INTRODUCTION
While the novel coronavirus outbreak has profoundly altered health care systems across the globe, it has also affected similar change and devastation on the social, educational, and cultural communities upon which many rely, including communities of choral singers, solo performers, conductors, voice teachers, and the professionals that collaborate with them. Even as citizens of the most heavily impacted countries took to their balconies to serenade first responders and health care workers, singers of most genres who typically perform in ensembles have been told to not carry on their beloved practice. This message, promoted by the lay media and by early reports of “super-spreading” of the virus at choral events in the United States and abroad,1 , 2 has been reinforced by expert and nonexpert opinion regarding the transmissibility of SARS-CoV-2 through droplets and aerosols, the generation of respiratory particles when singing, and concern about the interpersonal interactions that routinely accompany vocal performance, both in groups and in one-on-one teaching situations.
Unfortunately, there is a paucity of data about both how SARS-CoV-2 is transmitted by singing and how to bring communities of singers back together safely. The data available about disease spread through vocalization, most of which preceded the current pandemic, address primarily transmission of disease through droplets and aerosols and are specific neither to this virus nor to singing.3, 4, 5, 6 Specifically, there is a lack of data addressing how to congregate and sing safely in choral environments such as churches, concert halls and practice spaces, as well as stages, theatres and other venues. In addition, protective measures such as safest distancing between singers, wearing masks or other personal protective equipment (PPE), using larger rehearsal or performance spaces, reducing the number of singers inside a certain enclosed space, reducing the duration of rehearsals or performances, and using real time air and surface cleaning methods such as increased ventilation, UV-C light and HEPA filtration specific to a singing environment among other topics have not been studied well enough to provide evidence on which to base advice to the singing community.
Opinion on these matters is plentiful and often divergent. A recent webinar with a panel of experts in the world of voice care and singing left the audience with the message, “There is no safe way for singers to rehearse together until there is a COVID-19 vaccine and a 95% effective treatment in place.”7 , 8 Although, this may ultimately prove to be accurate, evidence-based practice (defined as an approach to health that integrates scientific research, patient preferences and values, and clinical expertise to make the best recommendations possible) does not allow for such a definitive conclusion to be made at this time.9 It must be understood, that these recommendations and decisions are made not only on what scientific information is available but on intuition and unsystematic experience that is often biased and inaccurate.
We do not understand all the risks posed by COVID 19 to ourselves, friends, family and colleagues, who wish to resume educational, performance, communal and congregational singing safely. Evidence-based practice demands that we critically evaluate our current state of knowledge to come up with the best possible information to disseminate. In this article, we review the information that exists relating to singing and COVID-19. This paper is intended to provide guidance based on what we know: the best available data, analyzed and scrutinized by a panel of experts in the medical, behavioral and basic science world of voice care, of whom many are professional singers, choir directors or teachers of singing. While it may not be able to afford any definitive answers, this work will offer suggestions of best practices for those singing groups that are willing to mitigate risk knowing that the risk cannot be brought to zero. Finally, this report will hopefully stimulate the larger voice research community to study the emerging consensus on safe resumption of singing and pursue scientific understanding of COVID-19.
COVID-19 IS LIKELY SPREAD BY RESPIRATORY PARTICLES
Given the relatively recent emergence of the SARS-CoV-2 virus, the precise mechanisms of its transmission have not been studied extensively. Early research showed presence of the virus in the respiratory tract.10 The World Health Organization (WHO) initially postulated that droplets and fomites were the primary modes of transmission, but that airborne transmission via aerosol was possible.11 The rapid spread of this disease around the world led many researchers to suspect that aerosols (particles smaller than droplets) might play a more important role than initially suspected. It was shown that SARS-CoV-2 virus can survive on surfaces and in aerosol form in laboratory simulations,12 a finding consistent with previous knowledge about other coronaviruses and influenza viruses.13, 14, 15 Clinically, hospitals in Wuhan, China demonstrated high concentrations of viral RNA in aerosol form in medical staff areas,16 but it is important to note that several other studies failed to corroborate that observation.17 , 18 Additionally, it appears that SARS-CoV-2 can be spread even by nonsymptomatic patients, and this spread is presumed to be from respiratory transmission.19, 20, 21 Many patients do not present with classic symptoms, including fever.22 Such nonsymptomatic transmission is crucially important to consider in planning return to occupational and recreational activities as nonsymptomatic patients will not know they are ill and will not isolate, unwittingly putting others at risk when they go out into society.
Considering the biology of the virus and epidemiology of the pandemic, respiratory particles are beyond doubt responsible for at least some transmission of disease, and aerosol transmission is plausible.23 However, the experimental results from preliminary SARS-CoV-2 research often are created in a laboratory. One commonly cited study12 demonstrating virus persistence in aerosols used nebulizers and a Goldberg drum to generate aerosols with viral particles. It has been emphasized by the WHO that these techniques do not reflect a clinical setting.24 Furthermore, the presence of virus in aerosol form does not necessarily lead to infection. This is likely to depend also on the infective potential of the virus,25 the viral load, ventilation, possibly individual susceptibility and other factors, all of which require further study. It should be emphasized that there is no consensus on the mechanisms of spread and their impact on clinically relevant disease transmission.
PHONATION PRODUCES DROPLETS AND AEROSOLS
Respiratory particles, including droplets and aerosols, exist in human exhaled breath.5 , 14 , 23 , 26 , 27 Other functions of the respiratory tract that also are thought to produce these particles at a higher level than breathing include phonation,4 sneezing,28 and coughing.6 Asadi et al5 demonstrated that certain individuals are “speech superemitters” who exude significantly more aerosol particles than others, and this has been corroborated by previous literature.29 The same group also demonstrated that louder phonation resulted in greater aerosol generation.5
These points raise questions about how the types of phonation in which humans engage (talking, cheering, singing, etc) affect the generation of aerosols. This is particularly relevant to the voice community attempting to safely engage in traditionally in-person activities such as instruction, rehearsals, and performances. Because airflow is a key element of all singing styles, one might expect the same risk of disease transmission among various singing styles and genres (ie, gospel, classical, barbershop, etc). Yet Konnai et al30 have shown that whispered and breathy phonation produce significantly greater airflow than normal phonation at all levels of loudness. Sound is transmitted by pressure waves, and theoretically viruses are buffeted on airflow. Singing with a more ‘resonant’ voice and less airflow, an accomplishment associated with trained singers of many genres who learn to manage breathing efficiency to sing long phrases, could in theory be less likely to transmit disease.31 , 32 Furthermore, airflow and subglottic pressure are known to vary between styles of singing.33 , 34 Microphone use may have the opposite effect, as it is known to lower voice intensity.34 , 35 However, it is still ultimately unknown how aerosol production varies according to voice type, vocal register, or vocal style (belt, growl, classical, choral, etc), and this topic deserves more study.
Regardless of the variation in types of singing, eliminating this aerosol burden entirely is unlikely. Johnson et al6 have noted that aerosols are generated in the alveoli of the lungs, but also in the larynx and the oral cavity. Fabian et al. suggest the re-opening of collapsed small airways after an exhalation plays a large role in aerosol particle production. Whether the greater use of the vital capacity in singing has any impact on the creation of aerosols as compared to singing techniques that utilize shallower breaths and a shorter vocal tract is unknown at this time.36
ENVIRONMENT AFFECTS RISK
Since the early days of the pandemic, governmental bodies across the world have recommended “social distancing” or “physical distancing” techniques as the optimal way to reduce the spread of SARS-CoV-2.37 Given the epidemiology of the pandemic, it is clear that transmission happens from person to person after close contact. Although very little definitive data exist for SARS-CoV-2, a systematic review considering other similar viruses demonstrated that physical distancing of 1 meter or more led to a significant 82% decrease in viral transmission, with protection increasing as the distance lengthened (risk decreased by half with each 1m interval studied).38 , 39 Anecdotally, clusters of transmission appear to happen more in environments with close quarters including schools, households, meat packing plants, gyms, choral practice rooms, and music clubs.1 , 40 , 41
Ventilation may play an important role in transmission. This has been established by a systematic review of the literature performed by Li et al, which showed strong evidence that there is an association between ventilation and air movement patterns in buildings, and the spread of infectious disease (SARS, measles, influenza, among others).42 However, the authors note that their data were not sufficient to recommend any minimum standards for ventilation of public and private spaces. There is some evidence to suggest that natural ventilation is an effective, low-cost strategy to reduce infection risk.43 A study comparing ventilating using open doors and windows versus mechanically ventilated rooms suggested that the former strategy is superior;44 and natural ventilation has been suggested as a strategy in the past by the WHO.45 Therefore, environments with increased distance between potentially infected subjects and with good ventilation are preferable to environments with close contact and poor ventilation; this should apply not only to hospital environments but also to public and private areas in which rehearsals or performances are held.
Advanced techniques for air filtration or sterilization should be considered when ventilation is not adequate. Ultraviolet (UV) radiation has also been employed during the COVID-19 pandemic, based on evidence from both influenza virus46 and other coronaviruses.47 UV induces damage in DNA and RNA of bacteria and viruses, leading to their inability to replicate.48 , 49 Likewise, filtration systems can remove viral particles from the air, including influenza and measles,50 , 51 and were used during the SARS outbreak. Finally, ionization of the air may help with bacteria52 and influenza virus.53 While it is likely that these measures are useful as part of a concerted effort towards reduction of viral spread, there is insufficient evidence to confirm that any one of these is sufficient. Additionally, in contrast to selection of outdoor environments or rooms with window/door ventilation, such filtration and sterilization techniques require significant expertise for proper functioning.
When considering the environment, one must also consider the natural climate. Given COVID-19′s emergence during winter months in the Northern hemisphere, much attention has been given to the effect of temperature and humidity on the virus. Preliminary data have suggested that high temperatures and relative humidity can decrease both the infectivity and mortality associated with the virus.54, 55, 56
PPE HELPS PREVENT VIRUS SPREAD
N95 masks, and to a lesser extent surgical masks, also have been shown to protect from coronavirus transmission, although most of these data come from other coronaviruses.38 , 57 Relative to the COVID-19 pandemic itself, models have demonstrated that even only moderately protective face mask could lead to lower infection rates.58 Cloth masks should fit well, be made of several layers and ideally has water-resistant fabric.39 , 59 Rather than filtering out small particles like N95 masks, cloth masks may reduce transmission by containing expired jets of respiratory particles when worn by infected patients. Globally, many countries have urged citizens to wear home-made masks for this reason.60 While there has been limited discussion of PPE use in singers, it is reasonable to assume that PPE use will help singers more safely interact with one another, though this will not be sufficient to establish safety.
Eye protection has been more controversial. Although there are known ocular manifestations of COVID-19,61 the SARS-CoV-2 virus has not been cultured from ocular secretions, and it is unclear whether ocular exposure is a source of transmission; regardless, eye protection has been recommended widely in healthcare environments with moderate risk of viral transmission.62 , 63 A recent systematic review of other viral outbreaks has shown benefit in reducing viral transmission when ocular protection is used.38
AGE, RACE, INCOME LEVEL, AND HEALTH COMORBIDITIES AFFECT RISK
We have also recognized demographic differences in terms of severity and fatalities. Since the early days of the outbreak, advanced age has been identified as a risk factor for both hospitalization and mortality.64 , 65 The specific underlying conditions of obesity and diabetes/metabolic syndrome have been correlated with worse outcomes.66 There are also disproportionate rates of death from COVID-19 in the African-American and Latinx communities that are likely more a manifestation of longstanding disparities than genetic predisposition.67 , 68 As such, the demographic of singing groups should be considered. Children's groups, for instance, are likely at less risk for suffering from severe COVID-19 disease than an elderly or African American ensemble, although transmission risk may be equivalent. Heightened vigilance in communities that may be more vulnerable appears paramount.
DISCUSSION
It cannot be overemphasized enough that the data we have about COVID-19 and the SARS-CoV-2 virus are still preliminary. While the existing research cited in this manuscript is helpful, much of it is based on observational, cross-sectional, or retrospective data. Some of it is likely underpowered to answer pressing questions about COVID-19 and some is anecdotal and experientially based. Decision-makers are appealing to the scientific community to inform the planning of activities and budgeting in the near- and long-term, but it is crucial that scientific claims are examined with great caution. Scientific studies provide evidence for or against theories and hypotheses, but often do not establish proof. Furthermore, science also takes time. Governments and organizations around the world have dedicated a tremendous amount of resources to further research towards vaccination,69 but other avenues of research (into contact tracing, PPE, and specific to singing and performance) should also be pursued. It should be our goal to facilitate efforts for ever-increasing adoption of evidence-based practice in our field.
Nevertheless, it is incumbent on the voice and performing arts communities to consider how to incorporate available evidence and scientific consensus, albeit evolving, into practice. Choosing a correct path is difficult and requires balancing objective and dispassionate review of information against emotionally charged or inherently biased reactions or instincts. Ultimately, we do not believe that all vocal performance should stop until herd immunity is achieved through vaccination or naturally or until a cure for COVID-19 is discovered.
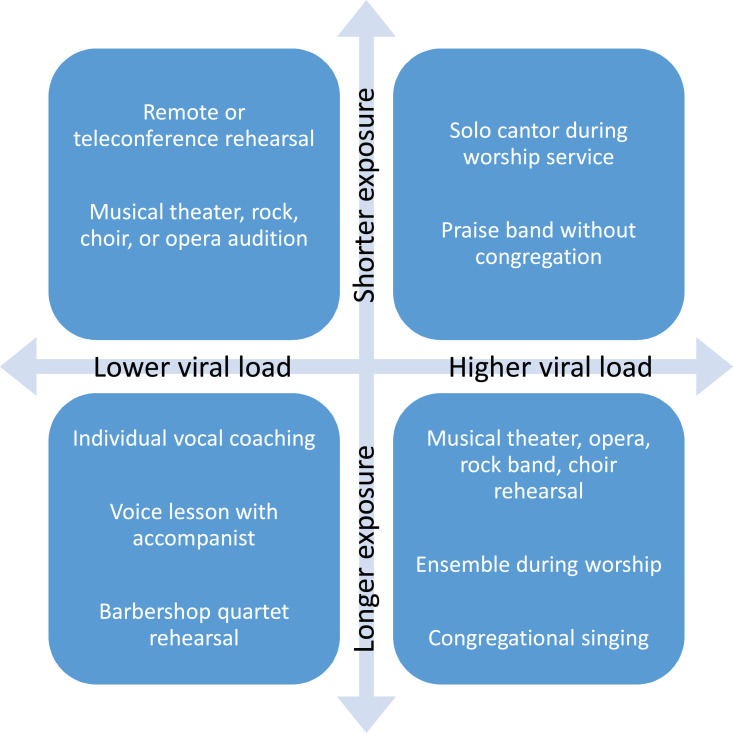
Different types of vocal performances will require different levels of complexity to optimize safety (Figure 1 ). Choral, educational and theatre organizations have the most to consider regarding safe-singing practices. Theatrical performance that requires the audience to see the faces and expressions of the performers (opera, musical theatre, plays, etc, as has evolved in and remains the primary style in the Western world) will face challenges beyond the scope of this paper.
FIGURE 1.
Examples of Potential Risk Associated With Types of Vocal Performance.
PPE will almost certainly play a central role in establishing safer environments for singing, regardless of the setting. Just as governments have encouraged face mask usage across American communities to reduce transmission through coughing, sneezing, coughing, and close contact, singing organizations should encourage PPE use to minimize the risk of spreading infection in all except remote (ie, teleconferencing) rehearsals and performances. It should be acknowledged that beyond the issues with speaking and singing leading to SARS-CoV-2 transmission as presented in this paper, the inability of the performer to wear a mask to deliver an effective and emotive performance will be a difficult, if not insurmountable, obstacle to returning to live theatre as it is known in the Western world without substantial concessions.
Additionally, social distancing recommendations and limitations on large gatherings restrict the ability to gather an audience in a traditional theater or concert venue.
Places of worship not only have choirs to consider but also congregations who gather and expect to participate. The aerosolized viral load risk increases by a) singing or congregating for longer periods of time, b) increasing the number of infected singers (choir or congregation member) in a closed space, and c) the limited ability or inability to clear the air in the space. Performance spaces, and, possibly more concerning, rehearsal spaces may not be ventilated well or even have windows to open. Private and educational voice teaching studios also may have the same limitations and must mitigate risk. Ideally, but not practically, one could determine if the size of the room, air filtration ability and air turnover time are sufficient to mitigate risk to even one teacher and his or her singing student together in a space.70 No evidence has emerged to date regarding the risk mitigation of transmitting SARS-CoV-2 by placing plexiglass shields in singing voice studios between teachers and students. It is likely barriers such as these can mitigate a sneeze or cough from showering a person on the other side of the plexiglass with droplets. However, if the plexiglass is not completely separating two people into two separate air spaces, a teacher and student are occupying the same air space and the above risks apply. Future studies are needed to determine if plexiglass shields between a teacher and student, singing with or without masks, reduce disease transmission in otherwise closed spaces.
While fans blowing air away from choirs in rooms with open doors may help, the risk mitigation in doing that will be individual to the duration of the rehearsal or performance and the space and the size of the group performing because singing with more than one person in a room carries increased risk of disease transmission. Safe-distance singing in the near future probably will require fewer singers than would typically fill the choir stalls or rehearsal space who are all wearing masks and singing for shorter periods of time. Rehearsing and performing outdoors, with social distancing and cloth masks worn appropriately might be the least risky of all environments for larger groups that must rehearse and perform en masse together, although there is no evidence to support this speculation.
Technology to improve our ability to make music collaboratively online falls into two broad categories: laggy and real-time. Laggy solutions include common video conferencing software at higher quality audio settings,71 or the use of a separate high quality audio only platform in conjunction with a video platform.72 Real-time solutions are more technology driven, but made possible through both higher tech network-based platforms73 and lower tech wireless audio setups.74 These approaches could eliminate the risk of virus transmission to a teacher or student, chamber ensemble, or possibly even choral group through the elimination of in-person or proximal activity. This may serve a purpose but also is less enjoyable than the preferred and traditional method of singing in spaces with better acoustics and the ability to see and hear cues and body language from fellow performers.
Choirs in faith-based and community-based ensembles with members who have health conditions as previously identified, as well as members of color or advanced age, should appreciate the statistically higher rate of COVID-19 fatalities when calculating their risk to benefit ratio. COVID-19 testing before each choir rehearsal, in addition to body temperature and other screening might be useful. However, specific conclusions regarding screening tests for SARS-CoV-2 in the singing community cannot be made. We do not anticipate that our vocal communities will have widespread access to testing in the near future that would allow for immediate testing prior to congregation, however symptom screening questions and temperature checks could be implemented.
In this spirit, we suggest the following points to decrease the risk of SARS-CoV-2 transmission, fully acknowledging that there is no certainty or scientific evidence regarding the risks specifically for the voice community, nor the efficacy of these interventions, all of which require research.
-
1.
Rehearse outside when possible. Inside, open windows and doors and use ventilation strategies such as fans to blow air away from the singer(s).
-
2.
Use PPE, at least cloth face masks. Singing is possible with a mask. Masks can cause breathing challenges for some, and this may affect especially singers with underlying pulmonary dysfunction.
-
3.
Rehearse alone, remotely, or in smaller groups. When with others, physical distancing of six feet or more appears paramount.
-
4.
Rehearse in shifts or smaller sections, if possible, ideally in separate locations. Consider having a few representatives from each voice part and, if necessary, change the repertoire to accommodate.
-
5.
Shorten rehearsal times. There is no absolutely “safe” duration of for rehearsal, and so organizations should do everything they can to limit rehearsals to the shortest possible time period.
-
6.
Limit extraneous activities (eg, breaks, socializing, food etc).
-
7.
Wipe down items that have been set up or touched by others before and after use (chairs, scores/paper music, instruments, music stands, etc).
-
8.
Screen for symptoms, including fever, upper respiratory infection symptoms such as coughing and nasal congestion, loss of smell and taste. Take temperatures of singers prior to entering the rehearsal space. Remember that some infected patients will not have any symptoms.
-
9.
Avoid direct contact (eg, hand-shaking, joining hands).
-
10.
Practice meticulous hygiene. Wash or disinfect hands before, during and after rehearsals. Singers should not touch their face as part of a warm up exercise or singing instruction method (or anytime unnecessarily).
-
11.
Sick singers should stay home as should singers who have been around a COVID-19 positive patient. Exposed singers should self-quarantine for two weeks.
These points must be taken as only recommendations. They are based on scant and often weak scientific evidence and on “common sense” that, while based on a wealth of experience, could still prove to be wrong. They may mitigate risk of transmitting SARS-CoV-2 but do not eliminate risk. It will be up to the singers themselves, teachers of singing, church and private choral leaders, performer guilds and unions and other entities to weigh the risks and decide when and how to resume their charge in their individual performance spaces. Ultimately, each person must evaluate his or her own risk and risk tolerance to make a personal decision as to whether or not to continue in his or her performance activities.
CONCLUSIONS
Scientific research has not examined safe singing practices in relation to the risk of SARS-CoV2 transmission. The evidence that exists is based on prior viral outbreaks and situations that may or may not translate to various singing environments. It is likely the risk can be mitigated with certain practices, but risks cannot yet be eliminated. Each individual community of singers and performers must do what it can to mitigate as much risk as possible and then decide if that risk mitigation of SARS-CoV-2 transmission is sufficient to resume each singing activity considered, taking into account all of the factors discussed above.
REFERENCES
- 1.Hamner L. High SARS-CoV-2 attack rate following exposure at a choir practice — Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69 doi: 10.15585/mmwr.mm6919e6. [DOI] [PubMed] [Google Scholar]
- 2.A choir decided to go ahead with rehearsal. Now dozens of members have COVID-19 and two are dead. Los Angeles Times. Available at:https://www.latimes.com/world-nation/story/2020-03-29/coronavirus-choir-outbreak. Accessed June 1, 2020.
- 3.Loudon RG, Roberts RM. Singing and the dissemination of tuberculosis. Am Rev Respir Dis. 1968;98:297–300. doi: 10.1164/arrd.1968.98.2.297. [DOI] [PubMed] [Google Scholar]
- 4.Morawska L, Johnson G, Ristovski Z, et al. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J Aerosol Sci. 2009;40:256–269. [Google Scholar]
- 5.Asadi S, Wexler AS, Cappa CD, et al. Aerosol emission and superemission during human speech increase with voice loudness. Sci Rep. 2019;9:2348. doi: 10.1038/s41598-019-38808-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson GR, Morawska L, Ristovski ZD, et al. Modality of human expired aerosol size distributions. J Aerosol Sci. 2011;42:839–851. [Google Scholar]
- 7.National Association of Teachers of Singing . 2020. A Conversation: What Do Science and Data Say About the Near Term Future of Singing.https://www.nats.org/cgi/page.cgi/_article.html/Featured_Stories_/NATS_COVID_Resources_Page Available at: [Google Scholar]
- 8.Enquirer DJH Cincinnati. Will COVID-19 silence singers until there's a vaccine? The Columbus Dispatch. Available at:https://www.dispatch.com/news/20200511/will-covid-19-silence-singers-until-theres-vaccine. Accessed June 1, 2020.
- 9.Sackett DL, Rosenberg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn't. BMJ. 1996;312:71–72. doi: 10.1136/bmj.312.7023.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO-China Joint Mission . 2020. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) p. 40.https://www.who.int/publications-detail/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19) Available at: Accessed June 2, 2020. [Google Scholar]
- 12.van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Booth TF, Kournikakis B, Bastien N, et al. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J Infect Dis. 2005;191:1472–1477. doi: 10.1086/429634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fabian P, McDevitt JJ, DeHaan WH, et al. Influenza virus in human exhaled breath: an observational study. PloS One. 2008;3:e2691. doi: 10.1371/journal.pone.0002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan J, Grantham M, Pantelic J, et al. Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc Natl Acad Sci USA. 2018;115:1081–1086. doi: 10.1073/pnas.1716561115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Ning Z, Chen Y, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020:1–4. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- 17.Faridi S, Niazi S, Sadeghi K, et al. A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Sci Total Environ. 2020;725 doi: 10.1016/j.scitotenv.2020.138401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ong SWX, Tan YK, Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu Z, Song C, Xu C, et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63:706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 22.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asadi S, Bouvier N, Wexler AS, et al. The coronavirus pandemic and aerosols: Does COVID-19 transmit via expiratory particles? Aerosol Sci Technol. 2020;54:635–638. doi: 10.1080/02786826.2020.1749229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Heath Organization . 2020. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. WHO/2019-nCoV/Sci_Brief/Transmission_modes/2020.1. [Google Scholar]
- 25.Fears AC, Klimstra WB, Duprex P, et al. Comparative dynamic aerosol efficiencies of three emergent coronaviruses and the unusual persistence of SARS-CoV-2 in aerosol suspensions. medRxiv. Published online April 18, 2020:2020.04.13.20063784. doi:10.1101/2020.04.13.20063784
- 26.Papineni RS, Rosenthal FS. The size distribution of droplets in the exhaled breath of healthy human subjects. J Aerosol Med. 1997;10:105–116. doi: 10.1089/jam.1997.10.105. [DOI] [PubMed] [Google Scholar]
- 27.Duguid JP. The size and the duration of air-carriage of respiratory droplets and droplet-nuclei. J Hyg (Lond) 1946;44:471–479. doi: 10.1017/s0022172400019288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Workman AD, Jafari A, Welling DB, et al. Airborne aerosol generation during endonasal procedures in the era of COVID-19: risks and recommendations. Otolaryngol Neck Surg. Published online May 26, 2020:0194599820931805. doi:10.1177/0194599820931805 [DOI] [PMC free article] [PubMed]
- 29.Nicas M, Nazaroff WW, Hubbard A. Toward understanding the risk of secondary airborne infection: emission of respirable pathogens. J Occup Environ Hyg. 2005;2:143–154. doi: 10.1080/15459620590918466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konnai R, Scherer RC, Peplinski A, et al. Whisper and phonation: aerodynamic comparisons across adduction and loudness. J Voice Off J Voice Found. 2017;31:773.e11–773.e20. doi: 10.1016/j.jvoice.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 31.Titze IR. Acoustic interpretation of resonant voice. J Voice Off J Voice Found. 2001;15:519–528. doi: 10.1016/S0892-1997(01)00052-2. [DOI] [PubMed] [Google Scholar]
- 32.Titze IR. A theoretical study of F0-F1 interaction with application to resonant speaking and singing voice. J Voice Off J Voice Found. 2004;18:292–298. doi: 10.1016/j.jvoice.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Borch DZ, Sundberg J. Some phonatory and resonatory characteristics of the rock, pop, soul, and Swedish dance band styles of singing. J Voice. 2011;25:532–537. doi: 10.1016/j.jvoice.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 34.Assad JP, Gama ACC, Santos JN, et al. The effects of amplification on vocal dose in teachers with dysphonia. J Voice. 2019;33:73–79. doi: 10.1016/j.jvoice.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 35.Morrow SL, Connor NP. Voice amplification as a means of reducing vocal load for elementary music teachers. J Voice Off J Voice Found. 2011;25:441–446. doi: 10.1016/j.jvoice.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Fabian P, Brain J, Houseman EA, et al. Origin of exhaled breath particles from healthy and human rhinovirus-infected subjects. J Aerosol Med Pulm Drug Deliv. 2011;24:137–147. doi: 10.1089/jamp.2010.0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.CDC. Social Distancing: Keep Your Distance to Slow the Spread. Centers for Disease Control and Prevention. Accessed June 2, 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/social-distancing.html
- 38.Chu DK, Akl EA, Duda S, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. The Lancet. 2020;0 doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacIntyre CR, Wang Q. Physical distancing, face masks, and eye protection for prevention of COVID-19. The Lancet. 2020;0 doi: 10.1016/S0140-6736(20)31183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leclerc Q, Fuller N, Knight L, et al. What settings have been linked to SARS-CoV-2 transmission clusters? Wellcome Open Res. 2020;5:83. doi: 10.12688/wellcomeopenres.15889.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Japan's live music clubs emerge as new coronavirus transmission sites. Reuters. Available at: https://www.reuters.com/article/us-health-coronavirus-japan-music-idUSKBN20X0WU. Accessed June 3, 2020.
- 42.Li Y, Leung GM, Tang JW, et al. Role of ventilation in airborne transmission of infectious agents in the built environment - a multidisciplinary systematic review. Indoor Air. 2007;17:2–18. doi: 10.1111/j.1600-0668.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 43.Qian H, Li Y, Seto WH, et al. Natural ventilation for reducing airborne infection in hospitals. Build Environ. 2010;45:559–565. doi: 10.1016/j.buildenv.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Escombe AR, Oeser CC, Gilman RH, et al. Natural ventilation for the prevention of airborne contagion. PLoS Med. 2007;4:e68. doi: 10.1371/journal.pmed.0040068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Heath Organization . World Health Organization; 2014. Infection Prevention and Control of Epidemic-and Pandemic Prone Acute Respiratory Infections in Health Care; p. 133.https://www.who.int/csr/bioriskreduction/infection_control/publication/en/ Accessed June 3, 2020. Available at: [PubMed] [Google Scholar]
- 46.Hollaender A, Oliphant JW. The inactivating effect of monochromatic ultraviolet radiation on influenza virus. J Bacteriol. 1944;48:447–454. doi: 10.1128/jb.48.4.447-454.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Darnell MER, Subbarao K, Feinstone SM, et al. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J Virol Methods. 2004;121:85–91. doi: 10.1016/j.jviromet.2004.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cadet J, Anselmino C, Douki T, et al. Photochemistry of nucleic acids in cells. J Photochem Photobiol B. 1992;15:277–298. doi: 10.1016/1011-1344(92)85135-h. [DOI] [PubMed] [Google Scholar]
- 49.Reed NG. The history of ultraviolet germicidal irradiation for air disinfection. Public Health Rep. 2010;125:15–27. doi: 10.1177/003335491012500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kormuth KA, Lin K, Prussin AJ, et al. Influenza virus infectivity is retained in aerosols and droplets independent of relative humidity. J Infect Dis. 2018;218:739–747. doi: 10.1093/infdis/jiy221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Azimi P, Keshavarz Z, Laurent J, et al. 2020. Estimating the Nationwide Transmission Risk of Measles in US Schools and Impacts of Vaccination and Supplemental Infection Control Strategies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shepherd SJ, Beggs CB, Smith CF, et al. Effect of negative air ions on the potential for bacterial contamination of plastic medical equipment. BMC Infect Dis. 2010;10:92. doi: 10.1186/1471-2334-10-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hagbom M, Nordgren J, Nybom R, et al. Ionizing air affects influenza virus infectivity and prevents airborne-transmission. Sci Rep. 2015;5:11431. doi: 10.1038/srep11431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma Y, Zhao Y, Liu J, et al. Effects of temperature variation and humidity on the death of COVID-19 in Wuhan, China. Sci Total Environ. 2020;724 doi: 10.1016/j.scitotenv.2020.138226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J, Tang K, Feng K, et al. High temperature and high humidity reduce the transmission of COVID-19. ArXiv E-Prints. 2020 2003:arXiv:2003.05003. [Google Scholar]
- 56.Qi H, Xiao S, Shi R, et al. COVID-19 transmission in Mainland China is associated with temperature and humidity: a time-series analysis. Sci Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei J, Li Y. Airborne spread of infectious agents in the indoor environment. Am J Infect Control. 2016;44(9, Supplement):S102–S108. doi: 10.1016/j.ajic.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ngonghala CN, Iboi E, Eikenberry S, et al. Mathematical assessment of the impact of non-pharmaceutical interventions on curtailing the 2019 novel Coronavirus. Math Biosci. 2020;325 doi: 10.1016/j.mbs.2020.108364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Covid-19: Should cloth masks be used by healthcare workers as a last resort? The BMJ. Available at: https://blogs.bmj.com/bmj/2020/04/09/covid-19-should-cloth-masks-be-used-by-healthcare-workers-as-a-last-resort/. Accessed June 2, 2020.
- 60.Leung NHL, Chu DKW, Shiu EYC, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26:676–680. doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138:575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen M-J, Chang K-J, Hsu C-C, et al. Precaution and prevention of coronavirus disease 2019 (COVID-19) infection in the eye. J Chin Med Assoc JCMA. doi:10.1097/JCMA.0000000000000334 [DOI] [PMC free article] [PubMed]
- 63.CDC. Infection Control Guidance for Healthcare Professionals about Coronavirus (COVID-19). Centers for Disease Control and Prevention. Accessed June 3, 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control.html
- 64.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. doi:10.1001/jama.2020.2648 [DOI] [PubMed]
- 65.Fauci AS, Lane HC, Redfield RR. Covid-19 — navigating the uncharted. N Engl J Med. 2020;382:1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dietz W, Santos‐Burgoa C. Obesity and its implications for COVID-19 mortality. Obesity. 2020;28 doi: 10.1002/oby.22818. 1005-1005. [DOI] [PubMed] [Google Scholar]
- 67.Price-Haywood EG, Burton J, Fort D, et al. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. doi:10.1056/NEJMsa2011686 [DOI] [PMC free article] [PubMed]
- 68.Rentsch CT, Kidwai-Khan F, Tate JP, et al. Covid-19 by race and ethnicity: a national cohort study of 6 Million United States Veterans. MedRxiv Prepr Serv Health Sci. May 18, 2020 doi: 10.1101/2020.05.12.20099135. Published online. [DOI] [Google Scholar]
- 69.Stevis-Gridneff M, Jakes L. World leaders join to pledge $8 billion for vaccine as U.S. Goes It Alone. The New York Times. Available at: https://www.nytimes.com/2020/05/04/world/europe/eu-coronavirus-vaccine.html. Accessed June 3, 2020.
- 70.Centers for Disease Control and Prevention. Appendices in the guideline for disinfection and sterilization in healthcare facilities (2008). Published July 22, 2019. Accessed June 15, 2020. Available at:https://www.cdc.gov/infectioncontrol/guidelines/environmental/appendix/air.html
- 71.Howell I, Gautereaux K, Glasner J, et al. Preliminary Report: Comparing the Audio Quality of Classical Music Lessons Over Zoom, Microsoft Teams, VoiceLessonsApp, and Apple FaceTime. Ian Howell, DMA
- 72.Source-Connect Now / Source Elements. Available at: https://now.source-elements.com/#!/. Accessed June 20, 2020.
- 73.SoundJack: Real Time Online Music. Ian Howell, DMA. Available at:https://www.ianhowellcountertenor.com/soundjack-real-time-online-music. Accessed June 20, 2020.
- 74.Setting up a realtime physically distant rehearsal. Art Song Central. Available at: https://artsongcentral.com/2020/setting-up-a-realtime-physically-distant-rehearsal/. Accessed June 20, 2020.