Abstract
We followed–up a mild COVID-19 patient for 91 days and serially monitored his serum antibodies to four SARS-CoV-2 related antigens (NP, RBD, S1 and ECD) and neutralization activities. Our data revealed a profile of serial antibody responses during the progress and a quick decline of neutralization activities after discharge.
Since December 2019, an outbreak of 2019 novel coronavirus disease (COVID-19) causing a severe pneumonia has been spreading globally [1]. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was revealed to be the causative agent [2]. Though, recent studies demonstrated that transfusion of convalescent plasma containing the neutralizing antibody responses resulted in clinical improvement [3]. The isotype, specificity and duration of protective antibodies in recovered COVID-19 patients were still not well-known. Antiviral treatment, infection control, epidemiological measures, and vaccination against SARS-CoV-2 were in urgent demand of reliable data for profiles of serial serum antibody responses during the progress of COVID-19. Here we retrospective studied a longitudinal profile of anti-SARS-CoV-2 antibody responses in a COVID-19 patient with mild clinical presentation. We serially monitored the IgA, IgM, IgG and IgG isotypes including IgG1 to IgG4 responses specific to four SARS-CoV-2 related antigens, including nucleocapsid protein (NP) and receptor binding domain (RBD), S1 protein, and ectodomain of spike protein (ECD) [4], from the 4th day he was symptom onset till the 91st day after symptom onset.
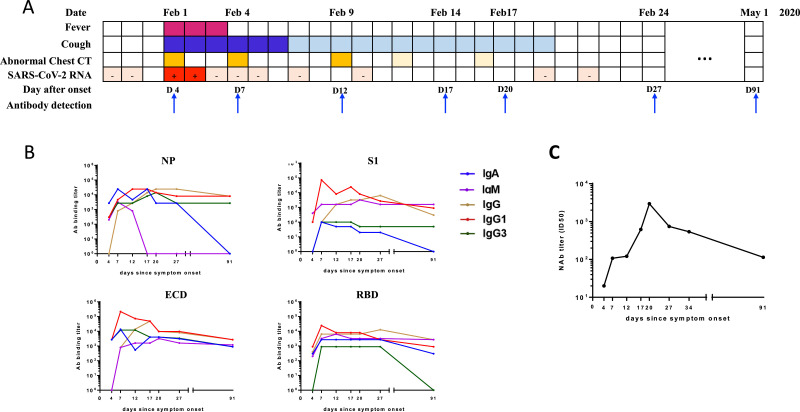
On February 1, 2020, a 27-year-old man sought medical advice for a fever of 38 °C and cough. Upon admission, his chest computed tomography (CT) scans showed focal ground-glass opacities and nasopharyngeal swab test was positive for SARS-CoV-2 by real-time reverse transcription-PCR (RT-PCR). He was diagnosed as mild symptomatic patients and admitted to the hospital. The RT-PCR tests for SARS-CoV-2 were positive for two days and symptoms were resolved except for mild cough in the next following days. For all RT-PCR results were negative since February 4, he was discharged on February 22. And we followed him up till the 91st day after symptom onset. The clinical course was summaried (Fig. 1 A).
Fig. 1.
The clinical course and the SARS-CoV-2 specific antibody responses in a COVID-19 patient with mild presentation. (A) Timeline of clinical symtoms, chest radiography findings and qRT-PCR results for the COVID-19 patient. His-cough was gradually allievated since Febuary 7, and chest radiographic improvement was observed on Febuary 12 and Febuary 16, respectively. (B) Longidudial IgA, IgM, IgG, IgG1 and IgG3 antibody titers in response to SARS-CoV-2 nucleocapsid protein (NP) and various subunits of spike protein including receptor binding domain (RBD), S1, and ectodomain (ECD), respectively. (C) Serial monition of serum antibodies neutralization activities of from January 29 to May 1, 2020.
Using four recombinant SARS-CoV-2 antigens, serial specific IgA, IgM, IgG and IgG isotypes including IgG1 to IgG4 responses were analyzed by an indirect enzyme-linked immunosorbent assay (ELISA) (Fig. 1B). Of note, the level of anti-SARS-CoV-2 IgG2 and IgG4 were almost undetectable. IgG specified to all the four antigens were peaked at 27 days after symptoms onset and decreased gradually until the 91 days after symptoms onset. Correlatively, IgG1 specified to ECD, S1 and RBD were peaked at 7 days and specified to NP was peaked at 14 days after symptoms onset. And IgG1 specified to all the four antigens stayed at relative high level till the 91st day. Though IgG3 responses to ECD, S1 and NP were increased since the 4th day and mildly declined until the 91st day, responses especially to RBD was almost undetectable on the 91st day. IgA reacted to ECD and RBD were increased from the 4th day and stayed sustainably at high level until the 91st day. On the contrary, NP and S1 specified IgA dropped rapidly after the peak point and was undetectable at 91st day after the symptoms onset. In addition, NP reacted IgM sharply decreased from 7th day till 17th day and stayed undetectable.
The neutralization activities were further determined by the pseudovirus microneutralization assay. The activity was rapidly increased from the 4th day to the 20th day after the symptoms onset, peaked with a titer of 2954 (ID50), and decreased obviously then. On the 91th day the titer was 114 (ID50), only 4% of the peak point (Fig. 1C).
Our case highlighted that the SARS-CoV-2 specific humoral immunity is critical during clinical recovery of COVID-19. Of note, antibody specific to RBD which is responsible for binding to angiotensin-converting enzyme 2 (ACE2) was correlated with neutralizing capability [5,6]. Early presence of anti-RBD antibody might facilitate virus clearance, contributing to a transient positive viral detection. The level of RBD-specific antibody might be associated with the favorable outcome of COVID-19 [7]. Secondly, high magnitude of antibody responses targeting at spike protein RBD region was identified, suggesting that RBD region is highly immunogenic, an ideal antigen candidate for vaccine design. Thirdly, our data suggested a rapidly declined neutralizing activity of COVID-19 clinical recovered patients 69 days after discharge, suggesting the circulating anti-SARS-CoV-2 neutralizing antibodies might have a relative short half-life. Fourth, this case validated the necessities of the combination of SARS-CoV-2 specific antibody responses with viral testing for diagnosis, especially for patients with minimal or mild presentation [8]. Such information is of immediate relevance and would assist clinical diagnosis, prognosis and vaccine design of COVID-19.
Acknowledgment
This study was supported by the Medical Science and technology Development Foundation, Nanjing Department of Health (Grant No. YKK19056), Nanjing Medical Science and Technique Development Foundation (QRX17141), Fundamental Research Funds for the Central Universities (No. 14380459).
Contributor Information
Rui Huang, Email: doctor_hr@126.com.
Yuxin Chen, Email: yuxin_chen2015@163.com.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel Coronavirus from patients with Pneumonia in China, 2019. New Engl. J. Med. 2020 Feb 20;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel Coronavirus-infected Pneumonia. New Engl. J. Med. 2020 Mar 26;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J. Treatment of 5 Critically Ill patients with COVID-19 with convalescent Plasma. JAMA. 2020 Mar 27;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.To K.K.-.W., Tsang O.T.-.Y., Leung W.-.S., Tam A.R., Wu T.-.C., Lung D.C. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect. Dis. 2020;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang W., Wang H., Deng Y., Song T., Lan J., Wu G. Characterization of anti-MERS-CoV antibodies against various recombinant structural antigens of MERS-CoV in an imported case in China. Emerg Microbes Infect. 2016 Nov 9;5(11):e113. doi: 10.1038/emi.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science (New York, NY) 2020 Mar 13;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu X.W., Wu X.X., Jiang X.G., Xu K.J., Ying L.J., Ma C.L. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ (Clin. Res. ed) 2020 Feb 19;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]