ABSTRACT
Background
Maternal status of long-chain PUFAs (LC-PUFAs) may be related to fetal growth. Maternal fish consumption exposes the mother to the neurotoxicant methylmercury (MeHg), which, in contrast, may restrict fetal growth.
Objective
Our aim was to examine relations between maternal LC-PUFA status at 28 wk and birth outcomes (birth weight, length, and head circumference), controlling for MeHg exposure throughout pregnancy, in the Seychelles Child Development Study Nutrition Cohort 2. Our secondary aim was to examine the influence of maternal variation in genes regulating the desaturation of LC-PUFAs [fatty acid desaturase (FADS)] on birth outcomes.
Methods
From nonfasting blood samples collected at 28 wk of gestation, we measured serum total LC-PUFA concentrations and FADS1 (rs174537, rs174561), FADS1–FADS2rs3834458, and FADS2rs174575 genotypes, with hair total mercury concentrations assessed at delivery. Data were available for n = 1236 mother–child pairs. Associations of maternal LC-PUFAs, MeHg, and FADS genotype with birth outcomes were assessed by multiple linear regression models, adjusting for child sex, gestational age, maternal age, BMI, alcohol use, socioeconomic status, and parity.
Results
In our cohort of healthy mothers, neither maternal LC-PUFA status nor MeHg exposure were significant determinants of birth outcomes. However, when compared with major allele homozygotes, mothers who were heterozygous for the minor allele of FADS1 (rs174537 and rs174561, GT compared with TT, β = 0.205, P = 0.03; TC compared with CC, β = 0.203, P = 0.04) and FADS1–FADS2 (rs3834458, Tdel compared with DelDel, β = 0.197, P = 0.04) had infants with a greater head circumference (all P < 0.05). Homozygosity for the minor allele of FADS2 (rs174575) was associated with a greater birth weight (GG compared with CC, β = 0.109, P = 0.04).
Conclusions
In our mother–child cohort, neither maternal LC-PUFA status nor MeHg exposure was associated with birth outcomes. The observed associations of variation in maternal FADS genotype with birth outcomes should be confirmed in other populations.
Keywords: maternal nutrition; long chain polyunsaturated fatty acids, methylmercury, birth outcomes, fatty acid desaturase (FADS) genotype, Seychelles Child Development Study
Introduction
Maternal nutritional status is an important determinant of infant birth size, which is hypothesized to predict health outcomes throughout the life span (1, 2). Of particular interest are the n–3 (ω-3) long-chain PUFAs (LC-PUFAs) EPA (20:5n–3) and DHA (22:6n–3), of which adequate prenatal availability has been linked to a longer gestation, greater birth weight, and a lower risk of pregnancy complications (3–7). Arachidonic acid (AA; 20:4n–6), a long chain n–6 PUFA, is transferred to the fetus in the third trimester for neurodevelopment and is a significant component of structural membrane lipids (8). Clinical evidence supports a relation between higher maternal AA status and fetal growth (8, 9). Yet, higher status of maternal AA, a precursor of proinflammatory eicosanoids, has also been inversely associated with birth weight by various prospective studies (3, 10, 11). Collectively the n–3 and n–6 LC-PUFAs have important roles in cell membrane function, energy storage, and oxygen transport. This may partially explain why greater prenatal concentrations have been most frequently reported to favorably modify birth outcomes (3). However, their circulating concentrations fluctuate throughout gestation and their effects on inflammation, blood viscosity, and platelet aggregation, which may also impact fetal growth, differ according to the balance of n–6:n–3 LC-PUFA–derived eicosanoids (12).
Studies that have examined associations between birth weight and maternal intake of fish, the richest dietary source of n–3 LC-PUFAs, have shown conflicting results. Some have reported increasing birth weight with increasing fish consumption (4, 13) and gestational age (14, 15), while others have reported a negative or null association with birth outcomes (14, 16–19). Other constituents of fish, which are not accounted for when examining fish consumption, may help explain these inconsistent findings. Several studies have suggested the balance between nutrients and potential contaminants in fish may be particularly important in determining fetal growth and birth size. Ramon et al. (20) analyzed mother–child data from the Spanish INfancia y Medio Ambiente (INMA) study, where the study population consumed a low to moderate intake of seafood (≤65 g/d). They reported a greater risk of infants being small for gestational age (SGA) when mothers consumed ≥2 portions/wk of large oily fish, but a lower risk of being SGA when mothers consumed ≥2 portions/wk of canned tuna and lean fish. Differential findings with the amount and type of fish have also been reported from the Danish National Birth Cohort Study (21). All fish contain small amounts of the potential neurotoxin methylmercury (MeHg), concentrations of which bioaccumulate in higher-order predatory fish species. MeHg readily crosses the placenta and detrimental associations have been reported between maternal MeHg exposure and both birth weight and head circumference (22–24). Others, however, including our own previous study in the Republic of Seychelles where there are few other marine pollutants, have shown no associations (25–28). With seafood as their primary dietary source, it is important to account for both the mother's exposure to n–3 LC-PUFAs and MeHg to avoid underestimating their respective associations with fetal growth.
There is a need to explore whether genetic variation may help explain some of the inconsistencies found between studies in this field. We, and others, have previously confirmed that variation through single nucleotide polymorphisms (SNPs) in genes encoding the Δ5 and Δ6 fatty acid desaturases (FADS1 and FADS2, respectively) determine maternal concentrations of LC-PUFAs (29). Two recent studies have suggested that FADS genotype might modify associations between LC-PUFA intake and birth weight. Minor allele carriers of rs174556 (TT) were reported to have shorter pregnancies and lower-weight infants than major allele carriers (30, 31). In the latter study, mothers who were minor allele carriers with high DHA intakes had significantly heavier infants than the homozygous reference genotype, suggesting that LC-PUFA intake and genetic makeup may interact in their associations with birth weight. This association was supported by results of a DHA supplementation trial, which showed differential responses to DHA supplementation on birth weight across FADS genotypes whereby mothers who received DHA gave birth to significantly heavier infants compared with those in the placebo group, but only if they were carriers of the minor FADS2rs174602 allele (32).
The primary aim of this study was to investigate relations between maternal LC-PUFA status at 28 wk and birth outcomes (birth weight, length, and head circumference), while adjusting for maternal MeHg exposure, in the high-fish-eating Seychelles Child Development Study (SCDS) Nutrition Cohort 2 (NC2). The secondary aim was to determine whether maternal FADS genotype is associated with birth outcomes.
Methods
Study population
The NC2 cohort is part of the SCDS, an ongoing multicohort observational study with the overall aim of investigating associations between prenatal MeHg exposure and child neurodevelopment. Mothers were recruited for NC2 during their first antenatal visit (from 14 wk of gestation) at 8 health centers on Mahé, the main island of the Republic of Seychelles, between January 2008 and January 2011. Inclusion criteria for NC2 included being native Seychellois, being ≥16 y of age, having a singleton pregnancy, and with no obvious health concerns (33). The study was reviewed and approved by the Seychelles Ethics Board and the Research Subjects Review Board at the University of Rochester.
Blood sampling and fatty acid analysis
Nonfasting blood samples were collected at 28 wk of gestation, from which serum was obtained and shipped at −80°C to Ulster for PUFA analysis. Serum lipids were extracted according to an adaptation of the Folch et al. method (1957) (34) as previously described (33). FAMEs were prepared using boron trifluoride methanol (BF3; Sigma Aldrich UK) and quantified by GC-MS (Agilent 7890A-5975C) with reference to the internal standard, which was added to each sample prior to extraction (heptadecaenoic acid, C17:0; Sigma Aldrich UK). The analysis was completed in split mode using a BPX70 capillary GC column (SGE Analytical Science) (length, 100 m; internal diameter, 250 μm; and film thickness, 0.25 μm) with helium as the carrier gas (constant flow at 1.0 mL/min). Samples were injected (1 μL) at a temperature of 130°C, which was then ramped at 15°C/min to 200°C and 30°C/min to 250°C where it was held for 5 min. MS was operated in positive-ion mode using electron ionization, and a mass range of 50–500 Da was selected for total ion chromatogram acquisition. In the current analysis we summed maternal concentrations of n–3 LC-PUFAs (EPA + DHA) and also assessed concentrations of the n–6 LC-PUFA AA. Absolute concentrations of serum total PUFAs are reported as milligrams per milliliter.
Prenatal mercury analysis
From maternal hair samples collected at delivery, concentrations of total mercury were determined by atomic absorption MS at the University of Rochester as previously described (33), where the longest hair segment available was taken to reflect exposure throughout pregnancy.
Birth outcomes
Birth weight (kilograms), length (centimeters), and head circumference (centimeters) were assessed at birth by trained nurses to the nearest 2 decimal places using routine clinical procedures and standardized scales.
Genotyping
Maternal whole-blood samples, collected at 28 wk of gestation, were shipped at −80°C to Lund University, Sweden, for genotyping. Four candidate SNPs—FADS1rs174537 and rs174561, FADS1–S2 intergenic rs3834458, and FADS2rs174575—were selected based on epidemiological evidence of having an association with LC-PUFA status (29). As previously described (29), DNA was extracted using the Qiagen DNA Blood Mini Kit (Qiagen), and genotyping was performed by iPLEX Gold assay on the MassARRAY platform (Sequenom) and by TaqMan allelic discrimination assay on an ABI 7900 instrument (Applied Biosystems), according to the manufacturer's instructions.
Covariates
We selected covariates a priori from our previous studies of birth outcomes (25), namely child sex, gestational age, maternal age, alcohol use, Hollingshead socioeconomic status (SES), parity (number of children), and maternal BMI. Due to its very low prevalence (0.9% of cohort), we did not adjust for smoking.
Mothers reported information about their age, parity, gestational diabetes, Hollingshead SES, and alcohol use during pregnancy (yes or no) using questionnaires administered by trained nurses at enrollment to the study. Child sex and gestational age (weeks) were recorded at delivery. When their infants were ∼20 mo of age, Hollingshead SES was assessed using a modified index relevant to the Republic of Seychelles (35). We combined occupational and educational codes as previously described into a continuous SES score (35). Height and weight of the mothers were obtained at the child's 20-mo examination, from which their postnatal BMI was calculated [BMI = weight (kg)/height (m2)]. Data on prenatal BMI were unavailable in this cohort. However, maternal postnatal BMI at 20 mo can be considered a reasonable surrogate for prepregnancy BMI in the Seychelles, based on data from our earlier Nutrition Cohort 1 (NC1), in which the correlation between BMI in early pregnancy and postnatal BMI, as measured at 19 mo, was 0.93 (36).
Statistical analysis
From a total cohort of n = 1536 we excluded mothers with an extreme gestational age (preterm birth defined as <34 wk; n = 13), mothers who had an infant with a very low birth weight (<1.5 kg; n = 4) or extreme head circumference (>38 cm; n = 2), mothers whose infants died at birth or neonatally (n = 9) or were born with a severe mental or physical disability (n = 2), mothers with serious gestational problems (including pre-eclamptic seizure and problems with insulin medication; n = 3), mothers without consent (n = 1), underage mothers (n = 1), those who gave birth to twins (n = 34), and those with >1 child in the cohort (n = 13). We excluded a further n = 6 observations missing all birth-outcome data and n = 13 missing gestational age data, leaving a total cohort of n = 1435. Due to a low number of mothers with gestational diabetes (n = 6), we did not exclude these from our analysis. A further 199 (14%) subjects were excluded due to missing data for model covariates. As suggested by Hoenig and Heisey (37), post hoc power calculations may lead to inaccurate interpretations of experimental data and, for this reason, were not conducted in our analysis.
Distributions of maternal and child characteristics were first examined, followed by Pearson's correlations to examine bivariate associations of each covariate with birth outcomes. Each of the 4 SNPs were treated as categorical variables with 3 levels. All other variables were treated as continuous variables, with the exception of alcohol and child sex, which were categorical variables (0 if mother did not drink and 1 if mother did drink alcohol during pregnancy; 0 if child is female and 1 if child is male).
Three categories of models were planned a priori. Within each model category, we fit a separate model for each birth outcome. Our primary models adjusted for AA and the sum of EPA and DHA (EPA + DHA), both without and with adjustment for MeHg. We also fit models examining the association between MeHg and each birth outcome that did not adjust for LC-PUFAs to determine whether LC-PUFAs confound the MeHg association (and vice versa). In secondary models that adjusted for the same covariates, but without LC-PUFAs, we examined each of the 4 FADS genotypes separately as primary predictors of birth outcomes, both with and without concurrent adjustment for maternal MeHg exposure. In each secondary model, the homozygous FADS genotype was the reference group. We fit an additional set of tertiary models to examine the interactions between maternal LC-PUFAs and each maternal FADS genotype on birth outcomes. Each tertiary model included an n–3 LC-PUFA interaction term (EPA + DHA × FADS) and an n–6 LC-PUFA interaction term (AA × FADS). Because each FADS genotype has 3 possible levels, the interaction model allows the slope for LC-PUFAs to differ for each FADS variant. For example, the FADS1rs174537 model included an AA × FADS (GG, GT, or TT) interaction term with the following 3 levels: AA × GG, AA × GT, and AA × TT, where GG is the homozygous major allele (or reference genotype). These models were re-parameterized to include a slope (β) for each interaction term level, which represented the true slope for observations with that corresponding FADS variant. This model did not include MeHg. All tests were 2-sided and statistical significance was determined using P < 0.05.
Where mothers were missing ≥1 covariate, we classified these observations as missing in the analysis; this resulted in a total of n = 1111 in models examining LC-PUFAs and MeHg, n = 1189 in FADS models without MeHg, and n = 1102 in FADS models with MeHg. The interaction model included n = 1178 mothers. Supplemental Figure 1 shows the distribution of participant numbers in our analysis.
Results
Table 1 shows the descriptive characteristics of the complete NC2 cohort of 1236 mother–child pairs with available data for any of the models in the current analysis. Table 2 presents the frequency of each FADS genotype and allele presentation among NC2 mothers. As we have previously reported for this cohort, the minor allele frequencies for the FADS SNPs are lower in the Seychellois population compared with European and African populations (29). The frequency of mothers homozygous for each FADS minor allele was ≤5.5%. Three of the SNPs (FADS1rs174561 and rs174537, FADS1–FADS2 intergenic rs3834458) exist in high linkage disequilibrium as previously reported (29).
TABLE 1.
Characteristics of mothers and infants in NC2 with complete data after missing covariate exclusions1
| Females (n = 588) | Males (n = 648) | |
|---|---|---|
| Maternal age at enrollment, y | 26.70 ± 6.12 [16.10–43.95] | 27.03 ± 6.42 [16.03–46.56] |
| Gestational age, wk | 39.07 ± 1.31 [34.00–41.00] | 39.08 ± 1.41 [34.00–41.00] |
| Parity | 0.85 ± 1.04 [0–6.00] | 0.93 ± 1.17 [0–8.00] |
| Hollingshead SES | 32.11 ± 10.25 [14.00–63.00] | 32.02 ± 10.51 [14.00–63.00] |
| BMI, kg/m2 | 26.66 ± 6.61 [14.67–49.60] | 27.12 ± 6.46 [14.71–48.88] |
| Prenatal hair mercury, ppm | 3.90 ± 3.47 [0–31.66] | 3.96 ± 3.52 [0.03–29.14] |
| Maternal serum EPA + DHA, mg/mL | 0.24 ± 0.09 [0.09–0.60] | 0.24 ± 0.09 [0.09–0.57] |
| Maternal serum AA, mg/mL | 0.20 ± 0.08 [0.04–0.38] | 0.20 ± 0.08 [0.04–0.38] |
| Birth weight, kg | 3.13 ± 0.44 [1.62–4.51] | 3.26 ± 0.48 [1.74–5.20] |
| Birth length, cm | 50.78 ± 3.21 [32.00–58.50] | 51.45 ± 3.13 [34.00–60.00] |
| Head circumference, cm | 33.57 ± 1.54 [28.00–38.00] | 33.97 ± 1.56 [28.00–38.00] |
The total number of participants was n = 1236; values are means ± SDs [range, minimum–maximum]. AA, arachidonic acid; NC2, Nutrition Cohort 2; SES, socioeconomic status.
TABLE 2.
Frequency of each FADS genotype in NC2 mothers1
| Homozygote reference | Heterozygote minor | Homozygote minor | |||||
|---|---|---|---|---|---|---|---|
| SNP | Gene | Allele | n (%) | Allele | n (%) | Allele | n (%) |
| rs174537 | FADS1 | GG | 800 (67) | GT | 348 (29) | TT | 41 (4) |
| rs174561 | FADS1 | TT | 854 (72) | TC | 300 (25) | CC | 35 (3) |
| rs174575 | FADS2 | CC | 732 (62) | CG | 391 (33) | GG | 65 (5) |
| rs38344582 | FADS1–FADS2 | TT | 833 (70) | Tdel | 321 (27) | DelDel | 35 (3) |
The FADS model included n = 1189 participants. FADS, fatty acid desaturase; NC2, Nutrition Cohort 2; SNP, single nucleotide polymorphism.
Intergenic SNP.
Maternal PUFA status and MeHg exposure
Table 3 presents the results of primary multiple regression models examining associations between maternal LC-PUFA status, MeHg exposure, and birth outcomes. Maternal LC-PUFA status was not found to be associated with birth weight, length, or head circumference, either with or without adjustment for MeHg. MeHg was not associated with any of the birth outcomes.
TABLE 3.
Associations between maternal LC-PUFA status and MeHg exposure on birth outcomes1
| Birth weight | Birth length | Head circumference | ||||
|---|---|---|---|---|---|---|
| Model and exposure | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P |
| LC-PUFAs only (n = 1111/1092/1091) | ||||||
| EPA + DHA | 0.124 (−0.214, 0.462) | 0.47 | 0.801 (−1.777, 3.379) | 0.54 | 0.132 (−1.108, 1.372) | 0.83 |
| AA | −0.053 (−0.415, 0.309) | 0.77 | 1.682 (−1.088, 4.452) | 0.23 | 0.219 (−1.114, 1.553) | 0.75 |
| LC-PUFAs and MeHg (n = 1111/1092/1091) | ||||||
| EPA+DHA | 0.122 (−0.219, 0.462) | 0.48 | 0.913 (−1.681, 3.507) | 0.49 | 0.074 (−1.174, 0.321) | 0.91 |
| AA | −0.051 (−0.414, 0.312) | 0.78 | 1.603 (−1.174, 4.381) | 0.26 | 0.261 (−1.076, 1.598) | 0.70 |
| MeHg | 0 (−0.006, 0.007) | 0.89 | −0.020 (−0.072, 0.031) | 0.44 | 0.01 (−0.014, 0.035) | 0.41 |
| MeHg only (n = 1111/1092/1091) | ||||||
| MeHg | 0.001 (−0.006, 0.007) | 0.83 | −0.019 (−0.070, 0.032) | 0.47 | −0.019 (−0.070, 0.032) | 0.47 |
In the primary model the numbers of participants were n = 1111 for all birth-weight models, n = 1092 for all birth-length models, and n = 1091 for all head-circumference models. All models adjusted for maternal age, gestational age, child sex, maternal BMI, parity, alcohol use in pregnancy, and socioeconomic status. LC-PUFA, long-chain PUFA; MeHg, methylmercury.
FADS genotype
Results of the secondary multiple regression models examining associations between maternal FADS genotype and birth outcomes are shown in Table 4. Results from the models without MeHg adjustment are available in Supplemental Table 1. Compared with the major allele homozygotes, having 1 copy of the minor allele for FADS1rs174537 (genotype GT), rs174561 (genotype TC), and FADS1–FADS2rs3834458 (genotype Tdel), as well as 2 copies of minor allele for FADS2rs174575 (genotype GG), was predictive of having significantly greater birth weight (Supplemental Table 1). Following adjustment for MeHg, only the association between FADS2rs174575 minor allele homozygosity and a greater birth weight than major allele homozygotes remained significant (GG compared with CC; β = 0.109; 95% CI: 0.005, 0.213; P = 0.04). There were no significant associations between FADS genotype and birth length, either with or without adjustment for MeHg exposure.
TABLE 4.
Associations between maternal FADS genotype and MeHg exposure on birth outcomes1
| Birth weight | Birth length | Head circumference | ||||
|---|---|---|---|---|---|---|
| FADS model and allele | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P |
| rs174537 | ||||||
| GT (n = 327) | 0.048 (−0.004, 0.100) | 0.07 | 0.127 (−0.268, 0.523) | 0.53 | 0.205 (0.015, 0.394) | 0.03 |
| TT (n = 37) | 0.084 (−0.047, 0.215) | 0.21 | 0.350 (−0.655, 1.354) | 0.49 | 0.170 (−0.311, 0.650) | 0.49 |
| MeHg | −0.001 (−0.007, 0.006) | 0.87 | −0.023 (−0.075, 0.029) | 0.39 | 0.008 (−0.017, 0.033) | 0.54 |
| rs174561 | ||||||
| TC (n = 284) | 0.053 (−0.001, 0.107) | 0.06 | 0.267 (−0.143, 0.678) | 0.20 | 0.203 (0.007, 0.399) | 0.04 |
| CC (n = 31) | 0.089 (−0.054, 0.231) | 0.22 | 0.361 (−0.732, 1.455) | 0.52 | 0.301 (−0.223, 0.824) | 0.26 |
| MeHg | −0.001 (−0.007, 0.006) | 0.88 | −0.025 (−0.077, 0.028) | 0.35 | 0.008 (−0.017, 0.033) | 0.54 |
| rs174575 | ||||||
| CG (n = 366) | 0.003 (−0.048, 0.053) | 0.91 | −0.061 (−0.445, 0.322) | 0.75 | 0.034 (−0.015, 0.218) | 0.72 |
| GG (n = 61) | 0.109 (0.005, 0.213) | 0.04 | 0.575 (−0.210, 1.360) | 0.15 | 0.266 (−0.110, 0.642) | 0.17 |
| MeHg | 0.000 (−0.007, 0.007) | 0.98 | −0.021 (−0.073, 0.030) | 0.42 | 0.011 (−0.014, 0.035) | 0.41 |
| rs3834458 | ||||||
| Tdel (n = 303) | 0.046 (−0.007, 0.099) | 0.09 | 0.186 (−0.217, 0.588) | 0.37 | 0.197 (0.005, 0.390) | 0.04 |
| DelDel (n = 31) | 0.104 (−0.039, 0.247) | 0.15 | 0.475 (−0.619, 1.570) | 0.39 | 0.276 (−0.248, 0.800) | 0.30 |
| MeHg | −0.001 (−0.007, 0.006) | 0.88 | −0.024 (−0.076, 0.028) | 0.37 | 0.008 (−0.017, 0.033) | 0.53 |
The secondary model of FADS included n = 1189 participants. Following adjustment for MeHg, this model included n = 1102. All models adjusted for maternal age, gestational age, child sex, maternal BMI, parity, alcohol use in pregnancy, and socioeconomic status. FADS, fatty acid desaturase; MeHg, methylmercury.
Each of the maternal FADS1 SNPs [rs174537 and rs174561 (GT compared with TT, β = 0.205, P = 0.03; TC compared with CC, β = 0.203, P = 0.04)] and FADS1–FADS2 [rs3834458 (Tdel compared with DelDel, β = 0.197, P = 0.03)] were statistically significant predictors of head circumference, in that having 1 copy of the minor allele was associated with a greater child head circumference at birth than being homozygote for the major allele (all P < 0.05). All of these associations were significant following adjustment for maternal MeHg exposure.
Across all models we found child sex and gestational age to be the most significant predictors of birth weight, length, and head circumference (P < 0.001; data not shown). Parity and maternal BMI were also significant covariates in models for birth weight (P < 0.01).
Interaction model
The slopes for EPA + DHA × FADS and for AA × FADS did not show significant differences across FADS groups for any birth outcome (all 2 df P values for each PUFA × FADS interaction >0.05, and all 4 df P values for the pair of interactions >0.05).
These results are shown in Supplemental Table 2. However, there was some suggestion of an adverse association between EPA + DHA and head circumference among the DelDel rs3834458 (FADS1–FADS2) genotype group (slope = −9.059; 95% CI: −17.708, −0.410; P = 0.04), in contrast to the other 2 genotypes where slopes (CI) were 0.281 (−1.167, 1.729) and 0.877 (−1.413, 3.167) for the TT and Tdel groups, respectively. This interaction is shown in Figure 1.
FIGURE 1.
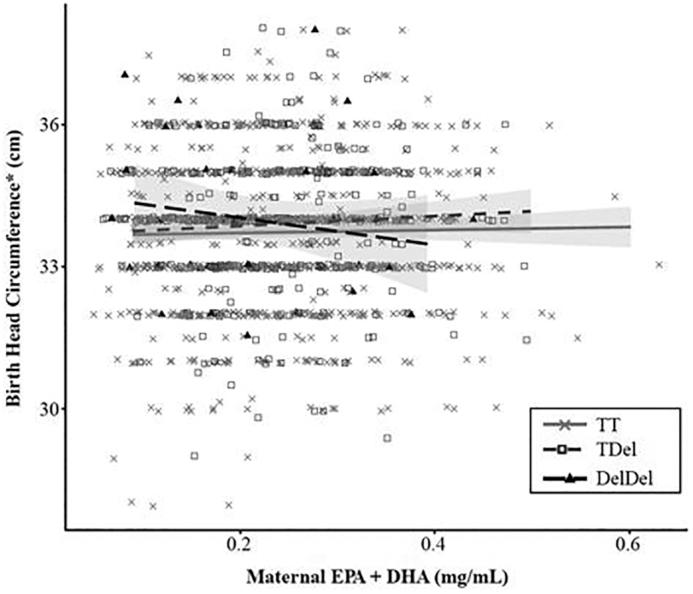
Interaction between maternal serum status of n–3 LC-PUFAs (EPA + DHA, mg/mL) and the rs3834458 FADS1–FADS2 genotype on infant head circumference at birth. FADS, fatty acid desaturase; LC-PUFA, long-chain PUFA.
Discussion
There is increasing interest in the influence of maternal nutrition, even among healthy, well-nourished populations, on birth outcomes and various health outcomes in later life. The n–3 LC-PUFAs, along with AA, are known to play critical roles in fetal growth and development (38, 39). In this large, carefully studied Seychellois mother–child cohort, maternal LC-PUFA status was not a determinant of infant birth weight, length, or head circumference. Simultaneously adjusting for concurrent exposure to MeHg in this high-fish-eating cohort did not influence these associations, nor was maternal hair MeHg itself associated with any birth outcomes. These results confirm those of our previous analysis of the smaller SCDS NC1, where we did not detect any relations between prenatal LC-PUFAs, MeHg, and birth weight (25). Some studies have reported positive associations between prenatal n–3 LC-PUFAs, birth weight (40), and gestational duration (38). However, the findings of observational mother–child cohorts, where maternal fish consumption has been the exposure variable, have been overall inconclusive (13, 14, 17, 41). Unlike most of these studies, we analyzed physiological biomarkers of maternal LC-PUFA status and MeHg exposure rather than dietary assessment of fish consumption. We believe it is a more accurate depiction of what the mother and fetus are exposed to during pregnancy. Our cohort is unique in that mothers consumed a mean of 8 fish meals/wk during pregnancy (33) and therefore have higher n–3 LC-PUFA status and MeHg exposure than lower-fish-consuming populations. Furthermore, our cohort of mothers is healthy with few preterm births or infants with low birth weight or extreme head circumference (n = 19). These mother–child pairs were excluded from our analysis. The factors above may partially explain the lack of associations found between LC-PUFAs and birth outcomes. In the SCDS we have consistently found no adverse associations between maternal MeHg exposure, at amounts ∼10 times higher than in the US population, and infant cognitive development (33, 42, 43). The results of the current study also found no detrimental associations with fetal growth, even when LC-PUFA status was accounted for. These findings are supportive of promoting fish consumption in pregnancy.
There are many differences across studies that may explain the inconsistency of findings, most notably the intake of n–3 LC-PUFAs. However, genetic background may also be important. Consequently, we examined associations between maternal FADS genotype and birth outcomes as a secondary aim of this study. Research by Molto-Puigmarti et al. (31) suggests that mothers who are minor allele carriers of FADS genotypes have infants of lower birth weight and shorter pregnancies overall, possibly as a result of having lower circulating concentrations of n–3 LC-PUFAs. In our study, we found maternal FADS genotype to be predictive of infant birth weight and head circumference, with different associations shown for the FADS1 and FADS2 genotypes. The most consistent associations were found with head circumference, where mothers who were heterozygous for the FADS1 (rs174537, rs174561) and FADS1–FADS2 (rs3834458) minor alleles, and therefore having less efficient conversion of LC-PUFAs compared with those homozygous for the major allele, were more likely to have infants with a greater head circumference. The 2 FADS1 SNPs and intergenic FADS1–FADS2rs3834458 are highly correlated (29) and therefore their similar associations with head circumference are expected.
Although the overall EPA + DHA × FADS interaction was not significant for any FADS genotype, Figure 1 shows that increasing concentrations of EPA + DHA are associated with lower head circumferences but only among mothers who are homozygous (DelDel) for the rs3834458 minor allele. Based on data from n = 35 mothers with this genotype, a 1-mg/mL increase in maternal serum EPA + DHA is associated with a mean birth head circumference decrease of −9.06 cm. This finding suggests that the relation between the FADS genotype and birth head circumference may be modified by the mothers’ LC-PUFA status. This finding is of particular interest given that our main effects model found greater head circumference among mothers heterozygous for the same FADS1–FADS2 minor allele. The possibility that this association differs by maternal EPA + DHA intake is worth further investigation, particularly given that head size at birth has been linked to cognitive function in childhood (44). However, when interpreting this finding it is important to recognize that the 95% CI for this estimate is quite wide (−17.708, −0.410) and that we did not find a direct association of maternal LC-PUFA status and head circumference. In addition, we did not adjust for multiple comparisons and this association may be a chance finding.
The FADS2rs174575 was not statistically associated with head circumference but was positively associated with birth weight, albeit in the opposite direction found by similar studies (30, 31). In our study, mothers homozygous for the minor allele of the FADS2 genotype (i.e., expected lower endogenous synthesis of LC-PUFAs) were found to have infants of a greater birth weight. This contrasting finding may be explained by our cohort having a low n–6 to n–3 PUFA ratio, likely due to high fish consumption. In NC2 we previously reported that maternal AA status was significantly lower among minor allele carriers of rs3834458, whereas the status of EPA and DHA was not significantly different (29). It is possible that a greater infant birth weight and head circumference among mothers who carry ≥1 copy of the minor allele for the FADS genotypes measured in our study may be related to lower maternal circulating concentrations of the AA-derived eicosanoids with vasoconstricting properties, prostaglandin F2 and prostaglandin E2 (45). We have previously hypothesized that AA may be a rate-limiting LC-PUFA in the Seychellois population due to high dietary intakes of n–3 LC-PUFAs, so that even a small decrease in AA relative to EPA and DHA exerts a more pronounced biological effect (33). Yet, we did not see a significant interaction between AA status and FADS genotype. In contrast, Molto-Puigmarti et al. (31) found a detrimental association of AA with birth weight but only among women who were homozygous for the minor allele of FADS1rs174556.
We did not find any relations between either LC-PUFAs, MeHg, or FADS genotype with birth length. This finding could be due to inherent difficulties associated with measuring infant length at birth, or to a different underlying biological mechanism. In their systematic review and meta-analysis of 26 studies, Li et al. (41) reported that a high prenatal dose of DHA (≥800 mg/d) was significantly associated with a greater birth length compared with a low dose (<800 mg/d), with a weighted mean difference of 0.26 cm. They did not find EPA supplementation to be associated with birth length. Although this was a subgroup analysis, it points to there being a potential optimal dosage of n–3 PUFAs for birth length and warrants further study.
Future research in this area should consider the additional measurement of maternal inflammatory or eicosanoid markers, which may also reveal further associations and improve mechanistic understanding in this area. Our robust biological measures of both serum LC-PUFA concentration and MeHg exposure represent a particular strength to our research, as well as the large cohort size and comprehensive adjustment for confounding variables. In all of our models, child sex and gestational age were the strongest covariate determinants of birth outcomes; these relations also warrant further study. This is the first study, to our knowledge, to present consistent associations between the particular maternal FADS SNPs studied and infant birth weight and head circumference. These findings need confirmation in other populations of varying fish consumption, LC-PUFA status, and genetic background.
In conclusion, in the SCDS where fish consumption is high throughout pregnancy, we did not find maternal LC-PUFA status, or exposure to MeHg, to be associated with infant birth outcomes as assessed by weight, length, and head circumference. Our observations of associations between the maternal FADS SNPs studied and infant birth weight and head circumference should be confirmed in other populations.
Supplementary Material
Acknowledgments
We acknowledge the participation of all women and children who took part in the study and the nursing staff from the Child Development Centre, Seychelles, for their assistance with data collection. The authors’ responsibilities were as follows—KB: conceived, designed, and conducted the research; PWD, GJM, EvW, CFS, GEW, JJS: conceived and designed the SCDS NC2 and conducted the research; AJY, MSM, EMM, AA, KE, KW: conducted the research; AZ, SWT: performed the statistical analyses; AJY, AZ, SWT, KB, EvW: interpreted the data; AJY: drafted the manuscript and has primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
This research was supported by grants R01-ES010219, P30-ES001247, R03-ES027514, and T32-ES007271 from the US National Institute of Environmental Health Sciences (NIH), the Swedish Research Council for Environmental, Agricultural Sciences and Spatial Planning (FORMAS), and by in-kind support from the Government of the Republic of Seychelles.
Author disclosures: The authors report no conflicts of interest. The study sponsors had no role in the design, collection, analysis, or interpretation of the data; in the writing of the report; or in the decision to submit the article for publication.
Supplemental Tables 1 and 2 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: AA, arachidonic acid; FADS, fatty acid desaturase; LC-PUFA, long-chain PUFA; MeHg, methylmercury; NC1, Nutrition Cohort 1; NC2, Nutrition Cohort 2; SCDS, Seychelles Child Development Study; SES, socioeconomic status; SGA, small for gestational age; SNP, single nucleotide polymorphism.
Contributor Information
Alison Jayne Yeates, Nutrition Innovation Centre for Food and Health (NICHE), Ulster University, Londonderry, United Kingdom.
Alexis Zavez, School of Medicine and Dentistry, University of Rochester, Rochester, NY, USA.
Sally W Thurston, School of Medicine and Dentistry, University of Rochester, Rochester, NY, USA.
Emeir M McSorley, Nutrition Innovation Centre for Food and Health (NICHE), Ulster University, Londonderry, United Kingdom.
Maria S Mulhern, Nutrition Innovation Centre for Food and Health (NICHE), Ulster University, Londonderry, United Kingdom.
Ayman Alhamdow, Unit of Metals and Health, Institute of Environmental Medicine, Metals and Health, Karolinska Institute, Stockholm, Sweden.
Karin Engström, Laboratory of Medicine, Division of Occupational and Environmental Medicine, Lund University, Lund, Sweden.
Karin Wahlberg, Laboratory of Medicine, Division of Occupational and Environmental Medicine, Lund University, Lund, Sweden.
J J Strain, Nutrition Innovation Centre for Food and Health (NICHE), Ulster University, Londonderry, United Kingdom.
Gene E Watson, School of Medicine and Dentistry, University of Rochester, Rochester, NY, USA.
Gary J Myers, School of Medicine and Dentistry, University of Rochester, Rochester, NY, USA.
Philip W Davidson, School of Medicine and Dentistry, University of Rochester, Rochester, NY, USA.
Conrad F Shamlaye, Child Development Centre, Ministry of Health, Victoria, Mahé, Republic of Seychelles.
Karin Broberg, Unit of Metals and Health, Institute of Environmental Medicine, Metals and Health, Karolinska Institute, Stockholm, Sweden; Laboratory of Medicine, Division of Occupational and Environmental Medicine, Lund University, Lund, Sweden.
Edwin van Wijngaarden, School of Medicine and Dentistry, University of Rochester, Rochester, NY, USA.
References
- 1. Belbasis L, Sawidou M, Kanu C, Evangelou E, Tzoulaki I. Birth weight in relation to health and disease in later life: an umbrella review of systematic reviews and meta-analyses. BMC Med. 2016;14:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301(6761):1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meher A, Randhir K, Mehendale S, Wagh G, Joshi S. Maternal fatty acids and their association with birth outcome: a prospective study. PLoS One. 2016;11(1):e0147359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Newberry SJ, Chung M, Booth M, Maglione MA, Tang AM, O'Hanlon CE, Wang DD, Okunogbe A, Huang C, Motala A et al.. Omega-3 fatty acids and maternal and child health: an updated systematic review. Evid Rep Technol Assess. 2016;224:1–826. [DOI] [PubMed] [Google Scholar]
- 5. Szajewska H, Horvath A, Koletzko B. Effect of n-3 long-chain polyunsaturated fatty acid supplementation of women with low-risk pregnancies on pregnancy outcomes and growth measures at birth: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2006;83:1337–44. [DOI] [PubMed] [Google Scholar]
- 6. Kulkarni A, Mehendale S, Pisal H, Kilari A, Dangat K, Salunkhe S, Tarlekar V, Joshi S. Association of omega-3 fatty acids and homocysteine concentrations in pre-eclampsia. Clin Nutr. 2011;30:60–4. [DOI] [PubMed] [Google Scholar]
- 7. Grootendorst-van M, Tiemeier H, Steenweg-de Graaff J, Koletzko B, Demmelmair H, Jaddoe VWV, Steegers EAP, Steegers-Theunissen RPM. Maternal plasma n-3 and n-6 polyunsaturated fatty acids during pregnancy and features of fetal health: fetal growth velocity, birth weight and duration of pregnancy. Clin Nutr. 2018;37:1367–74. [DOI] [PubMed] [Google Scholar]
- 8. Elias SL, Innis SM. Infant plasma trans, n-6 and n-3 fatty acids and conjugated linoleic acids are related to maternal plasma fatty acids, length of gestation and birth weight and length. Am J Clin Nutr. 2001;73:807–14. [DOI] [PubMed] [Google Scholar]
- 9. Van Eijsden M, Hornstra G, van der Wal MF, Vrijkotte TGM, Bonsel GJ. Maternal n-3, n-6 and trans fatty acid profile early in pregnancy and term birth weight: a prospective cohort study. Am J Clin Nutr. 2008;87(4):887–95. [DOI] [PubMed] [Google Scholar]
- 10. Koletzko B, Braun M. Arachidonic acid and early human growth: is there a relation?. Ann Nutr Metab. 1991;35(3):128–31. [DOI] [PubMed] [Google Scholar]
- 11. Dirix CEH, Kester AD, Hornstra G. Associations between term birth dimensions and prenatal exposure to essential and trans fatty acids. Early Hum Devel. 2009;85:525–30. [DOI] [PubMed] [Google Scholar]
- 12. Larque E, Pagan A, Prieto MT, Blanco JE, Gil-Sanchez A, Zornoza-Moreno M, Ruiz-Palacios M, Gazquez A, Demmelmair H, Parrilla JJ et al.. Placental fatty acid transfer: a key factor in fetal growth. Ann Nutr Metab. 2014;64(3–4):247–53. [DOI] [PubMed] [Google Scholar]
- 13. Brantsæter AL, Birgisdottir BE, Meltzer HM, Kvalem HE, Alexander J, Magnus P, Haugen M. Maternal seafood consumption and infant birth weight, length and head circumference in the Norweigan Mother and Child Cohort Study. Br J Nutr. 2012;107:436–44. [DOI] [PubMed] [Google Scholar]
- 14. Rogers I, Emmett P, Ness A, Golding J. Maternal fish intake in late pregnancy and the frequency of low birth weight and intrauterine growth retardation in a cohort of British infants. J Epidemiol Community Health. 2004;58:486–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buck GM, Tee GP, Fitzgerald EF, Vena JE, Weiner JM, Swanson M, Msall ME. Maternal fish consumption and infant birth size and gestation: New York State Angler Cohort Study. Environ Health. 2003;2:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oken E, Kleinman KP, Olsen SF, Rich-Edwards JW, Gillman MW. Associations of seafood and elongated n-3 fatty acid intake with fetal growth and length of gestation: results from a US pregnancy cohort. Am J Epidemiol. 2004;160:774–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guldner L, Monfort C, Rouget F, Garlantezec R, Cordier S. Maternal fish and shellfish intake and pregnancy outcomes: a prospective cohort study in Brittany, France. Environ Health. 2007;6:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mohanty AF, Thompson ML, Burbacher TM, Sisovick DS, Williams MA, Enquobahrie DA. Periconceptional seafood intake and fetal growth. Paediatr Perinat Epidemiol. 2015;29:376–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nykjaer C, Higgs C, Greenwood DC, Simpson NAB, Cade JE, Alwan NA. Maternal fatty fish intake prior to and during pregnancy and risks of adverse birth outcomes: findings from a British Cohort. Nutrients. 2019;11(3):E643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ramon R, Ballester F, Aguinagalde X, Amurrio A, Vioque J, Lacasana M, Rebagliato M, Murcia M, Iniguez C. Fish consumption during pregnancy, prenatal mercury exposure and anthropometric measures at birth in a prospectieve mother-infant cohort study in Spain. Am J Clin Nutr. 2009;90(4):1047–55. [DOI] [PubMed] [Google Scholar]
- 21. Halldorsson TI, Meltzer HM, Thorsdottir I, Knudsen V, Olsen SF. Is high consumption of fatty fish during pregnancy a risk factor for fetal growth retardation? A study of 44, 824 Danish pregnant women. Am J Epidemiol. 2007;166(6):687–96. [DOI] [PubMed] [Google Scholar]
- 22. Foldspang A, Hansen JC. Dietary intake of methylmercury as a correlate of gestational length and birth weight among newborns in Greenland. Am J Epidemiol. 1990;132:310–7. [DOI] [PubMed] [Google Scholar]
- 23. Tatsuta N, Kurokawa N, Nakai K, Suzuki K, Iwai-Shimada M, Murata K, Satoh H. Effects of intrauterine exposures to polychlorinated biphenyls, methylmercury and lead on birth weight in Japanese male and female newborns. Environ Health Prev Med. 2017;22:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Drouillet-Pinard P, Huel G, Slama R, Forhan A, Sahuquillo J, Goua V, Thiebaugeorges O, Foliguet B, Magnin G, Kaminski M et al.. Prenatal mercury contamination: relationship with maternal seafood consumption during pregnancy and fetal growth in the “EDEN mother-child” cohort. Br J Nutr. 2010;104(8):1096–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van Wijngaarden E, Harrington D, Kobrosly R, Thurston SW, O'Hara T, McSorley EM, Myers GJ, Watson GE, Shamlaye CF, Strain JJ et al.. Prenatal exposure to methylmercury and LCPUFA in relation to birth weight. Ann Epidemiol. 2014;24(4):273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taylor CM, Golding J, Emond AM. Blood mercury levels and fish consumption in pregnancy: Risks and benefits for birth outcomes in a prospective observational birth cohort. Int J Hygiene Environ Health. 2016;219:513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grandjean P, Bjerve KS, Weighe P, Steuerwald U. Birthweight in a fishing community: significance of essential fatty acids and marine food contaminants. Int J Epidemiol. 2001;30(6):1272–8. [DOI] [PubMed] [Google Scholar]
- 28. Lucas M, Dewailly E, Muckle G, Ayotte P, Bruneau S, Gingras S, Rhainds M, Holub BJ. Gestational age and birth weight in relation to n-3 fatty acids among Inuit (Canada) Lipids. 2004;39:617–26. [DOI] [PubMed] [Google Scholar]
- 29. Yeates AJ, Love TM, Engstrom K, Mulhern MS, McSorley EM, Grzesik K, Alhamdow A, Wahlberg K, Thurston SW, Davidson PW et al.. Genetic variation in FADS genes is associated with maternal long-chain PUFA status but not with cognitive development of infants in a high fish-eating observational study. Prostaglandins Leukot Essent Fatty Acids. 2015;102–103:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bernard JY, Pan H, Aris IM, Moreno-Betancur M, Soh SE, Tap F, Tan KH, Shek LP, Chong YS, Gluckman PD et al.. Long-chain polyunsaturated fatty acids, gestation duration and birth size: a Mendelian randomization study using fatty acid desaturase variants. Am J Clin Nutr. 2018;108(1):92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Molto-Puigmarti C, Van Dongen MC, Dagnelie PC, Plat J, Mensink RP, Tan FE, Heinrich J, Thujs C. Maternal but not fetal FADS gene variants modify the association between maternal long-chain PUFA intake in pregnancy and birth weight. J Nutr. 2014;144:1430–7. [DOI] [PubMed] [Google Scholar]
- 32. Gonzalez-Casanova I, Rzehak P, Stein AD, Garcia Feregrino R, Rivera Dommarco JA, Barraza-Villarreal A, Demmelmair H, Romiru I, Villalpando S, Martorell R et al.. Maternal single nucleotide polymorphisms in the fatty acid desaturase 1 and 2 coding regions modify the impact of prenatal supplementation with DHA on birth weight. Am J Clin Nutr. 2016;103:1171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Strain JJ, Yeates AJ, van Wijnggarden E, Thurston SW, Mulhern MS, McSorley EM, Watson GE, Love TM, Smith TH, Yost K et al.. Prenatal exposure to methyl mercury from fish consumption and polyunsaturated fatty acids: associations with child development at 20 months of age in an observational study in the Republic of Seychelles. Am J Clin Nutr. 2015;101:530–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissue. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 35. Davidson PW, Myers GJ, Cox C, Axtell C, Shamlaye C, Sloane-Reeves J, Cernichiari E, Needham L, Choi A, Wang Y et al.. Effects of prenatal and postnatal methylmercury exposure from fish consumption on neurodevelopment. JAMA. 1998;280:701–7. [DOI] [PubMed] [Google Scholar]
- 36. McSorley EM, Yeates AJ, Mulhern MS, van Wijngaarden E, Grzesik K, Thurston SW, Spence T, Crowe W, Davidson PW, Zareba G et al.. Associations of maternal immune response with MeHg exposure at 28 weeks’ gestation in the Seychelles Child Development Study. Am J Reprod Immunol. 2018;80:e13046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hoenig JM, Heisey DM. The abuse of power. Am Stat. 2001;55(1):19–24. [Google Scholar]
- 38. Innis SM. Metabolic programming of long-term outcomes due to fatty acid nutrition in early life. Matern Child Nutr. 2011;7(Suppl 2):112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Olsen SF, Halldorsson TI, Thorne-Lynam AL, Strom M, Gortz S, Granstrom C, Nelsen PH, Wohlfahrt J, Lykke JA, Langhoff-Roos J et al.. Plasma concentrations of long chain n-3 fatty acids in early and mid-pregnancy and risk of early preterm birth. BioMed. 2018;35:325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Van Wijngaarden E, Thurston SW, Myers GJ, Harrington D, Cory-Slechta DA, Strain JJ, Watson GE, Zareba G, Love T, Henderson J et al.. Methyl mercury exposure and neurodevelopmental outcomes in the Seychelles Child Development Study Main Cohort at age 22 and 24 years. Neurotoxicol Teratol. 2017;59:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li GL, Chen HJ, Zhang WX, Tong Q, Yan YE. Effects of maternal omega-3 fatty acids supplementation during pregnancy/lactation on body composition of the offspring: a systematic review and meta-analysis. Clin Nutr. 2018;37(5):1462–73. [DOI] [PubMed] [Google Scholar]
- 42. Olsen SF, Olsen J, Frische G. Does fish consumption during pregnancy increase fetal growth? A study of the size of the newborn, placental weight and gestational age in relation to fish consumption during pregnancy. Int J Epidemiol. 1990;19:971–7. [DOI] [PubMed] [Google Scholar]
- 43. Strain JJ, Davidson PW, Bonham MP, Duffy EM, Stokes-Riner A, Thurston SW, Wallace JMW, Robson PJ, Shamlaye CF, Georger LA et al.. Associations of maternal long-chain polyunsaturated fatty acids, methylmercury and infant development in the Seychelles Child Development Nutrition Study. Neurotoxicology. 2008;29:776–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Veena SR, Krishnaveni GC, Wills AK, Kurpad AV, Muthayya S, Hill JC, Karat SC, Nagarajaiah KK, Fall CHD, Srinivasan K. Association of birthweight and head circumference at birth to cognitive performance in 9–10 year old children in South India: prospective birth cohort study. Pediatr Res. 2010;67(4):424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Olsen SF, Osterdal ML, Salvig JD, Weber T, Tabor A, Secher NJ. Duration of pregnancy in relation to fish oil supplementation and habitual fish intake: a randomised clinical trial with fish oil. Eur J Clin Nutr. 2007;61:976–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


