Abstract
Coronavirus disease 2019 (COVID-19) results from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The clinical features and subsequent medical treatment, combined with the impact of a global pandemic, require specific nutritional therapy in hospitalised adults. This document aims to provide Australian and New Zealand clinicians with guidance on managing critically and acutely unwell adult patients hospitalised with COVID-19. These recommendations were developed using expert consensus, incorporating the documented clinical signs and metabolic processes associated with COVID-19, the literature from other respiratory illnesses, in particular acute respiratory distress syndrome, and published guidelines for medical management of COVID-19 and general nutrition and intensive care. Patients hospitalised with COVID-19 are likely to have preexisting comorbidities, and the ensuing inflammatory response may result in increased metabolic demands, protein catabolism, and poor glycaemic control. Common medical interventions, including deep sedation, early mechanical ventilation, fluid restriction, and management in the prone position, may exacerbate gastrointestinal dysfunction and affect nutritional intake. Nutrition care should be tailored to pandemic capacity, with early gastric feeding commenced using an algorithm to provide nutrition for the first 5–7 days in lower-nutritional-risk patients and individualised care for high-nutritional-risk patients where capacity allows. Indirect calorimetry should be avoided owing to potential aerosole exposure and therefore infection risk to healthcare providers. Use of a volume-controlled, higher-protein enteral formula and gastric residual volume monitoring should be initiated. Careful monitoring, particularly after intensive care unit stay, is required to ensure appropriate nutrition delivery to prevent muscle deconditioning and aid recovery. The infectious nature of SARS-CoV-2 and the expected high volume of patient admissions will require contingency planning to optimise staffing resources including upskilling, ensure adequate nutrition supplies, facilitate remote consultations, and optimise food service management. These guidelines provide recommendations on how to manage the aforementioned aspects when providing nutrition support to patients during the SARS-CoV-2 pandemic.
Keywords: COVID-19, Pandemic, Nutrition, Critical illness, Artificial feeding
1. Purpose
The purpose of this document is to provide evidence-based advice for nutrition management of critically ill and acutely unwell hospitalised patients during the coronavirus disease 2019 (COVID-19) pandemic. It provides key adaptations of usual best practice, taking into consideration staff safety, reduced staffing, resource utilisation, and the clinical condition of the patients. Optimal nutrition for these patients will require strong interdisciplinary collaboration, with flexible approaches to care to accommodate organisational changes resulting from this pandemic situation. As this pandemic is evolving rapidly, this document may be updated.
We recommend enacting this COVID-19 nutrition guideline when hospitals enter phase 2 management strategies as outlined in the Australian and New Zealand Intensive Care Society (ANZICS) COVID-19 Guideline (version 2, 15th April 2020).1 Phase 2 of the tiered intensive care unit (ICU) pandemic plan refers to a moderate impact on daily operations, with the ICU at or near maximum capacity but still able to meet demand and when up to 25% of beds are occupied by patients with pandemic illness.1
2. Impact of COVID-19 on nutrition
Patients with COVID-19 pneumonia, who develop respiratory failure, shock, or multiorgan failure, require intensive care management with mechanical ventilation (MV) and other organ supports. COVID-19 pneumonia is characterised by high fevers, which induce a catabolic state, resulting in impaired glucose utilisation and increased protein breakdown and energy utilisation.2 It has been reported that in addition to critical illness, there may be significant effects on appetite, conscious state, and direct gastrointestinal effects resulting in nausea, vomiting, diarrhoea, and feeding intolerance. These factors adversely impact nutritional intake and status. Patients with COVID-19 often require prolonged MV and ICU support, resulting in significant immobility, catabolic stress, and muscle wastage.2 These patients are at high risk of malnutrition during the period of critical care, as well as in the recovery phase of this illness, and may stay in hospital for a significant length of time. There are limited available data to guide the optimal nutritional management of patients with COVID-19, and as such, these guidelines are based on the available evidence from other similar conditions such as acute respiratory distress syndrome.3 , 4
3. Risk to staff
Although procedures such as nasogastric tube (NGT) insertion are not considered aerosol-generating procedures, there may be a risk to staff through the induction of coughing. The highest risk to staff is thought to relate to procedures involving the respiratory tract. Although studies suggest that RNA viral load is high in stool, the infectivity of faeces is not clear.5 , 6 It should be emphasised that personal protective equipment (PPE) is only one component in the hierarchy of hazard controls.
Whether viable virus is present in gastric secretions is unknown. Therefore, it should be considered that procedures such as aspirating gastric contents may pose a risk; although staff members are using PPE for airborne precautions, they should be adequately protected. To minimise any excess risk, we recommend the use of appropriate PPE before any procedure involving gastric aspirate and insertion or change of an enteral tube and consideration of the frequency or avoidance of these procedures if possible. Where staff members are required to individually review patients for nutrition care, this should be done remotely, or using the appropriate PPE, after appropriate training and according to hospital guidelines.
Staff should be aware of local guidelines regarding the use of PPE, which are broadly classified into categories for ‘airborne’ infections (P2/N95 respirator, eye protection, gloves, gown) and ‘droplet’ infections (surgical mask, eye protection, gloves, gown). Training should include the correct procedure of putting on and taking off PPE and careful attention to hand hygiene. PPE is only one component in the hierarchy of hazard controls.
4. Intensive care guidelines
4.1. Nutrition risk categories
For the purpose of the ICU guideline, patients at ‘high nutrition risk’ who are likely to require or benefit from individualised nutrition assessment on admission are defined as those with
-
•
Anaphylactic food allergy
-
•
Preexisting or suspected malnutrition (e.g., weight <50 kg, body mass index [BMI] <18.5 kg/m2, recent weight loss of ≥5%)
-
•
Weight >120 kg or BMI > 40 kg/m2
-
•
Requiring parenteral nutrition (PN)
-
•
Considered at high risk of refeeding
-
•
Type 1 diabetes mellitus
-
•
Cystic fibrosis
-
•
Inborn errors of metabolism
All other patients are considered to be at ‘low nutrition risk’, and the use of the standard nutrition algorithm should be considered safe unless otherwise indicated.
4.2. Recommendations
Energy and protein targets
-
1.
We do not recommend the use of indirect calorimetry (IC) in patients with COVID-19.
IC requires the disconnection of the ventilator circuit which risks exposing staff to the airborne virus. IC also takes considerable time to perform, which will also increase overall exposure to staff.
-
2.
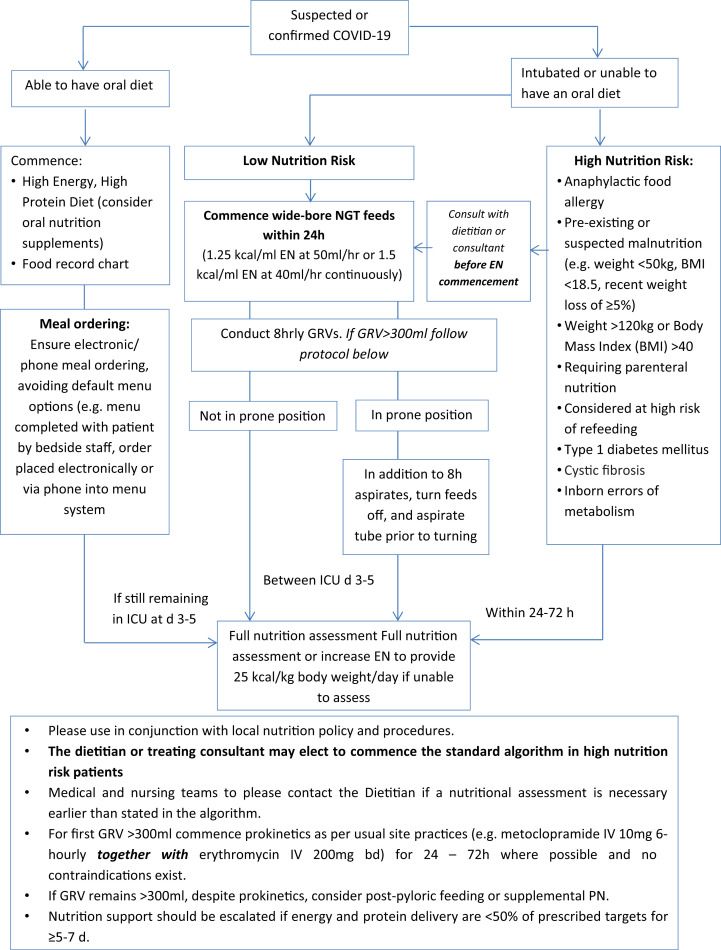
We recommend commencing enteral nutrition (EN) support in mechanically ventilated patients using an algorithm with a set rate for up to the first 5 d of ICU admission (see Fig. 1 )
-
3.We recommend providing 25 kcal/kg bodyweight/day after the first 5 d of ICU admission (and up to 30 kcal/kg bodyweight/day for severely unwell patients, those with malnutrition, or those who have a prolonged admission, e.g., extracorporeal membrane oxygenation (ECMO), continuous renal replacement therapy (CRRT), or length of MV > 7 d) and protein prescription of at least 1.2 g/kg bodyweight/day.
-
•Minimal evidence exists for the optimal nutritional targets in these patients, but in the absence of indirect calorimetry, we recommend calorie prescriptions to be based on 25 kcal/kg bodyweight/day after the first 5 d of ICU admission (and up to 30 kcal/kg bodyweight/day for severely unwell patients, e.g., ECMO, CRRT, or length of MV > 5 d or those with malnutrition) and protein prescription of at least 1.2 g/kg bodyweight/day.4 , 7 Actual bodyweight should be used for patients of normal BMI, and an adjusted bodyweight, for overweight and obese patients as per usual site method (e.g., ideal bodyweight + 25–50% [actual bodyweight – ideal bodyweight]).
-
•Current case reports state that fever of between 37.5 and 39.0 °C is common. The metabolic impact of increased temperature is said to be ∼10–13% for every 1 °C increase.8 This should be considered in the overall nutrition prescription.
-
•In obese patients, it is appropriate to commence nutrition according to the algorithm provided, but these patients should be considered at high nutrition risk and prioritised for nutrition review.
-
•Contribution of calories from propofol should be considered in the nutrition provision if more than 10% of daily calories are being provided from this source. EN calories should be reduced and adequate protein delivery, ensured while considering overall fluid provision.
-
•Recommendations for nutrition provision in patients admitted to the ICU and not ventilated within 24 h are provided in recommendation 18.
-
•
Fig. 1.
ICU Nutrition Algorithm for Management of Patients with COVID-19 in Australia and New Zealand. Algorithm to be enacted on instruction of senior medical and nutrition staff. EN, enteral nutrition; PN, parenteral nutrition; ICU, intensive care unit; GRV, gastric residual volume; BMI, body mass index; COVID-19, coronavirus disease 2019; NGT, nasogastric tube.
Insertion of a nasogastric tube for enteral feeding
-
4.We recommend following institutional guidelines regarding appropriate PPE during the insertion of nasogastric tubes (NGT), and unnecessary NGT changes should be avoided.
-
•The insertion of an NGT may induce coughing, and nasal and gastric sections may also contain virus. Guidelines for PPE are being constantly reviewed, and clinicians should be aware of, and refer to, national and institutional guidelines.
-
•The decision to insert an NGT should include consideration of the risk to staff, the benefit of providing nutrition support, and alternative modes of feeding including PN.
-
•
Commencement of nutrition support
-
5.
In patients receiving MV who are at low nutrition risk, we recommend commencing EN support within 24 h of ICU admission via the gastric route using an algorithm with a set rate for up to the first 5 d of ICU admission (Fig. 1).
This recommendation takes into consideration the safety of dietitians in the ICU (recognising that reducing exposure is a fundamental method of preventing COVID-19 infection),l preservation of PPE for clinical staff who have no choice but to be in contact with patients, the workload required for clinicians to calculate an individualised rate considering the high volume of patients anticipated, increased prevalence of hyperglycaemia related to the significant inflammatory response, and the likelihood of gastrointestinal dysmotility with early full feeding in this population. There is no critical care nutrition literature to demonstrate negative consequences of early hypocaloric feeding strategies for the first 5–7 d of ICU admission.3 , 9 This recommendation is also in keeping with recent international guidelines that recommend the introduction of hypocaloric nutrition over the first 5–7 d of illness.7
-
6.
In high-nutrition-risk patients, the dietitian or treating consultant should be contacted to determine if the standard feeding algorithm is appropriate to instate before commencement of feeding, and a dietetic consultation should be conducted within 24–72 h where possible (pending capacity and hospital COVID-19 phase).
This is to ensure the safe provision of appropriate nutrition support, minimise the risk of refeeding, anaphylactic reactions, and the risk of significant overfeeding or underfeeding.
-
7.
For low-nutrition-risk patients, a care plan should be provided between 3 and 5 d (based on dietetic capacity due to case load) if they are likely to require MV for greater than 5 d and are likely to survive.
-
8.We recommend the use of an energy-dense EN formula (1.25–1.5 kcal/ml).
-
•To reduce the volume of fluid provided to patients (in keeping with the ANZICS COVID-19 Guideline recommendations of avoiding high-volume EN as part of a restrictive fluid management strategy in patients with respiratory failure to reduce the risk of extravascular lung water),9 we recommend selecting an enteral formula that meets caloric needs, without compromising protein delivery.
-
•We recommend avoiding the prescription of a highly concentrated enteral formula (2 kcal/ml) unless essential for further fluid restriction. Highly concentrated enteral formula has been shown to delay gastric emptying, and therefore, they may exacerbate gastrointestinal dysfunction; in addition, these products usually have low protein content.
-
•
Continuing nutrition support
-
9.We recommend, where possible, keeping enteral tubes in place after extubation owing to the prolonged recovery anticipated for patients who survive COVID-19.
-
•This decision should be made in consultation with the dietitian. This takes into consideration the high metabolic demands and the challenges to achieving adequate oral nutrition (e.g., work of breathing, conscious state, potential eating and swallowing difficulties due to weakness, challenges with food selection and feeding with high workloads for bedside staff) and existing data in other populations informing of poor adequacy of nutrition with oral nutrition alone after critical illness.[10], [11], [12], [13]
-
•Where wide-bore NGT are in situ, consider changing to a fine-bore NGT before extubation if ongoing EN for >5 d is deemed likely. This should take into consideration the associated safety risks to staff and should be performed using appropriate precautions based on the infectivity status of the patient and coordinated with other clinical care.
-
•For commencement of postextubation oral nutrition, please refer to the statement on oral intake in the section ‘Nutrition for nonventilated patients and those receiving high-flow nasal oxygen’.
-
•
-
10.
We recommend the consideration of postpyloric feeding, using the appropriate level of infection prevention precautions, or PN if gastrointestinal intolerance remains an issue over 5–7 d despite the use of appropriate management strategies, and calorie and protein delivery is consistently <50% of prescribed targets.
-
11.
We recommend supplemental PN be considered after other measures to improve EN have been attempted or insertion of a postpyloric enteral feeding tube is deemed unsafe and calorie and protein intake remain significantly less than prescribed targets (i.e., <50% over a 5- to 7-d period). This should be assessed on a case-by-case basis, and the long-term impact of nutrition deficit should be considered.
Dietetic assessment and reviews
-
12.
For patients who are not at high nutrition risk, we recommend that a nutrition assessment be completed by day 3–5 of ICU admission in most circumstances, depending on staff capacity, or earlier if patients are at high nutritional risk (Fig. 1).
-
13.Where dietetic capacity is exhausted, and if a full dietetic review is not possible, we recommend increasing EN targets to meet 25–30 kcal/kg bodyweight/day after day 5 as a minimum.
-
•Nutritional monitoring should be maintained where possible, including assessment of calorie and protein delivery compared with prescribed targets, feeding intolerance, and other complications, to identify patients who may require an escalation in their nutritional care.
-
•It is anticipated there will be a reduction in dietetic workforce with staff illness and increased patient case load. Therefore, a delay in the conduct of an initial nutrition assessment and less frequent reviews of nutritionally stable patients should be anticipated. Where resources are limited, we recommended dedicating these to the
-
•first week of illness for high-nutrition-risk patients only
-
•first week of illness in low-nutrition-risk patients with feeding complications
-
•second week of illness in patients deemed low nutrition risk on admission
-
•ICU teams should be advised to escalate patients with nutritional concerns quickly to facilitate prioritisation.
-
•
Monitoring of gastric residual volumes
-
14.
We recommended continuing to measure gastric residual volumes (GRVs) in COVID-19 (using the appropriate level of infection control precautions but using a threshold of less than 300 ml and measuring every 8 h).
-
15.We recommend ceasing measurements when GRVs have been less than 300 ml for > 48 h in patients who are not prone.
-
•These recommendations are made as the viral load of gastric contents is unknown; however, the risk of not measuring GRVs is increased vomiting which also places staff at risk, and hence, strategies to avoid vomiting should be taken.
-
•Where applicable, management of feeding intolerance as per ICU protocols should be instated (e.g., use of prokinetics) (Fig. 1).
-
•Aspirated GRVs should be discarded rather than returned to reduce the risk of splash injury to staff.
-
•
-
16.
We recommend that the EN is paused and the NGT be aspirated before any position changes. EN should recommence as soon as possible to avoid unnecessary interruption to feeding.
-
17.We recommend GRV monitoring continue every 8 h while in the prone position, even if intolerance is not an issue.
-
•Patients in the prone position should commence EN as per the previous recommendations, with consideration that the prone position is associated with increased GRVs and risk of vomiting.14
-
•Assessing the position of the NGT at the site of entry into the nasal cavity after placing the patient in the prone position is important to assess the potential risk of pressure injury.
-
•
Nutrition for nonventilated patients and those receiving high-flow nasal oxygen
-
18.
We recommend routine provision of an appropriate oral diet (e.g., high energy, high protein) and oral nutrition supplements (e.g., 1.5 or 2 kcal/ml oral supplement) as soon as oral intake is commenced.
-
19.We recommend advocating for escalation to EN, with consideration given to the safety risk of NGT placement, for patients not receiving MV and meeting <50% of energy and protein targets orally for ≥5–7 d, despite provision of oral nutritional supplements, or if intubation is expected.
-
•The provision of nutrition in patients receiving high-flow nasal oxygen (HFNO) is difficult owing to fasting for potential intubation, and oral intake is often poor owing to nausea, delirium, fatigue, poor appetite, and difficulty breathing.15 These symptoms are commonly reported in critically ill patients.16 Specifically for patients with COVID-19, loss of taste and smell has been reported as a consequence, which may influence oral intake across the spectrum of illness (including recovery).17 This recommendation also considers the high patient numbers restricting timely individualised assessment.
-
•
5. Acute ward guidelines
5.1. Nutrition risk categories
For the purpose of the acute ward guideline, patients at ‘high nutrition risk’ who are likely to require or benefit from individualised nutrition assessment on admission are defined as those with
-
•
Requirements for EN or PN
-
•
Malnutrition or suspected malnutrition (Malnutrition Screening Tool [MST] ≥ 3, Malnutrition Universal Screening Tool [MUST] ≥ 2, BMI <18.5 kg/m2, recent weight loss ≥ 10%)
-
•
Anaphylactic food allergy
-
•
Considered at high risk of refeeding
-
•
Type 1 diabetes mellitus
-
•
Cystic fibrosis
-
•
Inborn errors of metabolism
∗Refer to Ward algorithm to define low and moderate nutrition risk
5.2. Recommendations
Identifying nutrition risk
-
20.
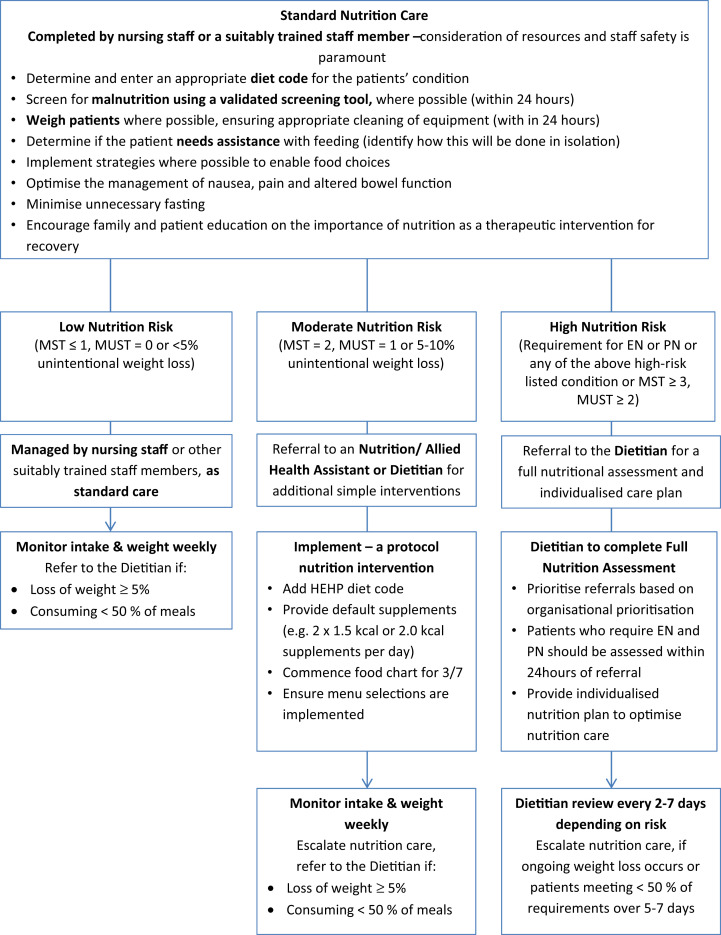
Where there is capacity, we recommend the use of a validated malnutrition screening tool to identify patients who are at risk of malnutrition (e.g., MUST, MST, Mini Nutritional Assessment–Short Form [MNA-SF]) although coordination of direct patient care should be considered to minimise staff exposure and PPE use.
-
•
Malnutrition screening is the most appropriate way to identify those who are most at risk and would benefit from nutrition interventions. This should be completed as part of standard care by staff members who are required to attend the bedside and are already utilising PPE.
Dietetic assessment and reviews
-
21.
For patients transferring to the ward from the ICU, we recommend that the ICU dietitian provides an appropriate handover to the ward dietitian within 24 h of ICU discharge.
-
•
This is to ensure the safe and appropriate transition of care from the ICU to the ward; this handover should include the nutritional status of the patients (if malnutrition is present, was it preexisting or hospital-acquired) and the assessed nutritional adequacy over the ICU admission.
-
22.
For patients admitted directly to the ward, we recommend the implementation of local pathways to optimise nutrition provision for patients as soon as possible, before full nutritional assessment, where appropriate (Fig. 2 ).
-
•
This takes into consideration the level of risk and also the availability of staffing and allows dietitians to focus on patients who require complex nutrition support and those at high nutritional risk.
-
23.
We recommend a dietetic consultation for high-nutrition-risk patients be conducted within 24 h.
-
•
High-risk patients include those requiring EN or PN or who have anaphylactic food allergy, cystic fibrosis, or inborn errors of metabolism.
-
•
Other patients at high nutritional risk should be seen within 24–72 h (e.g., patients at high risk of refeeding and/or severe malnutrition or patients with medical conditions in which specific nutrition therapy is required) based on dietetic capacity.
-
24.
We recommend that nutritional monitoring is maintained, including the monitoring of intake and weight (where possible), and high-nutritional-risk patients are reviewed at least twice weekly and lower-risk patients at least weekly.
Fig. 2.
Acute Ward Nutrition Algorithm for Management of Patients with COVID-19 in Australia and New Zealand. Algorithm to be enacted on instruction of senior medical and nutrition staff. EN, enteral nutrition; PN, parenteral nutrition; MST, Malnutrition Screening Tool; MUST, Malnutrition Universal Screening Tool.
Continuing nutrition support
-
25.
We recommend advocating for escalation to EN in patients who are meeting <50% of energy and protein targets orally for ≥5–7 days, or where a patient is assessed as malnourished and has a suboptimal oral intake (<65% of estimated requirements), despite provision of oral nutritional supplements or food fortification.
6. Contingency planning and additional workforce considerations
6.1. Recommendations
Dietetic workforce considerations
-
26.
We recommend that all dietitians treating patients with COVID-19 or entering a high-risk space have formal instruction on the use of PPE (including training, practice, and supervision).
-
27.
We recommend conducting nutrition consultations remotely, utilising family to obtain nutrition history where possible, limiting the number of staff in the patient space and the utilisation of PPE.
-
28.
We recommend nutrition departments are familiar with the hierarchy of hazard control. 1
-
29.
We recommend the utilisation of nutrition assistants or allied health assistants (AHAs) where possible for non–face-to-face management activities.
-
•
Potential nutrition assistant/AHA tasks could include assistance with monitoring of oral intake, quantification of oral nutrition supplement compliance, liaison with bedside staff regarding menu preferences, assisting with food service tasks, assistance with facilitating ICU transfer, obtaining weight history, etc.
Food service considerations
-
30.
We recommend developing food service systems to enable electronic or phone meal ordering to minimise contact with the patient at the bedside while enabling patient menu selection and ensuring optimal nutrition provision.
Other contingency planning
In combination with these protocols, we recommend consideration of the following:
-
•
Ensuring adequate equipment for EN is available, given the expected increase in bed numbers and patients, including feeding pumps, giving sets, and EN formula (including consideration of strategies for management, where some of the ICUs may be isolated from the rest and planning for potential EN or delivery system shortages with an appropriate contingency plan, such as equipment to facilitate gravity or bolus feeding).
-
•
Local instructions should be developed to communicate to staff where all nutritional products (e.g., pumps, giving sets, formulae) are stored and how to access additional stock.
-
•
Providing appropriate upskilling to non-ICU dietetic staff in the ICU or nonacute staff in other ward areas, including the necessary IT access.
-
•
Ensuring dietitians are able to facilitate nutrition by being competent at pump operation and changing of EN formula and giving sets to reduce the workload expectation on nursing staff. This should include non-COVID-19 patients within the ICU.
-
•
Minimising workload and risk of foodborne infection by avoiding the use of decanting of formula unless absolutely necessary.
-
•
Reviewing contingency processes with food service, to ensure optimal food choices are available and the maintenance of compliance with hospital food service guidelines and to ensure nutritional adequacy, with considerations for staff shortages.
-
•
Adapting workspace and team structure where possible to facilitate COVID-19 vs non-COVID-19 areas and staff.
-
•
Planning for an occurrence of exposure within the nutrition team and how this will be managed at an operational level.
-
•
Consideration of a 7-d service in the ICU and on-call service for out-of-hours support may be of benefit in some centres.
-
•
Formalising communication pathways with bedside clinicians and food service to enable remote nutrition assessment and reviews where possible to limit clinician contact at the bedside such as alternatives for attendance at ward rounds.
-
•
Considering areas for advanced scope of practice to support medical and nursing staff where appropriate, e.g., postpyloric tube insertion.
7. Conclusions and application to practice
The global pandemic caused by SARS-CoV-2 has resulted in a large number of patients requiring admission to intensive care for management of symptoms relating to COVID-19 infection. The metabolic consequences and symptoms associated with COVID-19, as well as the medical therapy required in intensive care, have potential nutrition implications for consideration. Optimal nutrition therapy, both in the ICU and after ICU stay, should involve careful management of glycaemic control, fluid balance, and gastrointestinal function, prioritising high-nutrition-risk patients and ensuring appropriate nutrition support to aid in recovery in long-stay patients and ICU survivors. Planning around safe working practices for infection control, staff resourcing, supply demands, and working remotely are key elements required to ensure nutrition support can be provided to the patients.
Ethical approval
Ethical approval was not required for this work.
Declaration of Competing Interest
Andrea P. Marshall is the Editor-in-Chief and Emma J. Ridley is an Editor of Australian Critical Care. This manuscript has been managed throughout the review process by Consulting Editor, Professor Gavin Leslie. This process prevents authors who also hold an editorial role to influence the editorial decisions made. All authors have read and approved the final version of the manuscript submitted.
CRediT authorship contribution statement
Lee-anne S. Chapple: Conceptualisation, Writing - original draft, Writing - review & editing. Kate Fetterplace: Conceptualisation, Writing - review & editing. Varsha Asrani: Conceptualisation, Writing - review & editing. Aidan Burrell: Conceptualisation, Writing - review & editing. Allen C. Cheng: Conceptualisation, Writing - review & editing. Peter Collins: Conceptualisation, Writing - review & editing. Ra'eesa Doola: Conceptualisation, Writing - review & editing. Suzie Ferrie: Conceptualisation, Writing - review & editing. Andrea P. Marshall: Conceptualisation, Writing - review & editing. Emma J. Ridley: Conceptualisation, Writing - original draft, Writing - review & editing.
Endorsement
This guideline is endorsed by the Australasian Society of Parenteral and Enteral Nutrition.
Acknowledgements
The authors would like to acknowledge the members of the Australasian Society of Parenteral and Enteral Nutrition Council for their review and endorsement and others who provided expert input.
References
- 1.Australian and New Zealand intensive care society . ANZICS; Melbourne: 2020. ANZICS COVID-19 guidelines. 15 April 2020. [Google Scholar]
- 2.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. May 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Heart L, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials N. Rice T.W., Wheeler A.P., Thompson B.T., Steingrub J., Hite R.D., Moss M., et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. J Am Med Assoc. 2012;307(8):795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McClave S.A., Taylor B.E., Martindale R.G., Warren M.M., Johnson D.R., Braunschweig C., et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: society of critical care medicine (SCCM) and American society for parenteral and enteral nutrition (A.S.P.E.N.) JPEN J Parenter Enter Nutr. 2016;40(2):159–211. doi: 10.1177/0148607115621863. [DOI] [PubMed] [Google Scholar]
- 5.Wolfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Muller M.A., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. May 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 6.Xiao F., Sun J., Xu Y., Li F., Huang X., Li H., et al. Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerg Infect Dis. 2020;26(8) doi: 10.3201/eid2608.200681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singer P., Blaser A.R., Berger M.M., Alhazzani W., Calder P.C., Casaer M.P., et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48–79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 8.Del Bene V.E. Clinical methods: the history, physical, and laboratory examinations. 3rd ed. Butterworths; Boston: 1990. Temperature. [PubMed] [Google Scholar]
- 9.TARGET Investigators for the ANZICS Clinical Trials Group. Chapman M., Peake S.L., Bellomo R., Davies A., Deane A., Horowitz M., et al. Energy-dense versus routine enteral nutrition in the critically ill. N Engl J Med. 2018;379(19):1823–1834. doi: 10.1056/NEJMoa1811687. [DOI] [PubMed] [Google Scholar]
- 10.Chapple L.S., Deane A.M., Heyland D.K., Lange K., Kranz A.J., Williams L.T., et al. Energy and protein deficits throughout hospitalization in patients admitted with a traumatic brain injury. Clin Nutr. Dec 2016;35(6):1315–1322. doi: 10.1016/j.clnu.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Ridley E.J., Parke R.L., Davies A.R., Bailey M., Hodgson C., Deane A.M., et al. What happens to nutrition intake in the post-intensive care unit hospitalization period? An observational cohort study in critically ill adults. JPEN J Parenter Enter Nutr. 2019;43(1):88–95. doi: 10.1002/jpen.1196. [DOI] [PubMed] [Google Scholar]
- 12.Merriweather J., Smith P., Walsh T. Nutritional rehabilitation after ICU - does it happen: a qualitative interview and observational study. J Clin Nurs. 2014;23(5–6):654–662. doi: 10.1111/jocn.12241. [DOI] [PubMed] [Google Scholar]
- 13.Wittholz K., Fetterplace K., Clode M., George E.S., MacIsaac C.M., Judson R., et al. Measuring nutrition-related outcomes in a cohort of multi-trauma patients following intensive care unit discharge. J Hum Nutr Diet. Jun 2020;33(3):414–422. doi: 10.1111/jhn.12719. [DOI] [PubMed] [Google Scholar]
- 14.Reignier J., Thenoz-Jost N., Fiancette M., Legendre E., Lebert C., Bontemps F., et al. Early enteral nutrition in mechanically ventilated patients in the prone position. Crit Care Med. 2004;32(1):94–99. doi: 10.1097/01.CCM.0000104208.23542.A8. [DOI] [PubMed] [Google Scholar]
- 15.Chapple L., Gan M., Louis R., Yaxley A., Murphy A., Yandell R. Nutrition-related outcomes and dietary intake in non-invasively mechanically ventilated critically ill adult patients: a pilot observational descriptive study. Aust Crit Care. 2020;33(3):300–308. doi: 10.1016/j.aucc.2020.02.008. Accepted 18.02.2020. [DOI] [PubMed] [Google Scholar]
- 16.Ridley E.J., Chapple L.S., Chapman M.J. Nutrition intake in the post-ICU hospitalization period. Curr Opin Clin Nutr Metab Care. 2020;23(2):111–115. doi: 10.1097/MCO.0000000000000637. [DOI] [PubMed] [Google Scholar]
- 17.Yan C.H., Faraji F., Prajapati D.P., Boone C.E., DeConde A.S. Association of chemosensory dysfunction and Covid-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. Jul 2020;10(7):806–813. doi: 10.1002/alr.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]