I. INTRODUCTION AND BACKGROUND
1.1. Overview
Brown adipocytes, which reside in specific depots called brown adipose tissues (BAT), produce heat in a process called non-shivering thermogenesis. Thermogenesis in BAT is stimulated mainly by the sympathetic nervous system in response to cold exposure, and it helps maintain body temperature (euthermia) in placental mammals. The acquisition of BAT in early mammalian evolution is considered one key evolutionary advantage that allowed for the successful expansion of mammals, and its functional importance in newborn humans and small rodents has long been appreciated. More recently, it has become apparent that adult humans also have functionally relevant BAT, and possibly the additional capacity to induce the formation of brown-like adipocytes within white adipose tissues (WAT) (called brite or beige adipocytes) under certain conditions. Because these thermogenic cells, when active, have a high rate of nutrient consumption and energy expenditure, their existence in adult humans not only correlates with improved metabolic profiles (Betz and Enerback, 2018), but has stimulated interest in targeting them therapeutically to fight obesity and improve glycemic control (Hanssen et al., 2015, Ouellet et al., 2012, Yoneshiro et al., 2011b, Yoneshiro et al., 2011c). This has gone hand-in-hand with renewed interest in understanding the basic biological mechanisms of brown fat development and metabolic regulation, which includes understanding the cellular lineages and precursor cell pools that give rise to brown and brite/beige adipocytes, and the signals that govern their fuel selection and unique metabolism. Identifying brown adipocyte stem and progenitor cells, and elucidating the mechanisms that stimulate their differentiation into mature thermogenic adipocytes, could have important implications in developing brown fat based therapeutics. Here, we will discuss our present understanding of brown adipocyte development and function, the related topic of brite/beige adipocytes, and key future goals and unanswered questions especially as they relate to potential therapies.
1.2. Basics of Non-shivering thermogenesis
Cold-stimulated non-shivering thermogenesis (NST) in the brown adipocyte is dependent upon the intrinsic expression and function of uncoupling protein 1 (UCP1), an inner mitochondrial membrane transporter that dissipates the energy stored in the mitochondrial electrochemical gradient as heat, “uncoupled” from ATP synthesis (Betz and Enerback, 2018). In the absence of thermal stress, brown adipocyte UCP1 is thought to be inhibited by purine nucleotides (Nicholls, 2006, Sluse et al., 2006). During cold stress, brown fat thermogenesis is classically stimulated by norepinephrine released from the sympathetic nervous system (SNS), which activates β3-adrenergic receptors on brown adipocytes to stimulate intracellular synthesis of the second messenger cyclic AMP (cAMP), leading to cAMP-driven protein kinase A (PKA) signaling activation. This stimulates lipid catabolism processes such as lipolysis which liberates free fatty acids from triacylglycerol lipid storage droplets, and increases expression of a thermogenic gene expression program that includes UCP1 mRNA (Nicholls, 2006, Sluse et al., 2006, Fedorenko et al., 2012, Lehr et al., 2006).
Exactly how brown adipocytes choose and utilize fuel remains an important and open question. Recent studies suggest that active lipolysis in brown adipocytes may not be required for sustaining thermogenesis so long as exogenous lipids are available; nevertheless, cellular free fatty acids reportedly directly activate UCP1 (Fedorenko et al., 2012, Shin et al., 2017, Schreiber et al., 2017). Active brown adipocytes also take up glucose from circulation, and synthesize free fatty acids de novo from glucose and possibly other lipogenic precursors (i.e. the process of de novo lipogenesis) to continuously fuel NST or to provide other yet to be appreciated metabolic advantages (Sanchez-Gurmaches et al., 2018, McCormack and Denton, 1977, Mottillo et al., 2014, Shimazu and Takahashi, 1980, Trayhurn, 1979, Yu et al., 2002). In addition, BAT thermogenesis is fueled by liver-derived plasma lipid metabolites (acyl-carnitines), the release of which is stimulated by cold-induced lipolysis in the WAT (Simcox et al., 2017). It has also been suggested recently that UCP1-independent mechanisms of thermogenesis might exist under certain circumstances (Bertholet et al., 2017, Ikeda et al., 2018, Kazak et al., 2015). Brown adipocytes might also have key metabolic functions in addition to thermogenesis, such as secreting special adipokines (called BATokines) and exosome containing miRNAs that might have both autocrine function and paracrine functions on nearby immune cells, as well as endocrine functions related to glucose homeostasis and cardiovascular health (Thomou et al., 2017, Villarroya et al., 2013, Hansen et al., 2014, Svensson et al., 2016, Long et al., 2016, Wang et al., 2014a, Villarroya and Giralt, 2015).
1.3. Brown Fat Anatomy and Morphology
The color distinction between a “brown” and a “white” adipocyte largely reflects the many more mitochondria (which are high in iron) in brown adipocytes compared to white adipocytes (Figure 1). A stimulated brown adipocyte actively generating heat also contains many small lipid droplets and is referred to as being multi-locular, while white adipocytes, such as those in subcutaneous and visceral depots (sWAT and vWAT, respectively) typically have a single large unilocular lipid droplet (Figure 1). Having many small lipid droplets increases lipid droplet surface area and presumably promotes metabolite exchange with mitochondria (Blanchette-Mackie and Scow, 1983, Benador et al., 2018). A less active brown adipocyte that is not engaged in thermogenesis (e.g. after acclimation to thermoneutrality) adopts a morphology more similar to a white adipocyte although it retains an epigenetic cellular identity that differentiates it from a white adipocyte (Roh et al., 2018).
Figure 1. General characteristics of brown, white and brite/beige adipocytes.
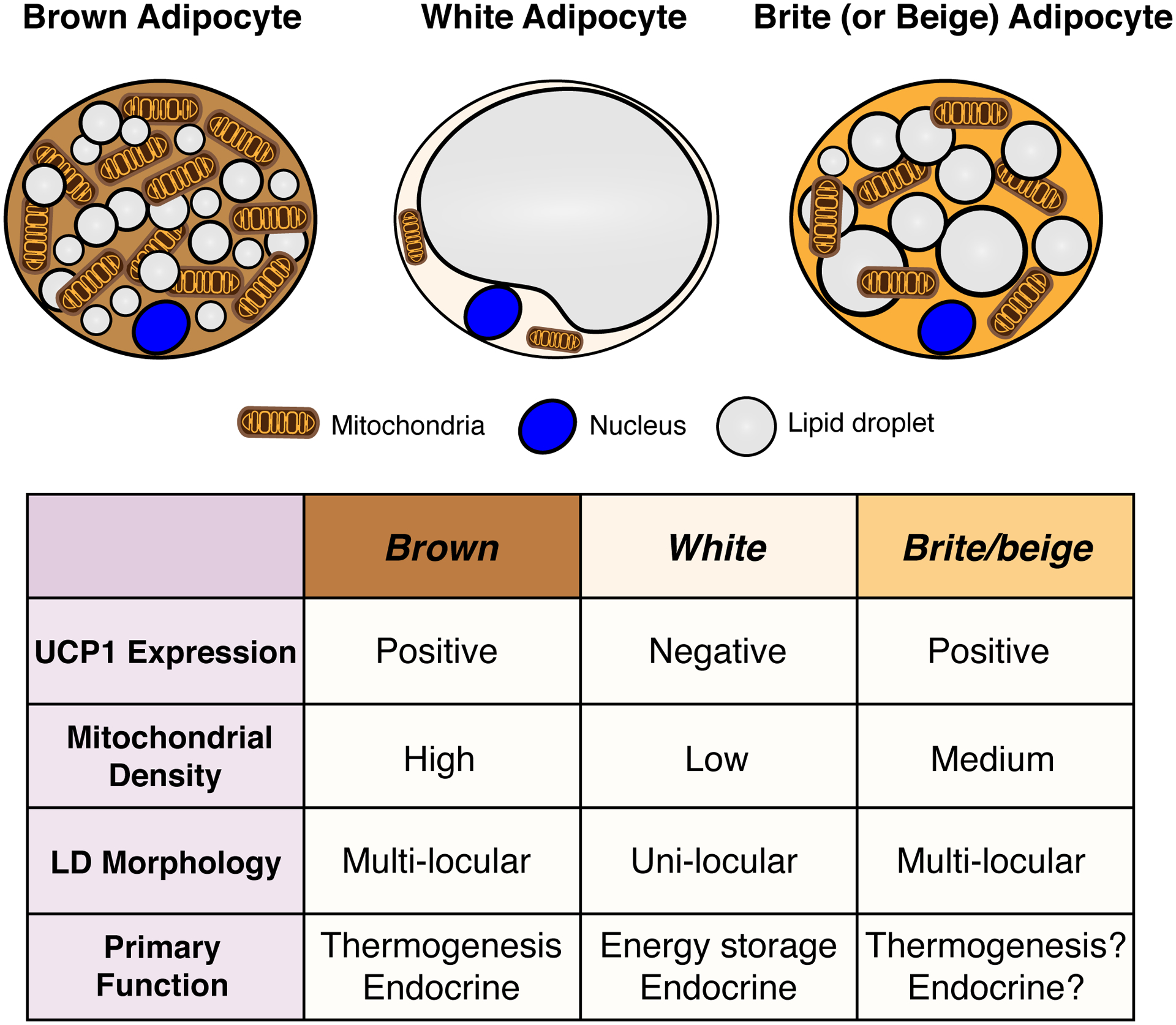
A stimulated brown adipocyte (left) contains numerous small lipid droplets, many mitochondria, and expresses high levels of uncoupling protein 1 (UCP1), which is embedded in the inner mitochondrial membrane and required for thermogenesis. The color of brown fat reflects the high iron content of mitochondria. A white adipocyte (middle) in contrast contains a single large lipid droplet, fewer mitochondria, and does not express UCP1. A Brite/beige adipocyte (right) is characteristically intermediate between brown and white adipocyte, having multiple lipid droplets (though often larger than those seen in a brown adipocyte), more mitochondria than a white adipocyte, and it expresses UCP1.
As indicated above, brown adipocytes exist in defined BAT depots in the mouse, which is the main model organism used to study brown fat. Notably, the size and composition of each BAT depot differs with age, gender and mouse strain background (Frontini and Cinti, 2010, Murano et al., 2009). The largest BAT depots are clustered in the dorsal anterior regions of the mouse body and include the inter-scapular (iBAT), sub-scapular (sBAT) and cervical depots (cBAT) (Frontini and Cinti, 2010, de Jong et al., 2015, Walden et al., 2012, Cinti, 2005) (Figure 2). In addition, there are several small BAT depots proximal to major blood vessels and specific organs, such as the peri-aortic BAT depot (paBAT) that aligns to aortic vessels, and the peri-renal BAT depot (prBAT) that localizes in a fibrous capsule of the kidney (Frontini and Cinti, 2010) (Figure 2). Recent studies using 18F-FDG PET/CT or (123/125I)-β-methyl-p-iodophenyl-pentadecanoic acid with SPECT/CT imaging, which traces glucose and lipid uptake respectively, suggests additional small pockets of cold responsive fat depots exist in suprascapular, supraspinal, infrascapular, and ventral spinal regions (Zhang et al., 2018, Mo et al., 2017).
Figure 2. Adipose tissue anatomy and plasticity.
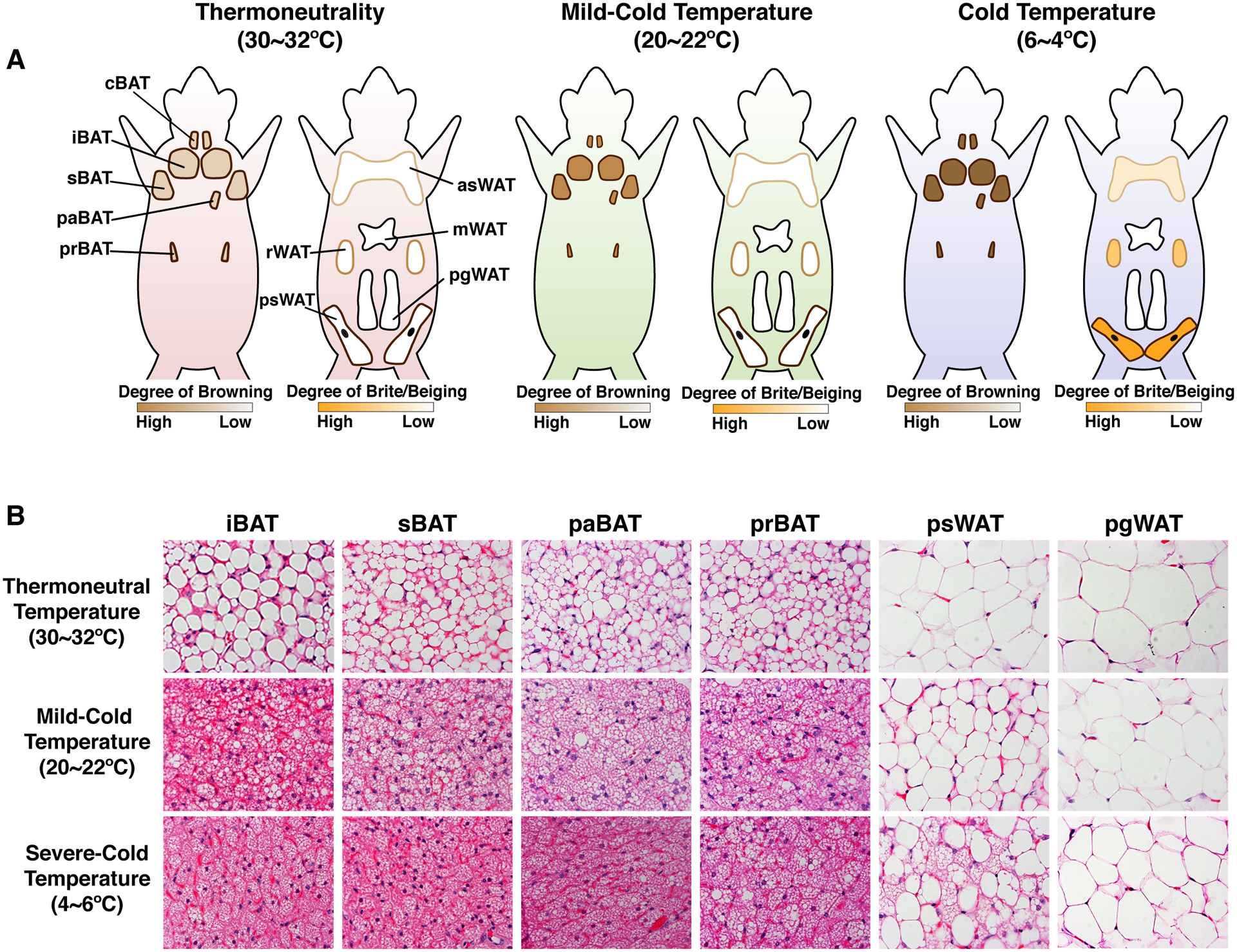
(A) Cartoons showing brown and white fat depots in mice that are acclimated to thermoneutrality (30°C ~ 32°C), mild cold (20°C ~ 22°C), and severe cold (6°C ~ 10°C). The color and size of each depot is modeled such that it reflects the observed differences in mice acclimated to each temperature. A key showing the gradient of “browning” or “britening/beiging” is provided below each model. (B) Hematoxylin and Eosin staining of the indicated brown and white fat depots at each temperature. Note that at thermoneutrality, brown adipocytes contain larger single lipid droplets. At 20–22°C, the standard mouse facility temperature, brown adipocytes exhibit their stimulated morphology of being multi-locular (see Figure 1) while white adipocytes remain unilocular though SWAT adipocyte size is reduced likely reflecting in part a higher level of lipolysis that is necessary to fuel the active brown fat depots. At severe cold temperatures, (6–10°C), additional morphological changes can been see in BAT (i.e. lipid droplets become more uniform), and under these conditions, brite/beige adipocytes also from in the subcutaneous WAT. Of note, the browning capacity of WAT depots is not dependent on a depot being subcutaneous or visceral because, for example, the retroperitoneal visceral WAT depot has high britening/beiging capacity (not shown) while the perigonadal visceral WAT (shown) does not. [Abbreviations] iBAT, interscapular BAT; sBAT, subscapular BAT; cBAT, cervical BAT; paBAT, peri-aortic BAT; prBAT, peri-renal BAT; asWAT, anterior subcutaneous WAT; psWAT, posterior subcutaneous WAT; mWAT, mesenteric WAT; rWAT, retroperitoneal WAT; pgWAT, perigonadal WAT. The images in this figure are based primarily on experiments with C57Bl/6 mice.
Similar to the mouse, newborn humans have active brown adipocytes present at birth in large interscapular BAT depots and peri-renal depots (Figure 3A), which presumably helps maintain core body temperature though could also have other neonatal functions not yet appreciated. Until recently, it was widely believed that after neonatal BAT recedes, adult humans lacked brown fat. However, about a decade ago the widespread existence of active BAT in adults was revealed by retrospective analyses of 18F-fluodeoxyglucose (FDG) uptake assays, which uses positron emission tomography/computed tomography (PET-CT) to measure glucose uptake into organs (Yoneshiro et al., 2011a, Yoneshiro et al., 2013, van der Lans et al., 2013, Ouellet et al., 2012, Hanssen et al., 2015, Nedergaard et al., 2007, Cypess et al., 2009, van Marken Lichtenbelt et al., 2009, Saito et al., 2009, Virtanen et al., 2009, Kortelainen et al., 1993). These studies also revealed a correlation between BAT activity/amount and metabolic fitness. More recent studies show that BAT depots in adult humans exist in the supraclavicular, axillar and paravertebral regions, though the variability across individuals and populations is still being worked out (Zhang et al., 2018, Nedergaard et al., 2007, Cypess et al., 2009, van Marken Lichtenbelt et al., 2009, Virtanen et al., 2009, Ouellet et al., 2012) (Figure 3B). There are also small BAT depots in perivascular regions (aorta, common carotid artery), and near the heart wall (epicardium), lung bronchia, and some solid organs (hilum of kidney and spleen, adrenal, pancreas, liver) (Sacks and Symonds, 2013) (Figure 3B).
Figure 3. Brown fat locations in humans.
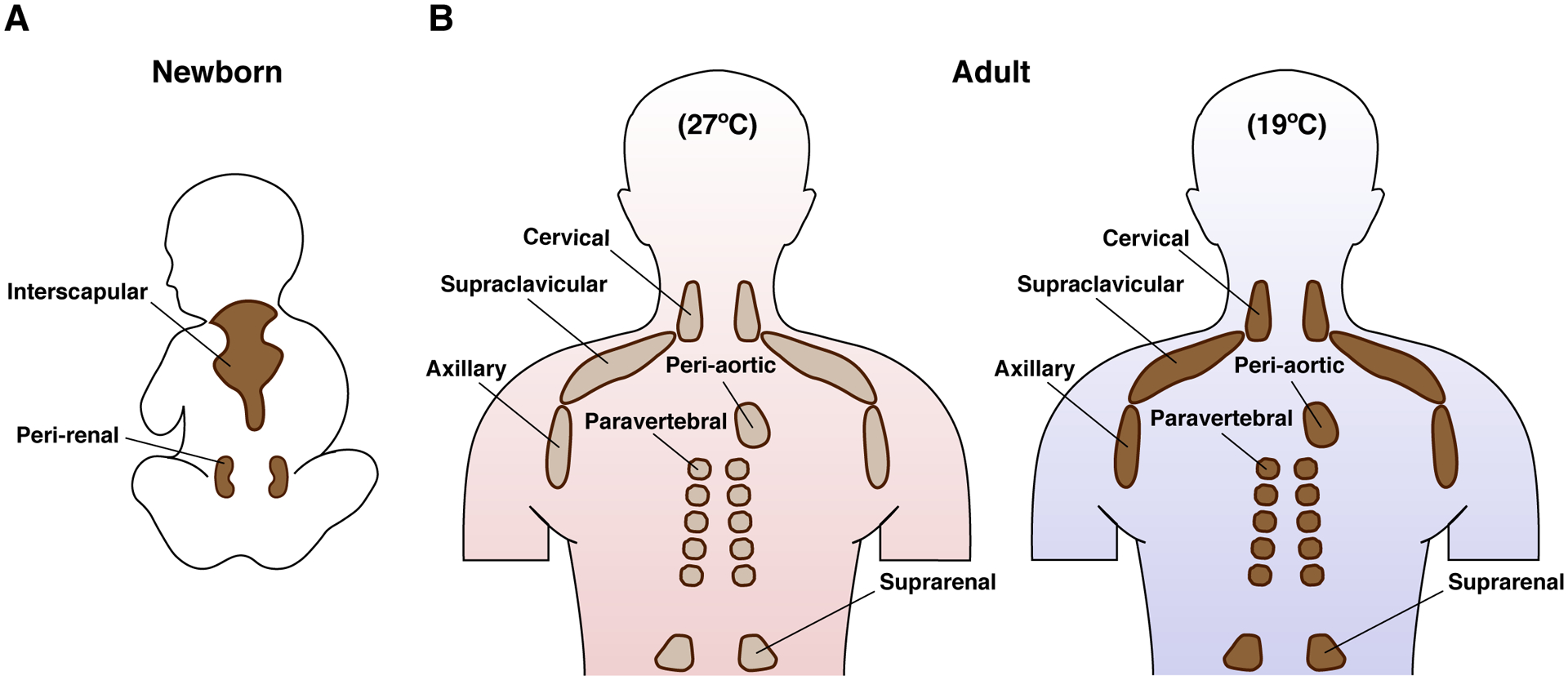
(A) Newborn infants have large interscapular and peri-renal BAT depots. (B) In adults, smaller BAT depots are located in the cervical, supraclavicular, axillary, peri-aortic, paravertebral and suprarenal regions. The mapping of these depots in adults is largely based on glucose uptake measurements by 18F-FDG-PET/CT imaging, which shows increased glucose flux at colder temperatures (shown in figure) and on post-mortem resections. The molecular and functional nature of individual (putative) BAT depots remains unclear in humans. Also note that the amount of BAT is highly variable between individuals, but when active BAT is present, it has been shown to correlate with improved metabolism (not shown, discussed in text). Emerging advances in BAT imaging will likely confirm additional depots.
1.4. BAT vascularization and innervation
Brown fat depots are also highly vascularized, which facilitates the exchange of oxygen and nutrients and the dissipation of heat and release of BATokines into circulation (Bartelt et al., 2011, Labbe et al., 2015, Sacks and Symonds, 2013). In fact, BAT requires increased blood infusion rate during BAT recruitment (i.e. cold stimulation) to obtain sufficient metabolic substrates and oxygen. Brown adipocytes also generate vascular endothelial growth factor-A (VEGF-A) and nitric oxide (NO), which facilitates BAT angiogenesis and vascularization (Xue et al., 2009, Sun et al., 2014, Nisoli et al., 1998, Mahdaviani et al., 2016), a process that is reduced in obese mice resulting in loss of thermogenic activity (Shimizu et al., 2014). Other recent work suggests that brown adipocytes may have a vasoprotective role that might be mediated by the secretion of hydrogen peroxide (H2O2), which inhibits vessel contractions in nearby vascular cells (Friederich-Persson et al., 2017).
In addition to being highly vascularized, BAT is extensively innervated allowing for its rapid stimulation by the sympathetic nervous system (SNS). The SNS releases catecholamines such as norepinephrine that activate G-protein coupled β3-adrenergic receptors that are highly expressed on mature brown adipocytes, and β1-adrenergic receptors on brown adipocyte precursors (Cannon and Nedergaard, 2004, Morrison et al., 2012, Bukowiecki et al., 1986, Bronnikov et al., 1992). While β3-adrenergic receptor signaling stimulates mature brown adipocyte lipid catabolic activity and thermogenesis, β1-adrenergic receptor signaling stimulates brown fat adipogenesis upon prolonged cold challenge (Bronnikov et al., 1992). Classic denervation studies reveal the indispensability of the SNS connections for thermogenesis (Silva and Larsen, 1983, Rothwell and Stock, 1984, Takahashi et al., 1992, Labbe et al., 2015). Emerging research also suggests that innervation may also be critical for BAT to communicate directly with other non-SNS tissues, such as the WAT (Schulz et al., 2013, Garretson et al., 2016, Nguyen et al., 2018).
In summary, BAT is a dynamic and heterogeneous tissue, and the extensive networks of vessels and nerves found in BAT suggests that during brown fat development, there is tight coordination between brown adipocyte precursors (discussed below), endothelial lineages, and nerve cell lineages, and likely immune cells too (Lumeng and Saltiel, 2011, Olefsky and Glass, 2010, Villarroya et al., 2018). The signaling and metabolic interactions between different cell lineages during brown fat development has not yet been extensively studied by systems based approaches.
1.5. Transcriptional control of brown adipocyte differentiation
Much of the general transcriptional cascade that promotes adipogenesis is shared between brown and white adipocytes, and has been studied at length using in vitro models (e.g. 3T3–L1 cells). The master regulator of adipogenesis, PPARγ, is both necessary and sufficient for adipogenesis (Rosen et al., 1999, Tontonoz et al., 1994, Wang et al., 2013a). Other key components of the general adipogenesis transcriptional cascade also important in brown and brite/beige adipocyte differentiation include the members of the C/EBP family (C/EBPα, C/EBPβ, C/EBPδ)(Farmer, 2006). While PPARγ is the dominant factor, overexpression of all C/EBP family members induces adipocyte formation. In culture, C/EBPβ and C/EBPδ function in the first wave of adipogenic transcription factors (hours after adipogenic induction) that eventually triggers a second wave (days after adipogenic induction) that includes C/EBPα and PPARγ, which feed-forward activate themselves (Farmer, 2006).
More recently, efforts to identify brown adipocyte lineage specific transcription factors has identified new additional components that may contribute to the brown (or brite/beige) adipocyte fate. PRDM16 (PRD1-BF1-RIZ1 homologous domain containing 16) was originally described as a BAT transcriptional determination factor that induces a robust thermogenic adipocyte phenotype in white adipocytes both in vitro and in vivo, and can direct muscle precursors to differentiate into brown adipocytes in vitro (Seale et al., 2008, Seale et al., 2007, Seale et al., 2011). In vivo, other PRDM family members can compensate for the loss of PRDM16 in BAT precursors to maintain normal BAT formation (Harms et al., 2014). In addition, the EBF2 (early B-cell factor 2) transcription factor is selectively expressed in both BAT and brite/beige precursors, and it is required for BAT identity and efficient brite/beige cell formation (Rajakumari et al., 2013, Stine et al., 2016, Wang et al., 2014b). Recent studies also identified zinc finger protein 516 (Zfp516), whose expression in brown fat is markedly increased in response to cold exposure or β-adrenergic stimulation via β-AR-cAMP pathway, and it directly interacts with PRDM16 to promote BAT development and WAT browning while suppressing myogenesis (Dempersmier et al., 2015, Sambeat et al., 2016). Whether there are brite/beige specific transcription factors that do not function in brown adipocyte lineages remains an important area of investigation.
In contrast to pro-thermogenic transcription factors, less is known about the transcriptional machinery that promotes and/or maintains the white adipocyte phenotype. One interesting candidate is Zfp423, which has recently emerged as a critical brake that prevents white adipocytes from converting to thermogenic adipocytes. Zfp423 is expressed in white adipocyte precursor cells and functions to block the brite/beige thermogenic program by inhibiting the EBF2 and PRDM16 (Gupta et al., 2010, Gupta et al., 2012, Shao and Gupta, 2018, Shao et al., 2016). While these studies are opening the door to our understanding of adipocyte fate determination at the level of gene expression, there is still much to be learned especially if this information is to be harnessed for therapeutic opportunities. Moreover, other key gene expression factors that contribute to fate decisions, such as epigenetic marks and higher order chromatin regulation, are just beginning to be explored (Roh et al., 2017, Zhao et al., 2016, Carrer et al., 2017, Roh et al., 2018) making this an important area of investigation for many years to come.
II. BROWN FAT GROWTH
2.1. Techniques for studying BAT development
Understanding how brown fat grows begins with understanding its developmental origins. We begin this section with a brief commentary on the two main methods that have been instrumental in beginning to elucidate the developmental origins of both brown and white adipocytes; (1) fluorescence activated cell sorting (FACS), in which stem and progenitor cells are isolated based on their expression of cell surface markers or engineered genetic labels, then tested for their ability to function as adipocyte precursors; and (2) lineage tracing, in which stem and progenitor cells are indelibly labelled with a genetic mark that can be followed, or traced, throughout development in all descendant cells.
FACS
Adipocyte precursors reside within whole adipose tissue depots in a highly heterogeneous non-adipocyte cell population commonly referred to as the stromal vascular fraction or “SVF”. In addition to adipocyte stem and progenitor cells, the SVF contains endothelial, immune, nerve, and other cells that support tissue function. Adipocyte precursors are necessary not only for establishing fat depots, but also for expanding and regenerating adipocytes. Starting with only the SVF population from white adipocytes, several studies have used FACS technology with cell surface markers thought to label the adipocyte precursor population to enrich for pools of adipocyte stem and progenitor cells (ASPCs) (Berry et al., 2014). Although a single marker for prospective isolation of adipocyte precursors has not been found, combinations of surface markers have been use in this regard to isolate white ASPCs (Berry and Rodeheffer, 2013, Rodeheffer et al., 2008). One common example in mice is the CD31neg, CD45neg,Ter119neg, CD29pos, Sca1pos, CD34pos, CD24pos population, which has enhanced adipogenic potential compared to the total SVF. Although brown and white adipocytes have many functional, anatomical, and morphological differences, a similar population of ASPCs can be isolated from BAT depots (Sanchez-Gurmaches et al., 2012, Wang et al., 2014b).
Recently, PDGFRα was also reported to be a marker for ASPCs. PDGFRα can be used to isolate ASPCs using flow cytometry from the CD31neg;CD45neg population within the SVF of all WAT and BAT (Church et al., 2014, Berry and Rodeheffer, 2013). These findings have been further validated using lineage tracing approaches (discussed below), which confirm that adipocyte lineages express Cre recombinase driven by the PDGFRα promoter (Berry and Rodeheffer, 2013, Vishvanath et al., 2016, Lee et al., 2012, Lee et al., 2015). From a technical perspective, this finding is important because it simplifies the enrichment protocol for ASPCs. Interestingly, PDGFRα also labels a fibro/adipogenic precursor cell population within skeletal muscles and skin (Joe et al., 2010, Rivera-Gonzalez et al., 2016) suggesting PDGFRα may be a broadly relevant marker of ASPCs, and recent studies further conclude that PDGFRα signaling may functionally contribute to ASPCs fate and adipose tissue organogenesis (Rivera-Gonzalez et al., 2016, Sun et al., 2017). However, PDGFRα also expresses in many non-adipocyte cells and it will be important to delineate its different roles within the heterogeneous SVF population of adipose tissues.
A current key challenge of using FACS isolated adipocyte precursors is that the ASPCs, although enriched for adipogenic precursors, are still a heterogeneous population containing subpopulations of cells that remain largely undefined by molecular approaches, and whether a true adipocyte stem cell can be purified is still an open question. Recent studies using single cell RNA-seq are beginning to provide key insights into this problem (discussed below). Other studies have identified markers of differentiated brown or beige adipocytes (Ussar et al., 2014). However, highly specific and reliable surface markers that can differentiate between brown, beige, or white adipocyte progenitors have not yet been identified. On the other hand, the prospective nature of using FACS to isolated adipocyte precursors may facilitate the isolation and application of human ASPCs for use in cell-based therapies. Several different protocols for the isolation of human adipocyte progenitors are being developed (van Harmelen et al., 2005, Baglioni et al., 2009, Baglioni et al., 2012, Perrini et al., 2013).
Lineage tracing
Lineage tracing is a classic developmental biology technique that has been used to study adipose tissue development mainly in mouse models due to its genetic nature. In classic lineage tracing experiments, an indelible mark, often a fluorescent reporter, is expressed in a specific population of precursor cells by homologous recombination or by a transgenic approach, and the permanently modified cells then transmit the reporter to all of their descendant cells, or lineages. A common strategy for studying adipocyte lineages in mice is to use a cell-specific Cre recombinase that activates the reporter’s expression. Cre-drivers can be always on in the specific population being studied, called constitutive, or stimulated to be active only transiently in a cell population, called inducible. The latter requires the administration of a stimulus to turn on Cre activity.
Both methods have advantages and disadvantages that need to be considered for data interpretation of adipocyte lineages. For starters, there is currently no known Cre driver that only expresses in ASPCs. Inducible Cre recombinases have the advantage that they allow the timing of activation to be regulated such that cells are only labeled for a brief moment, then those specific cells can be followed. This is not achievable with constitutive Cre drivers, making it difficult to determine precisely which cells first express the Cre in a particular lineage using these drivers. However, the inducers used to turn on the inducible Cre drivers, typically tamoxifen or doxycycline, can have unintended toxic effects on cells (Ye et al., 2015, Moullan et al., 2015). Even when pools of cells are inducibly labeled, it is difficult to distinguish whether two descendant labelled cells originate from common or distinct Cre expressing precursors. Another consideration is whether a particular Cre driver reflects the expression of the actual endogenous gene/protein whose promoter is used to drive the Cre, or whether it only reflects the promoter activity uncoupled from the normal expression of the associated gene and/or protein. The use of knock-in Cre drivers, which are expressed from endogenous promoters, can help mitigate against this concern. Related to this point, caution should be taken in inferring whether the activity of a specific Cre (i.e. promoter) reflects a functional role for the associated gene in lineage specification.
The choice of a reporter (often a fluorescent reporter) is also important when performing lineage tracing in adipocytes. One issue with adipocytes relative to non-adipocytes is the small amount of cytoplasm and large quantity of lipid droplets, which both makes the use of cytoplasmic fluorescent reporters challenging to detect, and makes it difficult to obtain high quality frozen sections. Thus, the reporter of choice for adipocytes is typically a membrane targeted reporter, such as the dual fluorescent membrane targeted Tomato;membrane targeted GFP or mTmG reporter (Muzumdar et al., 2007). This reporter has two major advantages; (1) all cells are labelled, the mGFP reporter only being activated in Cre-positive lineages, and (2) both fluorescent reporters are membrane targeted. Its utility in adipose tissue both for lineage tracing as well as for use in FACS-based studies has been demonstrated in many reports (Berry and Rodeheffer, 2013, Sanchez-Gurmaches and Guertin, 2014, Shao et al., 2016, Wang et al., 2014b). Related to lineage tracing is cell-labeling or cell-marking, which is a common technique to study mature adipocyte dynamics. By this strategy, only mature adipocytes are labeled (rather than precursors), which allows single mature adipocytes to be followed over time especially when combined with inducible Cre-drivers of reporter expression.
2.2. Brown Adipocyte Origins
Brown adipocytes are thought to originate from the mesoderm during embryonic development and thus share a very early developmental origin with skeletal muscle, bone, white adipocytes, and connective tissues (Wang et al., 2014b, Atit et al., 2006, Seale et al., 2008, Lepper and Fan, 2010, Sanchez-Gurmaches et al., 2012). However, the pathways that specify the brown adipocyte developmental lineage is not fully clear. In accordance with a mesodermal origin, a population of cells within the central dermomyotome that is labelled at E9.5 by expression of the homeobox transcription factor Engrailed 1 (En1) gives rise to iBAT, dermis, and epaxial muscles (Figure 2A, 4 and Table 1) (Atit et al., 2006). However, these E9.5 En1+ progenitors do not appear to give rise to sBAT, or any of the major white fat depots (Atit et al., 2006)(Atit personal communication) suggesting that some brown and white adipocyte origins may differ, and that not all brown adipocytes share a common origin (see below). This concept of adipocyte heterogeneity within and between depots, as we will discuss, is now a central tenet of adipocyte biology.
Figure 4. Model of the heterogeneity and complexity in brown and brite/beige adipocyte development.
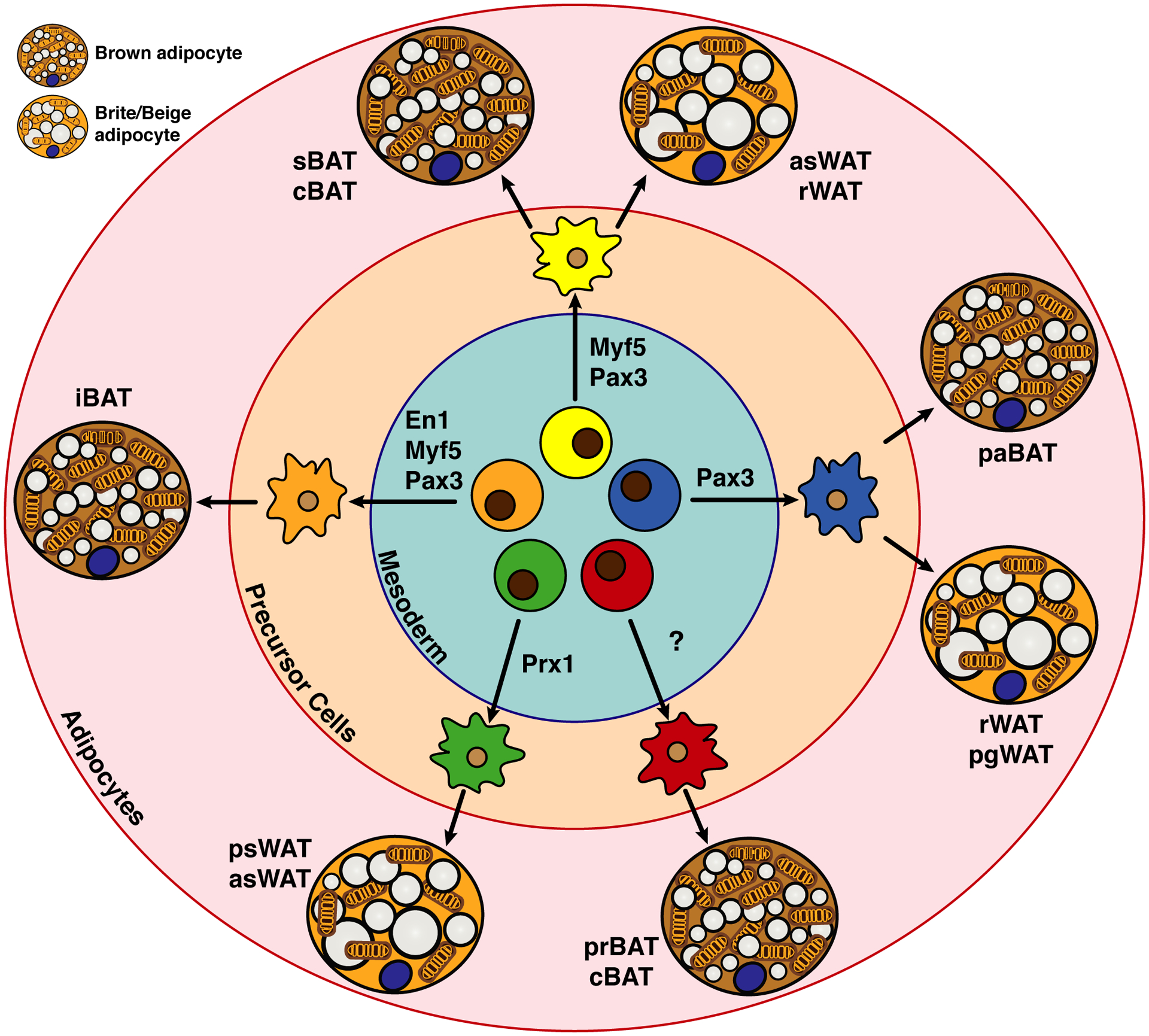
Several multi-potent cell populations that are mainly mesodermal and express specific transcription factors (e.g. En1, Myf5, Pax3, Prx1) appear to give rise heterogeneously to thermogenic adipocytes in different depots. Note that there is overlap shared with some markers but not with others. For example, Pax3 and Myf5 together may mark a pool of early precursors that give rise to iBAT, but only Pax3 marks a precursor pool that gives rise to some visceral pgWAT adipocytes (discussed in text). The significance of this heterogeneity is not understood. Additionally, there are several populations of brown and brite/beige adipocytes for which potential lineage markers remain unidentified. Also note that the brown and brite/beige adipocytes shown in this figure are depicted in their active state (i.e. upon β-adrenergic stimulation), but in vivo brown and brite/beige adipocytes are not necessarily present at the same time, such as in mild cold conditions (see Figure 2).
Table 1.
Depot-specific developmental origins of BAT
| Tissue Type | Anatomical Locations (Human) | Anatomical Locations (Mouse) | Developmental Origins (Lineage Tracing Study) |
|---|---|---|---|
| Brown adipocyte | Supraclavicular/Paravertebral Perivasicular: (aorta, artery) Periviscus: (heart, lung bronchia) Solid organs: kidney/spleen hilum pancreas, liver |
Interscapular | En1+, Myf5+, Pax7+, Pax3+, Prx1− |
| Subscapular | En1−, Myf5+, Pax7+, Pax3+, Prx1− | ||
| Cervical | En1?, Myf5+/−, Pax7+, Pax3+/−, Prx1− | ||
| Peri-renal | En1?, Myf5−, Pax7+, Pax3+/−, Prx1− | ||
| Peri-aortic | En1?, Myf5−, Pax7+, Pax3−, Prx1+/− | ||
| Brite/beige adipocyte | Supraclavicular/Subcutaneous | Posterior-Subcutaneous (Inguinal) | En1?, Myf5−, Pax3−, Prx1+ |
The model tilted toward brown fat and skeletal muscle sharing a common developmental origin with the finding that brown adipocytes in the iBAT and the skeletal muscles, but not certain populations of WAT, share a common cellular origin in the dermomyotome defined by the expression of Myf5-Cre (Seale et al., 2008). Using the constitutively expressing Myf5-Cre knock-in driver with a cytoplasmic reporter, this study found that Cre recombinase activity labels mature brown adipocytes in the iBAT in addition to skeletal muscles (Seale et al., 2008). Myf5 is a classic myogenic determination factor from the basic helix loop helix (bHLH) family, and thus the labeling of brown adipocytes with Myf5-Cre (Tallquist et al., 2000) was predicated to explain the metabolic similarities between brown fat and skeletal muscle with respect to high oxygen consumption and fuel usage, and conversely the metabolic difference between BAT and the less metabolically active and energy storing WAT depots (Harms and Seale, 2013). Notably, at the time most studies used mice that were mildly cold stressed in which the BAT is hyperactive, rather than mice living at thermoneutrality, when brown adipocytes are more similar morphologically and metabolically to white adipocytes. Nevertheless, in support of this model, an inducible Cre driver under control of the Pax7 promoter (the PAX transcription factor family member 7 collaborates with Myf5 and other myogenic factors during skeletal myogenesis) showed that Pax7+ progenitors that arise between E9.5 and E10.5 (but not later in development) also give rise to interscapular brown adipocytes (Lepper and Fan, 2010). This also suggested an early divergence between BAT and muscle lineages.
While the Myf5-lineage model of BAT specification was elegant in its simplicity, studies challenging its uniformity soon after revealed that the brown adipocyte developmental landscape is more complicated. Similar fate mapping experiments using the same Myf5-Cre driver, but more broadly examining brown and white fat depots, and using the mTmG reporter, showed that many white adipocytes are also Myf5-Cre lineage positive, and unexpectedly that many brown adipocytes are Myf5-Cre lineage negative (Sanchez-Gurmaches and Guertin, 2014). For example, Myf5-Cre labelled precursors appear to give rise to nearly all brown adipocytes in iBAT and sBAT depots, but only about half of the brown adipocytes in the cervical BAT, and none of the brown adipocytes in prBAT or paBAT. Moreover, Myf5-Cre positive adipocytes populate the asWAT and rWAT depots (Figure 2A), indicating that Myf5-Cre neither uniformly nor specifically labels brown adipocytes. Other studies have replicated these findings confirming the heterogeneous labeling of adipocytes with Myf5-Cre (Sanchez-Gurmaches and Guertin, 2014, Sanchez-Gurmaches et al., 2012, Shan et al., 2013, Wang et al., 2014b)
Interestingly, lineage tracing using a Pax3-Cre driver, (Pax3 is another myogenic Pax family transcription factor that expresses just prior to Myf5) labels similar populations of cells with a few key differences. Notably, Pax3-Cre cells give rise to most of the brown adipocyte in iBAT, sBAT, cBAT and prBAT, but none of the brown adipocytes in the paBAT (Sanchez-Gurmaches and Guertin, 2014, Liu et al., 2013), and also to nearly 50% of the white adipocytes in the large visceral pgWAT depot. For comparison, MyoD-Cre (another classic myogenic transcription factor) does not label any brown or white adipocytes, but importantly does label skeletal muscles (Sanchez-Gurmaches and Guertin, 2014). Thus, there may be specificity within skeletal muscle lineages in which some precursors (i.e. Pax3/Myf5/Pax7positive) can also become adipocytes while others (i.e. MyoDpositive) cannot, or rather that some adipocyte and muscle precursors can independently express Pax3/Myf5/Pax7-Cre (see discussion above on the challenges of lineage tracing studies)(Sanchez-Gurmaches and Guertin, 2014, Haldar et al., 2008, Gensch et al., 2008). The most interesting possibility is that there is a temporal or spatial separation between certain lineages and understanding this may help in understanding the commitment phase to brown adipocytes. Regardless, these studies conclusively revealed an unanticipated heterogeneity in both brown and white adipocyte development that suggests brown adipocytes residing in different depots could have different embryonic origins.
The developmental heterogeneity observed between brown adipocyte lineages is not likely due to low efficiency or specificity of the Cre-drivers because independent experiments with Myf5-Cre, Pax3-Cre and Pax7-CreER lines are remarkably similar (Lepper and Fan, 2010, Sanchez-Gurmaches and Guertin, 2014, Liu et al., 2013, Sanchez-Gurmaches et al., 2012, Seale et al., 2008, Shan et al., 2013, Wang et al., 2014b). Moreover, heterogenous Myf5 labeling is also observed in skeletal muscle lineages in which Myf5 only labels around 50% of the satellite cells in the limb muscles but around 80% in epaxial muscles (Haldar et al., 2008, Gensch et al., 2008). An unanswered question is whether developmentally distinct brown (or white) adipocytes differ only in their anatomical location, or whether they have unique functions (e.g. metabolic efficiency, BATokine production, exosome secretion, etc.) that might be specific by their developmental origins. Answering these questions will require an improved ability to isolate and study single brown adipocytes, a deeper understanding of the regulatory mechanisms of BAT development, and markers that label the unidentified (Myf5-Cre;Pax3-Crenegative) brown adipocyte lineages.
2.3. Postnatal and Adult Brown Fat Growth and Metabolism
In older laboratory mice (i.e. juveniles and adults), individual BAT depots can expand their mass by either increasing brown adipocyte number (hyperplasia) or by increasing individual cell size (hypertrophy) depending upon their initial housing temperature and the duration and degree of cold exposure. For example, hypertrophic growth of brown adipocytes is observed when mice living in standard housing conditions (22°C) are acclimated to their thermoneutral zone (e.g., 30~32 °C). Under these conditions, the sympathetic tone is reduced by removing thermal stress, and the brown adipocytes decrease their thermogenic activity. This results in lipids accumulating and coalescing into a single large unilocular lipid droplet, thereby increasing individual cell size. Notably, while thermoneutral BAT displays a WAT-like morphology and gene expression signature, it maintains its BAT epigenetic signature (Hung et al., 2014, Veniant et al., 2015, Roh et al., 2018). Nevertheless, the net result of increasing cell size is in an increase in total depot size compared to mice living in the mild cold temperatures of most mouse facilities (Figure 2).
Conversely, if mice living at thermoneutrality are moved to the mild cold (21–22°C) and BAT thermogenesis is activated, the mobilization and metabolism of lipids and other metabolites reduce individual adipocyte cell size and thereby overall BAT depot size (Figure 2). However, if these mice are then further adapted to more severely cold temperatures (e.g. in 6–10°C range), additional new active brown adipocytes are recruited into the BAT depots (presumably from the brown ASPC pool described above), which increases BAT mass, but by hyperplastic growth (Bukowiecki et al., 1982, Rehnmark and Nedergaard, 1989, Geloen et al., 1992, Lee et al., 2015, Razzoli et al., 2018). Indeed, de novo adipogenesis of brown adipocyte precursor cells occurs in response to chronic cold (Rosenwald et al., 2013, Lee et al., 2015). Brown adipocyte size also increases by denervation, during extended high caloric (fat) feeding, or with aging (Hung et al., 2014, Roberts-Toler et al., 2015). Thus, while there is an underlying natural turnover of brown adipocytes (Sakaguchi et al., 2017), the iBAT depots in laboratory mice are smallest when mice are acclimated to standard lab conditions (mild cold), and it grows with increased or decreased temperature mainly by hypertrophic or hyperplasic growth, respectively.
Gene expression profiling of BAT tissue reveals greater differences between mice acclimated to thermoneutrality (30~32°C) and mild cold (21~22°C) than between mice acclimated to mild cold (21~22°C) and severe cold (6°C) (Sanchez-Gurmaches et al., 2018). This is consistent with brown fat morphology at these temperatures, which shows individual brown adipocytes in an “off” state (unilocular) in thermoneutrality and an “on” state (multilocular lipid droplets) at 22°C. Further reductions in temperature (e.g. to 6°C) increase the magnitude of thermogenesis and many genes associated with thermogenesis, and this is additionally reflected by morphological “ordering” of the lipid droplets (Figure 2). A survey of metabolic genes upregulated in the mild cold indicates that genes encoding regulators fatty acid oxidation and de novo lipogenesis are both upregulated as are genes whose products function in respiratory metabolism (e.g. the tricarboxylic (TCA) cycle, electron transport chain) and thermogenesis (Sanchez-Gurmaches et al., 2018, McCormack and Denton, 1977, Mottillo et al., 2014, Shimazu and Takahashi, 1980, Townsend and Tseng, 2015, Trayhurn, 1979, Yu et al., 2002). This emphasizes an interesting metabolic paradox of brown adipocytes; that increasing BAT catabolic activity by cold is also associated with induction of anabolic lipid synthesis pathways. This may be another BAT characteristic futile cycling mechanism or alternatively, the stimulation of de novo lipogenesis may have other metabolic implications since many intermediates in the de novo lipogenesis pathway, such as acetyl-coA, also function as second messengers (Pietrocola et al., 2015).
III. OTHER THERMOGENIC ADIPOCYTES
3.1. Brite/Beige adipocytes
A second type of UCP1-expressing adipocyte called a brite (brown-like in white) adipocyte, also known as a beige adipocyte, is also attracting interest as a potential therapeutic target in obesity and metabolic disease. As the synonymous names imply, brite/beige adipocytes appear within specific WAT depots under certain stresses, and their morphology (lipid droplet size and mitochondria content) is intermediary between that of classic brown and white adipocytes (Figure 1). There have been two experimental methods used to drive brite/beige cell formation in sWAT. Acclimation to severe cold temperatures (6–10°C) may be the most physiological approach, which strongly induces BAT thermogenesis along with the formation of brite/beige adipocytes that express UCP1 in sWAT. Similar to how brown adipocytes change their appearance between thermoneutrality and mild cold, brite/beige adipocytes undergo morphological changes between mild cold and severe cold that include the typical multilocular morphology in severe cold (Figure 2A–2B). A second common method to induce brite/beige cell formation is to treat mice with the β3-adrenergic agonists CL-316243, which resembles the effects of cold exposure on mature adipocytes. Many other stresses can also lead to the formation of brite/beige adipocytes including exercise, cancer cachexia, and peripheral tissue injury (Ikeda et al., 2018, Singh and Dalton, 2018) suggesting brite/beige adipocyte formation may reflect a general stress response rather than specifically the response to cold. Whether these alternative modes of browning indicate a physiologically relevant role for thermogenesis, or reflect a secondary consequence of altered adipocyte state is not yet clear. Nevertheless, increasing brite/beige adipocyte number could also be a strategy to fight obesity, and thus there is strong interest in understanding the biology of how brite/beige adipocytes develop.
The location and number of brite/beige adipocytes in adult humans is less clear. Studies suggest that UCP1-positive brown-like adipocytes purified from human supraclavicular BAT depots have a similar gene expression pattern to murine brite/beige adipocytes (Lidell et al., 2013, Wu et al., 2012, Sharp et al., 2012, Shinoda et al., 2015), whereas brown-like adipocytes isolated from other human BAT depots (neck, cervical, perirenal) appear to more closely resemble classic brown adipocytes in mice (Cypess et al., 2013, Xue et al., 2015). More recent studies using 18F-fluodeoxyglucose (FDG) PET-CT imaging noticed that several additional metabolically active adipocytes reside in the abdominal and subcutaneous areas of adult humans, which could be brown or brite/beige adipocytes (Leitner et al., 2017). Other studies of BAT in the supraclavicular region of adult humans have shown more mixed transcriptional profiling representative of both brown and brite/beige adipocytes (Leitner et al., 2017). One caveat of comparing human studies to mouse studies is that often the comparisons are made between thermoneutral humans and cold stressed mice, and it is possible that some of the human brite/beige adipocytes could be less-stimulated brown adipocytes. Another open question is whether brite/beige adipocytes make significant contributions to overall thermogenesis (Singh and Dalton, 2018, Kajimura et al., 2015, Nedergaard and Cannon, 2014). Nevertheless, these studies support the idea that stimulating brite/beige adipocyte formation in humans could be another way to improve glucose homeostasis.
3.2. Brite/beige adipocyte origins
Understanding brite/beige adipocyte origins is important because it may provide insight into therapeutic strategies to induce their formation. Currently, there are two main competing theories to explain where brite/beige adipocytes originate from that are not necessarily mutually exclusive. The first theory posits that brite/beige adipocytes form de novo upon stimulation from a precursor cell pool (Wang et al., 2013b); the second argues that they inter-covert from existing adipocytes between a dormant to active state depending upon the presence of stimulus (Lee et al., 2015, Barbatelli et al., 2010). A third likely possibility is that both mechanisms occur, perhaps in a context dependent manner dependent upon many factors including type of stimulation, its duration, the depot analyzed, and proximity to the sympathetic nervous system input.
Using strategies to fluorescently mark individual adipocytes, it has been shown that around 60% of the total UCP1+ adipocytes that form in the sWAT after cold acclimation (7 days) are generated de novo by the process of adipogenesis (Wang et al., 2013b, Berry et al., 2016). These new brite/beige adipocytes originate from smooth muscle actin (SMA) positive progenitors and require β1-adrenergic receptor signaling similar to how nascent brown adipocytes form upon cold exposure (Berry et al., 2016, Jiang et al., 2017, Bukowiecki et al., 1986, Bronnikov et al., 1992, Bukowiecki et al., 1982, Rehnmark and Nedergaard, 1989, Geloen et al., 1992, Razzoli et al., 2018). However, SMA+ progenitors also give rise to all white adipocytes in both subcutaneous and visceral fats (Jiang et al., 2014). Thus, whether these reflect two distinct sub-pools of SMA+ progenitors for white and brite/beige adipocytes, or a common precursor pool, is unclear. The other implication of these data is that the other 40% of the UCP1+ adipocytes that formed originate, or interconvert, from preexisting white adipocytes, which has also been referred to as transdifferentiation to reflect the fundamental changes in gene expression and morphology (Cinti, 2002). However, there is inconsistency between these and other studies that may be related to the lack of a standard experimental approach across studies, or differences in strain background, age, and/or previous exposure to environmental or dietary variables (Lee et al., 2015).
Administering the β3-adrenergic agonist CL-316243, which is widely used to induce brite/beige cell formation, appears to induce brite/beige adipocyte formation from preexisting mature white adipocytes (Jiang et al., 2017, de Jong et al., 2017). However, because CL-316,243 acts only on the mature cells, a systemically derived signal that might act on the precursors may be absent. Notably, lack of β3-adrenergic receptor activity does not prevent the “browning” capacity of sWAT by cold further suggesting that multiple pathways to brite/beige adipocyte formation exist that could have compensatory capability (Jiang et al., 2017). Again, these results must be interpreted carefully because distinct responses to β3-adrenergic receptor inactivation are observed depending on mouse background (Barbatelli et al., 2010). An additional confusing factor is that the browning capacity of the psWAT depends on the genetic background. For instance, the A/J mouse strain shows higher UCP1 induction upon β3-adrenergic stimulation (in the psWAT) than the more commonly used C57Bl6 mice (Chabowska-Kita and Kozak, 2016, Collins et al., 1997). More research is clearly needed to fully understand which modality of brite/beige adipocyte formation is most tractable for therapeutic targeting.
An interesting question is whether all white adipocytes can become brite/beige under certain conditions, or whether there is a fundamental cell intrinsic feature of like the sWAT adipocytes that give them their “britening” capacity. A related question is whether there is a specific brite/beige adipocyte cell lineage that is different from the lineages that give rise to the white adipocytes that do not become brite/beige and the brown adipocytes (Nedergaard and Cannon, 2014, Kozak, 2011). Originally it was suggested that Myf5 expression could delineate between the brown adipocyte lineage and the brite/beige adipocyte lineage, which was thought to be Myf5-negative. However, more comprehensive Myf5-lineage tracing studies later showed that the brite/beige adipocytes that form in the Myf5-positive asWAT and retroperitoneal WAT depots are also Myf5-positive (Shan et al., 2013, Sanchez-Gurmaches and Guertin, 2014) (Figure 2A). In contrast, none of the brite/beige adipocytes that form in psWAT are Myf5-positive, suggesting Myf5 expression likely delineates between anatomical positioning rather than function; however, this has not yet been fully resolved and it is unknown if Myf5-positive brite/beige adipocytes are functionally identical to Myf5-negative brite/beige adipocytes (Berry et al., 2016, Sanchez-Gurmaches and Guertin, 2014, Sanchez-Gurmaches et al., 2012, Seale et al., 2008).
One study searching for markers of a brite/beige adipocyte lineage found that CD137 positive precursors isolated from psWAT have a greater propensity to induce UCP1 mRNA in culture compared to CD137-negative precursors, suggesting an intrinsic heterogeneity in the capacity to adopt different metabolic profiles (Wu et al., 2012). However, it has not yet been shown that this population is specific to a brite/beige adipocyte lineage in vivo such as by lineage tracing studies. Ribosome profiling studies have also shown that brite/beige adipocytes in the psWAT possess a gene expression signature that has similarity to smooth muscle-like cells, which is not observed in brown adipocytes and it is independent of anatomical position (Long et al., 2014). However, this appears to only represent a subset of the total brite/beige adipocyte population because lineage tracing experiments with a Cre recombinase driven by the Myh11 promoter, which is a marker of smooth muscle cells, only labels ~10% of the UCP1+ brite/beige adipocytes following prolonged cold acclimation (Long et al., 2014, Berry et al., 2016), and deleting PPARγ in this lineage does prevent not WAT browning (Berry et al., 2016). These data may reflect the inherent heterogeneity in adipocytes and thus, a specific brite/beige adipocyte lineage marker remains elusive. High expression of Ebf2 is found in precursor cells with high thermogenic capacity in psWAT (Wang et al., 2014b, Stine et al., 2016). It will be interesting to see whether Ebf2 functionally commits progenitors to a thermogenic lineage.
In contrast to the intra-depot heterogeneity seen with other brite/beige cell markers, all psWAT and bone marrow adipocytes are homogeneously labeled by a Cre recombinase driven from the paired related homeobox transcription factor 1 (Prx1) promoter, which expresses in mesenchymal precursors during development in what appears to be a multi-potent precursor population that also gives rise to limb and head tissues (Sanchez-Gurmaches et al., 2015, Krueger et al., 2014, Ambrosi et al., 2017). Interestingly, the Prx1 transcription factor itself has been linked to cell-fate decisions including adipocyte specification (Logan et al., 2002, Cserjesi et al., 1992, Du et al., 2013, Hu et al., 1998, Lu et al., 1999, Peterson et al., 2005, ten Berge et al., 1998). What is noteworthy about Prx1-Cre is that it does not significantly label any other WAT or BAT depots, allowing for some degree of depot specificity when used for targeting WAT that cannot be achieved with Adiponectin-Cre (Eguchi et al., 2011). Because Prx1-Cre does not label the brite/beige adipocytes that form in other depots, such as the rWAT, it is not a universal brite/beige marker, and it likely expresses very early before adipocyte specification. However, its labelling pattern could be a clue to understanding inter-depot heterogeneity, but this remains to be seen.
One factor to consider in studying brite/beige lineages is not only the potential heterogeneity of the ASPC pool or the individual adipocyte functional identities (which is still mysterious), but also the morphological heterogeneity across the depot with respect to where these cells form. For example, cold can induce browning in an irregular “patchy” pattern in sWAT such that distinct islands of brite/beige adipocytes can sometimes be seen. It is possible that there are differences in the local concentration of adrenaline/noradrenaline that is dependent upon proximity to nerves or neurite density and could explain the erratic patterning. However, recent discoveries suggest that almost all sWAT adipocytes are in direct contact with sympathetic innervation (Chi et al., 2018, Jiang et al., 2017) suggesting the alternative possibilities that the pattering is cell-autonomously regulated or could reflect different yet to be defined niches within the depot. In sum, a distinct lineage or ASPC population that exclusively gives rise to the brite/beige cells is still lacking, suggesting their formation is likely more complex and multifactorial.
IV. GOING FORWARD
4.1. Unanswered questions and future goals
Understanding the developmental origins of brown and brite/beige adipocytes, and the cell intrinsic and extrinsic signals that specify their fate and metabolic properties, is not only of biological interested, but critical to advancing potential therapies that target thermogenesis as a means to increase energy expenditure. One of the major themes in adipose tissue biology that has emerged in recent years, driven by both developmental and metabolic studies, is that adipose tissues are highly heterogeneous. Developmental studies suggest brown and white adipocytes in different depots, as well as brown and white adipocytes within the same depot, may have different embryonic origins. Metabolic studies indicate that different white fat depots have different metabolic properties; for example, excess vWAT is metabolically unhealthy while excess sWAT can be protective against metabolic disease (Reaven, 1988, Snijder et al., 2003, Snijder et al., 2004, Van Pelt et al., 2005). Even within WAT depots, some neighboring adipocytes may have different metabolic activity (Lee et al., 2017). Perhaps an interesting comparison is to skeletal muscle, which can have both fast and slow twitch fibers. Whether different BAT depots or brown adipocytes within single BAT depots have different metabolic properties or other functions is less clear. Understanding the functional significance of BAT heterogeneity, both at the mature adipocyte level and within the ASPC pool, is one important future goal.
While adipose tissue heterogeneity can be visualized by imaging studies, understanding the biochemical significance of BAT heterogeneity, and adipose tissue heterogeneity in general, has been more complicated by the fact that whole depots (as well as FACS-isolated ASPC pools) contain many non-adipocyte cells that can “contaminate” experiments that are focused on the adipocyte linages. Thus, key unanswered questions include whether there are genetic or epigenetic differences between lineages, whether there are lineage specific transcription factors, receptors, or other factors, and whether different lineages produce different amounts of adipokines/BATokines or other transmissible signals. Exciting technological advances in single-cell RNA/DNA-sequencing, metabolomics and proteomics, combined with emerging tools that can purify organelles (including nuclei) and translating RNA away from non-adipocyte cells (Roh et al., 2017, Chen et al., 2016, Abu-Remaileh et al., 2017) are opening the door to a much higher-resolution view of adipocyte heterogeneity.
Single cell profiling, for example, will allow us to define the cellular heterogeneity of ASPCs (and mature adipocyte populations) based on their genetic and epigenetic expression profiles. Single cell analysis on cells captured at different differentiation stages has been useful in other systems to understand the differentiation path of a particular cell type (following what has been called pseudotime). In one application for lineage tracing purposes, mutations or single nucleotide polymorphism can be introduced in precursors in a manner that will accumulate over time such that unique individual cell sequences can be followed cumulatively in descendent cells (McKenna et al., 2016). Additionally, algorithms capable of deciphering spatial differences in gene expression are being developed to help understand region specific functions within a tissue (Potter, 2018, Griffiths et al., 2018, Kumar et al., 2017, McKenna et al., 2016).
In a recent study focusing on adipocytes that will undoubtedly usher more, single-cell RNA-sequencing of the ASPCs population in WAT identified at least three different cell populations involved in adipocyte regulation, one of which surprisingly secretes an unknown signal that inhibits adipogenesis (Schwalie et al., 2018). Another recent study performed single cell transcriptomic analysis with human sWAT precursor cells and demonstrated that ASPCs are largely clustered in a single population (Acosta et al., 2017). Combining such studies with new tools that can isolate adipocytes away from the many non-adipocytes in a whole depot will be powerful. For example, a novel mouse model called NuTRAP, when combined with a fat-specific Cre driver, enables transcriptional and epigenomic profiling of only the Cre-marked adipocytes isolated from whole fat tissues (Roh et al., 2017, Roh et al., 2018). Advances such as these will undoubtedly refine our understanding of the adipocyte lineages and will be a major focus area for the near future. As such, it will be important to standardize strains, diets, temperature, sex across experiments as well as consider each depot as separate and functionally distinct entities so that results are comparable across laboratories.
Another important goal is to understand BAT fuel utilization, and whether developmental origins have any role in specifying metabolic activities. Since thermogenesis requires free fatty acid exchange with mitochondria, it will be important to understand how BAT handles lipids. For example, why does BAT have both catabolic and anabolic lipid pathways working simultaneously? (Sanchez-Gurmaches et al., 2018, McCormack and Denton, 1977, Mottillo et al., 2014, Shimazu and Takahashi, 1980, Townsend and Tseng, 2015, Trayhurn, 1979, Yu et al., 2002) What is the significance of BAT lipolysis (Schreiber et al., 2017, Shin et al., 2017)? Recent studies also suggest that mitochondria proximal to lipid droplets, called peridroplet mitochondria, are functionally different from cytoplasmic mitochondria not associated with lipid droplets (Benador et al., 2018, Rambold et al., 2015, Nguyen et al., 2017, Stone et al., 2009, Wang et al., 2011). The prospect of mitochondria heterogeneity within a single cell opens up a whole new avenue of interest in understanding how organelles communicate with each other and the genome to control BAT metabolism. For example, although brown adipocytes have lower endoplasmic reticulum compared to other cell types, a recent study shows that brown adipocyte thermogenesis is regulated by an ER-membrane-embedded transcription factor (Bartelt et al., 2018). Sorting out intracellular BAT metabolism, and the influence of anatomical positioning, developmental patterning, innervation/vascularization, and immune cell communication on these processes will be critical in guiding the development of better therapeutic models.
While the role of the SNS in stimulating brown fat activity has long been understood (Kawate et al., 1994, Muzik et al., 2017, Owen et al., 2014), there are many interesting future questions about the role of the SNS in brite/beige adipocyte formation, as well as in the ability of BAT to communicate back to the brain and to other WAT depots. For example, it was recently proposed that iBAT cross-talks to the sWAT through an “sWAT sensory neuron—Brain—iBAT” SNS connection (Garretson et al., 2016, Nguyen et al., 2018). According to this model, cold-induced sWAT lipolysis activates local afferent neurons triggering a neuronal circuit from sWAT to iBAT that controls iBAT thermogenesis, and this effect is abolished when the sWAT is denervated (Garretson et al., 2016, Nguyen et al., 2018). These findings emphasize that BAT development is likely tightly coordinated with nerve development, and the concept of “neurometabolism” remains and understudied aspect of BAT growth and overall metabolic homeostasis.
Regarding potential connections between brown (and white) adipocyte origins and human fat disorders, a curious observation is that many lipodystrophy disorders present as selective adipose tissue atrophy, in which some depots shrink or disappear while others expand possibly as a compensatory response (Garg, 2011). A similar type of fat body redistribution is observed when Myf5-Cre is used to genetically ablate regulators of the insulin signaling pathway in mice (Sanchez-Gurmaches et al., 2012, Sanchez-Gurmaches and Guertin, 2014). For example, deleting PTEN (a negative regulator of insulin signaling) in the Myf5-lineage expands Myf5-positive brown and white fat, converts the brown fat into a white-fat like tissue, and causes the non Myf5-lineage positive adipocytes to disappear (Sanchez-Gurmaches et al., 2012); in contrast, deleting insulin receptor-beta (IR-β) with Myf5-Cre redistributes body fat in the other direction and reduces individual adipocyte size (Sanchez-Gurmaches and Guertin, 2014, Gesta et al., 2007). The former model is strikingly similar to a rare fat disorder called multiple symmetric lipomatosis or Madelung’s disease (Guastella et al., 2002, Ramos et al., 2010, Herbst, 2012). Thus, another key question is whether differences in body fat distribution, whether pathological--such as in lipodystrophy or obesity--or even normal fat distribution across the population may have some link to the developmental heterogeneity of fat.
4.2. Prospects for BAT-based therapeutics.
There are a number of key issues that if resolved could help inform the development of BAT-based therapies. First, the commitment of progenitor cells to the thermogenic lineage is not understood. Knowing the mechanisms of brown fat specification could greatly aid in promoting the conversion of non-thermogenic cells to brown adipocytes. Whether better to focus on BAT or brite/beige adipocytes as a target for increasing energy expenditure remains unknown. Brite/beige fat may be promising because many obese or overweight adults seem to have a low abundance of BAT, at least based on classic BAT descriptions; however, better detection methods are needed (Hanssen et al., 2015, Ouellet et al., 2012, van der Lans et al., 2013, Yoneshiro et al., 2011a, Yoneshiro et al., 2011b, Betz and Enerback, 2018). Moreover, fully activated individual brite/beige adipocytes seem to have the same amount of UCP1 protein as an individual brown adipocyte even though total depot levels are quite different (Shabalina et al., 2013) and perhaps even slight increases in energy expenditure could have large effects over time. On the other hand, it may be possible to “train” adults to increase BAT activity (van der Lans et al., 2013, Hanssen et al., 2015). Understanding the development of thermogenic adipocytes is also relevant to stem cell-based models of thermogenesis such as in isogenic cell therapy programs (Singh and Dalton, 2018). For example, it may be possible to generate human induced pluripotent stem cells from patient derived somatic cells that are reprogrammed to have a thermogenic adipocyte fate when transplanted into recipients (Ahfeldt et al., 2012, Kishida et al., 2015, Guenantin et al., 2017, Pisani et al., 2011). Chemical/hormonal induction protocols to generate such cells have not yet been described but would be of interest. Directly transplanting patient derived brown adipocytes into obese individuals to improve metabolism may also be possible, and has been demonstrated in rodent models (Min et al., 2016). At this point, there is not a clear consensus as to the best strategy for increasing brown fat activity to fight obesity, and both classic brown and brite/beige adipocytes should be considered until we know more about BAT and brite/beige adipocyte development and function.
It is remarkable that it was only about a decade ago that it became widely appreciated that adults have brown and brite/beige adipocytes, and thus while excitement about the therapeutic potential of targeting these amazing cells to increase energy expenditure is high, there is much research to done to better understand their biology, and in particular, understanding their development and metabolic control, which are major challenge areas ahead.
Acknowledgments
SMJ is supported by a postdoctoral fellowship award from the American Diabetes Association (1-18-PDF-128). JSG is supported by an American Heart Association Career Development award (18CDA34080527). DAG is supported by grants from the NIH (R01DK094004 and R01CA196986) and a Leukemia and Lymphoma Society Career Development Award.
References
- ABU-REMAILEH M, WYANT GA, KIM C, LAQTOM NN, ABBASI M, CHAN SH, FREINKMAN E & SABATINI DM 2017. Lysosomal metabolomics reveals V-ATPase- and mTOR-dependent regulation of amino acid efflux from lysosomes. Science, 358, 807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACOSTA JR, JOOST S, KARLSSON K, EHRLUND A, LI X, AOUADI M, KASPER M, ARNER P, RYDEN M & LAURENCIKIENE J 2017. Single cell transcriptomics suggest that human adipocyte progenitor cells constitute a homogeneous cell population. Stem Cell Res Ther, 8, 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AHFELDT T, SCHINZEL RT, LEE Y-K, HENDRICKSON D, KAPLAN A, LUM DH, CAMAHORT R, XIA F, SHAY J, RHEE EP, CLISH CB, DEO RC, SHEN T, LAU FH, COWLEY A, MOWRER G, AL-SIDDIQI H, NAHRENDORF M, MUSUNURU K, GERSZTEN RE, RINN JL & COWAN CA 2012. Programming human pluripotent stem cells into white and brown adipocytes. Nature Cell Biology, 14, 209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMBROSI TH, SCIALDONE A, GRAJA A, GOHLKE S, JANK AM, BOCIAN C, WOELK L, FAN H, LOGAN DW, SCHURMANN A, SARAIVA LR & SCHULZ TJ 2017. Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration. Cell Stem Cell, 20, 771–784.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATIT R, SGAIER SK, MOHAMED OA, TAKETO MM, DUFORT D, JOYNER AL, NISWANDER L & CONLON RA 2006. beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Developmental Biology, 296, 164–176. [DOI] [PubMed] [Google Scholar]
- BAGLIONI S, CANTINI G, POLI G, FRANCALANCI M, SQUECCO R, DI FRANCO A, BORGOGNI E, FRONTERA S, NESI G, LIOTTA F, LUCCHESE M, PERIGLI G, FRANCINI F, FORTI G, SERIO M & LUCONI M 2012. Functional differences in visceral and subcutaneous fat pads originate from differences in the adipose stem cell. PLoS One, 7, e36569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAGLIONI S, FRANCALANCI M, SQUECCO R, LOMBARDI A, CANTINI G, ANGELI R, GELMINI S, GUASTI D, BENVENUTI S, ANNUNZIATO F, BANI D, LIOTTA F, FRANCINI F, PERIGLI G, SERIO M & LUCONI M 2009. Characterization of human adult stem-cell populations isolated from visceral and subcutaneous adipose tissue. FASEB J, 23, 3494–505. [DOI] [PubMed] [Google Scholar]
- BARBATELLI G, MURANO I, MADSEN L, HAO Q, JIMENEZ M, KRISTIANSEN K, GIACOBINO JP, DE MATTEIS R & CINTI S 2010. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab, 298, E1244–53. [DOI] [PubMed] [Google Scholar]
- BARTELT A, BRUNS OT, REIMER R, HOHENBERG H, ITTRICH H, PELDSCHUS K, KAUL MG, TROMSDORF UI, WELLER H, WAURISCH C, EYCHMULLER A, GORDTS PL, RINNINGER F, BRUEGELMANN K, FREUND B, NIELSEN P, MERKEL M & HEEREN J 2011. Brown adipose tissue activity controls triglyceride clearance. Nat Med, 17, 200–5. [DOI] [PubMed] [Google Scholar]
- BARTELT A, WIDENMAIER SB, SCHLEIN C, JOHANN K, GONCALVES RLS, EGUCHI K, FISCHER AW, PARLAKGUL G, SNYDER NA, NGUYEN TB, BRUNS OT, FRANKE D, BAWENDI MG, LYNES MD, LEIRIA LO, TSENG YH, INOUYE KE, ARRUDA AP & HOTAMISLIGIL GS 2018. Brown adipose tissue thermogenic adaptation requires Nrf1-mediated proteasomal activity. Nat Med, 24, 292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENADOR IY, VELIOVA M, MAHDAVIANI K, PETCHERSKI A, WIKSTROM JD, ASSALI EA, ACIN-PEREZ R, SHUM M, OLIVEIRA MF, CINTI S, SZTALRYD C, BARSHOP WD, WOHLSCHLEGEL JA, CORKEY BE, LIESA M & SHIRIHAI OS 2018. Mitochondria Bound to Lipid Droplets Have Unique Bioenergetics, Composition, and Dynamics that Support Lipid Droplet Expansion. Cell Metab, 27, 869–885 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERRY DC, JIANG Y & GRAFF JM 2016. Mouse strains to study cold-inducible beige progenitors and beige adipocyte formation and function. Nat Commun, 7, 10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERRY R, JEFFERY E & RODEHEFFER MS 2014. Weighing in on adipocyte precursors. Cell Metab, 19, 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERRY R & RODEHEFFER MS 2013. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol, 15, 302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTHOLET AM, KAZAK L, CHOUCHANI ET, BOGACZYNSKA MG, PARANJPE I, WAINWRIGHT GL, BETOURNE A, KAJIMURA S, SPIEGELMAN BM & KIRICHOK Y 2017. Mitochondrial Patch Clamp of Beige Adipocytes Reveals UCP1-Positive and UCP1-Negative Cells Both Exhibiting Futile Creatine Cycling. Cell Metab, 25, 811–822 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BETZ MJ & ENERBACK S 2018. Targeting thermogenesis in brown fat and muscle to treat obesity and metabolic disease. Nat Rev Endocrinol, 14, 77–87. [DOI] [PubMed] [Google Scholar]
- BLANCHETTE-MACKIE EJ & SCOW RO 1983. Movement of lipolytic products to mitochondria in brown adipose tissue of young rats: an electron microscope study. J Lipid Res, 24, 229–44. [PubMed] [Google Scholar]
- BRONNIKOV G, HOUSTEK J & NEDERGAARD J 1992. Beta-adrenergic, cAMP-mediated stimulation of proliferation of brown fat cells in primary culture. Mediation via beta 1 but not via beta 3 adrenoceptors. J Biol Chem, 267, 2006–13. [PubMed] [Google Scholar]
- BUKOWIECKI L, COLLET AJ, FOLLEA N, GUAY G & JAHJAH L 1982. Brown adipose tissue hyperplasia: a fundamental mechanism of adaptation to cold and hyperphagia. Am J Physiol, 242, E353–9. [DOI] [PubMed] [Google Scholar]
- BUKOWIECKI LJ, GELOEN A & COLLET AJ 1986. Proliferation and differentiation of brown adipocytes from interstitial cells during cold acclimation. Am J Physiol, 250, C880–7. [DOI] [PubMed] [Google Scholar]
- CANNON B & NEDERGAARD J 2004. Brown adipose tissue: function and physiological significance. Physiol Rev, 84, 277–359. [DOI] [PubMed] [Google Scholar]
- CARRER A, PARRIS JL, TREFELY S, HENRY RA, MONTGOMERY DC, TORRES A, VIOLA JM, KUO YM, BLAIR IA, MEIER JL, ANDREWS AJ, SNYDER NW & WELLEN KE 2017. Impact of a High-fat Diet on Tissue Acyl-CoA and Histone Acetylation Levels. J Biol Chem, 292, 3312–3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHABOWSKA-KITA A & KOZAK LP 2016. The critical period for brown adipocyte development: Genetic and environmental influences. Obesity (Silver Spring), 24, 283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN WW, FREINKMAN E, WANG T, BIRSOY K & SABATINI DM 2016. Absolute Quantification of Matrix Metabolites Reveals the Dynamics of Mitochondrial Metabolism. Cell, 166, 1324–1337 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHI J, WU Z, CHOI CHJ, NGUYEN L, TEGEGNE S, ACKERMAN SE, CRANE A, MARCHILDON F, TESSIER-LAVIGNE M & COHEN P 2018. Three-Dimensional Adipose Tissue Imaging Reveals Regional Variation in Beige Fat Biogenesis and PRDM16-Dependent Sympathetic Neurite Density. Cell Metab, 27, 226–236.e3. [DOI] [PubMed] [Google Scholar]
- CHURCH CD, BERRY R & RODEHEFFER MS 2014. Isolation and study of adipocyte precursors. Methods Enzymol, 537, 31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CINTI S 2002. Adipocyte differentiation and transdifferentiation: plasticity of the adipose organ. J Endocrinol Invest, 25, 823–35. [DOI] [PubMed] [Google Scholar]
- CINTI S 2005. The adipose organ. Prostaglandins Leukot Essent Fatty Acids, 73, 9–15. [DOI] [PubMed] [Google Scholar]
- COLLINS S, DANIEL KW, PETRO AE & SURWIT RS 1997. Strain-specific response to beta 3-adrenergic receptor agonist treatment of diet-induced obesity in mice. Endocrinology, 138, 405–13. [DOI] [PubMed] [Google Scholar]
- CSERJESI P, LILLY B, BRYSON L, WANG Y, SASSOON DA & OLSON EN 1992. MHox: a mesodermally restricted homeodomain protein that binds an essential site in the muscle creatine kinase enhancer. Development, 115, 1087–101. [DOI] [PubMed] [Google Scholar]
- CYPESS AM, LEHMAN S, WILLIAMS G, TAL I, RODMAN D, GOLDFINE AB, KUO FC, PALMER EL, TSENG YH, DORIA A, KOLODNY GM & KAHN CR 2009. Identification and importance of brown adipose tissue in adult humans. N Engl J Med, 360, 1509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CYPESS AM, WHITE AP, VERNOCHET C, SCHULZ TJ, XUE R, SASS CA, HUANG TL, ROBERTS-TOLER C, WEINER LS, SZE C, CHACKO AT, DESCHAMPS LN, HERDER LM, TRUCHAN N, GLASGOW AL, HOLMAN AR, GAVRILA A, HASSELGREN PO, MORI MA, MOLLA M & TSENG YH 2013. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med, 19, 635–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE JONG JM, LARSSON O, CANNON B & NEDERGAARD J 2015. A stringent validation of mouse adipose tissue identity markers. Am J Physiol Endocrinol Metab, 308, E1085–105. [DOI] [PubMed] [Google Scholar]
- DE JONG JMA, WOUTERS RTF, BOULET N, CANNON B, NEDERGAARD J & PETROVIC N 2017. The beta3-adrenergic receptor is dispensable for browning of adipose tissues. Am J Physiol Endocrinol Metab, 312, E508–e518. [DOI] [PubMed] [Google Scholar]
- DEMPERSMIER J, SAMBEAT A, GULYAEVA O, PAUL SM, HUDAK CS, RAPOSO HF, KWAN HY, KANG C, WONG RH & SUL HS 2015. Cold-inducible Zfp516 activates UCP1 transcription to promote browning of white fat and development of brown fat. Mol Cell, 57, 235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DU B, CAWTHORN WP, SU A, DOUCETTE CR, YAO Y, HEMATI N, KAMPERT S, MCCOIN C, BROOME DT, ROSEN CJ, YANG G & MACDOUGALD OA 2013. The transcription factor paired-related homeobox 1 (Prrx1) inhibits adipogenesis by activating transforming growth factor-beta (TGFbeta) signaling. J Biol Chem, 288, 3036–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGUCHI J, WANG X, YU S, KERSHAW EE, CHIU PC, DUSHAY J, ESTALL JL, KLEIN U, MARATOS-FLIER E & ROSEN ED 2011. Transcriptional control of adipose lipid handling by IRF4. Cell Metab, 13, 249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARMER SR 2006. Transcriptional control of adipocyte formation. Cell Metab, 4, 263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEDORENKO A, LISHKO PV & KIRICHOK Y 2012. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell, 151, 400–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDERICH-PERSSON M, NGUYEN DINH CAT A, PERSSON P, MONTEZANO AC & TOUYZ RM 2017. Brown Adipose Tissue Regulates Small Artery Function Through NADPH Oxidase 4-Derived Hydrogen Peroxide and Redox-Sensitive Protein Kinase G-1alpha. Arterioscler Thromb Vasc Biol, 37, 455–465. [DOI] [PubMed] [Google Scholar]
- FRONTINI A & CINTI S 2010. Distribution and Development of Brown Adipocytes in the Murine and Human Adipose Organ. Cell Metabolism, 11, 253–256. [DOI] [PubMed] [Google Scholar]
- GARG A 2011. Clinical review#: Lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab, 96, 3313–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARRETSON JT, SZYMANSKI LA, SCHWARTZ GJ, XUE B, RYU V & BARTNESS TJ 2016. Lipolysis sensation by white fat afferent nerves triggers brown fat thermogenesis. Mol Metab, 5, 626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GELOEN A, COLLET AJ & BUKOWIECKI LJ 1992. Role of sympathetic innervation in brown adipocyte proliferation. Am J Physiol, 263, R1176–81. [DOI] [PubMed] [Google Scholar]
- GENSCH N, BORCHARDT T, SCHNEIDER A, RIETHMACHER D & BRAUN T 2008. Different autonomous myogenic cell populations revealed by ablation of Myf5-expressing cells during mouse embryogenesis. Development, 135, 1597–604. [DOI] [PubMed] [Google Scholar]
- GESTA S, TSENG YH & KAHN CR 2007. Developmental origin of fat: tracking obesity to its source. Cell, 131, 242–56. [DOI] [PubMed] [Google Scholar]
- GRIFFITHS JA, SCIALDONE A & MARIONI JC 2018. Using single-cell genomics to understand developmental processes and cell fate decisions. Mol Syst Biol, 14, e8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUASTELLA C, BORSI C, GIBELLI S & DELLA BERTA LG 2002. Madelung’s lipomatosis associated with head and neck malignant neoplasia: a study of 2 cases. Otolaryngol Head Neck Surg, 126, 191–2. [DOI] [PubMed] [Google Scholar]
- GUENANTIN AC, BRIAND N, CAPEL E, DUMONT F, MORICHON R, PROVOST C, STILLITANO F, JEZIOROWSKA D, SIFFROI JP, HAJJAR RJ, FEVE B, HULOT JS, COLLAS P, CAPEAU J & VIGOUROUX C 2017. Functional Human Beige Adipocytes From Induced Pluripotent Stem Cells. Diabetes, 66, 1470–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUPTA RK, ARANY Z, SEALE P, MEPANI RJ, YE L, CONROE HM, ROBY YA, KULAGA H, REED RR & SPIEGELMAN BM 2010. Transcriptional control of preadipocyte determination by Zfp423. Nature, 464, 619–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUPTA RK, MEPANI RJ, KLEINER S, LO JC, KHANDEKAR MJ, COHEN P, FRONTINI A, BHOWMICK DC, YE L, CINTI S & SPIEGELMAN BM 2012. Zfp423 Expression Identifies Committed Preadipocytes and Localizes to Adipose Endothelial and Perivascular Cells. Cell Metabolism, 15, 230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALDAR M, KARAN G, TVRDIK P & CAPECCHI MR 2008. Two cell lineages, myf5 and myf5-independent, participate in mouse skeletal myogenesis. Dev Cell, 14, 437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSEN IR, JANSSON KM, CANNON B & NEDERGAARD J 2014. Contrasting effects of cold acclimation versus obesogenic diets on chemerin gene expression in brown and brite adipose tissues. Biochim Biophys Acta, 1841, 1691–9. [DOI] [PubMed] [Google Scholar]
- HANSSEN MJ, HOEKS J, BRANS B, VAN DER LANS AA, SCHAART G, VAN DEN DRIESSCHE JJ, JORGENSEN JA, BOEKSCHOTEN MV, HESSELINK MK, HAVEKES B, KERSTEN S, MOTTAGHY FM, VAN MARKEN LICHTENBELT WD & SCHRAUWEN P 2015. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med. [DOI] [PubMed] [Google Scholar]
- HARMS M & SEALE P 2013. Brown and beige fat: development, function and therapeutic potential. Nat Med, 19, 1252–63. [DOI] [PubMed] [Google Scholar]
- HARMS MJ, ISHIBASHI J, WANG W, LIM HW, GOYAMA S, SATO T, KUROKAWA M, WON KJ & SEALE P 2014. Prdm16 is required for the maintenance of brown adipocyte identity and function in adult mice. Cell Metab, 19, 593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERBST KL 2012. Rare adipose disorders (RADs) masquerading as obesity. Acta Pharmacol Sin, 33, 155–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HU YS, ZHOU H, KARTSOGIANNIS V, EISMAN JA, MARTIN TJ & NG KW 1998. Expression of rat homeobox gene, rHOX, in developing and adult tissues in mice and regulation of its mRNA expression in osteoblasts by bone morphogenetic protein 2 and parathyroid hormone-related protein. Mol Endocrinol, 12, 1721–32. [DOI] [PubMed] [Google Scholar]
- HUNG CM, CALEJMAN CM, SANCHEZ-GURMACHES J, LI H, CLISH CB, HETTMER S, WAGERS AJ & GUERTIN DA 2014. Rictor/mTORC2 loss in the Myf5 lineage reprograms brown fat metabolism and protects mice against obesity and metabolic disease. Cell Rep, 8, 256–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IKEDA K, MARETICH P & KAJIMURA S 2018. The Common and Distinct Features of Brown and Beige Adipocytes. Trends Endocrinol Metab, 29, 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIANG Y, BERRY DC & GRAFF JM 2017. Distinct cellular and molecular mechanisms for beta3 adrenergic receptor-induced beige adipocyte formation. Elife, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIANG Y, BERRY DC, TANG W & GRAFF JM 2014. Independent stem cell lineages regulate adipose organogenesis and adipose homeostasis. Cell Rep, 9, 1007–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOE AW, YI L, NATARAJAN A, LE GRAND F, SO L, WANG J, RUDNICKI MA & ROSSI FM 2010. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol, 12, 153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAJIMURA S, SPIEGELMAN BM & SEALE P 2015. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab, 22, 546–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWATE R, TALAN MI & ENGEL BT 1994. Sympathetic nervous activity to brown adipose tissue increases in cold-tolerant mice. Physiol Behav, 55, 921–5. [DOI] [PubMed] [Google Scholar]
- KAZAK L, CHOUCHANI ET, JEDRYCHOWSKI MP, ERICKSON BK, SHINODA K, COHEN P, VETRIVELAN R, LU GZ, LAZNIK-BOGOSLAVSKI D, HASENFUSS SC, KAJIMURA S, GYGI SP & SPIEGELMAN BM 2015. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell, 163, 643–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISHIDA T, EJIMA A, YAMAMOTO K, TANAKA S, YAMAMOTO T & MAZDA O 2015. Reprogrammed Functional Brown Adipocytes Ameliorate Insulin Resistance and Dyslipidemia in Diet-Induced Obesity and Type 2 Diabetes. Stem Cell Reports, 5, 569–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORTELAINEN ML, PELLETIER G, RICQUIER D & BUKOWIECKI LJ 1993. Immunohistochemical detection of human brown adipose tissue uncoupling protein in an autopsy series. J Histochem Cytochem, 41, 759–64. [DOI] [PubMed] [Google Scholar]
- KOZAK LP 2011. The genetics of brown adipocyte induction in white fat depots. Front Endocrinol (Lausanne), 2, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRUEGER KC, COSTA MJ, DU H & FELDMAN BJ 2014. Characterization of Cre recombinase activity for in vivo targeting of adipocyte precursor cells. Stem Cell Reports, 3, 1147–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUMAR P, TAN Y & CAHAN P 2017. Understanding development and stem cells using single cell-based analyses of gene expression. Development, 144, 17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LABBE SM, CARON A, BAKAN I, LAPLANTE M, CARPENTIER AC, LECOMTE R & RICHARD D 2015. In vivo measurement of energy substrate contribution to cold-induced brown adipose tissue thermogenesis. FASEB J, 29, 2046–58. [DOI] [PubMed] [Google Scholar]
- LEE KY, SHARMA R, GASE G, USSAR S, LI Y, WELCH L, BERRYMAN DE, KISPERT A, BLUHER M & KAHN CR 2017. Tbx15 Defines a Glycolytic Subpopulation and White Adipocyte Heterogeneity. Diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE YH, PETKOVA AP, KONKAR AA & GRANNEMAN JG 2015. Cellular origins of cold-induced brown adipocytes in adult mice. FASEB J, 29, 286–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE YH, PETKOVA AP, MOTTILLO EP & GRANNEMAN JG 2012. In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell Metab, 15, 480–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEHR L, CANOLA K, ASENSIO C, JIMENEZ M, KUEHNE F, GIACOBINO JP & MUZZIN P 2006. The control of UCP1 is dissociated from that of PGC-1alpha or of mitochondriogenesis as revealed by a study using beta-less mouse brown adipocytes in culture. FEBS Lett, 580, 4661–6. [DOI] [PubMed] [Google Scholar]
- LEITNER BP, HUANG S, BRYCHTA RJ, DUCKWORTH CJ, BASKIN AS, MCGEHEE S, TAL I, DIECKMANN W, GUPTA G, KOLODNY GM, PACAK K, HERSCOVITCH P, CYPESS AM & CHEN KY 2017. Mapping of human brown adipose tissue in lean and obese young men. Proc Natl Acad Sci U S A, 114, 8649–8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEPPER C & FAN C-M 2010. Inducible Lineage Tracing of Pax7-Descendant Cells Reveals Embryonic Origin of Adult Satellite Cells. Genesis, 48, 424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIDELL ME, BETZ MJ, LEINHARD OD, HEGLIND M, ELANDER L, SLAWIK M, MUSSACK T, NILSSON D, ROMU T, NUUTILA P, VIRTANEN KA, BEUSCHLEIN F, PERSSON A, BORGA M & ENERBACK S 2013. Evidence for two types of brown adipose tissue in humans. Nat Med. [DOI] [PubMed] [Google Scholar]
- LIU W, SHAN T, YANG X, LIANG S, ZHANG P, LIU Y, LIU X & KUANG S 2013. A heterogeneous lineage origin underlies the phenotypic and molecular differences of white and beige adipocytes. J Cell Sci, 126, 3527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOGAN M, MARTIN JF, NAGY A, LOBE C, OLSON EN & TABIN CJ 2002. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis, 33, 77–80. [DOI] [PubMed] [Google Scholar]
- LONG JZ, SVENSSON KJ, BATEMAN LA, LIN H, KAMENECKA T, LOKURKAR IA, LOU J, RAO RR, CHANG MR, JEDRYCHOWSKI MP, PAULO JA, GYGI SP, GRIFFIN PR, NOMURA DK & SPIEGELMAN BM 2016. The Secreted Enzyme PM20D1 Regulates Lipidated Amino Acid Uncouplers of Mitochondria. Cell, 166, 424–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONG JZ, SVENSSON KJ, TSAI L, ZENG X, ROH HC, KONG X, RAO RR, LOU J, LOKURKAR I, BAUR W, CASTELLOT JJ JR., ROSEN ED & SPIEGELMAN BM 2014. A smooth muscle-like origin for beige adipocytes. Cell Metab, 19, 810–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LU MF, CHENG HT, KERN MJ, POTTER SS, TRAN B, DIEKWISCH TG & MARTIN JF 1999. prx-1 functions cooperatively with another paired-related homeobox gene, prx-2, to maintain cell fates within the craniofacial mesenchyme. Development, 126, 495–504. [DOI] [PubMed] [Google Scholar]
- LUMENG CN & SALTIEL AR 2011. Inflammatory links between obesity and metabolic disease. J Clin Invest, 121, 2111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAHDAVIANI K, CHESS D, WU Y, SHIRIHAI O & APRAHAMIAN TR 2016. Autocrine effect of vascular endothelial growth factor-A is essential for mitochondrial function in brown adipocytes. Metabolism, 65, 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCORMACK JG & DENTON RM 1977. Evidence that fatty acid synthesis in the interscapular brown adipose tissue of cold-adapted rats is increased in vivo by insulin by mechanisms involving parallel activation of pyruvate dehydrogenase and acetyl-coenzyme A carboxylase. Biochem J, 166, 627–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCKENNA A, FINDLAY GM, GAGNON JA, HORWITZ MS, SCHIER AF & SHENDURE J 2016. Whole-organism lineage tracing by combinatorial and cumulative genome editing. Science, 353, aaf7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIN SY, KADY J, NAM M, ROJAS-RODRIGUEZ R, BERKENWALD A, KIM JH, NOH HL, KIM JK, COOPER MP, FITZGIBBONS T, BREHM MA & CORVERA S 2016. Human ‘brite/beige’ adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat Med, 22, 312–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MO Q, SALLEY J, ROSHAN T, BAER LA, MAY FJ, JAEHNIG EJ, LEHNIG AC, GUO X, TONG Q, NUOTIO-ANTAR AM, SHAMSI F, TSENG YH, STANFORD KI & CHEN MH 2017. Identification and characterization of a supraclavicular brown adipose tissue in mice. JCI Insight, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRISON SF, MADDEN CJ & TUPONE D 2012. Central control of brown adipose tissue thermogenesis. Front Endocrinol (Lausanne), 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOTTILLO EP, BALASUBRAMANIAN P, LEE YH, WENG C, KERSHAW EE & GRANNEMAN JG 2014. Coupling of lipolysis and de novo lipogenesis in brown, beige, and white adipose tissues during chronic beta3-adrenergic receptor activation. J Lipid Res, 55, 2276–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOULLAN N, MOUCHIROUD L, WANG X, RYU D, WILLIAMS EG, MOTTIS A, JOVAISAITE V, FROCHAUX MV, QUIROS PM, DEPLANCKE B, HOUTKOOPER RH & AUWERX J 2015. Tetracyclines Disturb Mitochondrial Function across Eukaryotic Models: A Call for Caution in Biomedical Research. Cell Rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURANO I, BARBATELLI G, GIORDANO A & CINTI S 2009. Noradrenergic parenchymal nerve fiber branching after cold acclimatisation correlates with brown adipocyte density in mouse adipose organ. J Anat, 214, 171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUZIK O, MANGNER TJ, LEONARD WR, KUMAR A & GRANNEMAN JG 2017. Sympathetic Innervation of Cold-Activated Brown and White Fat in Lean Young Adults. J Nucl Med, 58, 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUZUMDAR MD, TASIC B, MIYAMICHI K, LI L & LUO L 2007. A global double-fluorescent Cre reporter mouse. Genesis, 45, 593–605. [DOI] [PubMed] [Google Scholar]
- NEDERGAARD J, BENGTSSON T & CANNON B 2007. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab, 293, E444–52. [DOI] [PubMed] [Google Scholar]
- NEDERGAARD J & CANNON B 2014. The browning of white adipose tissue: some burning issues. Cell Metab, 20, 396–407. [DOI] [PubMed] [Google Scholar]
- NGUYEN NLT, XUE B & BARTNESS TJ 2018. Sensory denervation of inguinal white fat modifies sympathetic outflow to white and brown fat in Siberian hamsters. Physiol Behav, 190, 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NGUYEN TB, LOUIE SM, DANIELE JR, TRAN Q, DILLIN A, ZONCU R, NOMURA DK & OLZMANN JA 2017. DGAT1-Dependent Lipid Droplet Biogenesis Protects Mitochondrial Function during Starvation-Induced Autophagy. Dev Cell, 42, 9–21 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHOLLS DG 2006. The physiological regulation of uncoupling proteins. Biochim Biophys Acta, 1757, 459–66. [DOI] [PubMed] [Google Scholar]
- NISOLI E, CLEMENTI E, TONELLO C, SCIORATI C, BRISCINI L & CARRUBA MO 1998. Effects of nitric oxide on proliferation and differentiation of rat brown adipocytes in primary cultures. Br J Pharmacol, 125, 888–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLEFSKY JM & GLASS CK 2010. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol, 72, 219–46. [DOI] [PubMed] [Google Scholar]
- OUELLET V, LABBE SM, BLONDIN DP, PHOENIX S, GUERIN B, HAMAN F, TURCOTTE EE, RICHARD D & CARPENTIER AC 2012. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. Journal of Clinical Investigation, 122, 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OWEN BM, DING X, MORGAN DA, COATE KC, BOOKOUT AL, RAHMOUNI K, KLIEWER SA & MANGELSDORF DJ 2014. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab, 20, 670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRINI S, FICARELLA R, PICARDI E, CIGNARELLI A, BARBARO M, NIGRO P, PESCHECHERA A, PALUMBO O, CARELLA M, DE FAZIO M, NATALICCHIO A, LAVIOLA L, PESOLE G & GIORGINO F 2013. Differences in gene expression and cytokine release profiles highlight the heterogeneity of distinct subsets of adipose tissue-derived stem cells in the subcutaneous and visceral adipose tissue in humans. PLoS One, 8, e57892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSON RE, HOFFMAN S & KERN MJ 2005. Opposing roles of two isoforms of the Prx1 homeobox gene in chondrogenesis. Dev Dyn, 233, 811–21. [DOI] [PubMed] [Google Scholar]
- PIETROCOLA F, GALLUZZI L, BRAVO-SAN PEDRO JM, MADEO F & KROEMER G 2015. Acetyl coenzyme A: a central metabolite and second messenger. Cell Metab, 21, 805–21. [DOI] [PubMed] [Google Scholar]
- PISANI DF, DJEDAINI M, BERANGER GE, ELABD C, SCHEIDELER M, AILHAUD G & AMRI EZ 2011. Differentiation of Human Adipose-Derived Stem Cells into “Brite” (Brown-in-White) Adipocytes. Front Endocrinol (Lausanne), 2, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POTTER SS 2018. Single-cell RNA sequencing for the study of development, physiology and disease. Nat Rev Nephrol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAJAKUMARI S, WU J, ISHIBASHI J, LIM HW, GIANG AH, WON KJ, REED RR & SEALE P 2013. EBF2 determines and maintains brown adipocyte identity. Cell Metab, 17, 562–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMBOLD AS, COHEN S & LIPPINCOTT-SCHWARTZ J 2015. Fatty acid trafficking in starved cells: regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev Cell, 32, 678–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMOS S, PINHEIRO S, DIOGO C, CABRAL L & CRUZEIRO C 2010. Madelung disease: a not-so-rare disorder. Ann Plast Surg, 64, 122–4. [DOI] [PubMed] [Google Scholar]
- RAZZOLI M, EMMETT MJ, LAZAR MA & BARTOLOMUCCI A 2018. beta-Adrenergic receptors control brown adipose UCP-1 tone and cold response without affecting its circadian rhythmicity. Faseb j, fj201800452R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REAVEN GM 1988. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes, 37, 1595–607. [DOI] [PubMed] [Google Scholar]
- REHNMARK S & NEDERGAARD J 1989. DNA synthesis in mouse brown adipose tissue is under beta-adrenergic control. Exp Cell Res, 180, 574–9. [DOI] [PubMed] [Google Scholar]
- RIVERA-GONZALEZ GC, SHOOK BA, ANDRAE J, HOLTRUP B, BOLLAG K, BETSHOLTZ C, RODEHEFFER MS & HORSLEY V 2016. Skin Adipocyte Stem Cell Self-Renewal Is Regulated by a PDGFA/AKT-Signaling Axis. Cell Stem Cell, 19, 738–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS-TOLER C, O’NEILL BT & CYPESS AM 2015. Diet-induced obesity causes insulin resistance in mouse brown adipose tissue. Obesity (Silver Spring), 23, 1765–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODEHEFFER MS, BIRSOY K & FRIEDMAN JM 2008. Identification of White Adipocyte Progenitor Cells In Vivo. Cell, 135, 240–249. [DOI] [PubMed] [Google Scholar]
- ROH HC, TSAI LT, LYUBETSKAYA A, TENEN D, KUMARI M & ROSEN ED 2017. Simultaneous Transcriptional and Epigenomic Profiling from Specific Cell Types within Heterogeneous Tissues In Vivo. Cell Rep, 18, 1048–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROH HC, TSAI LTY, SHAO M, TENEN D, SHEN Y, KUMARI M, LYUBETSKAYA A, JACOBS C, DAWES B, GUPTA RK & ROSEN ED 2018. Warming Induces Significant Reprogramming of Beige, but Not Brown, Adipocyte Cellular Identity. Cell Metab, 27, 1121–1137.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEN ED, SARRAF P, TROY AE, BRADWIN G, MOORE K, MILSTONE DS, SPIEGELMAN BM & MORTENSEN RM 1999. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell, 4, 611–7. [DOI] [PubMed] [Google Scholar]
- ROSENWALD M, PERDIKARI A, RULICKE T & WOLFRUM C 2013. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol, 15, 659–67. [DOI] [PubMed] [Google Scholar]
- ROTHWELL NJ & STOCK MJ 1984. Effects of denervating brown adipose tissue on the responses to cold, hyperphagia and noradrenaline treatment in the rat. J Physiol, 355, 457–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SACKS H & SYMONDS ME 2013. Anatomical locations of human brown adipose tissue: functional relevance and implications in obesity and type 2 diabetes. Diabetes, 62, 1783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO M, OKAMATSU-OGURA Y, MATSUSHITA M, WATANABE K, YONESHIRO T, NIO-KOBAYASHI J, IWANAGA T, MIYAGAWA M, KAMEYA T, NAKADA K, KAWAI Y & TSUJISAKI M 2009. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes, 58, 1526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKAGUCHI M, FUJISAKA S, CAI W, WINNAY JN, KONISHI M, O’NEILL BT, LI M, GARCIA-MARTIN R, TAKAHASHI H, HU J, KULKARNI RN & KAHN CR 2017. Adipocyte Dynamics and Reversible Metabolic Syndrome in Mice with an Inducible Adipocyte-Specific Deletion of the Insulin Receptor. Cell Metab, 25, 448–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMBEAT A, GULYAEVA O, DEMPERSMIER J, THARP KM, STAHL A, PAUL SM & SUL HS 2016. LSD1 Interacts with Zfp516 to Promote UCP1 Transcription and Brown Fat Program. Cell Rep, 15, 2536–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANCHEZ-GURMACHES J & GUERTIN DA 2014. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat Commun, 5, 4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANCHEZ-GURMACHES J, HSIAO WY & GUERTIN DA 2015. Highly selective in vivo labeling of subcutaneous white adipocyte precursors with Prx1-Cre. Stem Cell Reports, 4, 541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANCHEZ-GURMACHES J, HUNG CM, SPARKS CA, TANG Y, LI H & GUERTIN DA 2012. PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell Metab, 16, 348–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANCHEZ-GURMACHES J, TANG Y, JESPERSEN NZ, WALLACE M, MARTINEZ CALEJMAN C, GUJJA S, LI H, EDWARDS YJK, WOLFRUM C, METALLO CM, NIELSEN S, SCHEELE C & GUERTIN DA 2018. Brown Fat AKT2 Is a Cold-Induced Kinase that Stimulates ChREBP-Mediated De Novo Lipogenesis to Optimize Fuel Storage and Thermogenesis. Cell Metab, 27, 195–209.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHREIBER R, DIWOKY C, SCHOISWOHL G, FEILER U, WONGSIRIROJ N, ABDELLATIF M, KOLB D, HOEKS J, KERSHAW EE, SEDEJ S, SCHRAUWEN P, HAEMMERLE G & ZECHNER R 2017. Cold-Induced Thermogenesis Depends on ATGL-Mediated Lipolysis in Cardiac Muscle, but Not Brown Adipose Tissue. Cell Metab, 26, 753–763.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULZ TJ, HUANG P, HUANG TL, XUE R, MCDOUGALL LE, TOWNSEND KL, CYPESS AM, MISHINA Y, GUSSONI E & TSENG YH 2013. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature, 495, 379–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWALIE PC, DONG H, ZACHARA M, RUSSEIL J, ALPERN D, AKCHICHE N, CAPRARA C, SUN W, SCHLAUDRAFF K-U, SOLDATI G, WOLFRUM C & DEPLANCKE B 2018. A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature. [DOI] [PubMed] [Google Scholar]
- SEALE P, BJORK B, YANG W, KAJIMURA S, CHIN S, KUANG S, SCIME A, DEVARAKONDA S, CONROE HM, ERDJUMENT-BROMAGE H, TEMPST P, RUDNICKI MA, BEIER DR & SPIEGELMAN BM 2008. PRDM16 controls a brown fat/skeletal muscle switch. Nature, 454, 961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEALE P, CONROE HM, ESTALL J, KAJIMURA S, FRONTINI A, ISHIBASHI J, COHEN P, CINTI S & SPIEGELMAN BM 2011. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest, 121, 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEALE P, KAJIMURA S, YANG W, CHIN S, ROHAS LM, ULDRY M, TAVERNIER G, LANGIN D & SPIEGELMAN BM 2007. Transcriptional control of brown fat determination by PRDM16. Cell Metabolism, 6, 38–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHABALINA IG, PETROVIC N, DE JONG JM, KALINOVICH AV, CANNON B & NEDERGAARD J 2013. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep, 5, 1196–203. [DOI] [PubMed] [Google Scholar]
- SHAN T, LIANG X, BI P, ZHANG P, LIU W & KUANG S 2013. Distinct populations of adipogenic and myogenic Myf5-lineage progenitors in white adipose tissues. J Lipid Res, 54, 2214–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAO M & GUPTA RK 2018. Transcriptional brakes on the road to adipocyte thermogenesis. Biochim Biophys Acta. [DOI] [PubMed] [Google Scholar]
- SHAO M, ISHIBASHI J, KUSMINSKI CM, WANG QA, HEPLER C, VISHVANATH L, MACPHERSON KA, SPURGIN SB, SUN K, HOLLAND WL, SEALE P & GUPTA RK 2016. Zfp423 Maintains White Adipocyte Identity through Suppression of the Beige Cell Thermogenic Gene Program. Cell Metab, 23, 1167–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARP LZ, SHINODA K, OHNO H, SCHEEL DW, TOMODA E, RUIZ L, HU H, WANG L, PAVLOVA Z, GILSANZ V & KAJIMURA S 2012. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One, 7, e49452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIMAZU T & TAKAHASHI A 1980. Stimulation of hypothalamic nuclei has differential effects on lipid synthesis in brown and white adipose tissue. Nature, 284, 62–3. [DOI] [PubMed] [Google Scholar]
- SHIMIZU I, APRAHAMIAN T, KIKUCHI R, SHIMIZU A, PAPANICOLAOU KN, MACLAUCHLAN S, MARUYAMA S & WALSH K 2014. Vascular rarefaction mediates whitening of brown fat in obesity. J Clin Invest, 124, 2099–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIN H, MA Y, CHANTURIYA T, CAO Q, WANG Y, KADEGOWDA AKG, JACKSON R, RUMORE D, XUE B, SHI H, GAVRILOVA O & YU L 2017. Lipolysis in Brown Adipocytes Is Not Essential for Cold-Induced Thermogenesis in Mice. Cell Metab, 26, 764–777.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHINODA K, LUIJTEN IH, HASEGAWA Y, HONG H, SONNE SB, KIM M, XUE R, CHONDRONIKOLA M, CYPESS AM, TSENG YH, NEDERGAARD J, SIDOSSIS LS & KAJIMURA S 2015. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat Med, 21, 389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVA JE & LARSEN PR 1983. Adrenergic activation of triiodothyronine production in brown adipose tissue. Nature, 305, 712–3. [DOI] [PubMed] [Google Scholar]
- SIMCOX J, GEOGHEGAN G, MASCHEK JA, BENSARD CL, PASQUALI M, MIAO R, LEE S, JIANG L, HUCK I, KERSHAW EE, DONATO AJ, APTE U, LONGO N, RUTTER J, SCHREIBER R, ZECHNER R, COX J & VILLANUEVA CJ 2017. Global Analysis of Plasma Lipids Identifies Liver-Derived Acylcarnitines as a Fuel Source for Brown Fat Thermogenesis. Cell Metab, 26, 509–522 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGH AM & DALTON S 2018. What Can ‘Brown-ing’ Do For You? Trends Endocrinol Metab, 29, 349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLUSE FE, JARMUSZKIEWICZ W, NAVET R, DOUETTE P, MATHY G & SLUSE-GOFFART CM 2006. Mitochondrial UCPs: new insights into regulation and impact. Biochim Biophys Acta, 1757, 480–5. [DOI] [PubMed] [Google Scholar]
- SNIJDER MB, DEKKER JM, VISSER M, BOUTER LM, STEHOUWER CD, KOSTENSE PJ, YUDKIN JS, HEINE RJ, NIJPELS G & SEIDELL JC 2003. Associations of hip and thigh circumferences independent of waist circumference with the incidence of type 2 diabetes: the Hoorn Study. Am J Clin Nutr, 77, 1192–7. [DOI] [PubMed] [Google Scholar]
- SNIJDER MB, DEKKER JM, VISSER M, BOUTER LM, STEHOUWER CD, YUDKIN JS, HEINE RJ, NIJPELS G, SEIDELL JC & HOORN S 2004. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care, 27, 372–7. [DOI] [PubMed] [Google Scholar]
- STINE RR, SHAPIRA SN, LIM HW, ISHIBASHI J, HARMS M, WON KJ & SEALE P 2016. EBF2 promotes the recruitment of beige adipocytes in white adipose tissue. Mol Metab, 5, 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STONE SJ, LEVIN MC, ZHOU P, HAN J, WALTHER TC & FARESE RV JR. 2009. The endoplasmic reticulum enzyme DGAT2 is found in mitochondria-associated membranes and has a mitochondrial targeting signal that promotes its association with mitochondria. J Biol Chem, 284, 5352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUN C, BERRY WL & OLSON LE 2017. PDGFRalpha controls the balance of stromal and adipogenic cells during adipose tissue organogenesis. Development, 144, 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUN K, KUSMINSKI CM, LUBY-PHELPS K, SPURGIN SB, AN YA, WANG QA, HOLLAND WL & SCHERER PE 2014. Brown adipose tissue derived VEGF-A modulates cold tolerance and energy expenditure. Mol Metab, 3, 474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVENSSON KJ, LONG JZ, JEDRYCHOWSKI MP, COHEN P, LO JC, SERAG S, KIR S, SHINODA K, TARTAGLIA JA, RAO RR, CHEDOTAL A, KAJIMURA S, GYGI SP & SPIEGELMAN BM 2016. A Secreted Slit2 Fragment Regulates Adipose Tissue Thermogenesis and Metabolic Function. Cell Metab, 23, 454–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI A, SHIMAZU T & MARUYAMA Y 1992. Importance of sympathetic nerves for the stimulatory effect of cold exposure on glucose utilization in brown adipose tissue. Jpn J Physiol, 42, 653–64. [DOI] [PubMed] [Google Scholar]
- TALLQUIST MD, WEISMANN KE, HELLSTROM M & SORIANO P 2000. Early myotome specification regulates PDGFA expression and axial skeleton development. Development, 127, 5059–5070. [DOI] [PubMed] [Google Scholar]
- TEN BERGE D, BROUWER A, KORVING J, MARTIN JF & MEIJLINK F 1998. Prx1 and Prx2 in skeletogenesis: roles in the craniofacial region, inner ear and limbs. Development, 125, 3831–42. [DOI] [PubMed] [Google Scholar]
- THOMOU T, MORI MA, DREYFUSS JM, KONISHI M, SAKAGUCHI M, WOLFRUM C, RAO TN, WINNAY JN, GARCIA-MARTIN R, GRINSPOON SK, GORDEN P & KAHN CR 2017. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature, 542, 450–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TONTONOZ P, HU E & SPIEGELMAN BM 1994. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell, 79, 1147–56. [DOI] [PubMed] [Google Scholar]
- TOWNSEND KL & TSENG YH 2015. Of mice and men: novel insights regarding constitutive and recruitable brown adipocytes. Int J Obes Suppl, 5, S15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAYHURN P 1979. Fatty acid synthesis in vivo in brown adipose tissue, liver and white adipose tissue of the cold-acclimated rat. FEBS Lett, 104, 13–6. [DOI] [PubMed] [Google Scholar]
- USSAR S, LEE KY, DANKEL SN, BOUCHER J, HAERING MF, KLEINRIDDERS A, THOMOU T, XUE R, MACOTELA Y, CYPESS AM, TSENG YH, MELLGREN G & KAHN CR 2014. ASC-1, PAT2, and P2RX5 are cell surface markers for white, beige, and brown adipocytes. Sci Transl Med, 6, 247ra103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DER LANS AA, HOEKS J, BRANS B, VIJGEN GH, VISSER MG, VOSSELMAN MJ, HANSEN J, JORGENSEN JA, WU J, MOTTAGHY FM, SCHRAUWEN P & VAN MARKEN LICHTENBELT WD 2013. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest, 123, 3395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN HARMELEN V, SKURK T & HAUNER H 2005. Primary culture and differentiation of human adipocyte precursor cells. Methods Mol Med, 107, 125–35. [DOI] [PubMed] [Google Scholar]
- VAN MARKEN LICHTENBELT WD, VANHOMMERIG JW, SMULDERS NM, DROSSAERTS JM, KEMERINK GJ, BOUVY ND, SCHRAUWEN P & TEULE GJ 2009. Cold-activated brown adipose tissue in healthy men. N Engl J Med, 360, 1500–8. [DOI] [PubMed] [Google Scholar]
- VAN PELT RE, JANKOWSKI CM, GOZANSKY WS, SCHWARTZ RS & KOHRT WM 2005. Lower-body adiposity and metabolic protection in postmenopausal women. J Clin Endocrinol Metab, 90, 4573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENIANT MM, SIVITS G, HELMERING J, KOMOROWSKI R, LEE J, FAN W, MOYER C & LLOYD DJ 2015. Pharmacologic Effects of FGF21 Are Independent of the “Browning” of White Adipose Tissue. Cell Metab, 21, 731–8. [DOI] [PubMed] [Google Scholar]
- VILLARROYA F, CEREIJO R, VILLARROYA J, GAVALDA-NAVARRO A & GIRALT M 2018. Toward an Understanding of How Immune Cells Control Brown and Beige Adipobiology. Cell Metab, 27, 954–961. [DOI] [PubMed] [Google Scholar]
- VILLARROYA F & GIRALT M 2015. The Beneficial Effects of Brown Fat Transplantation: Further Evidence of an Endocrine Role of Brown Adipose Tissue. Endocrinology, 156, 2368–70. [DOI] [PubMed] [Google Scholar]
- VILLARROYA J, CEREIJO R & VILLARROYA F 2013. An endocrine role for brown adipose tissue? Am J Physiol Endocrinol Metab, 305, E567–72. [DOI] [PubMed] [Google Scholar]
- VIRTANEN KA, LIDELL ME, ORAVA J, HEGLIND M, WESTERGREN R, NIEMI T, TAITTONEN M, LAINE J, SAVISTO NJ, ENERBACK S & NUUTILA P 2009. Functional brown adipose tissue in healthy adults. N Engl J Med, 360, 1518–25. [DOI] [PubMed] [Google Scholar]
- VISHVANATH L, MACPHERSON KA, HEPLER C, WANG QA, SHAO M, SPURGIN SB, WANG MY, KUSMINSKI CM, MORLEY TS & GUPTA RK 2016. Pdgfrbeta+ Mural Preadipocytes Contribute to Adipocyte Hyperplasia Induced by High-Fat-Diet Feeding and Prolonged Cold Exposure in Adult Mice. Cell Metab, 23, 350–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALDEN TB, HANSEN IR, TIMMONS JA, CANNON B & NEDERGAARD J 2012. Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am J Physiol Endocrinol Metab, 302, E19–31. [DOI] [PubMed] [Google Scholar]
- WANG F, MULLICAN SE, DISPIRITO JR, PEED LC & LAZAR MA 2013a. Lipoatrophy and severe metabolic disturbance in mice with fat-specific deletion of PPARgamma. Proc Natl Acad Sci U S A, 110, 18656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG GX, ZHAO XY, MENG ZX, KERN M, DIETRICH A, CHEN Z, COZACOV Z, ZHOU D, OKUNADE AL, SU X, LI S, BLUHER M & LIN JD 2014a. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat Med, 20, 1436–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG H, SREENIVASAN U, HU H, SALADINO A, POLSTER BM, LUND LM, GONG DW, STANLEY WC & SZTALRYD C 2011. Perilipin 5, a lipid droplet-associated protein, provides physical and metabolic linkage to mitochondria. J Lipid Res, 52, 2159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG QA, TAO C, GUPTA RK & SCHERER PE 2013b. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med, 19, 1338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG W, KISSIG M, RAJAKUMARI S, HUANG L, LIM HW, WON KJ & SEALE P 2014b. Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc Natl Acad Sci U S A, 111, 14466–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU J, BOSTROM P, SPARKS LM, YE L, CHOI JH, GIANG AH, KHANDEKAR M, VIRTANEN KA, NUUTILA P, SCHAART G, HUANG K, TU H, VAN MARKEN LICHTENBELT WD, HOEKS J, ENERBACK S, SCHRAUWEN P & SPIEGELMAN BM 2012. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell, 150, 366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XUE R, LYNES MD, DREYFUSS JM, SHAMSI F, SCHULZ TJ, ZHANG H, HUANG TL, TOWNSEND KL, LI Y, TAKAHASHI H, WEINER LS, WHITE AP, LYNES MS, RUBIN LL, GOODYEAR LJ, CYPESS AM & TSENG YH 2015. Clonal analyses and gene profiling identify genetic biomarkers of the thermogenic potential of human brown and white preadipocytes. Nat Med, 21, 760–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XUE Y, PETROVIC N, CAO R, LARSSON O, LIM S, CHEN S, FELDMANN HM, LIANG Z, ZHU Z, NEDERGAARD J, CANNON B & CAO Y 2009. Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell Metab, 9, 99–109. [DOI] [PubMed] [Google Scholar]
- YE R, WANG QA, TAO C, VISHVANATH L, SHAO M, MCDONALD JG, GUPTA RK & SCHERER PE 2015. Impact of tamoxifen on adipocyte lineage tracing: Inducer of adipogenesis and prolonged nuclear translocation of Cre recombinase. Mol Metab, 4, 771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YONESHIRO T, AITA S, MASTUSHITA M, OGAWA T, OKAMATSU-OGURA Y, KAWAI Y & SAITO M 2011a. Age-Related Decrease in Brown Adipose Tissue and Obesity in Humans. Obesity, 19, S79–S79. [DOI] [PubMed] [Google Scholar]
- YONESHIRO T, AITA S, MATSUSHITA M, KAMEYA T, NAKADA K, KAWAI Y & SAITO M 2011b. Brown Adipose Tissue, Whole-Body Energy Expenditure, and Thermogenesis in Healthy Adult Men. Obesity, 19, 13–16. [DOI] [PubMed] [Google Scholar]
- YONESHIRO T, AITA S, MATSUSHITA M, KAYAHARA T, KAMEYA T, KAWAI Y, IWANAGA T & SAITO M 2013. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest, 123, 3404–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YONESHIRO T, AITA S, MATSUSHITA M, OKAMATSU-OGURA Y, KAMEYA T, KAWAI Y, MIYAGAWA M, TSUJISAKI M & SAITO M 2011c. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity (Silver Spring), 19, 1755–60. [DOI] [PubMed] [Google Scholar]
- YU XX, LEWIN DA, FORREST W & ADAMS SH 2002. Cold elicits the simultaneous induction of fatty acid synthesis and beta-oxidation in murine brown adipose tissue: prediction from differential gene expression and confirmation in vivo. Faseb j, 16, 155–68. [DOI] [PubMed] [Google Scholar]
- ZHANG F, HAO G, SHAO M, NHAM K, AN Y, WANG Q, ZHU Y, KUSMINSKI CM, HASSAN G, GUPTA RK, ZHAI Q, SUN X, SCHERER PE & OZ OK 2018. An Adipose Tissue Atlas: An Image-Guided Identification of Human-like BAT and Beige Depots in Rodents. Cell Metab, 27, 252–262.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHAO S, TORRES A, HENRY RA, TREFELY S, WALLACE M, LEE JV, CARRER A, SENGUPTA A, CAMPBELL SL, KUO YM, FREY AJ, MEURS N, VIOLA JM, BLAIR IA, WELJIE AM, METALLO CM, SNYDER NW, ANDREWS AJ & WELLEN KE 2016. ATP-Citrate Lyase Controls a Glucose-to-Acetate Metabolic Switch. Cell Rep, 17, 1037–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]


