Summary
Integration of disease diagnosis and therapy in vivo by nanotechnology is a challenge in the design of multifunctional nanocarriers. Herein, we report an intelligent and degradable nanoreactor, an assembly of the 4-mercaptobenzonitrile-decorated silver nanoparticles (AgNPs@MBN) and the glucose oxidase (GOx)-loaded metal-organic-framework (ZIF-8@GOx), which can be activated by tumor microenvironment to start the catalytic cascade-enhanced chemo-starvation synergistic therapy and simultaneous self-sense of cellular glucose level. Under the mild acidic microenvironment of tumor, the nanoreactor will collapse to release GOx that triggers a catalytic cascade reaction in vivo, depleting glucose, etching AgNPs@MBN, and producing toxic H2O2, Ag+, and Zn2+ ions, all of which work together to inhibit tumor growth. The AgNPs@MBN as SERS nanoprobe reads out glucose concentration noninvasively in tumor to achieve instant feedback of therapeutic progression. This work proposes a promising example of using enzyme-encapsulated biomineralized MOFs as an effective anticarcinogen for clinical applications.
Subject Areas: Biomaterials, Biomedical Engineering, Nanostructure
Graphical Abstract
Highlights
-
•
This nanoreactor integrates in vivo sensing and synergistic therapy capabilities
-
•
The ZIF-8 nanocarrier protects GOx from deactivation and immune clearance
-
•
The nanoreactor is biodegradable, avoiding the side effects on tumor-bearing mice
-
•
Instant non-invasive glucose feedback capability realized by in vivo SERS
Biomaterials; Biomedical Engineering; Nanostructure
Introduction
Cancer remains a major threat to human health. To combat it, a variety of cancer therapeutic approaches have been developed (Lin et al., 2018; Yang et al., 2019; 2019b, ) and widely employed in the clinic including surgery, chemotherapy, and ultrasound, photodynamic, photothermal, and radiotherapy. However, these strategies may cause severe damages to the surrounding normal tissues (Cheng et al., 2014; Liang et al., 2019) and/or induce undesired tumor metastasis (Song et al., 2016; Zhang et al., 2015). Therefore, minimally invasive or noninvasive precise therapeutic modalities, with high spatial/temporal controllability and less toxicity to healthy tissues, are still highly desired (Li et al., 2014; Ge et al., 2014; Han et al., 2018; Kotagiri et al., 2015).
Recently, glucose oxidase (GOx)-based cancer starvation therapy that depletes glucose and engenders toxic H2O2/gluconic acid in the presence of oxygen has been explored (Chang et al., 2017; Wang et al., 2016; Zhang et al., 2018a) as a targeting therapeutic strategy for cancers due to its powerful ability to change tumor microenvironments. Moreover, the generated H2O2 not only markedly enhances tumor oxidative stress but also can be converted into ·OH radicals to kill cancer cells (Wang et al., 2019b). Thus this strategy can be further integrated with other therapeutic methods to achieve enhanced synergistic therapeutic effects (Wang et al., 2019c; Ma et al., 2019; Tang et al., 2019). The naked GOx exposed in biological environments is particularly prone to inactivation. Also, it has the weaknesses of short in vivo half-life, immunogenicity, and systematic toxicity (Fu et al., 2018). Thus, nanocarriers (inorganic or organic) for GOx (Wang et al., 2016; Zhang et al., 2018a) are highly suggested in many GOx-based starvation therapeutic strategies. However, these nanocarriers are usually undegradable in vivo, and hence may cause biotoxicity. Therefore, a degradable and intelligent nanocarrier for precise GOx-based starvation therapy is urgently desired.
Metal-organic frameworks (MOFs) are a class of highly crystalline, porous, and degradable solid-state materials constructed by metal ions and organic linkers (Wang et al., 2019a; Schoedel et al., 2016). It affords a promising biomedical nanocarrier platform for encapsulating drugs, antibodies, genes, enzymes, etc. (Feng et al., 2018; Chen et al., 2019) for in vivo drug delivery. These MOF cages can protect proteins from the attack of proteases and the clearance of the mononuclear phagocyte system in physiological environments (Cheng et al., 2019; Gao et al., 2019; Lin et al., 2019; Zhang et al., 2018b). Recent reports showed that some of them could react with the cellular microenvironments of cancers, e.g., ZIF-8 and UiO-66 for pH, ZIF-90 for ATP (Cai et al., 2019). These intelligent responses of the MOF-based nanocarriers endow them with microenvironment-switchable drug-releasing ability, achieving effective tumor-targeted killing.
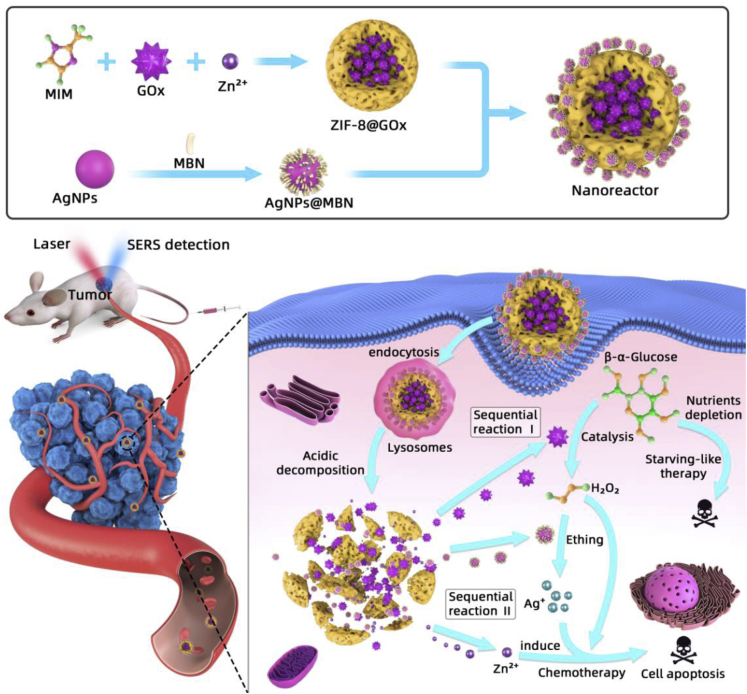
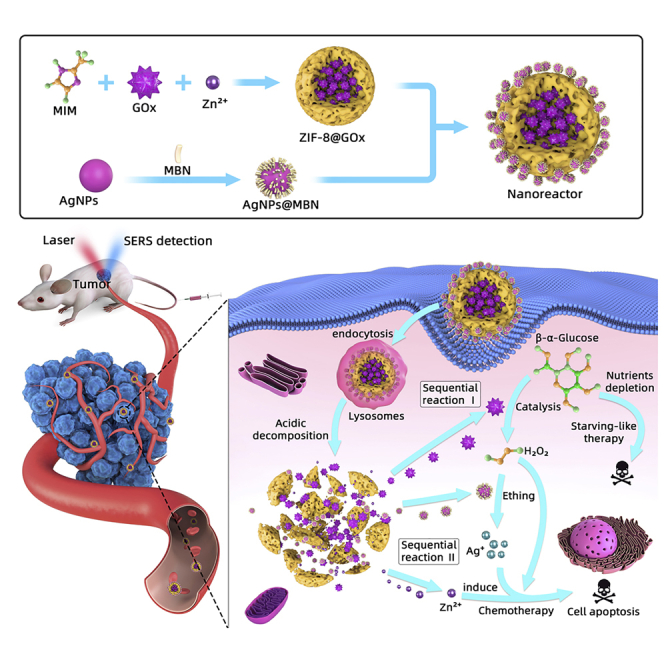
In this study, we developed a biomimetic, degradable, and intelligent nanoreactor (ZIF-8@GOx-AgNPs@MBN) for the catalytic cascade-enhanced chemo-starvation synergistic therapy of tumor. The nanoreactor was fabricated by the electrostatic assembly of the surface-enhanced Raman scattering (SERS) nanoprobes (AgNPs@MBN) on the GOx-encapsulated ZIF-8 MOF nanoparticle (ZIF-8@GOx). After the internalization of the nanoreactors by cancer cells, the GOx encapsulated in MOF can be gradually released according to the intercellular microenvironment of cancerous cells, to trigger a catalytic cascade reaction that can collapse the ZIF-8 cage, consume glucose, etch the AgNPs@MBN, and produce toxic H2O2, Zn2+, and Ag+ ions, realizing the chemo-starvation synergistic therapy of cancer cells. Many studies have proved that Ag+ and Zn2+ ions have cytotoxicity on various cancer cell lines through the induction of oxidative stress, mitochondrial damage, autophagy, and cell apoptosis (Soenen et al., 2015; Skulachev et al., 1967; Manev et al., 1997; Link and Jagow, 1995; Gazaryan et al., 2007). And the nanoreactor can be gradually degraded in the lysosomes due to the mild acidic environment of lysosomes of cancer cells. Moreover, the SERS nanoprobes loaded on the nanoreactors can self-sense and provide a feedback of the glucose level simultaneously during the therapeutic progress due to the decreasing SERS intensity of the Raman reporter (MBN) caused by the H2O2-etching effect on AgNPs. We applied the nanoreactor for the treatment of cervical carcinoma cells (HeLa) and mice planted with the cervical carcinoma tumors. The chemo-starvation synergistic therapeutic effect of the nanoreactor for tumors was assessed, and the systemic toxicity was also evaluated. The merits of the designer multifunctional nanoreactor are obvious. (1) It integrates in vivo sensing and chemo-starvation synergistic therapy capabilities. (2) As the main element of the nanoreactor, the ZIF-8 nanocarrier protects GOx from deactivation and immune clearance. More importantly, it has an intelligent acid response to the tumor microenvironment, and its fragmentation triggers on-demand drug (GOx) release, which is a key step for the cancer-specific therapy. (3) The nanoreactor is biodegradable, avoiding the long-term accumulation of the nanomaterials and the side effects on tumor-bearing mice. (4) The nanoreactor has instant non-invasive glucose feedback capability for therapeutic effectiveness evaluation, realized by in vivo SERS. (5) The high payloads of GOx and SERS nanoprobes improve the therapeutic effect and strengthen the sensing ability.
Results and Discussions
Characterization of ZIF-8@GOx-AgNPs@MBN
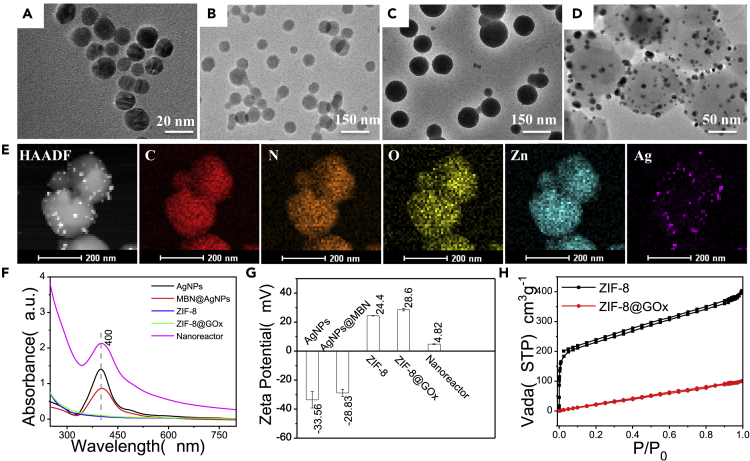
As displayed in Scheme 1, the hierarchical nanoreactor was constructed by the assembly of the AgNPs@MBN on the ZIF-8@GOx nanoparticle. Figures 1A–1D show the transmission electron microscopic (TEM) images of the components and the resulting nanoreactors. AgNPs with a diameter of ~13 ± 2.4 nm (Figure 1A and S1) were used for constructing the SERS nanoprobe, and our SERS measurements indicate that such a size of AgNPs affords acceptable SERS intensity for the Raman reporter MBN (Figure S2C). We chose MBN as the reporter because it has a unique band in the silent range of bio tissues, minimizing the interference of the living body during in vivo SERS detections The successful preparation of the AgNPs@MBN was evidenced by the UV-visible (UV-vis) spectra and zeta potential characterizations. The plasmonic band of AgNPs centered at 400 nm (Figure 1F) has a red shift after the MBN coating, whereas the zeta potential of the NPs also switches from −33.56 to −28.83 mV after the coating (Figure 1G).
Scheme1.
The Design of the Nanoreactor and Its Catalytic Cascade-enhanced Synergistic Chemo-Starvation Therapy for Cancer Cells
Figure 1.
Characterization of Nanoreactors
(A–D) Typical TEM images of (A) AgNPs, (B) ZIF-8, (C) ZIF-8@GOx, and (D) the resulting ZIF-8@GOx-AgNPs@MBN nanoreactors.
(E) High-angle annular dark-field-scanning transmission electron microscopic and elemental mapping of the ZIF-8@GOx-AgNPs@MBN for C, N, O, Zn, and Ag.
(F and G) (F) The UV-vis absorption spectra and (G) zeta potentials of the AgNPs, AgNPs@MBN, ZIF-8, ZIF-8@GOx, and ZIF-8@GOx-AgNPs@MBN.
(H) Nitrogen adsorption analysis isotherms of ZIF-8 and ZIF-8@GOx.
The ZIF-8@GOx was prepared by the crystallization of the ZIF-8 precursors (zinc nitrate and 2-methylimidazole) with GOx under stirring. The average size distribution of the pure ZIF-8 is about 84 ± 4.3 nm (Figure 1B). The encapsulation of GOx in ZIF-8 NPs (ZIF-8@GOx) caused a size increase to 99 ± 6.9 nm (Figure 1C), ascribing to a promoted growth kinetics mediated by the GOx-seeded clusters. The zeta potential of the resulting ZIF-8@GOx is 28.6 mV, which is ~4 mV higher than that of pure ZIF-8 (24.4 mV, Figure 1G). Figure 1H shows the nitrogen adsorption analysis of the ZIF-8@GOx, manifested as Type 1 in shape. The Brunauer-Emmett-Teller analysis exhibits that the ZIF-8@GOx has a surface area of 841.27 m2/g, which is smaller than that of the pure ZIF-8 NPs (1,014.36 m2/g). The loading ratio of GOx in ZIF-8@GOx analyzed by thermogravimetric analysis was ~55 wt % (Figure S4). Moreover, the X-ray diffraction data confirm that ZIF-8@GOx maintains the same crystalline form as the pure ZIF-8 NPs (Figure S2B), suggesting that the doping of GOx does not alter the crystalline structure of ZIF-8.
Figure 1D shows the TEM image of the obtained nanoreactor. It can be observed that the AgNPs@MBN are randomly attached to the ZIF-8@GOx surface. The existence and uniform distributions of C, N, O, Zn, and Ag were revealed by both elemental mapping (Figure 1E) and X-ray photoelectron spectroscopic analysis (Figure S3), affirming the successful assembly of the nanoreactors. The UV-vis spectrum of the ZIF-8@GOx-AgNPs@MBN shows a band at ~ 402 nm, which is mainly originated from the plasmon band of AgNPs (from AgNPs@MBN). The resulting nanoreactor gives a surface potential at 4.82 mV, which differs from either AgNPs@MBN (−28.83 mV) or ZIF-8@GOx (28.6 mV). The aforementioned data further verified the successful decoration of the AgNPs@MBN on the ZIF-8@GOx.
Tumor Microenvironment-Responsive Catalytic Cascade Reaction of the Nanoreactor
Owing to the acidic degradable feature of ZIF-8, the tailor-made nanoreactor could trigger a catalytic cascade reaction in an acidic tumor microenvironment, which can be employed to perform the synergistic tumor therapy based on the fast glucose metabolism and consumption of cancer cells (Hsu and Sabatini, 2008; Ying et al., 2012; Huo et al., 2017; Qi et al., 2018) and the poisoning of released metal ions (Scheme 1). Since the pH of the subcellular organelles in the cancer cells is more acidic as compared to that in the normal cells (Shen et al., 2018; Qi et al., 2019), the nanosensor starts degradation under an acidic environment within lysosomes of cancer cells; the fracture of the ZIF-8 skeleton structure produces zinc (Zn2+) and releases the loaded GOx as well. So, this synergistic therapy is tumor cell selective and normal cell ineffective, realizing the targeting and selective therapy.
Although Zn2+ is an essential element for humans (Gao et al., 2017), it has toxic effects on mammalian cells at elevated concentrations as the elevation of Zn2+ can trigger the breakdown of the mitochondrial membrane potential, caspase activation, and cell apoptosis (Skulachev et al., 1967; Manev et al., 1997; Link and Jagow, 1995; Gazaryan et al., 2007). Moreover, the content of glucose in the cancerous cells is more than in normal cells (Huo et al., 2017; Chen et al., 2015), so that the exposed GOx could effectively deplete the glucose in cancerous cells, producing H2O2 that subsequently etches AgNPs into toxic Ag+. Therefore, the deleterious substances (H2O2, Zn2+, and Ag+) produced by this catalytic cascade reaction of the nanoreactor, together with the starvation effect, will cooperatively kill tumor cells. Also, this synergistic therapy is selective for tumors due to the difference in the pH microenvironments between cancer and normal cells.
Equally importantly, in addition to the synergistic therapy capability, the designed nanoreactor also has the capability of glucose self-sensing, which is vital not only for the feedback of the intracellular glucose environment but also for the assessment of GOx activity of the nanoreactor. The sensing performance of the nanoreactor was assessed by using the SERS spectroscopy based on a “turn-off” mechanism (intensity decrease) of the Raman reporter (MBN) induced by the H2O2 etching effect on the AgNPs (described as Equations 1 and 2), in which the enzymatically produced H2O2 is glucose relevant.
| (Equation 1) |
| 2Ag + H2O2 + 2H+ → 2Ag+ + 2H2O | (Equation 2) |
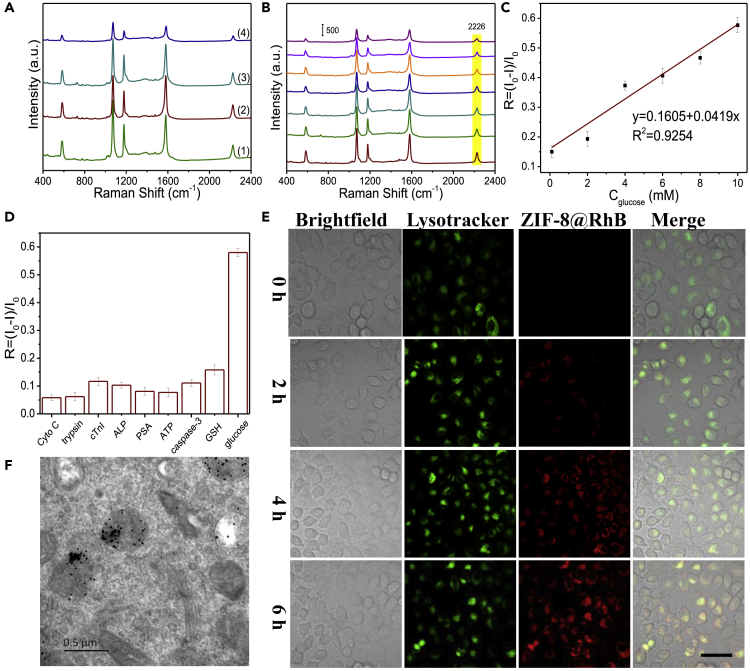
Clearly and as confirmed experimentally, only in the presence of glucose and acidic conditions, the SERS intensity of MBN was reduced (Figure 2A), proving enzymatic activity of GOx and degradability of the nanoreactor in an acidic environment.
Figure 2.
Tumor Microenvironment-Responsive Catalytic Cascade Reaction of the Nanoreactors
(A) SERS spectra of the ZIF-8@GOx-AgNPs@MBN nanoreactors without (1 and 3) or with 5 mM glucose (2 and 4) at pH = 7.0 (1 and 2) and pH = 4.5 (3 and 4).
(B) SERS spectra of the nanoreactors reacted with different concentrations of glucose (bottom to top: 0–10 mM) for 3 h at pH = 4.5.
(C) The plot of the SERS peak intensity with the concentrations of glucose.
(D) The selectivity of the nanoreactors for glucose over other negative controls according to the SERS intensity of 4-MBN at 2,226 cm−1. The error bars are calculated from three trials.
(E) Fluorescence colocalization images of HeLa cells after incubation with the rhodamine B(RhB)-labeled (BSA)-encapsulated ZIF-8 NPs (red, ZIF-8@RhB) for different incubation times, while the lysosomes were stained with LysoTracker (green). Scale bar, 50 μm.
(F) Bio-TEM images of HeLa cells after incubation with the ZIF-8@GOx-AgNPs@MBN for 6 h.
ZIF-8 is considered as a good delivery carrier for enzyme, antibody, gene, and so on. ZIF-8 can protect these biomolecules from inactivity and immune clearance and maintain their biological function, which is key role for a nanocarrier (Lin et al., 2019; Zhang et al., 2018a, 2018b; Chen et al., 2018). In the present design, ZIF-8 is an acid-responsive material, and its drug release condition is proved to be 4.5 (Figure 2A). One concern is whether the enzymatic activity of GOx under acidic condition would be affected. To test this, a chromogenic reaction of tetramethyl benzidine (TMB) was conducted. In this sensing process, the hydroxyl radicals produced from the disproportionation of the enzymatically produced H2O2 under catalysis by horseradish peroxidase will oxidize colorless TMB to chromogenic TMB cation-free radicals, which can be assayed at 650 nm with a UV-vis spectrometer. The results (Figures S5A and S5B) indicate that the GOx maintained its good catalytic activity irrespective of whether they were encapsulated into ZIF-8 or were under acidic condition (pH = 4.5) (Figures S5C and S5D). In addition, the optimum pH for GOx activity is ∼4 in vitro and the activity of GOx is about 616.6 U/mL in the acidic environment of the tumor (Figure S6). Fortunately, the activity of GOx is also not affected by the high temperature during the synthesis of the ZIF-8@GOx (Figure S7).
The AgNPs@MBN element on the nanoreactor shows a good linear response to the GOx/glucose reaction in a glucose concentration range of 0–10 mM (Figures 2B and 2C) with the lowest detectable concentration of 0.1 mM. This sensing shows high selectivity to glucose (Figure 2D) and fewer interferences from other substances in the biological environments.
Positioning of the Nanoreactor in Cells
The distribution of the nanoreactors within cells was disclosed by using confocal laser scanning microscopic imaging by using rhodamine B (RhB)-encapsulated ZIF-8 NPs (Figures S8 and 2E). From the fluorescence co-localization of the ZIF-8@RhB and the LysoTracker (green) in HeLa cells, we can observe that by increasing the culture time, more and more ZIF-8@RhB enters into cells, giving brighter red fluorescence; after 6-h cell incubation, the ZIF-8@RhB lights the whole cells and the cells still keep their morphology. The colocalization coefficient (Pearson's correlation coefficient) of the ZIF-8@RhB and the LysoTracker was calculated as 0.9914, indicating their high spatial consistency. Furthermore, the location of nanoreactors in the lysosomes was affirmed by bio-TEM (Figure 2F).
Therapeutic Effect of Nanoreactors for HeLa Cells
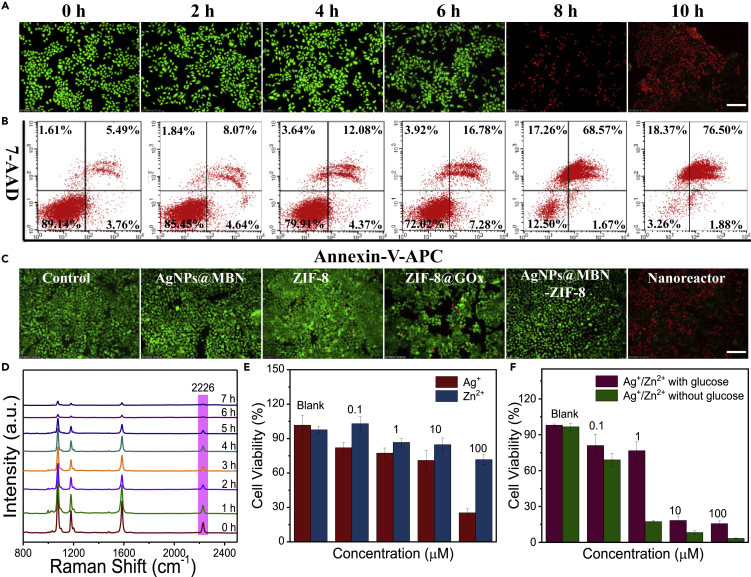
The treatment effect of the nanoreactors after being incubated with HeLa cells was then examined. The dose of the nanoreactor for cells was first evaluated. A safe concentration of the nanoreactor for HeLa cells for 24 h was optimized as less than 0.16 mg/mL by using MTT assay (Figure S9A). Figure 3A shows the fluorescence images of the live/dead cells stained by calcein-AM/propidium iodide after the cells were incubated with the nanoreactor (0.08 mg/mL) for different culture times. We can observe that ~95% cells are dead after incubation for 6 h and the percentage of the dead cells increases further with incubation time (Figure S9B). The therapeutic efficacy of the nanoreactors was further quantified by the flow cytometry experiments (the typical Annexin V-APC/7-AAD staining protocol, Figure 3B). The results were consistent with those of the aforementioned live/dead cell staining assay, indicating that HeLa cells died by the apoptotic pathway.
Figure 3.
Therapeutic Effect of Nanoreactors for HeLa Cells
(A) Confocal fluorescent images of HeLa cells after incubation with ZIF-8@GOx-AgNPs@MBN at different times (n = 3). All scale bars, 80 μm.
(B) Flow apoptosis assay of HeLa cells after different treatment times (n = 3).
(C) The confocal fluorescent images of live and dead HeLa cells that underwent different treatments, including control (PBS), ZIF-8, ZIF-8@GOx, AgNPs@MBN, and ZIF-8@GOx-AgNPs@MBN. Cells were stained by Calcein-AM/propidium iodide, and the green and red colors stand for the alive and dead cells, respectively (n = 3). All scale bars, 80 μm.
(D) SERS spectra of HeLa cells incubated with ZIF-8@GOx-AgNPs@MBN for different times.
(E and F) Relative cell viabilities of HeLa cells incubated with different concentrations of Ag+, Zn2+ (E), and Ag+/Zn2+ with or without glucose for 24 h (F), determined by the MTT assay.
To confirm the synergy of multi-factors that drive cancerous cells to death, the cell viabilities of various forms of the nanocomposites including the control group (PBS), AgNPs@MBN, ZIF-8, ZIF-8@GOx, and ZIF-8@GOx-AgNPs@MBN, incubated with HeLa cells for 24 h, were comparatively examined via the standard MTT assay and the fluorescence live/dead cell staining as well. As clearly shown in Figures 3C and S9C, all the control groups show no significant cell damage. Comparatively, the cell viability rate of the nanoreactor group decreases to 5.97%, demonstrating the strong cell-killing ability of the nanoreactors. We also found experimentally that the same concentration of the nanoreactors (0.08 mg/mL) is non-toxic to the normal H8 cells (Figures S10A, S10B and S11), which confirms our expectation that the normal cells (H8 cell) with a higher pH microenvironment are hard to trigger the catalytic cascade reactions of the nanoreactors.
To check the synergistic effects of the H2O2, Ag+, Zn2+, and glucose consumption on cancerous cell killing, we first performed the glucose consumption test in HeLa cells after they were incubated with the nanoreactor for 6 h, by taking advantage of the glucose self-sensing capability of the nanoreactors. As seen from Figure 3D, the SERS intensity of MBN gradually weakened over time, indicating that intracellular glucose was gradually consumed to the starvation state. To testify the generation of hydrogen peroxide in the catalytic cascade reaction within HeLa cells, an H2O2 kit assay was used for the HeLa cells incubated with the nanoreactor. Figure S12 shows the fluorescence images of H2O2 within the tested HeLa cells after incubation with the nanoreactors for different time. The fluorescence intensity increased gradually, indicating that more and more H2O2 was generated in the catalytic cascade reactions. The toxicities of individual Ag+ and Zn2+ to HeLa cells at different concentrations were further studied. The cell survival rate was ~70% when the concentration of Zn2+ is 100 μM (Figure 3E), whereas the same concentration of Ag+ made the cell survival rate lower than ~25%, proving that Ag+ has a higher toxicity than Zn2+ to tumor cells. The viabilities of HeLa cells treated with the two kinds of metal ions (Ag+ and Zn2+) under the different concentrations with or without glucose were also tested by the MTT assay (Figure 3F). Compared with those in the presence of glucose, the tumor cells exhibited a starved stage when glucose has been removed from the culture medium, and lower concentrations of Ag+/Zn2+ in the absence of glucose can kill more cancer cells (Figure 3F), confirming the enhanced synergistic therapeutic effect by combined treatment of starvation and the poisoning of metal ions.
In Vivo Tumor Therapy
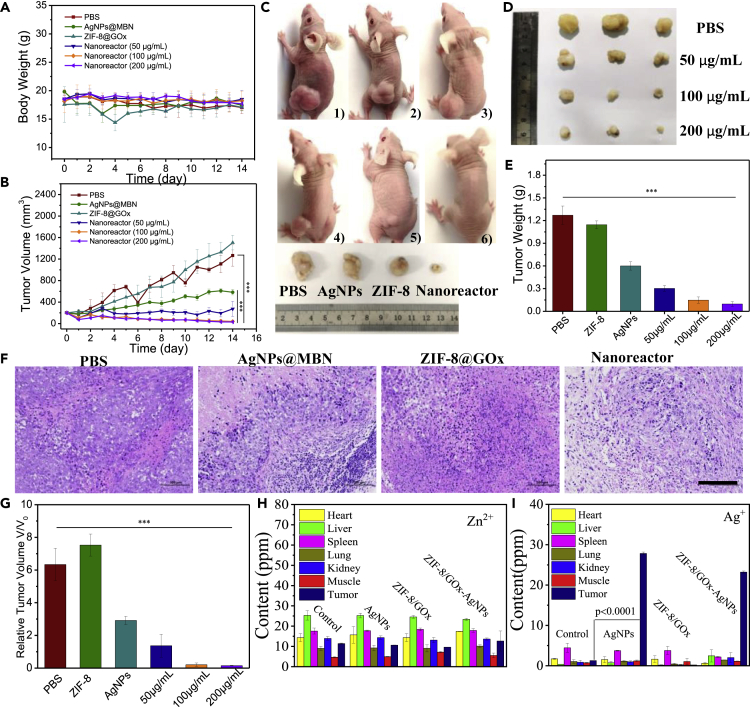
Motivated by the outstanding biocompatibility and excellent therapeutic performance of the nanoreactor in vitro, we proceeded to assess its in vivo therapeutic behavior by examining the cervical tumor xenografted on the specific pathogen-free BALB/c nude mice (Figure S13). The cervical tumor-bearing mice with initial tumor volumes of 200 mm3 were randomly divided into the following six groups: treated by (1) PBS, (2) AgNPs@MBN, (3) ZIF-8@GOx, and (4–6) nanoreactors with different concentrations (50, 100, and 200 μg/mL, respectively). During a therapeutic period of 14 days, the body weights of mice in control and all therapeutic groups show no significant variations, which indicates that the nanoreactor provides excellent biocompatibility in the whole therapeutic process (Figure 4A). Compared with other groups, the nanoreactor (200 μg/mL) presents satisfactory suppression effects on the tumor (Figures 4B–4E). The suppression rate, in terms of the variation of the relative tumor volume (Figure 4G), was calculated as 96.8%, implying the excellent in vivo therapeutic performance of the nanoreactor.
Figure 4.
In Vivo Tumor Therapy and Biosafety of the Nanoreactors
(A and B) (A) The body weight changes and tumor volume curve (B) of HeLa tumor-bearing mice in each group, which underwent various treatments including AgNPs@MBN, ZIF-8@GOx, and ZIF-8@GOx-AgNPs@MBN at different concentrations (50, 100, and 200 μg/mL), recorded every day.
(C–E) (C) Representative digital photographs of the tumor-bearing mice after different therapies for 14 days. 1–6: PBS, AgNPs@MBN, ZIF-8@GOx, and ZIF-8@GOx-AgNPs@MBN at different concentrations (50, 100, and 200 μg/mL). The photographs (D) and weights (E) of the tumors in each group were collected on day 14.
(F) H&E staining of the tumor slides after the different treatments. All scale bars, 25 μm.
(G–I) (G) The relative tumor volume 14 d after treatment at different conditions. The biodistribution of Zn2+ (H) and Ag+ (I) in main tissues and tumors in 48 h of intravenous administrations of the ZIF-8@GOx-MBN@AgNPs.
p values in (B, E, and G) were calculated by the t test, where ∗∗∗p < 0.001 indicate that the two being compared have a very high degree of discrimination.
The tumor tissues of mice in different groups after the treatment were collected and analyzed by hematoxylin and eosin (H&E) staining assays, and the results are presented in Figure 4F. In the AgNPs@MBN and the nanoreactor groups, the apparent deformation, shrinking of the nuclei, and destruction of the membrane integrity were all observed, indicating severe damage to tumor cells. For tumors in other groups, we observed no distinct injury.
Biosafety of the Nanoreactors
To evaluate the biosafety of the nanoreactors, the biodistributions of silver and zinc elements in the main organs and tumors were determined using the inductively coupled plasma mass spectrometry (ICP-MS). As shown in Figures 4H, 4I, and S15, the silver element was observed mainly accumulated in the tumor, without serious side effects on other organs (heart, liver, spleen, lung, kidney, and muscle), whereas the contents of the zinc element were almost unchanged in all tested organs and tumors. The histopathological evaluation of these major organs and the hematology-related assays were further carried out after the treatment. As shown in Figure S14, the major organs stained by H&E show no significant pathological changes between the control groups and the treatment groups.
The blood chemistry analyses were also conducted by checking standard eight hematological biomarkers (Figure S16). The results manifest no distinct abnormality in blood chemistry before and after treatment for 14 days, indicating negligible side effects of the nanoreactor. In addition, analyses of the microelements within blood show that the zinc element has no abnormal change between the control groups and treatment groups (Table S1). These results demonstrate that this intelligent nanoreactor not only can effectively suppress tumor growth but also displays satisfactory low toxicity.
Biodegradability of the Nanoreactors
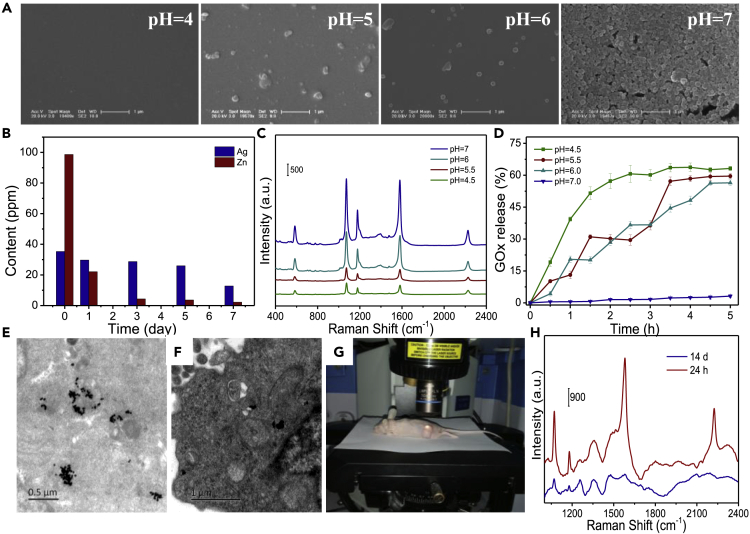
Biodegradation of the nanoreactors was evaluated under neutral (pH = 7.0) and acidic (pH = 4.0–6.0) media, which imitate the in vivo neutral healthy body fluids and intratumoral mildly acidic environment, respectively. As shown by scanning electron microscopic images in Figure 5A, the nanoreactors can be degraded under an acid environment. Simultaneously, the degradation time of the nanoreactor was determined by using the ICP-MS (Figures S17 and 5B). The content of Zn2+ from the extracted lysosomes within the glucose solution (1.0 mM. pH = 5.0) is gradually reduced, and the nanoreactors can be basically degraded in an acid environment within ~3 days.
Figure 5.
Biodegradability of the Nanoreactors
(A) Scanning electron microscopic images of the nanoreactors under different pH values.
(B) The biodistribution of Zn2+ and Ag+ in the extracted lysosomes of HeLa cells with treatment time, assessed by ICP-MS.
(C) SERS spectra of the nanoreactor after reacting with 5.0 mM glucose for 3 h at different pH values.
(D) GOx release from the nanoreactors, measured by SERS method.
Bio-TEM images of the tumor tissues 6 h postinjection of the nanoreactor (E) or 14 days after treatment with this nanoreactor (F).
(G) Photograph shows in situ SERS measurements of a mouse by a confocal Raman microspectrometer. A laser beam is focusing on the tumor.
(H) SERS spectra recorded from the tumor site of a mouse with intravenous preinjection of the nanoreactor for 24 h and 14 days, respectively.
Figures 5C and 5D show that the SERS signal of the nanoreactor gradually weakens as the acidity of the medium increases, indicating gradual degradation of the ZIF-8 skeleton structure of the nanoreactors under acidic condition, causing GOx's leakage eventually. The released GOx catalyzes the hydrolysis of glucose to generate H2O2, which can further etch AgNPs gradually, resulting in the weakening of the SERS signal of MBN located on AgNPs. Figure 5D shows the time evolution with the GOx releasing, derived from the time-dependent SERS intensities (at 2,226 cm−1) of the nanoreactors, indicating that GOx release is pH dependent and that this process can be completed in 2.5 h at pH 4.5. No GOx release is observed at pH = 7.0 (Figures 5D and S18). All these results prove that our nanoreactor is degradable under a mild acid condition.
The biodegradation of the nanoreactors was further evaluated in HeLa cells and tumor-bearing nude mice by using bio-TEM and in vivo SERS spectra. Figures 5E, 5F and S19 show the distributions of the nanoreactors within the tumor tissue of a mouse 6 h postinjection and 14 days after treatment, respectively. It can be observed that the nanoreactors, previously located in lysosomes of the tumor tissue, have been completely degraded 14 days after treatment. Figure 5G shows the photograph of a Raman laser beam (785 nm) focusing on the tumor site of a mouse at 24 h postinjection, to acquire its Raman spectra in vivo. As shown in Figure 5H, the tumor area of a mouse treated with the nanoreactors for 24 h exhibits strong SERS signals. However, almost no SERS signal was observed for the cancer tissue 14 days after treatment. This proves that the nanoreactors in the mice can be degraded after the therapy, which solves the common problem of the long-term accumulation in vivo for most nanocarriers or nanodrugs.
Conclusion
In summary, we developed an intelligent degradable nanocomposite (ZIF-8@GOx-AgNPs@MBN) composed of the GOx-enveloped ZIF-8 nanoparticles and the MBN-modified AgNPs. The nanoreactor possesses the catalysis-enhanced synergistic starvation/metal ion poisoning cancer therapy triggered in situ by the tumor microenvironment of lysosomes. Differentiated therapy toward tumor cells from normal cells was achieved by this strategy. This ZIF-8 nanocarrier has a high loading of GOx to ~55 wt %, and this encapsulation structure well protects GOx from the enzyme-mediated degradation in vivo. Meanwhile, the AgNPs@MBN component on the nanoreactor can sense glucose and GOx activity during the therapeutic process via SERS feedback. The signal readout in the Raman silent range is beneficial to probe pharmacodynamics and tumor microenvironment changes in a living body. In addition, this nanoreactor keeps long-persistent activity under the 4 oC storage for at least one month (Figure S20). More interestingly, this nanoreactor can be cleared effectively from the mouse body due to its excellent biodegradability, which avoids long-term toxicity and endows it with many in vivo applicable potentials. This work highlights a unique multi-functional nanocomposite, the MOF-supported GOx, for combating solid tumors. It also provides an extensible route to design smart catalytic cascade nanosystems for anticancer and extensive applications in biomedical fields.
Limitations of the Study
Some limitations to the findings of this study must be acknowledged. First, the limitation of this work is that the sample size of the mice studied is not large enough, so that the results obtained cannot fully accurately reflect the therapeutic effect of the nanoreactor. Second, in this study we worked with nude mice. Although its physiological characteristics are similar to those of humans, there are certain differences. Therefore, there are still some challenges in using this method for real clinical diagnosis and cancer therapy.
Electronic Supplementary Information (ESI) available: Experimental Section, characterizations of the ZIF-8@GOx-AgNPs@MBN, catalytic performance of GOx, characterization of ZIF-8@RhB, treatment effect of the nanoreactor for HeLa cells, cell viabilities of H8 cells under different treatments, comparison of H8 cells viability under 2D and 3D cell culture, evaluation of H2O2 content in HeLa cells, establishment of cervical tumor xenograft, H&E staining of main organs, biodistributions of Zn2+ and Ag+ in main organs, blood chemistry analyses of the tested mice, biodistributions of Zn2+ and Ag+ in extracted lysosomes, GOx release self-sensing, long-term stability of the nanoreactor.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Shuping Xu (xusp@jlu.edu.cn).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
This study did not generate datasets/code.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by National Natural Science Foundation of China (grant Nos. 21873039, 21675146, 21573087,and 21573092).
Author Contributions
D.S. conducted the experiments, generated figures, performed analysis and wrote the manuscript. G.Q. designed and conducted the experiments and collected and analyzed the data. K.M. performed animal experiments and collected the data. X.Q. performed animal experiments. W.X. participated in the discussion and contributed to manuscript preparation. S.X. conceptualized the project and contributed to manuscript preparation. Y.J. conceived the project, participated in the discussion, and contributed to manuscript preparation. All authors read and approved the final manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: July 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101274.
Contributor Information
Shuping Xu, Email: xusp@jlu.edu.cn.
Yongdong Jin, Email: ydjin@ciac.ac.cn.
Supplemental Information
References
- Cai W., Wang J.Q., Chu C.C., Chen W., Wu C.S., Liu G. Metal-organic framework-based stimuli-responsive systems for drug delivery. Adv. Sci. 2019;6:1801526. doi: 10.1002/advs.201801526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K., Liu Z., Fang X., Chen H., Men X., Yuan Y., Sun K., Zhang X., Yuan Z., Wu C. Enhanced phototherapy by nanoparticle-enzyme via generation and photolysis of hydrogen peroxide. Nano Lett. 2017;17:4323–4329. doi: 10.1021/acs.nanolett.7b01382. [DOI] [PubMed] [Google Scholar]
- Cheng H., Jiang X.Y., Zheng R.R., Zuo S.J., Zhao L.P., Fan G.L., Xie B.R., Yu X.Y., Li S.Y., Zhang X.Z. A biomimetic cascade nanoreactor for tumor targeted starvation therapy amplified Chemotherapy. Biomaterials. 2019;195:75–85. doi: 10.1016/j.biomaterials.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Cheng L., Liu J.J., Gu X., Gong H., Shi X.Z., Liu T., Wang C., Wang X.Y., Liu G., Xing H.Y. PEGylated WS2 nanosheets as a multifunctional theranostic agent for in vivo dual-modal CT/photoacoustic imaging guided photothermal therapy. Adv. Mater. 2014;26:1886–1893. doi: 10.1002/adma.201304497. [DOI] [PubMed] [Google Scholar]
- Chen G.S., Huang S.M., Kou X.X., Wei S.B., Huang S.Y., Jiang S.Q., Shen J., Zhu F., Ouyang G.F. A convenient and versatile amino-acid-boosted biomimetic strategy for the nondestructive encapsulation of biomacromolecules within metal–organic frameworks. Angew. Chem. Int. Ed. 2019;58:1463–1467. doi: 10.1002/anie.201813060. [DOI] [PubMed] [Google Scholar]
- Chen L.M., Li H.J., He H.L., Wu H.X., Jin Y.D. Smart plasmonic glucose nanosensors as generic theranostic agents for targeting-free cancer cell screening and killing. Anal. Chem. 2015;87:6868–6874. doi: 10.1021/acs.analchem.5b01260. [DOI] [PubMed] [Google Scholar]
- Chen T.T., Yi J.T., Zhao Y.Y., Chu X. Biomineralized metal−organic framework nanoparticles enable intracellular delivery and endo-lysosomal release of native active proteins. J. Am. Chem. Soc. 2018;140:9912–9920. doi: 10.1021/jacs.8b04457. [DOI] [PubMed] [Google Scholar]
- Feng Y.F., Wang H.R., Zhang S.N., Zhao Y., Gao J., Zheng Y.Y., Zhao P., Zhang Z.J., Zaworotko M.J., Cheng P. Antibodies@MOFs: an in vitro protective coating for preparation and storage of biopharmaceuticals. Adv. Mater. 2018;140:1805148. doi: 10.1002/adma.201805148. [DOI] [PubMed] [Google Scholar]
- Fu L.H., Qi C., Lin J., Huang P. Catalytic chemistry of glucose oxidase in cancer diagnosis and treatment. Chem. Soc. Rev. 2018;47:6454–6472. doi: 10.1039/c7cs00891k. [DOI] [PubMed] [Google Scholar]
- Gao H., Zhao L., Wang H., Xie E.J., Wang X.H., Wu Q., Yu Y.Y., He X.Y., Ji H.B., Rink L. Metal transporter Slc39a10 regulates susceptibility to inflammatory stimuli by controlling macrophage survival. Proc. Natl. Acad. Sci. U S A. 2017;114:12940–12945. doi: 10.1073/pnas.1708018114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L.H., Chen Q., Gong T.T., Liu J.H., Li C.X. Recent advancement of imidazolate framework (ZIF-8) based nanoformulations for synergistic tumor therapy. Nanoscale. 2019;11:21030–21045. doi: 10.1039/c9nr06558j. [DOI] [PubMed] [Google Scholar]
- Gazaryan I.G., Krasinskaya I.P., Kristal B.S., Brown A.M. Zinc irreversibly damages major enzymes of energy production and antioxidant defense prior to mitochondrial permeability transition. J. Biol. Chem. 2007;282:24373–24380. doi: 10.1074/jbc.M611376200. [DOI] [PubMed] [Google Scholar]
- Ge J., Lan M., Zhou B., Liu W., Guo L., Wang H., Jia Q., Niu G., Huang X., Zhou H. A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nat. Commun. 2014;5:4596–4603. doi: 10.1038/ncomms5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Huang J., Jing X., Yang D., Lin H., Wang Z., Li P., Chen Y. Oxygen-deficient black titania for synergistic/enhanced sonodynamic and photoinduced cancer therapy at near infrared-II biowindow. ACS Nano. 2018;12:4545–4555. doi: 10.1021/acsnano.8b00899. [DOI] [PubMed] [Google Scholar]
- Hsu P.P., Sabatini D.M. Cancer cell metabolism: warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Huo M.F., Wang L.Y., Chen Y., Shi J.L. Tumor-selective catalytic nanomedicine by nanocatalyst delivery. Nat. Commun. 2017;8:357–369. doi: 10.1038/s41467-017-00424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotagiri N., Sudlow G.P., Akers W.J., Achilefu S. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nat. Nanotechnol. 2015;10:370–379. doi: 10.1038/nnano.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S., Deng X.R., Chang Y., Sun C.Q., Shao S., Xie Z.X., Xiao X., Ma P., Zhang H.Y., Cheng Z.Y. Intelligent hollow Pt-CuS Janus architecture for synergistic catalysis-enhanced sonodynamic and photothermal cancer therapy. Nano Lett. 2019;19:4134–4145. doi: 10.1021/acs.nanolett.9b01595. [DOI] [PubMed] [Google Scholar]
- Lin H., Chen Y., Shi J. Nanoparticle-triggered in situ catalytic chemical reactions for tumour-specific therapy. Chem. Soc. Rev. 2018;47:1938–1958. doi: 10.1039/c7cs00471k. [DOI] [PubMed] [Google Scholar]
- Link T.A., Jagow G. Zinc ions inhibit the QP center of bovine heart mitochondrial bc1 complex by blocking a protonatable group. J. Biol. Chem. 1995;270:25001–25006. doi: 10.1074/jbc.270.42.25001. [DOI] [PubMed] [Google Scholar]
- Lin L.S., Huang T., Song J.B., Ou X.Y., Wang Z.T., Deng H.Z., Tian R., Liu Y.J., Wang J.F., Liu Y. Synthesis of copper peroxide nanodots for H2O2 self-supplying chemodynamic therapy. J. Am. Chem. Soc. 2019;141:9937–9945. doi: 10.1021/jacs.9b03457. [DOI] [PubMed] [Google Scholar]
- Li Y., Wen T., Zhao R., Liu X., Ji T., Wang H., Shi X., Shi J., Wei J., Zhao Y. Localized electric field of plasmonic nanoplatform enhanced photodynamic tumor therapy. ACS Nano. 2014;8:11529–11542. doi: 10.1021/nn5047647. [DOI] [PubMed] [Google Scholar]
- Manev H., Kharlamov E., Uz T., Mason R.P., Cagnoli C.M. Characterization of zinc-induced neuronal death in primary cultures of rat cerebellar granule cells. Exp. Neurol. 1997;146:171–178. doi: 10.1006/exnr.1997.6510. [DOI] [PubMed] [Google Scholar]
- Ma Y.C., Zhao Y.Y., Bejjanki N.K., Tang X.F., Jiang W., Dou J.X., Khan M.I., Wang Q., Xia J.X., Liu H. Nanoclustered cascaded enzymes for targeted tumor starvation and deoxygenation-activated chemotherapy without systemic toxicity. ACS Nano. 2019;13:8890–8902. doi: 10.1021/acsnano.9b02466. [DOI] [PubMed] [Google Scholar]
- Qi G.H., Zhang Y., Xu S.P., Li C.P., Wang D.D., Li H.J., Jin Y.D. Nucleus and mitochondria targeting theranostic plasmonic surface-enhanced Raman spectroscopy nanoprobes as a means for revealing molecular stress response differences in hyperthermia cell death between cancerous and normal Cells. Anal. Chem. 2018;90:13356–13364. doi: 10.1021/acs.analchem.8b03034. [DOI] [PubMed] [Google Scholar]
- Qi G.H., Li H.J., Zhang Y., Li C.P., Xu S.P., Wang M.M., Jin Y.D. Smart plasmonic nanorobot for real-time monitoring cytochrome c release and cell acidification in apoptosis during electrostimulation. Anal. Chem. 2019;91:1408–1415. doi: 10.1021/acs.analchem.8b04027. [DOI] [PubMed] [Google Scholar]
- Schoedel A., Li M., Li D., O’Keeffe M., Yaghi O.M. Structures of metal−organic frameworks with rod secondary building Units. Chem. Rev. 2016;116:12466–12535. doi: 10.1021/acs.chemrev.6b00346. [DOI] [PubMed] [Google Scholar]
- Shen Y.T., Liang L.J., Zhang S.Q., Huang D.S., Zhang J., Xu S.P., Liang C.Y., Xu W.Q. Organelle-targeting surface-enhanced Raman scattering (SERS) nanosensors for subcellular pH sensing. Nanoscale. 2018;10:1622–1630. doi: 10.1039/c7nr08636a. [DOI] [PubMed] [Google Scholar]
- Skulachev V.P., Chistyakov V.V., Jasaitis A.A., Smirnova E.G. Inhibition of the respiratory chain by zinc ions. Biochem. Biophys. Res. Commun. 1967;26:1–6. doi: 10.1016/0006-291x(67)90242-2. [DOI] [PubMed] [Google Scholar]
- Soenen S.J., Parak W.J., Rejman J., Manshian B. (Intra)Cellular stability of inorganic nanoparticles: effects on cytotoxicity, particle functionality, and biomedical applications. Chem. Rev. 2015;115:2109–2135. doi: 10.1021/cr400714j. [DOI] [PubMed] [Google Scholar]
- Song G.S., Chen Y.Y., Liang C., Yi X., Liu J.J., Sun X.Q., Shen S.D., Yang K., Liu Z. Catalase-loaded TaOx nanoshells as bio-nanoreactors combining high-Z element and enzyme delivery for enhancing radiotherapy. Adv. Mater. 2016;28:7143–7148. doi: 10.1002/adma.201602111. [DOI] [PubMed] [Google Scholar]
- Tang Z.M., Liu Y.Y., He M.Y., Bu W.B. Chemodynamic therapy: tumour microenvironment-mediated fenton and fenton-like reactions. Angew. Chem. Int. Ed. 2019;58:946–956. doi: 10.1002/anie.201805664. [DOI] [PubMed] [Google Scholar]
- Wang C., Ye Y., Hochu G.M., Sadeghifar H., Gu Z. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody. Nano Lett. 2016;16:2334–2340. doi: 10.1021/acs.nanolett.5b05030. [DOI] [PubMed] [Google Scholar]
- Wang M.M., Tang Y.F., Jin Y.D. Modulating catalytic performance of metal−organic framework composites by localized surface plasmon resonance. ACS Catal. 2019;9:11502–11514. [Google Scholar]
- Wang M., Wang D.M., Chen Q., Li C.X., Li Z.Q., Lin J. Recent advances in glucose-oxidase-based nanocomposites for tumor therapy. Small. 2019;15:e1903895. doi: 10.1002/smll.201903895. [DOI] [PubMed] [Google Scholar]
- Wang W.G., Cheng Y.H., Yu P., Wang H.R., Zhang Y., Xu H.H., Ye Q.S., Yuan A.H., Hu Y.Q., Wu J.H. Perfluorocarbon regulates the intratumoural environment to enhance hypoxia-based agent efficacy. Nat. Commun. 2019;10:1580–1590. doi: 10.1038/s41467-019-09389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Chen Y., Shi J. Reactive oxygen species (ROS)-based nanomedicine. Chem. Rev. 2019;119:4881–4985. doi: 10.1021/acs.chemrev.8b00626. [DOI] [PubMed] [Google Scholar]
- Ying H.Q., Kimmelman A.C., Lyssiotis C.A., Hua S.J., Chu G.C., Sananikone E., Locasale J.W., Son J.Y., Zhang H.L., Coloff J.L. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656–670. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Zhao K., Bu W.B., Ni D.L., Liu Y.Y., Feng J.W., Shi J.L. Marriage of scintillator and semiconductor for synchronous radiotherapy and deep photodynamic therapy with diminished oxygen dependence. Angew. Chem. Int. Ed. 2015;54:1770–1774. doi: 10.1002/anie.201408472. [DOI] [PubMed] [Google Scholar]
- Zhang L., Wang Z.Z., Zhang Y., Cao F.F., Dong K., Ren J.S., Qu X.G. Erythrocyte membrane cloaked metal–organic framework nanoparticle as biomimetic nanoreactor for starvation-activated colon cancer therapy. ACS Nano. 2018;12:10201–10211. doi: 10.1021/acsnano.8b05200. [DOI] [PubMed] [Google Scholar]
- Zhang L., Wan S.S., Li C.X., Xu L., Cheng H., Zhang X.Z. An adenosine triphosphate-responsive autocatalytic fenton nanoparticle for tumor ablation with self-supplied H2O2 and acceleration of Fe(III)/Fe(II) conversion. Nano Lett. 2018;18:7609–7618. doi: 10.1021/acs.nanolett.8b03178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate datasets/code.