Abstract
Background
Our hospital experienced an outbreak of OXA-48-producing Enterobacteriaceae, triggering this study. We aimed to describe the population with carbapenemase-producing Enterobacteriaceae (CPE) in our hospital from 2014 to 2018, the phenotypic and genotypic characteristics of isolates, and strategies to stop the outbreak.
Methods
We performed a retrospective study, including every patient with CPE species in a clinical sample. Epidemiology, risk factors, treatment and outcomes were gathered from medical records.
Results
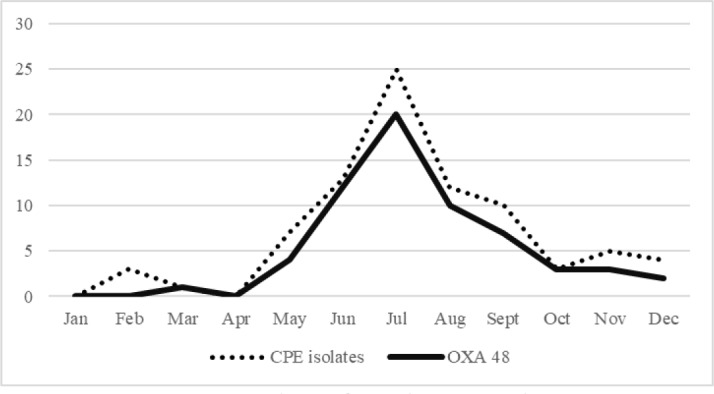
A total 113 patients were included, ranging from 5 in 2015 to 83 in 2018. In 2018 the number of CPE went from 4 in May to 20 in July. With the implemented measures, propagation stopped. Implantable devices were present in 36% of patients and open wounds in 34%. Antibiotics had been prescribed to 71% of patients in the prior 30 days and most of the patients had been hospitalized for more than 5 days prior to sample collection or had a hospital stay in the previous year.
Klebsiella pneumoniae was the most common species (87%). OXA-48 (62%) and Klebsiella pneumoniae-carbapenemase (KPC) (15%) were the most common carbapenemases, with OXA-48 being implicated in the 2018 outbreak. The case fatality rate at 30 days was 32%. Combination therapy resulted in less mortality.
Conclusions
While KPC is the most common carbapenemase in Europe and Portugal, we experienced an important OXA-48 outbreak. Surveillance should be in place as these isolates are probably spreading. Effective communication, multidisciplinary team work and proper infection control measures are some of the best strategies during outbreaks.
Keywords: Carbapenemase, drug-resistance, OXA-48, outbreak, infection control
Introduction
Ever since the first steps in antibiotic treatment, there has been emergence of resistances alongside new treatment discoveries.1
Enterobacteriaceae like Escherichia coli, Klebsiella pneumoniae and Enterobacter are some of the most common causes of community- and healthcare-associated infections.2-4 The development of extended spectrum beta-lactamases (ESBL), plasmid-mediated Amp enzymes and deficiency of porins in the outer membrane, has led to important resistances to beta-lactams.5,6 Therefore, carbapenems currently play a critically important role. However, in the last two decades, we have watched the emergence and spread of carbapenemase-producing Enterobacteriaceae (CPE) worldwide, with few antibiotics remaining active.7,8 These multidrug-resistant Enterobacteriaceae have a significant impact on multiple sectors: they cause millions of deaths annually, are more difficult to treat, require longer treatment duration and increase in-hospital length of stay, with great repercussion on healthcare costs.9,10
K. pneumoniae in particular has been regarded as a critical priority across the globe for its growing expansion of carbapenem resistance, being listed as a top threat by WHO.11,13 In Europe, K. pneumoniae carbapenemase (KPC) is the most common carbapenemase, with ongoing transmission across the continent.12 Mediterranean countries, namely Turkey, Greece and Spain, have first witnessed the rise of OXA-48-producing isolates but these currently have a global distribution.12,14 Portugal still lacks detailed and updated information on its situation regarding CPE. Reports from 2014 listed KPC-positive K. pneumoniae as the most frequent CPE, but OXA-48-producing species have been described in some local reports.15-17
Colistin and tigecycline remain some of the most important therapeutic options in CPE infections but their efficacy (even in combination with other agents) is uncertain and resistance is also a threat, leaving us with few therapeutic options.18-21 New agents such as ceftazidime-avibactam, ceftolozane-tazobactam, meropenem-vaborbactam and plazomicin have been developed and are promising but rational use must remain key.22
We describe the CPE epidemiology in a Portuguese regional hospital. During the year of 2018 a largely growing number of CPE isolates was detected while previously they occurred only sporadically. This raised suspicion of nosocomial transmission and triggered our Infection Control Committee to reinforce infection control measures. In this context, we initiated a retrospective analysis of patient epidemiology, microbiology and resistance data, clinical measures taken and outcomes. Here we describe the findings of a five-year retrospective analysis.
Methods
Patients and setting
This study was conducted in a regional hospital, located in Cascais, Portugal, which comprises 277 beds for acute care, an 18-bed ICU and Emergency Care, and provides care for adult and pediatric patients from a population of 211,302 people. A retrospective analysis of CPE isolates between 2015 and 2018 was made, based on the records of our Microbiology Laboratory and our Infection Control Committee. We included every clinical sample analyzed in the microbiology laboratory, whether it was from patients in the Emergency Room, outpatient or inpatient care. For each patient, we only included the first isolate, since multiple patients became colonized and had subsequent CPE-positive clinical samples. In July 2018, we implemented rectal swab screenings for CPE and patients with positive screenings for CPE were also included. This screening depended on the physician decision but was recommend in patients with multiple antibiotic cycles in the previous year, institutionalized in nursing-homes, hospitalized in the previous year or who had contact with a patient with CPE.
Microbiology sample analysis
Regarding microbiologic methods, samples prior to 2018 were sent to the Portuguese National Health Institute that would later inform us on carbapenemase identification. The Institute used disk diffusion to analyze antimicrobial susceptibility, according to EUCAST guidelines. Clinical isolates with resistance or decreased susceptibility to ertapenem were submitted to PCR and sequencing to detect and identify CPE and ESBL-encoding genes.
In our microbiology laboratory, Vitek MS/Vitek 2 (bioMérieux, Marcy-l’Étoile, France) was used for automated bacterial identification and antibiotic susceptibility testing. Each sample with a minimal inhibitory concentration (MIC) by E-TEST (bioMérieux), corresponding to intermediate susceptibility or resistance to any carbapenem (according to EUCAST criteria) was tested for carbapenemase production with a immunochromatography test from Coris (Coris, Genbloux, Belgium).
The rectal screening samples were inoculated in Brilliance CRE Agar (Oxoid, Cheshire, England), a chromogenic screening plate for CPE, and positive results went through VITEK-MS for automated mass spectrometry microbial identification.
In July of 2018, regarding the possibility of a nosocomial outbreak of CPE, more strict infection control measures were implemented in order to stop transmission.
Results
Between 2015 and 2018, CPE were detected in a total of 113 patients. Of these 113, nine were diagnosed after being selected for CPE screening with rectal sample. The clinicians ordered a total of 91 screening tests (nine positive, 10%). The number of isolates per year ranged from 5 in 2015 to 83 in 2018.
Patients were considered colonized if they had CPE isolates on clinical samples but showed no signs of active infection (fever, elevated inflammatory markers, clinical symptoms related with infection, etc.), according to the clinician best judgement. Seventy-two patients (64%) were considered infected and the remaining colonized.
We only had two OXA-48 isolates before 2018. From May 2018 an outbreak was suspected (see Figure 1).
Figure 1. Number of total CPE isolates per month in 2018 (dots) and number of OXA-48 isolates per month in 2018 (full line).
Characteristics of patients
The incidence of infection/colonization with CPE was higher in males (57%). The average age was 75.6 years (range 0-100 years), with 47% being 80 or older. Sixty-six percent of the patients lived at home while 31% were institutionalized (nursing home or rehabilitation center). One of our patients was a preterm neonate born at our hospital.
The main causes of admission were urinary tract infection (UTI) (31%), respiratory tract infection (17%) and diabetic foot infection (8%). Other less common causes of admission were heart disease, abdominal infections, oncologic problems, skin infections, amongst others.
Medical devices such as urinary catheters, central venous catheters, tracheostomy tubes, chest tubes; or invasive procedures such as mechanical ventilation, recently implanted hip prosthesis or endourological procedures, were present in 36% of our patients. Urinary catheters were present, or had been for more than two days in the same hospitalization, in 25% of the patients, being the most frequent device. Open wounds such as pressure ulcers, postoperative wounds or even burns were present in 33.6% of patients.
Seventy-one percent of the patients had taken antibiotics in the 30 days preceding the CPE diagnosis and 58% of those had two or more antibiotic courses. The antibiotic classes most prescribed were penicillins (60%), third-generation cephalosporins (20%), carbapenems (19%) and vancomycin (10%). Most of the patients (88%) had been hospitalized for more than five days prior to the sample collection or had a previous hospital stay in the 12 months before.
Microbiology
The infection site most affected was the urinary tract, in 69% (n=78) of the patients and 8% (n=6) of these also had bacteremia. The sputum was positive in 9% (n=10) (10% of these with bacteremia), wound exudates/surgical aspirates in 6% (n=7), ascitic fluid in 1% (n=1) and 8% (n=9) of the patients had isolated bacteremia.
Eighty-seven percent (n=90) of the isolates from were K. pneumoniae (Table 1), 10% Enterobacter cloacae, 3% E. coli and only one isolate was Citrobacter freundii. There was no E. coli with carbapenemase activity before 2017. The nine screenings for CPE corresponded to K. pneumoniae isolates but we have no further details from antibiotic susceptibility and will further describe the results from the 104 isolates from other clinical samples.
Table 1. Number of isolates (percentage) for each species.
| Species isolated | Number of isolates (%) |
|---|---|
| K. pneumoniae | 90 (87%) |
| Enterobacter cloacae | 10 (10%) |
| E. coli | 3 (3%) |
| Citrobacter freundii | 1 (1%) |
| Rectal screening detecting K. pneumoniae | 9 (8%) |
| TOTAL | 113 |
OXA-48 was the most common carbapenemase, found in 62% (n=64) of our isolates, followed by KPC, present in 15% (n=16) (Table 2).
Table 2. Number of isolates (percentage) for each resistance mechanism.
| Resistance mechanism | Number of isolates (%) |
|---|---|
| OXA 48 | 64 (62%) |
| KPC | 15 (14%) |
| MBL | 2 (2%) |
| MBL + AmpC | 2 (2%) |
| KPC + MBL | 1 (1%) |
| Unknown or inconclusive | 20 (19%) |
| TOTAL | 104 (100%) |
Co-production of ESBL was present in 63% of the total of CPE isolates and in 81% of the OXA 48 isolates.
From the 104 CPE isolates, 13% had intermediate susceptibility and 88% were resistant to ertapenem. Regarding meropenem, 74% were susceptible, 5% had intermediate susceptibility and 21% were resistant.
From the OXA-48 producers only 6% (n=4) of the isolates had a meropenem MIC >8 mg/L, while in the KPC group this happened in 31% (n=5) (but we did not have access to the meropenem MIC of 19% of the KPC isolates). From the OXA-48 isolates, 89% had an ertapenem MIC >1 mg/L but the vast majority remained susceptible to meropenem: 94% (n=60) had a meropenem MIC ≤2 mg/L and only 6% (n=4) had a meropenem MIC >2 mg/L (one with MIC 16 mg/L and three with MIC 32 mg/L).
Resistance to colistin was present in only one of the isolates (K. pneumoniae with AmpC enzyme and ESBL-positive with intermediate susceptibility to ertapenem, that maintained susceptibility to meropenem with MIC<2).
Treatment and outcomes
Ninety-one percent (n=103) of our patients required in-hospital care whether because of clinical instability or for the need of intravenous antibiotic treatment. The average hospital stay was 20 days, being longer in patients considered infected than in colonized ones (27 days vs. 13.5 days).
Susceptibility-guided treatment was only possible for 75% of patients with infection (total of 54 treatments). This happened mainly because patients died before the antimicrobial susceptibility was available.
The case fatality rate (that we considered at 30 days after the first CPE detection, for any cause of death) was 32% (n=36) in the global population of CPE infected/colonized, 41% in those considered infected and 17% in those colonized (Table 3).
Table 3. Case fatality rate depending on infected or colonized status.
| Status | Case fatality rate |
|---|---|
| Global study population | 32% |
| Patients considered infected | 41% |
| Patients considered colonized | 17% |
| Patients with OXA-48 isolates | 28% |
| Patients with KPC isolates | 25% |
The case fatality rate was 28% in the patients with OXA-48 isolates and 25% in the KPC group. In the OXA-48 group we had 41 patients considered infected and 23 colonized with the case fatality rate being 39% in the infected and 9% in the colonized. In the KPC group we had ten patients infected and six colonized with the case fatality rate being 40% in the infected and 0% in the colonized patients.
From the patients considered infected, those who had guided antibiotic had a lower case fatality rate of 25%.
Regarding treatment, from the 54 cases in which susceptibility was available, the adequate treatment was only implemented in 52 patients. In the two cases in which the treatment regimen was not adequate, one included meropenem alone, even though the MIC was >8 mg/L, and in one case an aminoglycoside alone was used. Only 32 patients received combination treatment. Mortality rate was inferior in patients with adequate antimicrobial regimen and even more if the treatment regimen included two or more antibiotic classes (Table 4).
Table 4. Adequacy of treatment regimen and mortality rate associated.
| n | % | Mortality rate | |
|---|---|---|---|
| Adequate antimicrobial regimen (according to susceptibility results) | 52 | 96% | 25.0% |
| Combined treatment | 32 | 61.5% | 18.8% |
| Not combined treatment | 20 | 38.5% | 35.0% |
| Not adequate | 2 | 4% | 50% |
Single agent antibiotic treatment was used in cases with meropenem MIC <2 or in cases before 2017 and the main agents were meropenem, ceftazidime-avibactam or colistin.
Included in the monotherapy group were five patients treated exclusively with ceftazidime-avibactam with no deaths registered. Amongst patients treated with only meropenem or only amikacin, the case fatality rate was 45%.
The treatment most used was meropenem with amikacin, in 11 patients, mainly patients with K. pneumoniae OXA 48 with ESBL production (Table 5). Two patients treated this way died. Meropenem with colistin was used in nine patients, with two deaths in the 30 days after the detection of CPE.
Table 5. Case fatality rate according to antibiotic therapy used.
| Guided treatment | Number treated | Deaths at 30 days | % |
|---|---|---|---|
| Meropenem + amikacin | 11 | 2 | 18% |
| Meropenem + colistin | 9 | 2 | 22% |
| Meropenem | 9 | 3 | 25% |
| Ceftazidime-avibactam | 5 | 0 | 0% |
| Colistin | 4 | 4 | 100% |
| Tigecycline + colistin | 3 | 1 | 33% |
| Amikacins + colistin | 2 | 1 | 50% |
| Meropenem + gentamicin | 2 | 0 | 0% |
| Tigecycline + piperacillin/tazobactam + clindamycin | 1 | Unknown | Unknown |
| Meropenem + ceftazidime | 1 | 1 | 100% |
| Amikacin + colistin + ceftazidime-avibactam | 1 | 0 | 0% |
| Ceftazidime-avibactam + linezolid | 1 | 0 | 0% |
| Colistin + ampicillin | 1 | 0 | 0% |
| Colistin + tigecycline | 1 | 0 | 0% |
| Colistin + gentamicin | 1 | 0 | 0% |
| Gentamicin + cefotaxime | 1 | 0 | 0% |
| Ciprofloxacin | 1 | 0 | 0% |
| Unknown | 1 | 0 | 0% |
| Total | 54 | 13 | 24% |
Meropenem monotherapy was used in nine patients, six with UTI and three with CPE isolates on blood samples, mainly before 2018. It was used in seven K. pneumoniae that were resistant to ertapenem but susceptible to meropenem. Three of these patients died. It was also used in one Enterobacter cloacae resistant to ertapenem but susceptible to meropenem and in one E. coli OXA-48 and ESBL-positive, with no mortality at 30 days.
Ceftazidime-avibactam became available at our hospital in July 2018, and we used it to treat four K. pneumoniae OXA 48 and one KPC with good results and no mortality at 30 days. Another patient was considered colonized with OXA-48 K. pneumoniae and MRSA but afterwards became ill and was treated with ceftazidime-avibactam and linezolid and did not die during the 30 day-period after the screening test but died before ending antibiotic treatment. Another patient with diabetic foot ulcer infection with K. pneumoniae OXA 48 was treated with amikacin plus colistin plus ceftazidime-avibactam with success.
Treatment with colistin alone was used in four patients, one with Citrobacter freundii with KPC production, one with K. pneumoniae with KPC, one with Enterobacter cloacae with VIM and AmpC and one with K. pneumoniae with resistance to meropenem and ertapenem in which we did not have the carbapenemase identification. All of the four patients died within 30 days. Colistin in combination therapy was used with success with ampicillin, tigecycline and gentamicin respectively, in three patients. One patient with K. pneumoniae OXA 48 maintained susceptibility to ciprofloxacin and was treated accordingly with success.
Discussion
Carbapenemases are proliferating worldwide and Portugal is not an exception.13
While our hospital has registered a relevant increase in CPE numbers in 2018, this was integrated in a nosocomial outbreak with numbers returning close to the pre-outbreak incidence. A continuous surveillance and infection control strategy should be in place to understand how these numbers evolve throughout the years. During this outbreak, our healthcare workers were intensively re-educated on infection control measures. Between July and September 2018, patients with CPE infection/colonization were isolated in a cohort with a specific team of healthcare workers assigned exclusively to them. An online communication group was formed between the microbiology laboratory, the cohort task force and the team responsible for bed-occupancy management, so that patients with CPE isolates were isolated as fast as possible. Patients and families were also educated on infection control measures and instructed to warn the hospital staff, in subsequent hospital visits, of their previous infection/colonization with CPE. Effective communication and (multidisciplinary) team work, with mandatory and fast reports, were some of the most important features of our experience towards outbreak control. This goes in hand with previous studies that highlight the interdisciplinary team importance in infection control.23
As we describe an outbreak with nosocomial transmission, it would be highly relevant to analyze risk factors inherent to in-hospital transmission such as ward distribution, staying in single-bed rooms vs. in multiple-bed rooms, or even common nursing staff but, retrospectively, that information was not available. The most relevant patient characteristics, that may point to risk factors of CPE infection, were age, open wounds, medical devices, prior antibiotic use (in the previous 30 days), especially if two or more antibiotic courses and hospitalization in the previous 12 months. Medical devices have been largely known as a risk factors for acquiring CPE and are, at times, avoidable.24 Reducing unnecessary devices is essential to patient safety, particularly regarding urinary catheters which played a significant role in our study. Urinary catheters must be carefully considered before their placement and their utility should be reassessed daily.14,25 Open wounds, namely surgical wounds and pressure ulcers, were of utmost relevance. Being associated with severe illness, they probably also represent increased susceptibility to infection. Wound care should be carefully managed regarding infection control measures and early detection of these isolates on surgical wards and antibiotic stewardship measures are crucial.
OXA-48 was the most frequently detected carbapenemase but the vast majority of these isolates had ESBL co-production. Since OXA-48 carbapenemases in isolation induce no hydrolysis of cephalosporins and only weak hydrolysis of carbapenems, the co-production of other beta-lactamases made their identification more evident.14 There is probably a larger population of OXA-48 isolates that goes undetected and may be spreading. KPC is still the most common carbapenemase in Portugal and our study is the first describing an OXA-48 outbreak and the first Portuguese Hospital with OXA 48 predominance.12,16
The fact that OXA-48 isolates, in the vast majority, maintained meropenem MIC inferior to 8 allowed us to keep it in our therapeutic options. Colistin resistance in Enterobacteriaceae is still very infrequent but reports have been spreading.19 Until the end of 2018 we only had one case of colistin resistance.
Community acquired carbapenemases are also worrisome, even more because most Enterobacteriaceae are very common in the community.14 Most of our patients (88%) had nosocomial infection or had a prior admission to the hospital within the prior 12 months. It is our belief that these carbapenemases were mainly hospital-acquired but studies regarding the prevalence of these resistances in the community are lacking. K. pneumoniae was by far the most common species with carbapenemase production, which was expected giving the international results.26 E. coli with carbapenemase activity is still infrequent but, while it was never detected before 2017 at hour hospital, in 2018 we had two cases.26 Spreading of carbapenem-resistant E. coli in the community may lead to the emergence of untreatable community-acquired infections.
The global case fatality rate at 30 days of 32% is in accordance with other studies.27,28 Even though colonized patients had a lower case fatality rate of 17% (vs. 41% in infected), this is still considerable. It is difficult to compare to other studies since the definition of colonization vs infection is not uniform. Another limitation to our study is that we did not analyze the proportion of patients considered colonized that went on to develop infections and this would be an area for future work.
Regarding treatment, we acknowledge our high portion of patients treated with monotherapy which is not the most advisable strategy.7 One explanation is that, in the majority of the cases, those were strains that remained susceptible to meropenem with MIC <2 and monotherapy with meropenem was implemented. However, our study, with its limitations and small number of treated patients, points to better results with combination therapy.
We acknowledge that this study has numerous limitations, namely having a rather high proportion of isolates in which phenotypically there was resistance to carbapenems but in which no carbapenemase was identified. This happened mainly before 2017 and is a consequence of the previous centralization of microbiologic analysis and poor communication between institutions. It is also an exclusively descriptive report but we highlight the importance of tight infection control measures on the termination of a nosocomial outbreak of CPE. Case-control studies and further statistical analysis to identify risk factors for acquiring CPE and for worse outcomes would be relevant. As we show small numbers of patients treated for each treatment modality, these numbers do not allow us to make solid conclusions on outcomes for each antimicrobial regimen.
Conclusions
While KPC seems to be the most common carbapenemase found in Mediterranean countries, our hospital registered an important outbreak of OXA-48 isolates. This should warn us for the probable rise of these strains in the next years. Patients at risk were elderly, had medical devices inserted or open wounds, had taken antibiotics in the prior 30 days or had had contact with medical institutions. This should make us rethink some of our practices in order to prevent new infections/colonizations.
Case fatality rate is high (global case fatality rate of 32%), even in patients only considered colonized. Combination therapy and the rational use of new antimicrobial agents such as ceftazidime-avibactam lead to better outcomes.
Regarding outbreaks, we hereby recommend permanent effective communication and multidisciplinary team work as some of the best strategies for tackling propagation.
Footnotes
Authors’ contributions statement: AG was the main contributor to study design, made the biggest contribution to collection, analysis and interpretation of data and was the most involved in drafting the manuscript. AMG made substantial contributions to study design, collection, analysis and interpretation of data and manuscript review. MF and JS were involved in reviewing data and the manuscript. All authors have read and approved the final version of the manuscript.
Conflicts of interest: All authors - none to declare.
Funding: None to declare.
References
- 1.Dodds DR. Antibiotic resistance: a current epilogue. Biochem Pharmacol. 2017;134:139–46. doi: 10.1016/j.bcp.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC). Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep. 2013;62:165–70. [PMC free article] [PubMed] [Google Scholar]
- 3.Kontula KSK, Skogberg K, Ollgren J, Järvinen A, Lyytikäinen O. The outcome and timing of death of 17,767 nosocomial bloodstream infections in acute care hospitals in Finland during 1999-2014. Eur J Clin Microbiol Infect Dis. 2018;37:945–52. doi: 10.1007/s10096-018-3211-0. [DOI] [PubMed] [Google Scholar]
- 4.Rank EL, Lodise T, Avery L, et al. Antimicrobial susceptibility trends observed in urinary pathogens obtained from New York State. Open Forum Infect Dis. 2018;5:ofy297. doi: 10.1093/ofid/ofy297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iredell J, Brown J, Tagg K. Antibiotic resistance in Enterobacteriaceae: mechanisms and clinical implications. BMJ. 2016;352:h6420. doi: 10.1136/bmj.h6420. [DOI] [PubMed] [Google Scholar]
- 6.Jacoby GA, Munoz-Price LS. The new beta-lactamases. N Engl J Med. 2005;352:380–91. doi: 10.1056/NEJMra041359. [DOI] [PubMed] [Google Scholar]
- 7.Doi Y, Paterson DL. Carbapenemase-producing Enterobacteriaceae. Semin Respir Crit Care Med. 2015;36:74–84. doi: 10.1055/s-0035-1544208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brolund A, Lagerqvist N, Byfors S, et al. Worsening epidemiological situation of carbapenemase-producing Enterobacteriaceae in Europe, assessment by national experts from 37 countries, July 2018. Euro Surveill. 2019;24:1900123. doi: 10.2807/1560-7917.ES.2019.24.9.1900123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson H, Torok ME. Extended-spectrum beta-lactamase-producing and carbapenemase-producing Enterobacteriaceae. Microb Genom. 2018;4:e000197. doi: 10.1099/mgen.0.000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu L, Sun X, Ma X. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann Clin Microbiol Antimicrob. 2017;16:18. doi: 10.1186/s12941-017-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization . Prioritization of pathogens to guide discovery, research and development of new antibiotics for drug resistant bacterial infections, including tuberculosis. Geneva, Switzerland: WHO; 2017. [Google Scholar]
- 12.David S, Reuter S, Harris SR, et al. Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nat Microbiol. 2019;4:1919–29. doi: 10.1038/s41564-019-0492-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Centre For Disease Prevention and Control. Surveillance of antimicrobial resistance in Europe - Annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) 2017. Stockholm, Sweden: ECDC; 2018. [Google Scholar]
- 14.van Duin D, Doi Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence. 2017;8:460–9. doi: 10.1080/21505594.2016.1222343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manageiro V, Romão R, Moura IB, et al. Molecular epidemiology and risk factors of carbapenemase-producing Enterobacteriaceae isolates in Portuguese hospitals: Results from European Survey on Carbapenemase-Producing Enterobacteriaceae (EuSCAPE). Front Microbiol. 2018;9:2834. doi: 10.3389/fmicb.2018.02834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manageiro V, Ferreira E, Almeida J, et al. Predominance of KPC-3 in a survey for carbapenemase-producing Enterobacteriaceae in Portugal. Antimicrob Agents Chemother. 2015;59:3588–92. doi: 10.1128/AAC.05065-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aires-de-Sousa M, Ortiz de la Rosa JM, Gonçalves ML, Pereira AL, Nordmann P, Poirel L. Epidemiology of Carbapenemase-producing Klebsiella pneumoniae in a Hospital, Portugal. Emerg Infect Dis. 2019;25:1632–8. doi: 10.3201/eid2509.190656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Duin D, Doi Y. Outbreak of colistin-resistant, carbapenemase-producing Klebsiella pneumoniae: are we at the end of the road? J Clin Microbiol. 2015;53:3116–7. doi: 10.1128/JCM.01399-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du H, Chen L, Tang YW, Kreiswirth BN. Emergence of the mcr-1 colistin resistance gene in carbapenem-resistant Enterobacteriaceae. Lancet Infect Dis. 2016;16:287–8. doi: 10.1016/S1473-3099(16)00056-6. [DOI] [PubMed] [Google Scholar]
- 20.Du X, He F, Shi Q, et al. The rapid emergence of tigecycline resistance in blaKPC-2 harboring Klebsiella pneumoniae, as mediated in vivo by mutation in tetA during tigecycline treatment. Front Microbiol. 2018;9:648. [Google Scholar]
- 21.Doi Y. Treatment options for carbapenem-resistant Gram-negative bacterial infections. Clin Infect Dis. 2019;69:S565–75. doi: 10.1093/cid/ciz830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humphries RM, Yang S, Hemarajata P, et al. First report of ceftazidime-avibactam resistance in a KPC-3-expressing Klebsiella pneumoniae isolate. Antimicrob Agents Chemother. 2015;59:6605–7. doi: 10.1128/AAC.01165-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uchida M, Stone PW, Conway LJ, Pogorzelska M, Larson EL, Raveis VH. Exploring infection prevention: policy implications from a qualitative study. Policy Polit Nurs Pract. 2011;12:82–9. doi: 10.1177/1527154411417721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cronin KM, Poy Lorenzo YS, Olenski ME, et al. Risk factors for KPC-producing Enterobacteriaceae acquisition and infection in a healthcare setting with possible local transmission: a case-control study. J Hosp Infect. 2017;96:111–5. doi: 10.1016/j.jhin.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Meddings J, Rogers MA, Krein SL, Fakih MG, Olmsted RN, Saint S. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf. 2014;23:277–89. doi: 10.1136/bmjqs-2012-001774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grundmann H, Glasner C, Albiger B, et al. Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): a prospective, multinational study. Lancet Infect Dis. 2017;17:153–63. doi: 10.1016/S1473-3099(16)30257-2. [DOI] [PubMed] [Google Scholar]
- 27.Tischendorf J, de Avila RA, Safdar N. Risk of infection following colonization with carbapenem-resistant Enterobactericeae: A systematic review. Am J Infect Control. 2016;44:539–43. doi: 10.1016/j.ajic.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balkan II, Aygün G, Aydin S, et al. Blood stream infections due to OXA-48-like carbapenemase-producing Enterobacteriaceae: treatment and survival. Int J Infect Dis. 2014;26:51–6. doi: 10.1016/j.ijid.2014.05.012. [DOI] [PubMed] [Google Scholar]


