ABSTRACT
Background
A protective association of dietary carotenoids with cognitive function has been suggested, but most studies have been relatively small with limited periods of follow-up.
Objectives
We examined prospectively long-term intakes of carotenoids in relation to subjective cognitive function (SCF), a self-reported, validated indicator of cognitive dysfunction.
Methods
Among 49,493 female registered nurses with a mean age of 48 y in 1984, we used multinomial logistic regression to estimate the ORs and 95% CIs relating intakes of carotenoids to self-reported SCF in 2012 and 2014. Mean intakes of carotenoids were calculated from 7 repeated FFQs collected in 1984, 1986, and every 4 y afterwards until 2006. Self-reported SCF was assessed by a 7-item questionnaire on changes in memory and cognition; validity was supported by strong associations with Apolipoprotein E (APOE) ε4 genotype and concurrent cognitive function and cognitive decline measured by telephone-based neuropsychological tests. The mean values of scores assessed in 2012 and 2014 were categorized as “good” (0 points, 40.8%), “moderate” (0.5–2.5 points, 46.9%), and “poor” (3–7 points, 12.3%).
Results
Higher intake of total carotenoids was associated with substantially lower odds of moderate or poor cognitive function after controlling for other dietary and nondietary risk factors and total energy intake. Comparing the top with the bottom quintile of total carotenoids, the multivariable ORs were 0.86 (95% CI: 0.80, 0.93; P-trend < 0.001) for moderate SCF and 0.67 (95% CI: 0.60, 0.75; P-trend < 0.001) for poor SCF. This lower OR was also seen for carotenoids consumed 28 y before SCF assessment. Similar associations were found for total β-carotene, dietary β-carotene, α-carotene, lycopene, lutein + zeaxanthin, and β-cryptoxanthin. The significant associations for β-cryptoxanthin, lycopene, and lutein + zeaxanthin persisted after mutual adjustment for each other.
Conclusions
Our findings support a long-term beneficial role of carotenoid consumption on cognitive function in women.
Keywords: dietary carotenoids, subjective cognitive function, age-related cognitive function, prospective study, α-carotene, β-carotene, β-cryptoxanthin, lutein + zeaxanthin, lycopene
Introduction
Despite reductions in age-specific incidence of dementia, the number of persons experiencing age-related cognitive decline is increasing dramatically worldwide and leading to significant personal suffering, high risk of dementia, and huge burdens on families and health care systems (1, 2). Because no effective treatment exists for dementia (3, 4), identification of risk factors to reduce or delay the onset of the early stages of dementia would have substantial public health and clinical impact (5, 6).
Carotenoids, which are naturally occurring pigments found in red, yellow, orange, and dark green fruits and vegetables, have been suggested to play a role in prevention of cognitive decline or dementia (7–9). Although the underlying mechanism is not fully understood, it may relate to antioxidant activity that may inhibit β-amyloid deposition and fibril formation (10–14). Nonetheless, previous observational studies and randomized controlled trials have produced equivocal results for carotenoid intake and cognitive function (7, 8, 15). In studies with relatively small sample sizes and limited periods of follow-up, no associations were observed (16–19). For several studies with >7 y of follow-up, protective associations were seen for intake of total and individual carotenoids (20–23) and for a carotenoid-rich dietary pattern (24).
In particular, a previous study based on 16,010 female participants in the Nurses’ Health Study (NHS) reported that higher mean intakes of lycopene, lutein + zeaxanthin, and total carotenoids over 15 y were related to better overall cognition at older ages and slower cognitive decline (only for dietary lycopene) measured by telephone-based neuropsychological testing (20). In line with the increasing interest in subjective cognitive measures as a sensitive precursor to mild cognitive impairment, the present study aimed to replicate and expand the study on carotenoids and cognitive function with an additional decade of follow-up and the collection of repeated subjective cognitive measures among 49,493 women in the NHS.
Methods
Study design
The NHS (25) included 121,701 female registered nurses aged 30–55 y in 1976 in the United States. Every 2 y, follow-up questionnaires have been sent to update information on potential risk factors and to identify newly diagnosed cases of chronic diseases. The 1984, 1986, and quadrennial follow-up questionnaires included comprehensive semiquantitative food-frequency questionnaires (SFFQs). Response rates have been ∼90% for each 2-y cycle. A total of 57,364 women with eligible dietary information in 1984 (energy intake range: 600–3500 kcal/d; number of food item blanks < 70) were alive in 2006 (Supplemental Figure 1). Among them, 49,693 participants reported their subjective cognitive function (SCF) in either 2012 or 2014. We further excluded 200 participants who developed Parkinson disease before 2012 and the final study included 49,493 women with a mean age of 48 y at baseline in 1984. The study was approved by the Human Subjects Committees of the Harvard TH Chan School of Public Health and Brigham and Women's Hospital.
Dietary assessment
For this analysis, we began follow-up in 1984, when the first comprehensive SFFQ with 131 items was administered to the NHS cohort. Participants reported how often, on average, they consumed each food using a specified portion size. Validation studies within the NHS from baseline to recent years have documented that the study SFFQ provides reasonably valid estimates for intakes of >140 nutrients, food/food groups, and diet patterns compared with weighed dietary records and nutrient biomarkers (26–30).
In a recent validation study, for intakes of specific carotenoids comparing the SFFQ with two 7-d weighed dietary records collected over a period of 1 y, the Spearman correlation coefficients (corrected for within-person variation in dietary records) were 0.64 for α-carotene, 0.63 for β-carotene, 0.68 for lutein + zeaxanthin, 0.61 for lycopene, and 0.54 for β-cryptoxanthin (28). Carotenoid intakes measured by the study SFFQ were also correlated with plasma concentrations (r = 0.51 for α-carotene, 0.41 for β-carotene, 0.46 for β-cryptoxanthin, 0.35 for lutein, and 0.33 for lycopene) (27).
Mean intakes of carotenoids were calculated from 7 repeated SFFQs collected in 1984, 1986, and every 4 y up to 2006, which was 6 y before the SCF measurement. The year of 2006 was chosen to make the best use of the repeated dietary data in order to represent long-term diet (31), and to minimize possible effects of altered cognitive function on diet. Each participant's nutrient intakes were adjusted for total energy intake using the residual method (32). In addition to total carotenoids, we considered the following individual carotenoids: β-carotene, α-carotene, lutein + zeaxanthin, lycopene, and β-cryptoxanthin. Of the carotenoids, only β-carotene was regularly included in vitamin supplements throughout the dietary reporting period; thus, we evaluated β-carotene in foods and supplements but only dietary intakes of α-carotene, lutein + zeaxanthin, lycopene, and β-cryptoxanthin. Mean total energy intake and alcohol consumption, and major nutrients from these dietary questionnaires were also calculated. The total number of returned SFFQs (range: 1–7) for each participant was calculated, and >87% participants completed ≥6 SFFQs. Although this percentage of completion was high, we adjusted for the number of SFFQs to account for the possibility that this might be a confounding factor.
Assessment of SCF
Assessment of SCF was based on 7 yes/no questions on recent change in general memory, executive function, attention, and visuospatial skills (33, 34): Have you recently experienced any change in your ability to remember things? Do you have more trouble than usual remembering recent events? Do you have more trouble than usual remembering a short list of items, such as a shopping list? Do you have trouble remembering things from one second to the next? Do you have any difficulty in understanding things or following spoken instructions? Do you have more trouble than usual following a group conversation or a plot in a TV program due to your memory? Do you have trouble finding your way around familiar streets? We then assigned equal value to each question, giving 1 point for every “yes,” and for each woman summed the points. If a woman completed only 1 of the 2 SCF assessments, we computed her SCF score using only 1 questionnaire. Otherwise, the mean value of the 2 questionnaires was used; 86% of participants completed both questionnaires. Based on the distribution of scores in the study population, we categorized scores as “good” (0 points), “moderate” (0.5–2.5 points), and “poor” (3–7 points).
SCF can serve as a preclinical indicator of dementia, and is associated with the brain pathology of dementia in clinically normal older individuals (34–40). We also reported inverse relations of Mediterranean diet, vegetables, and fruits with similar SCF scores in a large cohort of men in the United States (41, 42). Two validation studies conducted among 16,964 women enrolled in the NHS found robust and sensitive relations between SCF score and concurrently assessed change in cognitive function, measured by telephone-based neuropsychological testing during 6 y of follow-up (34, 43). The validity of the SCF score was further supported by strong associations with Apolipoprotein E (APOE) ε4 genotype in the NHS; the age-standardized prevalence of the homozygous APOE ε4 allele was 1.3% in the good-SCF group and 2.4% in the poor-SCF group (P-trend < 0.01). In addition, the mean baseline age was 52 y in the poor-SCF group compared with 47 y in the good-SCF group. Depression, heavy smoking, elevated blood cholesterol, high blood pressure, diabetes, cancer, and cardiovascular disease (CVD) were all associated with future low SCF (Supplemental Table 1). These multiple lines of evidence indicate that the SCF scores reflect the underlying pathology of dementia. For the present study, SCF was the primary outcome.
Assessment of other covariates
Information on covariates of interest, including lifestyle factors and medical history, was prospectively collected at NHS baseline and by follow-up questionnaires. Information from baseline at 1984 until 2006 was utilized to be consistent with the time frame of dietary assessment. Baseline age and height, race (white, other), education (registered nurse, bachelor's degree, graduate degree), husband education (high school degree or less, college degree, or graduate degree), parental history of dementia, averaged BMI (in kg/m2) and physical activity (metabolic equivalent of tasks, hours per week) from 1984–2006, and other self-reported covariates were each updated to 2006 (except where indicated differently). Other variables used as covariates, or for exclusion or stratification, included multivitamin use, smoking status in pack-years, diabetes, high blood pressure, elevated cholesterol, CVD (stroke, myocardial infarction, angina, or coronary artery surgery), cancer (excluding nonmelanoma skin cancer), postmenopausal status and hormone replacement therapy use, parity, depression (defined as use of antidepressants in 1996–2006 or self-reported depression in 2002–2006), missing indicator for SCF measurement at 2012 or 2014, number of dietary assessments from 1984–2006, APOE ε4 carrier status, self-reported moderate or severe hearing problems at 2012, self-reported eye disease (cataracts, age-related macular degeneration, and glaucoma) before or at 2014, and optimism level measured at 2004, 2008, and 2012. APOE ε4 carrier status was available in a subgroup of 14,108 women who had APOE ε4 directly genotyped or imputed from a genome-wide association analysis as part of case-control studies for various endpoints [imputation using single nucleotide polymorphisms rs429358 and rs7412 (44)].
Statistical analyses
We calculated age-standardized characteristics for all participants and according to quintiles (Q1 and Q5) of total and individual carotenoid intakes. To evaluate associations between intakes of carotenoids from 1984–2006 and odds of SCF status at 2012/2014, we used multinomial logistic regression to calculate ORs and 95% CIs. Intakes of carotenoids were divided into quintiles based on the distribution in the overall participants. Comparisons were made between the moderate and poor SCF categories and the good SCF category. We calculated estimates of associations, adjusting for baseline age and height; race; education; husband education; parental history of dementia; averaged total energy intake, alcohol intake, BMI, and physical activity in 1984–2006; smoking status; multivitamin use; self-reported diagnosis of hypercholesterolemia, hypertension, diabetes, cancer, and CVD; postmenopausal status and hormone replacement therapy use; parity; and depression updated until 2006; missing indicator for SCF measurement at 2012 or 2014; and number of dietary assessments during 1984–2006. To examine if the observed associations were independent of other nutrients, we further conducted analyses also adjusting for vitamins C, D, and E, anthocyanidins, trans-fat, saturated fat, and long-chain n–3 fatty acids. Missing indicators were created for variables with missing values. We also treated SCF as a continuous variable and performed multivariable linear regression with this outcome. Moreover, we conducted additional sensitivity analyses treating the 2012 SCF (n = 48,201) and 2014 SCF (n = 43,843) scores separately, and limited to those with both SCF questionnaires (n = 42,551). In the analyses of the 5 individual carotenoids, a Bonferroni-corrected P value (0.05/5 = 0.01) was used to account for multiple comparisons. In exploratory analyses, we investigated the independent associations of individual carotenoids selected by a stepwise method (entry criterion = 0.10, retention criterion = 0.10). We also conducted exploratory sensitivity analyses by including all participants who reported diet in 1984 and 2006 (n = 57,364) and then including those who died or did not respond into the poor-SCF category.
To evaluate the temporal relations of carotenoid intake with SCF, we examined diets at individual years (1984, 1986, 1990, 1994, 1998, 2002, 2006) in relation to SCF. We also mutually adjusted for the mean of carotenoid intakes for 1984–1990 and for 2002–2006 in a multivariable model to evaluate the independent associations of remote and recent dietary intakes with SCF. To address the possibility of residual confounding and outcome misclassification, we conducted sensitivity analyses by further adjusting for hearing problems, eye disease, and optimism level (45). We further examined whether the associations between diet and SCF varied by baseline age (<50 y, ≥50 y), disease status (self-reported CVD, depression, diabetes, elevated cholesterol, and high blood pressure), hearing problems (yes/no), eye disease (yes/no), parental history of dementia, smoking status (<5 pack-years, ≥5 pack-years), and APOE ε4 allele carrier status. In the stratified analyses, we also treated SCF as a continuous variable and calculated the difference in 1-y equivalents of SCF when comparing the 90th with the 10th percentile of total carotenoid intake (21.7 compared with 9.4 mg/d). All analyses were conducted using SAS software, version 9.3 (SAS Institute Inc.).
Results
Among the 49,493 women in this analysis, the mean age in 1984 was 48 y, and in 2012–2014 41% had good cognitive function, 47% had moderate function, and 12% had poor function. Intakes of α-carotene and lutein + zeaxanthin were highly correlated with β-carotene (Spearman correlation: r = 0.70 and 0.73, respectively) (Supplemental Table 2). Table 1 presents the age-standardized characteristics of study participants by quintiles (Q1 and Q5) of total carotenoids, total β-carotene, and β-cryptoxanthin (see Supplemental Table 3 for characteristics by quintiles of lutein + zeaxanthin, lycopene, and α-carotene). In general, characteristics were similar across increasing quintiles of total carotenoid intake. However, participants with higher carotenoid intake tended to be more engaged in physical activities, to have higher multivitamin use, to smoke less, and to be less depressed.
TABLE 1.
Characteristics in 1984–2006 of 49,493 women in the Nurses’ Health Study who completed SCF questions in 2012–2014, by quintiles (Q1 and Q5) of total and specific carotenoid intakes1
| Overall participants | Total carotenoids | Total β-carotene | Total β-cryptoxanthin | ||||
|---|---|---|---|---|---|---|---|
| Variables | Q1 | Q5 | Q1 | Q5 | Q1 | Q5 | |
| n | 49,493 | 9898 | 9898 | 9898 | 9899 | 9897 | 9898 |
| Age at study baseline, y | 48 ± 7 | 49 ± 7 | 48 ± 6 | 47 ± 7 | 49 ± 7 | 47 ± 6 | 51 ± 7 |
| Intakes (1984–2006) | |||||||
| Total calorie intake, kcal/d | 1740 ± 418 | 1750 ± 424 | 1670 ± 421 | 1710 ± 427 | 1720 ± 421 | 1690 ± 426 | 1680 ± 396 |
| Total alcohol use, g/d | 5.7 ± 8.4 | 5.1 ± 8.7 | 6.0 ± 8.0 | 5.3 ± 9.1 | 5.7 ± 7.7 | 7.1 ± 10.4 | 4.7 ± 6.9 |
| Total carotenoids, mg/d | 15.1 ± 5.2 | 9.1 ± 1.5 | 23.0 ± 4.3 | 9.9 ± 2.6 | 21.8 ± 5.0 | 12.8 ± 4.6 | 17.1 ± 5.8 |
| β-carotene, mg/d | 5.0 ± 2.3 | 2.8 ± 0.8 | 8.0 ± 2.6 | 2.5 ± 0.5 | 8.6 ± 2.1 | 3.9 ± 1.9 | 6.0 ± 2.7 |
| Without supplement | 4.4 ± 1.9 | 2.5 ± 0.7 | 6.8 ± 2.1 | 2.3 ± 5.4 | 6.9 ± 2.1 | 3.4 ± 1.4 | 5.3 ± 2.3 |
| α-carotene, mg/d | 0.81 ± 0.46 | 0.47 ± 0.22 | 1.21 ± 0.63 | 0.42 ± 0.17 | 1.29 ± 0.62 | 0.56 ± 0.29 | 1.03 ± 0.62 |
| Lutein + zeaxanthin, mg/d | 3.0 ± 1.5 | 1.7 ± 5.6 | 4.7 ± 1.8 | 1.7 ± 0.5 | 4.6 ± 1.9 | 2.4 ± 1.2 | 3.5 ± 1.7 |
| Lycopene, mg/d | 6.2 ± 2.7 | 4.0 ± 1.2 | 8.9 ± 3.5 | 5.2 ± 2.3 | 7.1 ± 3.1 | 5.9 ± 2.9 | 6.3 ± 2.7 |
| β-cryptoxanthin, mg/d | 0.17 ± 0.08 | 0.14 ± 0.08 | 0.20 ± 0.09 | 0.14 ± 0.07 | 0.21 ± 0.08 | 0.08 ± 0.02 | 0.29 ± 0.06 |
| Physical activity level, 1984–2006, METs-h/wk | 18.6 ± 16.1 | 13.8 ± 12.8 | 24.3 ± 19.8 | 13.1 ± 12.3 | 25.3 ± 20.6 | 15.2 ± 13.7 | 21.8 ± 19.0 |
| BMI, 1984–2006, kg/m2 | 26.2 ± 4.6 | 26.3 ± 4.8 | 25.9 ± 4.5 | 26.5 ± 4.9 | 25.6 ± 4.4 | 26.4 ± 4.8 | 25.5 ± 4.4 |
| Education level, 1992 | |||||||
| Registered nurse | 63.4 | 69.8 | 55.8 | 70.1 | 56.0 | 66.0 | 60.6 |
| Bachelor's degree | 20.2 | 16.3 | 23.7 | 15.7 | 23.6 | 18.3 | 21.8 |
| Graduate degree | 10.4 | 7.2 | 13.8 | 7.0 | 14.3 | 9.2 | 11.3 |
| Missing | 6.0 | 6.6 | 6.7 | 7.2 | 6.1 | 6.5 | 6.2 |
| Husband education level, 1992 | |||||||
| High school degree or less | 34.6 | 42.6 | 26.8 | 43.6 | 26.4 | 38.0 | 30.4 |
| College degree | 24.1 | 21.0 | 25.8 | 20.5 | 25.8 | 22.3 | 24.8 |
| Graduate degree | 21.0 | 15.2 | 25.2 | 14.3 | 26.5 | 18.4 | 24.0 |
| Missing | 20.3 | 21.1 | 22.1 | 21.6 | 21.3 | 21.3 | 20.7 |
| Smoking pack-years, 1984–2006 | |||||||
| Never smoked | 46.6 | 48.9 | 43.5 | 47.3 | 45.1 | 38.6 | 53.2 |
| ≤4 | 10.9 | 9.2 | 12.3 | 8.7 | 13.0 | 9.4 | 12.2 |
| 5–24 | 22.7 | 19.0 | 26.2 | 19.0 | 25.3 | 21.9 | 21.1 |
| ≥25 | 18.2 | 21.4 | 16.2 | 23.6 | 14.7 | 28.6 | 11.9 |
| Missing | 1.6 | 1.4 | 1.8 | 1.4 | 1.9 | 1.5 | 1.6 |
| Parental history of dementia, 1992–2004 | 20.6 | 20.7 | 19.7 | 20.3 | 20.4 | 21.0 | 20.0 |
| White, 1992–2004 | 97.9 | 98.0 | 97.3 | 98.4 | 96.9 | 98.5 | 96.9 |
| High blood pressure, 1984–2006 | 60.5 | 59.9 | 60.0 | 61.9 | 57.6 | 60.2 | 59.6 |
| Elevated cholesterol, 1984–2006 | 71.6 | 71.8 | 70.7 | 72.3 | 69.0 | 72.2 | 69.4 |
| Diabetes, 1984–2006 | 10.8 | 10.4 | 10.8 | 11.3 | 9.4 | 11.0 | 9.5 |
| Cardiovascular disease, 1984–2006 | 13.4 | 13.1 | 13.6 | 13.7 | 13.1 | 13.5 | 13.2 |
| Cancer, 1984–2006 | 18.5 | 17.3 | 19.1 | 17.7 | 19.6 | 18.0 | 19.2 |
| Depression, 1996–2006 | 19.4 | 21.2 | 17.4 | 20.6 | 18.7 | 21.8 | 16.8 |
| Multivitamin use during 1984–2006 | 90.6 | 86.4 | 93.4 | 84.1 | 94.9 | 87.9 | 91.5 |
| Number of dietary assessments, 1984–2006 | |||||||
| 1–5 | 12.9 | 13.9 | 14.2 | 15.2 | 13.3 | 13.9 | 12.5 |
| 6 | 19.2 | 19.5 | 20.9 | 19.7 | 20.3 | 20.0 | 18.3 |
| 7 | 67.9 | 66.5 | 64.8 | 65.2 | 66.4 | 66.1 | 69.2 |
| SCF, 2012–2014 | |||||||
| Good (score = 0) | 40.8 | 37.1 | 45.5 | 37.5 | 44.5 | 36.6 | 45.4 |
| Moderate (score ≥0.5, ≤2.5) | 46.9 | 47.9 | 44.8 | 47.7 | 45.2 | 49.0 | 44.4 |
| Poor (score ≥3) | 12.3 | 15.0 | 9.7 | 14.8 | 10.4 | 14.4 | 10.2 |
| Missing year of cognitive measurement | |||||||
| None | 85.9 | 85.2 | 85.6 | 84.3 | 86.4 | 85.1 | 86.2 |
| 2012 | 2.6 | 2.8 | 2.5 | 3.0 | 2.3 | 2.7 | 2.4 |
| 2014 | 11.5 | 12.0 | 11.8 | 12.7 | 11.2 | 12.2 | 11.4 |
| Postmenopausal status and hormone use | |||||||
| Premenopause | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| Postmenopause and never use hormone therapy | 20.9 | 22.9 | 19.9 | 23.9 | 19.4 | 21.5 | 21.5 |
| Postmenopause and ever use hormone therapy | 73.9 | 71.8 | 74.5 | 70.0 | 75.7 | 72.8 | 73.0 |
| Missing | 4.8 | 4.9 | 5.2 | 5.6 | 4.5 | 5.3 | 5.0 |
| Parity | |||||||
| Nulliparous | 5.2 | 5.5 | 5.2 | 4.9 | 5.6 | 5.4 | 5.2 |
| 1 | 6.6 | 7.1 | 6.9 | 7.0 | 6.8 | 7.2 | 6.6 |
| ≥2 | 86.3 | 85.8 | 85.8 | 86.2 | 85.7 | 85.7 | 86.4 |
| Missing | 1.8 | 1.5 | 2.2 | 1.9 | 1.8 | 1.7 | 1.8 |
Values are means ± SDs or percentages. Except for age at baseline, values were standardized to the age distribution of the study population. METs, metabolic equivalent of tasks; SCF, subjective cognitive function.
In the primary analyses, long-term higher intake of total carotenoids was related to lower odds of moderate and poor SCF after controlling for multiple nondietary confounding factors and total energy intake (Table 2). For the highest compared with the lowest quintile of total carotenoids, the OR for poor SCF was 0.51 (95% CI: 0.46, 0.56; P-trend < 0.001) in the age-adjusted model and 0.56 (95% CI: 0.51, 0.62; P-trend < 0.001) in the multivariable model. The associations were only modestly attenuated after further adjusting for intakes of vitamins C, D, and E, anthocyanidins, trans-fat, saturated fat, and long-chain n–3 fatty acids (OR: 0.67; 95% CI: 0.60, 0.75; P-trend < 0.001). The results were unchanged after further adjusting for optimism level, whereas they were slightly attenuated after also adjusting for hearing loss and eye disease (e.g., the OR for poor SCF comparing the extreme quintiles of total carotenoids became 0.72; 95% CI: 0.64, 0.81; P-trend < 0.001). Moreover, we observed similar results after including all participants who reported diet in 1984 and 2006 (n = 57,364) and then including those who died or did not respond in the poor-SCF category. In further sensitivity analyses using the 2012 SCF and 2014 SCF scores separately, or limited to those with both SCF questionnaires, the results for total carotenoids were similar to the primary analyses’ results (data not shown).
TABLE 2.
ORs (95% CIs) for moderate and poor SCF, compared with good SCF assessed in 2012–2014, associated with quintiles of the total carotenoid intake in 1984–2006 of 49,493 women in the Nurses’ Health Study1
| Quintile of intake | ||||||
|---|---|---|---|---|---|---|
| Total carotenoids (energy-adjusted) | Q1 | Q2 | Q3 | Q4 | Q5 | P-trend |
| n | 9898 | 9899 | 9899 | 9899 | 9898 | |
| Median, mg/d | 9.40 | 12.2 | 14.4 | 17.0 | 21.7 | |
| Moderate SCF | ||||||
| Age-adjusted model | Ref. | 0.94 (0.89, 1.00) | 0.92 (0.87, 0.98) | 0.84 (0.79, 0.90) | 0.75 (0.71, 0.80) | <0.001 |
| Model 12 | Ref. | 0.94 (0.89, 1.00) | 0.93 (0.87, 0.99) | 0.86 (0.80, 0.91) | 0.78 (0.74, 0.84) | <0.001 |
| Model 23 | Ref. | 0.96 (0.90, 1.03) | 0.96 (0.90, 1.03) | 0.91 (0.85, 0.97) | 0.86 (0.80, 0.93) | <0.001 |
| Poor SCF | ||||||
| Age-adjusted model | Ref. | 0.83 (0.76, 0.91) | 0.77 (0.70, 0.84) | 0.65 (0.59, 0.71) | 0.51 (0.46, 0.56) | <0.001 |
| Model 12 | Ref. | 0.84 (0.77, 0.93) | 0.80 (0.73, 0.88) | 0.69 (0.63, 0.76) | 0.56 (0.51, 0.62) | <0.001 |
| Model 23 | Ref. | 0.90 (0.82, 0.99) | 0.88 (0.80, 0.97) | 0.79 (0.71, 0.88) | 0.67 (0.60, 0.75) | <0.001 |
Values are ORs (95% CIs) unless otherwise indicated. Ref., reference; SCF, subjective cognitive function.
Multivariable model 1 was adjusted for age, race (white, other), education (registered nurse, bachelor's degree, graduate degree), husband education (high school degree or less, college degree, or graduate school), parental history of dementia (yes/no), smoking (never, ≤4, 5–24, >24 pack-years), cancer (yes/no), hypertension diagnosis (yes/no), depression, elevated cholesterol (yes/no), physical activity level (metabolic equivalent of tasks, h/wk; quintiles) and BMI (<23, 23-24.9, 25-29.9, ≥30 kg/m2) from 1984–2006, cardiovascular disease (yes/no), multivitamin use (yes/no), alcohol intake, total calorie intake, number of dietary assessments, postmenopausal status and hormone replacement therapy use, and parity (nulliparous, 1, ≥2).
Multivariable model 2 was also adjusted for intakes of vitamins C, D, and E, anthocyanidins, trans-fat, saturated fat, and long-chain n–3 fatty acids.
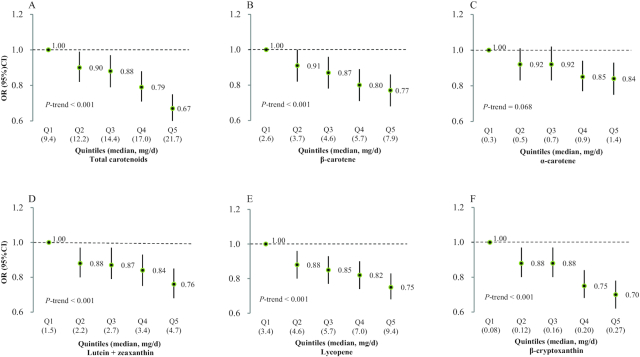
In the analyses for individual carotenoids, long-term greater intakes of total β-carotene, dietary β-carotene, α-carotene, lycopene, lutein + zeaxanthin, and β-cryptoxanthin were each associated with lower odds of moderate and poor SCF (Figure 1, Supplemental Table 4). For example, participants in the highest quintile of β-cryptoxanthin intake had 30% lower odds of poor SCF (OR: 0.70; 95% CI: 0.62, 0.78) than participants in the lowest quintile (Table 2). In the analysis in which we entered each individual carotenoid into the same model, the significant associations for β-cryptoxanthin, lycopene, and lutein + zeaxanthin persisted, whereas the associations for β-carotene and α-carotene, especially for α-carotene, were attenuated (Supplemental Table 5). Owing to the high correlations among β-carotene, α-carotene, and lutein + zeaxanthin, each of them was included in the mutually adjusted model separately. In an analysis using stepwise regression, β-cryptoxanthin, lycopene, and lutein + zeaxanthin were selected as independent predictors of future SCF status.
FIGURE 1.
ORs for poor subjective cognitive function assessed in 2012–2014, across increasing quintiles of total and individual carotenoid intakes in 1984–2006 in 49,493 women in the Nurses’ Health Study. (A) Total carotenoid intake, (B) β-carotene intake, (C) α-carotene, (D) lutein + zeaxanthin, (E) lycopene, (F) β-cryptoxanthin. The multivariable model was adjusted for age, race (white, other), education (registered nurse, bachelor's degree, graduate degree), husband education (high school degree or less, college degree, or graduate school), parental history of dementia (yes/no), smoking (never, ≤4, 5–24, >24 pack-years), cancer (yes/no), hypertension diagnosis (yes/no), depression, elevated cholesterol (yes/no), physical activity level (metabolic equivalent of tasks, h/wk; quintiles) and BMI (<23, 23-24.9, 25-29.9, ≥30 kg/m2) from 1984–2006, cardiovascular disease (yes/no), multivitamin use (yes/no), alcohol intake, total calorie intake, number of dietary assessments, postmenopausal status and hormone replacement therapy use, parity (nulliparous, 1, ≥2), and intakes of vitamins C, D, and E, anthocyanidins, trans-fat, saturated fat, and long-chain n–3 fatty acids. The numbers of participants across the increasing quintiles of total and individual carotenoids were ∼9898, 9899, 9899, 9899, and 9898, respectively.
In the evaluation of the temporal relations, we found that higher intake of total carotenoids at each of the 7 time points during follow-up was significantly associated with lower odds of moderate and poor SCF (Figure 2). The associations were more pronounced for recent years and the strongest association was with the mean of all dietary assessments. When both remote (22–28 y before SCF assessment) and recent (6–10 y before SCF assessment) intakes of total carotenoids were included in the model, the associations with SCF were independently significant for both time intervals, although stronger for more proximal years. In particular, for the highest compared with the lowest quintile of total carotenoids, the OR for poor SCF was 0.87 (95% CI: 0.78, 0.97) for remote years and 0.74 (95% CI: 0.66, 0.83) for recent years.
FIGURE 2.
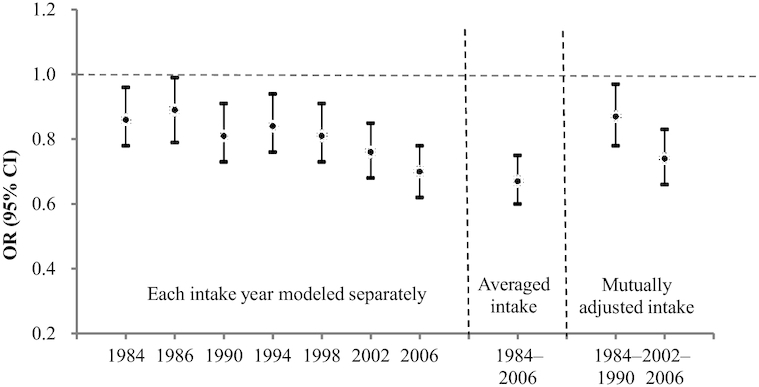
Intake of total carotenoids at each year of dietary assessment and odds of poor subjective cognitive function assessed in 2012–2014, comparing the highest quintile of intake with the lowest, in 49,493 women in the Nurses’ Health Study. The multivariable model was adjusted for age, race (white, other), education (registered nurse, bachelor's degree, graduate degree), husband education (high school degree or less, college degree, or graduate school), parental history of dementia (yes/no), smoking (never, ≤4, 5–24, >24 pack-years), cancer (yes/no), hypertension diagnosis (yes/no), depression, elevated cholesterol (yes/no), physical activity level (metabolic equivalent of tasks, h/wk; quintiles), BMI (<23, 23-24.9, 25-29.9, ≥30 kg/m2), cardiovascular disease (yes/no), multivitamin use (yes/no), alcohol intake, total calorie intake, number of dietary assessments, postmenopausal status and hormone replacement therapy use, parity (nulliparous, 1, ≥2), and intakes of vitamins C, D, and E, anthocyanidins, trans-fat, saturated fat, and long-chain n–3 fatty acids.
When we treated SCF as a continuous variable and examined associations between carotenoid intakes and the SCF score, we again found that higher intakes of total and individual carotenoids were each associated with lower SCF scores after adjusting for nondietary and dietary factors and total energy. The multivariable mean difference in the SCF score comparing the extreme quintiles of total carotenoids was −0.15 (95% CI: −0.19, −0.11), which was equivalent to ∼3 y younger in age.
The significant associations between total carotenoid intake and SCF were observed in almost all subgroups based on characteristics including baseline age (young and old), depression, elevated cholesterol, high blood pressure, hearing problems, eye disease, family history of dementia, and smoking status (Figure 3). The associations were stronger among women with depression and hearing problems (P-interaction < 0.05). In addition, the inverse associations between carotenoid intake and SCF appeared to be much stronger in the small group of women homozygous for APOE ε4.
FIGURE 3.
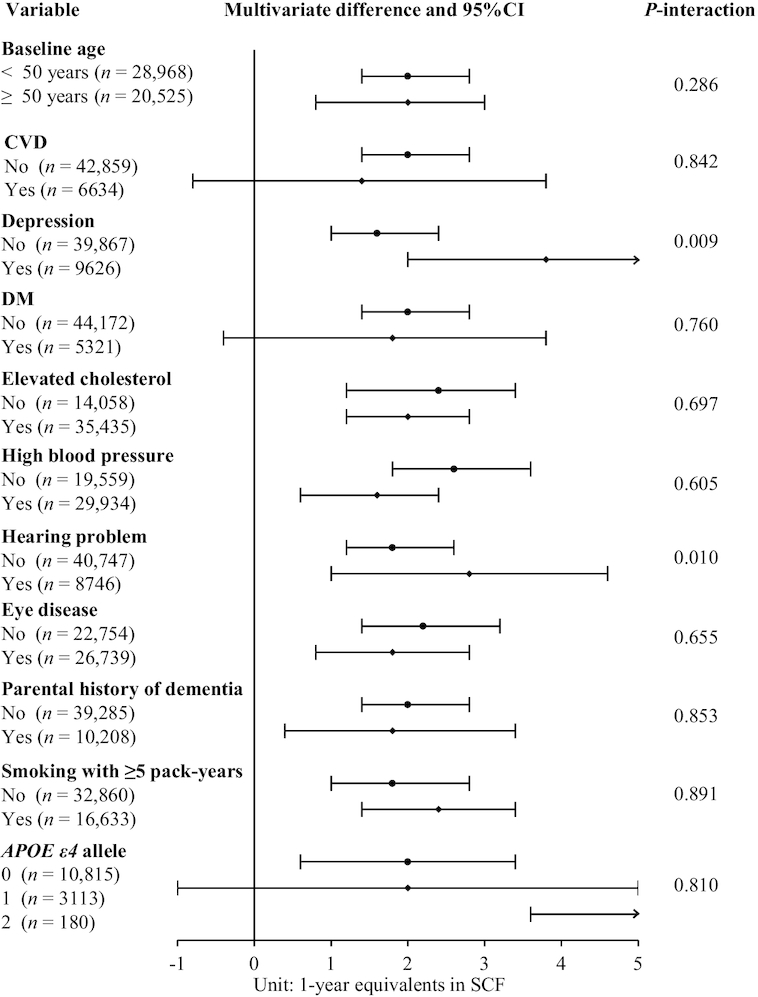
Subgroup multivariable-adjusted differences in 1-y equivalents of SCF in 2012–2014 comparing the 90th (21.7 mg/d) with the 10th (9.4 mg/d) percentile of total carotenoid intake between 1984 and 2006 in 49,493 women in the Nurses’ Health Study. The multivariable model was adjusted for age, race (white, other), education (registered nurse, bachelor's degree, graduate degree), husband education (high school degree or less, college degree, or graduate school), parental history of dementia (yes/no), smoking (never, ≤4, 5–24, >24 pack-years), cancer (yes/no), hypertension diagnosis (yes/no), depression, elevated cholesterol (yes/no), physical activity level (metabolic equivalent of tasks, h/wk; quintiles) and BMI (<23, 23-24.9, 25-29.9, ≥30 kg/m2) from 1984–2006, CVD (yes/no), multivitamin use (yes/no), alcohol intake, total calorie intake, number of dietary assessments, postmenopausal status and hormone replacement therapy use, parity (nulliparous, 1, ≥2), and intakes of vitamins C, D, and E, anthocyanidins, trans-fat, saturated fat, and long-chain n–3 fatty acids. Analyses within subgroups were adjusted for all other covariates and for the restricted covariate within the subgroup the analysis was restricted to. For the subgroups of participants with depression (OR: 2.8; 95% CI: 2.0, 5.6) and 2 APOE ε4 alleles (OR: 19; 95% CI: 4, 35), the corresponding lines were marked with arrows pointing to the right, indicating that the upper bound of the 95% CI exceeds the space provided. CVD, cardiovascular disease; DM, diabetes mellitus; SCF, subjective cognitive function.
Discussion
In this cohort of 49,493 US female nurses followed from middle to late adulthood, long-term higher intake of total carotenoids (median intake of 21.7 compared with 9.40 mg/d) was associated with 14% lower odds of moderate SCF and 33% lower odds of poor SCF in later life after extensive adjustment for dietary and nondietary variables. The associations were seen even with carotenoid intake at 28 y before SCF assessment, although they were stronger for more proximal years. Similar associations were observed for each individual carotenoid, including β-cryptoxanthin, lycopene, β-carotene, lutein + zeaxanthin, and α-carotene. Moreover, we found independent associations for intakes of β-cryptoxanthin, lycopene, and lutein + zeaxanthin.
These findings extend results of an earlier study in this cohort (20), based on a smaller number of women (n = 16,010) and a shorter period of dietary assessment, which indicated that higher consumption of carotenoids might be related to better objective cognitive function at older ages. Despite the use of 2 different methods of measuring cognition, the results were consistent for total carotenoids, lycopene, and lutein + zeaxanthin. With the advantage of repeated cognitive measures over a longer follow-up period from a larger number of cohort participants, we also observed a strong inverse association with intake of β-cryptoxanthin. Owing to high correlation with lutein + zeaxanthin, a possible benefit for β-carotene and α-carotene could not be excluded. The findings are consistent with the hypothesis that carotenoid intake over a period of many years may be most relevant to cognitive outcomes in later life because cognitive decline begins decades before symptom onset (46). Furthermore, the results are consistent with several studies reporting apparent protective associations with cognitive function of long-term intake of a carotenoid-rich (notably β-carotene, α-carotene, β-cryptoxanthin, and lutein) dietary pattern (24), lutein (20, 21), β-carotene (21, 22), and β-carotene supplementation (23). Importantly, in the Physicians’ Health Study II (n = 4052), the longest-term antioxidant randomized trial to date, β-carotene supplementation (50 mg every other day) for 18 y significantly reduced the rate of cognitive decline, but there was no effect of the β-carotene supplement after 1 y of supplementation (23). Moreover, the parallel analyses (unpublished) conducted among 27,842 US male health professionals with >2 decades of follow-up yielded results highly consistent with those of the present study. With the unprecedented large sample size and multiple repeated dietary assessments, these 2 long-running, large prospective cohort studies provide strong additional evidence to support the protective role of overall carotenoids in cognitive function.
Carotenoids have been suggested to have brain-protective function through their antioxidant properties in animal and cell culture studies, and may also reduce the influence of inflammation on brain function by interacting with inflammatory signaling cascades (12–14). Individual carotenoids may also act through other mechanisms relevant to cognitive function. Among them, β-carotene has been the most studied nutrient on cognitive function owing to its pro–vitamin A function, which has been suggested to modulate cerebral plasticity (47); lutein, as a major carotenoid specifically in the macula and the brain, has recently received increasing interest and was linked with cognitive function (10); and lycopene has been proposed to be related to cognitive function via multiple microvascular benefits (13). Our study, together with the findings in the Health Professionals Follow-up Study, was one of few that have reported an apparent beneficial role of β-cryptoxanthin. Although evidence is limited, lower plasma concentrations of β-cryptoxanthin have been reported among Alzheimer disease patients than among healthy controls (48) and subjects with mild cognitive impairment (49). Nonetheless, more research is needed to establish and confirm the cognitive effects of specific carotenoids. Although distinction among some specific carotenoids is challenging owing to intercorrelations, our findings strongly suggest that benefits are not limited to β-carotene or another single carotenoid.
To our knowledge, the present study is the largest and longest evaluation of long-term intakes of carotenoids in relation to SCF. The availability of a large sample allows deeper analyses into the specific carotenoids accounting for this finding. Strengths of the present study also include the detailed control of many potential dietary and nondietary confounders. Moreover, the mean dietary intakes calculated from multiple dietary assessments over time dampen within-subject variation and best represent long-term diet. Reverse causation is less of a concern in our prospective analyses because we stopped updating dietary data 6 y before SCF measurement, and inverse associations were seen with longer lags between intake and the outcome assessment. The present study also had limitations. Firstly, the study sample was limited to mostly Caucasian registered nurses, whose training and employment require relatively high cognitive function. Although this might limit generalizability, this ensures that the population had a relatively high level of cognitive function in early adult life and reduces the possibility of residual confounding. Moreover, the questions on SCF themselves are framed as changes compared with earlier function. Therefore, poor SCF at the time of our assessment can be interpreted as indicating long-term decline during adult life. Second, the SCF assessment may be subject to errors and the reporting may be influenced by personality traits. However, the SCF score has been validated against concurrent levels of cognitive function (34) and faster rates of subsequent cognitive decline (43) measured by neuropsychological testing. Moreover, we adjusted for depression in the multivariable model, which partly accounted for the potential influence of personality traits on the self-report of SCF. To further address this concern, our results remained the same with further adjustment of optimism level. Moreover, the highly consistent findings with the previous study in the NHS using objective measurement of cognition indirectly support the validity of the subjective measurement used in this study. Another limitation is that individuals who did not complete the 2014 follow-up questionnaire are more likely to have died or have had cognitive difficulty. However, if this is the case, this may bias the results toward the null. Lastly, the study findings may be subject to residual and unmeasured confounding by other disease and lifestyle factors. However, the only modest attenuation of associations after extensive adjustment for a wide range of behavioral and socioeconomic variables and for other aspects of diet suggests that residual confounding is not likely to be large. The most plausible confounding factors are other constituents of foods that are high in the carotenoids that we studied. These constituents include numerous phytochemicals that alone or in combination could exert neuroprotective effects. For these reasons caution is warranted in the interpretation of our findings. However, the fact that β-carotene as an isolated substance had an effect on cognitive function in the Physicians’ Health Study randomized trial supports a causal interpretation of our findings.
This prospective study supports a potential beneficial role of long-term intake of multiple carotenoids in maintaining late-life cognitive function in women. Our findings most strongly support benefits of lycopene, β-cryptoxanthin, and lutein + zeaxanthin. However, beneficial roles for other individual carotenoids could not be excluded owing to strongly intercorrelated intakes. Because increasing intakes of carotenoids is a promising and relatively simple means of maintaining brain health, further research is warranted to establish the optimal combination and intakes of carotenoids for the prevention of cognitive decline in populations worldwide.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—WCW and CY: designed the analysis and had the primary responsibility for the final study content; CY: conducted the analysis, interpreted the data, and wrote the manuscript; EF: assisted in the data analysis and completed the technical review of the results; EF, AA, FG, OIO, AH, and WCW: contributed to the interpretation of the results and revision of the manuscript for important intellectual content; and all authors: read and approved the final manuscript.
Notes
Supported by NIH grant UM1 CA186107 and a gift from the VoLo Foundation (to WCW).
Author disclosures: The authors report no conflicts of interest.
The funding agencies had no role in study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
Supplemental Figure 1 and Supplemental Tables 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn.
Abbreviations used: APOE, Apolipoprotein E; CI, confidence interval; CVD, cardiovascular disease; METs, metabolic equivalent of tasks; NHS, Nurses’ Health Study; OR, odds ratio; SCF, subjective cognitive function; SFFQ, semiquantitative food-frequency questionnaire.
Contributor Information
Changzheng Yuan, Department of Big Data and Health Science, School of Public Health, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China; Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
Elinor Fondell, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
Alberto Ascherio, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Olivia I Okereke, Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Psychiatry, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
Francine Grodstein, Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Albert Hofman, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Epidemiology, Erasmus MC University Medical Center, Rotterdam, Netherlands.
Walter C Willett, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
References
- 1. Schaller S, Mauskopf J, Kriza C, Wahlster P, Kolominsky-Rabas PL. The main cost drivers in dementia: a systematic review. Int J Geriatr Psychiatry. 2015;30(2):111–29. [DOI] [PubMed] [Google Scholar]
- 2. Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nelson L, Tabet N. Slowing the progression of Alzheimer's disease; what works?. Ageing Res Rev. 2015;23(Pt B):193–209. [DOI] [PubMed] [Google Scholar]
- 4. WHO [Internet] Dementia. Geneva: WHO 2019; [cited 2020 Apr 17]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/dementia. [Google Scholar]
- 5. Ramos Cordero P, Yubero R. [Non-pharmacological treatment of cognitive impairment]. Rev Esp Geriatr Gerontol. 2016;51(Suppl 1):12–21. [DOI] [PubMed] [Google Scholar]
- 6. Michel J-P. Is it possible to delay or prevent age-related cognitive decline?. Korean J Fam Med. 2016;37(5):263–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crichton GE, Bryan J, Murphy KJ. Dietary antioxidants, cognitive function and dementia – a systematic review. Plant Foods Hum Nutr. 2013;68(3):279–92. [DOI] [PubMed] [Google Scholar]
- 8. Morris MC. Nutrition and risk of dementia: overview and methodological issues. Ann N Y Acad Sci. 2016;1367(1):31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ames BN. Prolonging healthy aging: longevity vitamins and proteins. Proc Natl Acad Sci U S A. 2018;115:(43):10836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson EJ. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr Rev. 2014;72(9):605–12. [DOI] [PubMed] [Google Scholar]
- 11. Dias IH, Polidori MC, Li L, Weber D, Stahl W, Nelles G, Grune T, Griffiths HR. Plasma levels of HDL and carotenoids are lower in dementia patients with vascular comorbidities. J Alzheimers Dis. 2014;40(2):399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Katayama S, Ogawa H, Nakamura S. Apricot carotenoids possess potent anti-amyloidogenic activity in vitro. J Agric Food Chem. 2011;59(23):12691–6. [DOI] [PubMed] [Google Scholar]
- 13. Obulesu M, Dowlathabad MR, Bramhachari PV. Carotenoids and Alzheimer's disease: an insight into therapeutic role of retinoids in animal models. Neurochem Int. 2011;59(5):535–41. [DOI] [PubMed] [Google Scholar]
- 14. Rao AV, Balachandran B. Role of oxidative stress and antioxidants in neurodegenerative diseases. Nutr Neurosci. 2002;5(5):291–309. [DOI] [PubMed] [Google Scholar]
- 15. Rafnsson SB, Dilis V, Trichopoulou A. Antioxidant nutrients and age-related cognitive decline: a systematic review of population-based cohort studies. Eur J Nutr. 2013;52(6):1553–67. [DOI] [PubMed] [Google Scholar]
- 16. Kalmijn S, Feskens EJ, Launer LJ, Kromhout D. Polyunsaturated fatty acids, antioxidants, and cognitive function in very old men. Am J Epidemiol. 1997;145(1):33–41. [DOI] [PubMed] [Google Scholar]
- 17. Nooyens AC, Milder IE, van Gelder BM, Bueno-de-Mesquita HB, van Boxtel MP, Verschuren WM. Diet and cognitive decline at middle age: the role of antioxidants. Br J Nutr. 2015;113(9):1410–7. [DOI] [PubMed] [Google Scholar]
- 18. Peacock JM, Folsom AR, Knopman DS, Mosley TH, Goff DC Jr, Szklo M. Dietary antioxidant intake and cognitive performance in middle-aged adults. Public Health Nutr. 2000;3(3):337–43. [DOI] [PubMed] [Google Scholar]
- 19. Engelhart MJ, Geerlings MI, Ruitenberg A, van Swieten JC, Hofman A, Witteman JC, Breteler MM. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002;287(24):3223–9. [DOI] [PubMed] [Google Scholar]
- 20. Devore EE, Kang JH, Stampfer MJ, Grodstein F. The association of antioxidants and cognition in the Nurses’ Health Study. Am J Epidemiol. 2012;177(1):33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morris MC, Wang Y, Barnes LL, Bennett DA, Dawson-Hughes B, Booth SL. Nutrients and bioactives in green leafy vegetables and cognitive decline: prospective study. Neurology. 2018;90(3):e214–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wengreen HJ, Munger RG, Corcoran CD, Zandi P, Hayden KM, Fotuhi M, Skoog I, Norton MC, Tschanz J, Breitner JC et al.. Antioxidant intake and cognitive function of elderly men and women: the Cache County Study. J Nutr Health Aging. 2007;11(3):230–7. [PubMed] [Google Scholar]
- 23. Grodstein F, Kang JH, Glynn RJ, Cook NR, Gaziano JM. A randomized trial of beta carotene supplementation and cognitive function in men: the Physicians’ Health Study II. Arch Intern Med. 2007;167(20):2184–90. [DOI] [PubMed] [Google Scholar]
- 24. Kesse-Guyot E, Andreeva VA, Ducros V, Jeandel C, Julia C, Hercberg S, Galan P. Carotenoid-rich dietary patterns during midlife and subsequent cognitive function. Br J Nutr. 2014;111(5):915–23. [DOI] [PubMed] [Google Scholar]
- 25. Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6(1):49–62. [DOI] [PubMed] [Google Scholar]
- 26. Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. [DOI] [PubMed] [Google Scholar]
- 27. Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, Chavarro JE, Rood JC, Harnack LJ, Sampson LK et al.. Relative validity of nutrient intakes assessed by questionnaire, 24-hour recalls, and diet records as compared with urinary recovery and plasma concentration biomarkers: findings for women. Am J Epidemiol. 2018;187(5):1051–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, Chavarro JE, Subar AF, Sampson LK, Willett WC. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol. 2017;185(7):570–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18(4):858–67. [DOI] [PubMed] [Google Scholar]
- 30. Michaud DS, Giovannucci EL, Ascherio A, Rimm EB, Forman MR, Sampson L, Willett WC. Associations of plasma carotenoid concentrations and dietary intake of specific carotenoids in samples of two prospective cohort studies using a new carotenoid database. Cancer Epidemiol Biomarkers Prev. 1998;7(4):283–90. [PubMed] [Google Scholar]
- 31. Bernstein AM, Rosner BA, Willett WC. Cereal fiber and coronary heart disease: a comparison of modeling approaches for repeated dietary measurements, intermediate outcomes, and long follow-up. Eur J Epidemiol. 2011;26(11):877–86. [DOI] [PubMed] [Google Scholar]
- 32. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 Suppl):1220S–8S.; discussion 1229S–31S. [DOI] [PubMed] [Google Scholar]
- 33. Go RC, Duke LW, Harrell LE, Cody H, Bassett SS, Folstein MF, Albert MS, Foster JL, Sharrow NA, Blacker D. Development and validation of a Structured Telephone Interview for Dementia Assessment (STIDA): the NIMH Genetics Initiative. J Geriatr Psychiatry Neurol. 1997;10(4):161–7. [DOI] [PubMed] [Google Scholar]
- 34. Amariglio RE, Townsend MK, Grodstein F, Sperling RA, Rentz DM. Specific subjective memory complaints in older persons may indicate poor cognitive function. J Am Geriatr Soc. 2011;59(9):1612–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Amariglio RE, Becker JA, Carmasin J, Wadsworth LP, Lorius N, Sullivan C, Maye JE, Gidicsin C, Pepin LC, Sperling RA et al.. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012;50(12):2880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perrotin A, Mormino EC, Madison CM, Hayenga AO, Jagust WJ. Subjective cognition and amyloid deposition imaging: a Pittsburgh Compound B positron emission tomography study in normal elderly individuals. Arch Neurol. 2012;69(2):223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Scheef L, Spottke A, Daerr M, Joe A, Striepens N, Kolsch H, Popp J, Daamen M, Gorris D, Heneka MT et al.. Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology. 2012;79(13):1332–9. [DOI] [PubMed] [Google Scholar]
- 38. van der Flier WM, van Buchem MA, Weverling-Rijnsburger AW, Mutsaers ER, Bollen EL, Admiraal-Behloul F, Westendorp RG, Middelkoop HA. Memory complaints in patients with normal cognition are associated with smaller hippocampal volumes. J Neurol. 2004;251(6):671–5. [DOI] [PubMed] [Google Scholar]
- 39. Amariglio RE, Mormino EC, Pietras AC, Marshall GA, Vannini P, Johnson KA, Sperling RA, Rentz DM. Subjective cognitive concerns, amyloid-β, and neurodegeneration in clinically normal elderly. Neurology. 2015;85(1):56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Oijen M, de Jong FJ, Hofman A, Koudstaal PJ, Breteler MM. Subjective memory complaints, education, and risk of Alzheimer's disease. Alzheimers Dement. 2007;3(2):92–7. [DOI] [PubMed] [Google Scholar]
- 41. Yuan C, Fondell E, Bhushan A, Ascherio A, Okereke OI, Grodstein F, Willett WC. Long-term intake of vegetables and fruits and subjective cognitive function in US men. Neurology. 2018;92(1):e63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bhushan A, Fondell E, Ascherio A, Yuan C, Grodstein F, Willett W. Adherence to Mediterranean diet and subjective cognitive function in men. Eur J Epidemiol. 2018;33(2):223–34. [DOI] [PubMed] [Google Scholar]
- 43. Samieri C, Proust-Lima C, Glymour MM, Okereke OI, Amariglio RE, Sperling RA, Rentz DM, Grodstein F. Subjective cognitive concerns, episodic memory, and the APOE ε4 allele. Alzheimers Dement. 2014;10(6):752–9.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Radmanesh F, Devan WJ, Anderson CD, Rosand J, Falcone GJ; for the Alzheimer's Disease Neuroimaging Initiative. Accuracy of imputation to infer unobserved APOE epsilon alleles in genome-wide genotyping data. Eur J Hum Genet. 2014;22(10):1239–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carver CS, Scheier MF, Segerstrom SC. Optimism. Clin Psychol Rev. 2010;30(7):879–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Launer LJ. The epidemiologic study of dementia: a life-long quest?. Neurobiol Aging. 2005;26(3):335–40. [DOI] [PubMed] [Google Scholar]
- 47. McCaffery P, Zhang J, Crandall JE. Retinoic acid signaling and function in the adult hippocampus. J Neurobiol. 2006;66(7):780–91. [DOI] [PubMed] [Google Scholar]
- 48. Mecocci P, Polidori MC, Cherubini A, Ingegni T, Mattioli P, Catani M, Rinaldi P, Cecchetti R, Stahl W, Senin U et al.. Lymphocyte oxidative DNA damage and plasma antioxidants in Alzheimer disease. Arch Neurol. 2002;59(5):794–8. [DOI] [PubMed] [Google Scholar]
- 49. Rinaldi P, Polidori MC, Metastasio A, Mariani E, Mattioli P, Cherubini A, Catani M, Cecchetti R, Senin U, Mecocci P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer's disease. Neurobiol Aging. 2003;24(7):915–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.