letter generation (correct/min) | 38.54 (17.51) |
| STW HbO2 levels | 0.215 (1.43) |
| Alpha HbO2 levels | 1.110 (1.61) |
| DTW HbO2 levels | 1.096 (1.83) |
| Trial 2 | |
| STW stride velocity (cm/s) | 85.23 (19.29) |
| DTW stride velocity (cm/s) | 72.98 (18.91) |
| Alpha rate of letter generation (correct/min) | 37.84 (10.66) |
| DTW: rate of letter generation (correct/min) | 41.49 (17.97) |
| STW HbO2 levels | 0.190 (1.56) |
| Alpha HbO2 levels | 0.869 (1.05) |
| DTW HbO2 levels | 0.840 (1.71) |
| Trial 3 | |
| STW stride velocity (cm/s) | 84.13 (18.79) |
| DTW stride velocity (cm/s) | 73.20 (19.47) |
| Alpha rate of letter generation (correct/min) | 38.39 (11.48) |
| DTW: rate of letter generation (correct/min) | 43.52 (17.96) |
| STW HbO2 levels | 0.141 (1.54) |
| Alpha HbO2 levels | 0.898 (1.01) |
| DTW HbO2 levels | 0.773 (1.61) |
Note: DTW = Dual-Task-Walk; GDS = Geriatric Depression Scale; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; STW = Single-Task-Walk.
Table 2.
Performance and HbO2 Levels Stratified by Trial and Course
| Course 1 | Course 2 | Course 3 | Course 4 | Course 5 | Course 6 | |
|---|---|---|---|---|---|---|
| Trial 1 | ||||||
| STW Stride velocity (cm/s) | 83.07 ± 18.23 | 86.13 ± 17.91) | 84.53 ± 18.29 | 85.43 ± 18.81 | 83.41 ± 18.67 | 84.74 ± 18.46 |
| DTW Stride velocity (cm/s) | 72.44 ± 19.18 | 71.68 ± 19.57) | 67.77 ± 18.69 | 69.87 ± 19.44 | 68.70 ± 19.35 | 70.22 ± 19.83 |
| DTW Letter generation (corr/min) | 44.06 ± 17.88 | 39.17 ± 18.77) | 37.18 ± 15.90 | 37.81 ± 19.93 | 37.25 ± 15.47 | 35.79 ± 17.12 |
| STW HbO2 | 0.374 ± 1.019 | 0.393 ± 1.390) | 0.261 ± 1.372 | 0.062 ± 1.527 | 0.090 ± 1.537 | 0.114 ± 1.662 |
| DTW HbO2 | 0.895 ± 1.292 | 1.141 ± 1.700) | 1.190 ± 1.833 | 1.141 ± 1.953 | 1.163 ± 2.027) | 1.047 ± 2.092 |
| Trial 2 | ||||||
| STW Stride velocity (cm/s) | 84.41 ± 18.71 | 87.28 ± 19.18 | 84.72 ± 18.98 | 85.86 ± 19.57 | 83.93 ± 19.85 | 85.20 ± 19.86 |
| DTW Stride velocity (cm/s) | 74.27 ± 18.54 | 74.48 ± 18.84 | 71.65 ± 19.02 | 74.01 ± 19.64 | 70.90 ± 18.42 | 72.57 ± 19.30 |
| DTW Letter generation (corr/min) | 47.98 ± 16.38 | 42.88 ± 17.37 | 38.71 ± 19.50 | 40.96 ± 18.53 | 38.20 ± 16.24 | 40.25 ± 19.84 |
| STW HbO2 | 0.292 ± 1.128 | 0.396 ± 1.400 | 0.265 ± 1.517 | 0.121 ± 1.591 | 0.060 ± 1.822 | 0.011 ± 1.780 |
| DTW HbO2 | 0.621 ± 1.209 | 0.768 ± 1.550 | 0.915 ± 1.668 | 0.889 ± 1.803 | 0.920 ± 1.902 | 0.930 ± 2.022 |
| Trial 3 | ||||||
| STW Stride velocity (cm/s) | 84.25 ± 18.67 | 86.24 ± 18.56 | 82.44 ± 18.32 | 84.66 ± 18.89 | 83.18 ± 18.65 | 84.00 ± 19.99 |
| DTW Stride velocity (cm/s) | 75.29 ± 18.84 | 74.96 ± 20.59 | 71.20 ± 19.44 | 73.36 ± 19.80 | 71.69 ± 19.25 | 72.74 ± 19.15 |
| DTW Letter generation (corr/min) | 50.64 ± 17.18 | 47.55 ± 16.49 | 43.45 ± 18.90 | 40.29 ± 19.48 | 41.50 ± 18.08 | 37.70 ± 17.78 |
| STW HbO2 | 0.173 ± 1.243 | 0.229 ± 1.556 | 0.179 ± 1.570 | 0.164 ± 1.667 | 0.058 ± 1.596 | 0.041 ± 1.589 |
| DTW HbO2 | 0.614 ± 1.192 | 0.794 ± 1.536 | 0.789 ± 1.611 | 0.853 ± 1.761 | 0.853 ± 1.761 | 0.705 ± 1.725 |
Note: DTW = Dual-Task-Walk; STW = Single-Task-Walk.
Course and Repeated Trials Trajectories: Behavioral Performance
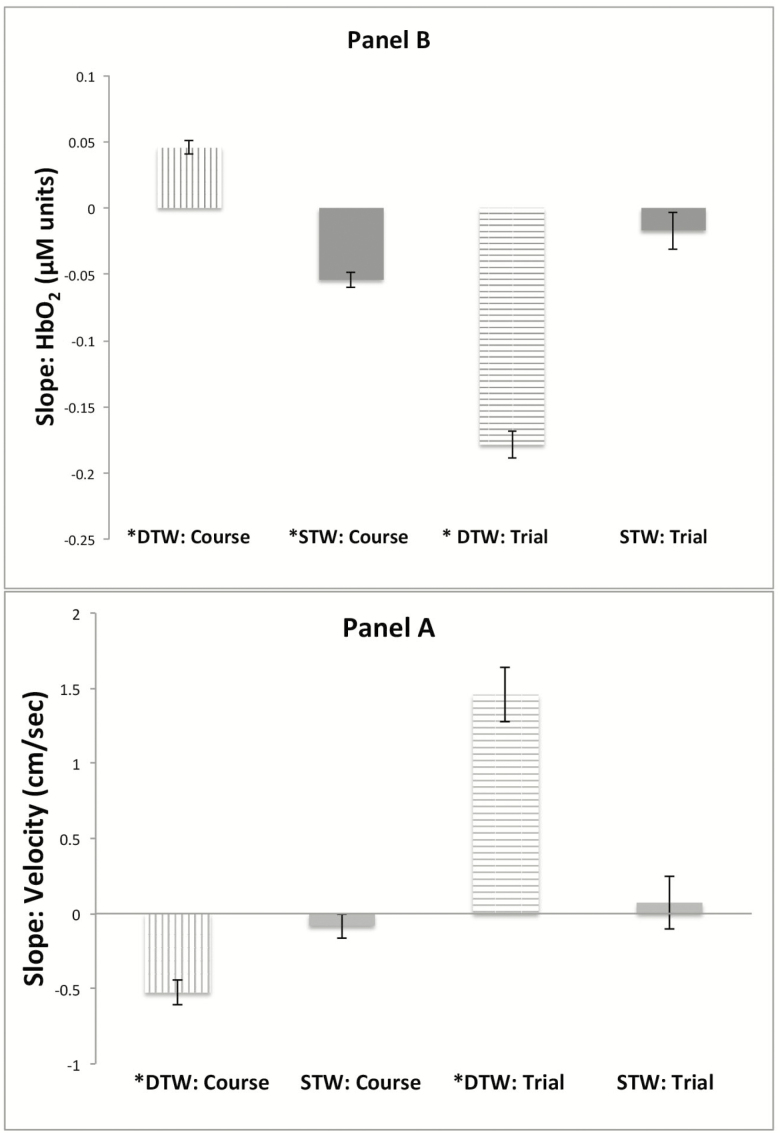
Changes in stride velocity during the course (estimate = −0.0826 cm/s, p = .3112, d = 0.11) and over repeated trials (estimate = 0.0727 cm/s, p = .6757, d = 0.04) of STW were not significant. Stride velocity declined during the course (estimate = −0.5259 cm/s, p <.0001, d = 0.67) but improved over repeated trials (estimate = 1.4577 cm/s per trial, p < .0001, d = 0.87) of DTW. The rate of correct letter generation decreased during the course (log estimate of rate ratio = −0.0377, p < .0001, d = 0.53) but increased over repeated trials (log estimate of rate ratio = 0.0578, p < .0001, d = 0.47) of DTW. Summary of the LME and GEE models is provided in Table 3.
Table 3.
Course and Trial Effects: Behavioral Performance
| Model: STW Velocity | ||||
|---|---|---|---|---|
| Variables | Estimate | SE | 95% CI | p |
| Course | −0.0826 | 0.0817 | −0.2427 to 0.0773 | .3112 |
| Trial | 0.0727 | 0.1740 | −0.2685 to 0.4141 | .6757 |
| Age | −0.7979 | 0.2935 | −1.3737 to −0.2222 | .0066 |
| Gender | 1.9323 | 3.7169 | −5.3594 to 9.2239 | .6032 |
| Education | 0.8493 | 0.6425 | −0.4111 to 2.1098 | .1864 |
| RBANS Index score | 0.4463 | 0.1464 | 0.1591 to 0.7335 | .0023 |
| Disease Comorbidity Index | −3.3641 | 1.8530 | −6.9992 to 0.2711 | .0697 |
| GDS | −1.0058 | 0.5650 | −2.1143 to 0.1026 | .0753 |
| Model: DTW Velocity | ||||
| Variables | ||||
| Course | −0.5259 | 0.0854 | −0.6935 to −0.3583 | <.0001 |
| Trial | 1.4577 | 0.1824 | 1.0999 to 1.8155 | <.0001 |
| Age | −0.4697 | 0.3199 | −1.0973 to 0.1578 | .1422 |
| Gender | −3.7970 | 4.0515 | −11.7450 to 4.1509 | .3488 |
| Education | 0.4791 | 0.7003 | −0.8948 to 1.8530 | .4940 |
| RBANS Total Index Score | 0.4002 | 0.1596 | 0.0871 to 0.7132 | .0123 |
| Disease Comorbidity Index | −2.4141 | 1.0198 | −6.3764 to 1.5483 | .2322 |
| GDS | −0.3750 | 0.6159 | −1.5832 to 0.8333 | .5428 |
| Model: DTW Correct Letter Generation Rate | ||||
| Variables | ||||
| Course | −0.0377 | 0.0078 | −0.0531 to −0.0224 | <.0001 |
| Trial | 0.0578 | 0.0134 | 0.0315 to 0.0841 | <.0001 |
| Age | 0.0011 | 0.0058 | −0.0102 to 0.0125 | .8478 |
| Gender | 0.0376 | 0.0662 | −0.0921 to 0.1673 | .5696 |
| Education | 0.0119 | 0.0120 | −0.0116 to 0.0355 | .3207 |
| RBANS Total Index Score | 0.0148 | 0.0029 | 0.0091 to 0.0204 | <.0001 |
| Disease Comorbidity Index | 0.0133 | 0.0343 | −0.0540 to 0.0806 | .6993 |
| GDS | −0.0154 | 0.0137 | −0.0422 to 0.0114 | .2602 |
Note: DTW = Dual-Task-Walk; GDS = Geriatric Depression Scale; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; STW = Single-Task-Walk.
The rate of correct letter generation increased significantly over repeated Alpha trials (log estimate of rate ratio = 0.0447, 95% CI = 0.0231–0.0663, p < .0001, d = 0.48).
Course and Repeated Trials Trajectories: HbO2
HbO2 declined during the course of STW (estimate = −0.0542 μM, p < .0001, d = 1.20) but did not change over repeated STW trials (estimate = −0.0171 μM, p = .1190, d = 0.17). HbO2 increased during the course of DTW (estimate = 0.0454 μM, p < .0001, d = 0.96) but declined over repeated DTW trials (estimate = −0.1786 μM, p < .0001, d = 1.75). Summary of the LMEs is provided in Table 4.
Table 4.
Course and Trial Effects: HbO2
| Model: STW HbO2 | ||||
|---|---|---|---|---|
| Variables | Estimate | SE | 95% CI | p |
| Course | −0.0542 | 0.005 | −0.064 to −0.0443 | <.0001 |
| Trial | −0.0171 | 0.0109 | −0.0386 to 0.0043 | .1190 |
| Age | −0.0150 | 0.0135 | −0.0420 to 0.0118 | .2680 |
| Gender | 0.0364 | 0.1744 | −0.3114 to 0.3842 | .8350 |
| Education | −0.0053 | 0.0295 | −0.0643 to 0.0536 | .8570 |
| RBANS Index score | 0.0033 | 0.0070 | −0.0107 to 0.0174 | .6400 |
| Disease Comorbidity Index | 0.0619 | 0.0852 | −0.1080 to 0.2319 | .4700 |
| GDS | −0.0109 | 0.0263 | −0.0635 to 0.0416 | .6790 |
| Model: DTW HbO 2 | ||||
| Variables | ||||
| Course | 0.0454 | 0.0052 | 0.0351 to 0.0557 | <.0001 |
| Trial | −0.1786 | 0.0113 | −0.2009 to −0.1564 | <.0001 |
| Age | −0.0160 | 0.0185 | −0.0530 to 0.0210 | .3910 |
| Gender | −0.0804 | 0.2399 | −0.5587 to 0.3978 | .6920 |
| Education | 0.0326 | 0.0334 | −0.0476 to 0.1145 | .4130 |
| RBANS Index score | 0.0164 | 0.0097 | −0.0029 to 0.0358 | .0950 |
| Disease Comorbidity Index | 0.0452 | 0.1172 | −0.1885 to 0.270 | .7010 |
| GDS | −0.0337 | 0.0362 | −0.1060 to 0.0385 | .3560 |
Note: DTW = Dual-Task-Walk; GDS = Geriatric Depression Scale; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; STW = Single-Task-Walk.
HbO2 declined over repeated Alpha trials (estimate = −0.086 μM, SE = 0.016, 95% CI = −0.117 to −0.054, p < .001, d = 0.59).
Figure 2 depicts separate slopes of stride velocity and HbO2 for course and trial per walking condition.
Figure 2.
Slopes, extracted from the LMEs, of stride velocity and HbO2 during the course and over repeated trials of STW and DTW. DTW = Dual-Task-Walk; LME = Linear mixed effects models; STW = Single-Task-Walk.
Sensitivity analyses
We examined the effect of trial on deoxygenated hemoglobin (Hb) across the three experimental conditions. Hb values were negative for STW (trial 1: M ± SD = −0.210 ± 1.099; trial 2: M ± SD = −0.159 ± 1.225; trial 3: M ± SD = −0.152 ± 1.567), Alpha (trial 1: M ± SD = −0.349 ± 0.789; trial 2: M ± SD = −0.254 ± 1.070; trial 3: M ± SD = −0.103 ± 1.020), and DTW (trial 1: M ± SD = −0.437 ± 1.309; trial 2: M ± SD = −0.450 ± 1.301; trial 3: M ± SD = −0.244 ± 1.247). Further, LME showed significant effects of repeated trials on Hb in DTW (estimate = 0.097, 95% CI = 0.047–0.146, p < .001) and Alpha (estimate = 0.134, 95% CI = 0.098–0.170, p < .001) but not on STW (estimate = 0.026, 95% CI = −0.025–0.078, p = .326).
We examined the moderating effects of DTW performance slopes (stride velocity, correct letter generation rate) on HbO2 decline over repeated DTW trials. Both performance slopes were dichotomized at 0 to empirically distinguish improvers (>0) from nonimprovers (<0). Descriptively, mean DTW HbO2 slope for improvers in DTW stride velocity (77.1%; M ± SD = −0.240 ± 0.579) was more negative than for nonimprovers (22.9%; M ± SD = 0.019 ± 0.409). Descriptively, mean DTW HbO2 slope for improvers in DTW correct letter generation rate (73.1%; M ± SD = −0.237 ± 0.557) was more negative than for nonimprovers (26.9%; M ± SD = 0.343 ± 0.405). In a fully-adjusted LME, both performance slopes, dichotomized at 0, served as moderators of change in HbO2 over repeated DTW trials. The interactions of both moderators with trials were significant whereby improvers in stride velocity (estimate = −.1705, p < .0001, 95% CI = −0.2267 to −0.1144) and correct letter generation rate (estimate = −.1789, P < 0.0001, 95% CI = −0.2342 to −0.1236) showed a greater decline in HbO2 over DTW trials compared to nonimprovers.
Discussion
We determined task-specific performance and PFC HbO2 trajectories during walking in older adults. The trajectories of stride velocity during the course and over repeated trials of STW were not significant. In contrast, the trajectories of stride velocity and rate of correct letter generation during the course and over repeated trials of DTW were significant and in opposite directions. Consistent with our hypothesis, both stride velocity and correct letter generation rate declined during the course of DTW. This finding replicates and extends previous work suggesting that decline in performance can be documented during the course of relatively brief but cognitively demanding tasks (21,24). In contrast, stride velocity and the rate of correct letter generation improved over repeated DTW trials. Improved performance was also observed over repeated Alpha trials. Evidence for these distinct behavioral trajectories provided the context to examining their respective HbO2 trajectories.
Learning was observed over repeated Alpha trials along with a reduction in HbO2 suggesting improved PFC efficiency. Consistent with previous findings (21) HbO2 declined during the course of STW. The change in HbO2 over repeated STW trials, however, was not significant given the relatively limited cognitive demands STW places on the brain. As predicted, HbO2 increased during the course of DTW. In contrast, HbO2 declined over repeated DTW trials, which along with practice-related improvement in DTW performance suggested improved PFC efficiency. This finding is consistent with a meta-analysis revealing that practice in cognitively demanding tasks resulted in reduced brain activations, notably in the PFC (33). Moreover, exploratory analysis suggested that participants whose DTW performance improved demonstrated greater HbO2 decline over repeated DTW trials (ie, better PFC efficiency) compared to participants who did not improve.
Clinical Implications
Poor DTW performance on this paradigm predicted increased risk of incident falls (34) as well as frailty, disability and mortality in older adults (35). Moreover, higher mean PFC HbO2, assessed with fNIRS during DTW, predicted increased incident falls risk among healthy older adults (36). Training improves DTW in older adults (37). Our findings suggest that DTW performance and PFC efficiency can be improved in one session. Rehabilitation efforts could focus on improving performance and automaticity of DTW, which in turn may improve brain efficiency thereby reducing the risk of adverse mobility outcomes.
Study Limitations and Strengths
Strengths of this investigation include the novelty of our experimental procedures, careful clinical characterization of the participants and multivariate analyses accounting for several possible confounders. Although channels contributed to the mean and variation in HbO2, the effects of task, course and trials were reported on overall activation patterns. Future studies could assess whether spatial distributions in PFC activation vary as a function individual differences and experimental manipulation. The current fNIRS system offers significant advantages in terms of portability and capability to assess cortical activation during locomotion but methodological consideration including depth of penetration, spatial resolution, and possible effects of superficial layers should be acknowledged (38). However, confounders such as skin, skull thickness, respiration, or physical fatigue could not explain the distinct neural trajectories observed during the course and over repeated STW and DTW trials given that experimental conditions were administered in a random order and had the same walking environment and physical requirements. Inferences regarding improved brain efficiency were based on data limited to the PFC. It is possible that the reduction in PFC HbO2, observed over repeated DTW trials, was accompanied by changes in neural activity in more posterior brain regions suggesting that a more global brain activation pattern was associated with learning and improved efficiency. Long-term retention of both learning and PFC efficiency gains will have to be evaluated in future research as well as their potential transfer to proximal and distal outcomes.
Sensitivity analyses using deoxygenated hemoglobin as an additional outcome revealed significant trial effects on Alpha and DTW that were in opposite direction to the observed trial effects on HbO2. This complementary analysis further supports the notion that improved PFC efficiency, inferred from the coupling of improved performance and the decline in HbO2 over DTW trials, represented a change in the hemodynamic signal and not systemic changes.
In summary, we provided evidence for distinct attention (course) and learning (repeated trials) trajectories and their corresponding PFC activity. Findings suggest that learning and improved PFC efficiency were demonstrated in one experimental session involving repeated DTW trials.
Funding
This research was supported by the National Institutes on Aging grants (R01AG036921 and R01AG044007).
Conflict of Interest
M.I. has a very minor share in the company that manufactures the fNIRS device used in this study. R.H. serves on the editorial board of Journal of Gerontology Medical Sciences. All other authors have no conflicts of interest to report in relation to the current article.
References
- 1. Holtzer R, Epstein N, Mahoney JR, Izzetoglu M, Blumen HM. Neuroimaging of mobility in aging: a targeted review. J Gerontol A Biol Sci Med Sci. 2014;69:1375–1388. doi: 10.1093/gerona/glu052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545 [DOI] [PubMed] [Google Scholar]
- 3. Goldman-Rakic PS, Bates JF, Chafee MV. The prefrontal cortex and internally generated motor acts. Curr Opin Neurobiol. 1992;2:830–835. doi: 10.1016/0959-4388(92)90141–7 [DOI] [PubMed] [Google Scholar]
- 4. Agbangla NF, Audiffren M, Albinet CT. Use of near-infrared spectroscopy in the investigation of brain activation during cognitive aging: a systematic review of an emerging area of research. Ageing Res Rev. 2017;38:52–66. doi: 10.1016/j.arr.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 5. Gramigna V, Pellegrino G, Cerasa A, et al. Near-infrared spectroscopy in gait disorders: is it time to begin?Neurorehabil Neural Repair. 2017;31:402–412. doi: 10.1177/1545968317693304 [DOI] [PubMed] [Google Scholar]
- 6. Mihara M, Miyai I, Hatakenaka M, Kubota K, Sakoda S. Role of the prefrontal cortex in human balance control. Neuroimage. 2008;43:329–336. doi: 10.1016/j.neuroimage.2008.07.029 [DOI] [PubMed] [Google Scholar]
- 7. Miyai I, Tanabe HC, Sase I, et al. Cortical mapping of gait in humans: a near-infrared spectroscopic topography study. Neuroimage. 2001;14:1186–1192. doi: 10.1006/nimg.2001.0905 [DOI] [PubMed] [Google Scholar]
- 8. Suzuki M, Miyai I, Ono T, Kubota K. Activities in the frontal cortex and gait performance are modulated by preparation. An fNIRS study. Neuroimage. 2008;39:600–607. doi: 10.1016/j.neuroimage.2007.08.044 [DOI] [PubMed] [Google Scholar]
- 9. Harada T, Miyai I, Suzuki M, Kubota K. Gait capacity affects cortical activation patterns related to speed control in the elderly. Exp Brain Res. 2009;193:445–454. doi: 10.1007/s00221-008-1643-y [DOI] [PubMed] [Google Scholar]
- 10. Mirelman A, Maidan I, Bernad-Elazari H, Shustack S, Giladi N, Hausdorff JM. Effects of aging on prefrontal brain activation during challenging walking conditions. Brain Cogn. 2017;115:41–46. doi: 10.1016/j.bandc.2017.04.002 [DOI] [PubMed] [Google Scholar]
- 11. Mihara M, Miyai I, Hatakenaka M, Kubota K, Sakoda S. Sustained prefrontal activation during ataxic gait: a compensatory mechanism for ataxic stroke?Neuroimage. 2007;37:1338–1345. doi: 10.1016/j.neuroimage.2007.06.014 [DOI] [PubMed] [Google Scholar]
- 12. Hernandez ME, Holtzer R, Chaparro G, et al. Brain activation changes during locomotion in middle-aged to older adults with multiple sclerosis. J Neurol Sci. 2016;370:277–283. doi: 10.1016/j.jns.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 13. Maidan I, Nieuwhof F, Bernad-Elazari H, et al. The role of the frontal lobe in complex walking among patients with Parkinson’s disease and healthy older adults: an fNIRS study. Neurorehabil Neural Repair. 2016;30:963–971. doi: 10.1177/1545968316650426 [DOI] [PubMed] [Google Scholar]
- 14. Maidan I, Bernad-Elazari H, Giladi N, Hausdorff JM, Mirelman A. When is higher level cognitive control needed for locomotor tasks among patients with Parkinson’s disease?Brain Topogr. 2017;30:531–538. doi: 10.1007/s10548-017-0564-0 [DOI] [PubMed] [Google Scholar]
- 15. Holtzer R, Wang C, Verghese J. Performance variance on walking while talking tasks: theory, findings, and clinical implications. Age (Dordr). 2014;36:373–381. doi: 10.1007/s11357-013-9570-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holtzer R, Mahoney JR, Izzetoglu M, Izzetoglu K, Onaral B, Verghese J. fNIRS study of walking and walking while talking in young and old individuals. J Gerontol A Biol Sci Med Sci. 2011;66:879–887. doi: 10.1093/gerona/glr068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fraser SA, Dupuy O, Pouliot P, Lesage F, Bherer L. Comparable cerebral oxygenation patterns in younger and older adults during dual-task walking with increasing load. Front Aging Neurosci. 2016;8:240. doi: 10.3389/fnagi.2016.00240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holtzer R, Mahoney JR, Izzetoglu M, Wang C, England S, Verghese J. Online fronto-cortical control of simple and attention-demanding locomotion in humans. Neuroimage. 2015;112:152–159. doi: 10.1016/j.neuroimage.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holtzer R, Verghese J, Allali G, Izzetoglu M, Wang C, Mahoney JR. Neurological gait abnormalities moderate the functional brain signature of the posture first hypothesis. Brain Topogr. 2016;29:334–343. doi: 10.1007/s10548-015-0465-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holtzer R, Schoen C, Demetriou E, et al. Stress and gender effects on prefrontal cortex oxygenation levels assessed during single and dual-task walking conditions. Eur J Neurosci. 2017;45:660–670. doi: 10.1111/ejn.13518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holtzer R, Yuan J, Verghese J, Mahoney JR, Izzetoglu M, Wang C. Interactions of subjective and objective measures of fatigue defined in the context of brain control of locomotion. J Gerontol A Biol Sci Med. 2017;72:417–423. doi: 10.1093/gerona/glw167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Salthouse TA, Nesselroade JR. Dealing with short-term fluctuation in longitudinal research. J Gerontol B Psychol Sci Soc Sci. 2010;65:698–705. doi: 10.1093/geronb/gbq060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sliwinski MJ, Almeida DM, Smyth J, Stawski RS. Intraindividual change and variability in daily stress processes: findings from two measurement-burst diary studies. Psychol Aging. 2009;24:828–840. doi: 10.1037/a0017925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holtzer R, Foley F, D’Orio V, Spat J, Shuman M, Wang C. Learning and cognitive fatigue trajectories in multiple sclerosis defined using a burst measurement design. Mult Scler. 2013;19:1518–1525. doi: 10.1177/1352458513477922 [DOI] [PubMed] [Google Scholar]
- 25. Neubauer AC, Fink A. Intelligence and neural efficiency. Neurosci Biobehav Rev. 2009;33:1004–1023. doi: 10.1016/j.neubiorev.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 26. Holtzer R, Verghese J, Wang C, Hall CB, Lipton RB. Within-person across-neuropsychological test variability and incident dementia. JAMA. 2008;300:823–830. doi: 10.1001/jama.300.7.823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. England SE, Verghese J, Mahoney JR, Trantzas C, Holtzer R. Three-level rating of turns while walking. Gait Posture. 2015;41:300–303. doi: 10.1016/j.gaitpost.2014.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Izzetoglu M, Devaraj A, Bunce S, Onaral B. Motion artifact cancellation in NIR spectroscopy using wiener filtering. IEEE Trans Biomed Eng. 2005;52:934–938. doi: 10.1109/TBME.2005.845243 [DOI] [PubMed] [Google Scholar]
- 29. Chen M, Blumen HM, Izzetoglu M, Holtzer R. Spatial coregistration of functional near-infrared spectroscopy to brain MRI. J Neuroimaging. 2017;27:453–460. doi: 10.1111/jon.12432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ayaz H, Izzetoglu M, Platek SM, et al. Registering fNIR data to brain surface image using MRI templates. Conf Proc IEEE Eng Med Biol Soc. 2006;1:2671–2674. doi: 10.1109/IEMBS.2006.260835 [DOI] [PubMed] [Google Scholar]
- 31. Duff K, Humphreys Clark JD, O’Bryant SE, Mold JW, Schiffer RB, Sutker PB. Utility of the RBANS in detecting cognitive impairment associated with Alzheimer’s disease: sensitivity, specificity, and positive and negative predictive powers. Arch Clin Neuropsychol. 2008;23:603–612. doi: 10.1016/j.acn.2008.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4 [DOI] [PubMed] [Google Scholar]
- 33. Chein JM, Schneider W. Neuroimaging studies of practice-related change: fMRI and meta-analytic evidence of a domain-general control network for learning. Brain Res Cogn Brain Res. 2005;25:607–623. doi: 10.1016/j.cogbrainres.2005.08.013 [DOI] [PubMed] [Google Scholar]
- 34. Ayers EI, Tow AC, Holtzer R, Verghese J. Walking while talking and falls in aging. Gerontology. 2014;60:108–113. doi: 10.1159/000355119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Verghese J, Holtzer R, Lipton RB, Wang C. Mobility stress test approach to predicting frailty, disability, and mortality in high-functioning older adults. J Am Geriatr Soc. 2012;60:1901–1905. doi: 10.1111/j.1532-5415.2012.04145.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Verghese J, Wang C, Ayers E, Izzetoglu M, Holtzer R. Brain activation in high-functioning older adults and falls: prospective cohort study. Neurology. 2017;88:191–197. doi: 10.1212/WNL.0000000000003421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Silsupadol P, Lugade V, Shumway-Cook A, et al. Training-related changes in dual-task walking performance of elderly persons with balance impairment: a double-blind, randomized controlled trial. Gait Posture. 2009;29:634–639. doi: 10.1016/j.gaitpost.2009.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vitorio R, Stuart S, Rochester L, Alcock L, Pantall A. fNIRS response during walking - artefact or cortical activity? A systematic review. Neurosci Biobehav Rev. 2017;83:160–172. doi: 10.1016/j.neubiorev.2017.10.002 [DOI] [PubMed] [Google Scholar]