Abstract
Background/Objective
This study examined relationships between health-related physical fitness indicators and clustered cardiometabolic risk factors in adolescents between 2014 and 2017.
Methods
The sample consisted of 93 students (60% girls), with complete data sets in both 2014 and 2017. The physical fitness components evaluated were: flexibility (sit and reach), muscular fitness (curl-up and push-up), cardiorespiratory fitness (progressive aerobic cardiovascular endurance run), and body fat (BMI). The cardiometabolic risk factors were: waist circumference, blood pressure, high-density lipoprotein cholesterol (HDL-C), triglycerides and fasting blood glucose. Z-scores were calculated for each risk factor, with the sum of risk factor z-scores values used to represent clustered cardiometabolic risk.
Results
The results of cross-sectional analysis indicated that muscle fitness (curl-up: β = −0.37, p < 0.001; push-up: β = −0.38, p < 0.005) and cardiorespiratory fitness (β = −0.56, p < 0.001) were inversely associated with clustered cardiometabolic risk, with BMI positively associated (β = 0.58, p < 0.001). In the longitudinal analysis, cardiorespiratory fitness (β = −0.33; p < 0.005) and body fat (β = 0.46, p < 0.001) demonstrated a significant association with clustered cardiometabolic risk. However, no significant associations between the health-related physical fitness and clustered cardiometabolic risk were observed after adjustment for baseline values.
Conclusion
Our cross-sectional findings highlight the importance of health-related physical fitness indicators to adolescents. In regarding the longitudinal analysis, further studies are needed in order to clarify the influence of physical fitness in the adolescence and cardiometabolic risk later in life.
Keywords: Cardiorespiratory fitness, Body composition, Muscle strength, Metabolic syndrome
Introduction
Cardiometabolic risk factors such as abdominal obesity, high blood pressure, hyperglycemia, and dyslipidemia have been observed in children and adolescents and are considered predictors of future health problems.1, 2, 3 Although there may be a genetic predisposition to these risk factors, their development is also a result of lifestyle, which can be influenced by physical fitness indicators.3,4
The literature has demonstrated that some fitness indicators are directly related to improvements in cardiovascular health.5,6 Cross-sectional and longitudinal studies indicate that cardiorespiratory fitness and muscular fitness are inversely associated to cardiometabolic risk factors while body fat is positively associated.7, 8, 9, 10, 11, 12
Potentially, physical fitness is an important health marker in childhood and adolescence,4 as well as, a predictor of future cardiovascular (CVD) risk factors in young adulthood.11 In this way, international and national organizations such as the World Health Organization13 and the United States Department of Health and Human Services Organization14 recommend the promotion of aerobic and muscle-strengthening activities as part of their physical activity guidelines for children and adolescents.
A recent systematic review and meta-analysis of longitudinal studies found moderate-to-large relationships between muscular fitness in childhood and adolescence and future levels of BMI, skinfold thickness, Homeostatic Model Assessment of Insulin Resistance (HOMA-IR), triglycerides, CVD risk score, and bone mineral density.15 Regarding cardiorespiratory fitness (CRF), Mintjens et al.16 reported a significant association between higher CRF in childhood and adolescence and lower BMI, body fatness, and metabolic syndrome incidence at least two years later. However, for blood pressure, lipid metabolism and glucose homeostasis, the evidence was unconvincing.
Among the longitudinal studies performed, there is great variation concerning methodology, length of follow-up, and adjustment for potential confounders such as maturational status and physical activity levels. Another problem is that many studies do not adjust the outcome variable of interest for baseline values, which have implications for the interpretation of the temporal sequence and thus causality.
Thus, because of known biological and behavioral alterations in this period of life, we need to better understand the association between health-related physical fitness indicators and clustered cardiometabolic risk whilst also controlling for baseline values of maturational status, cardiometabolic risk, and physical activity levels. Thus, the objective of the present study was to examine the relationships between health-related physical fitness indicators and clustered cardiometabolic risk factors in adolescents between 2014 and 2017.
Methods
Participants
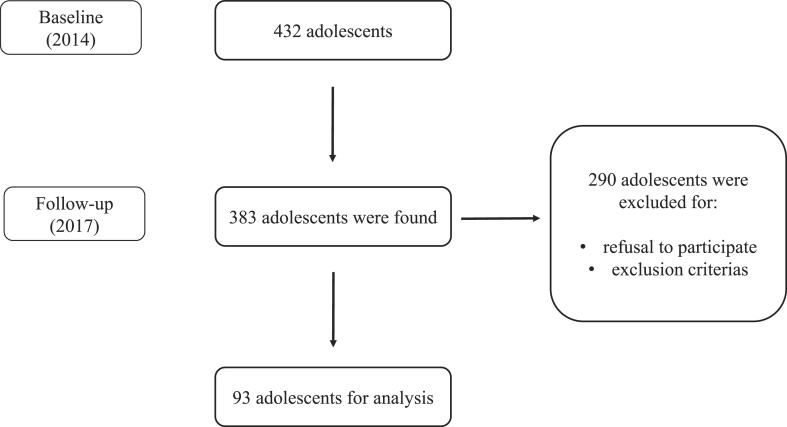
The first phase of data collection took place in 2014, with adolescents who were enrolled in elementary schools (2nd cycle) and high school in Jacarezinho, Paraná, Brazil. In 2014, 432 adolescents participated in baseline testing, with 89% or 383 adolescents followed-up three years later in 2017. Of these, 76% or 290/383 were loss to contact, refused to further participate, or were excluded for the following reasons: (a) any health problems that could have affected study participation; (b) pregnancy; or (c) use of any type of medication that could have affected study variables. The final sample comprised 93 adolescents (60% girls) who had complete data in both 2014 and 2017 (Fig. 1).
Fig. 1.
Selection of participants.
The protocols were approved by the Research Ethics Committee of the Northern University of Paraná - UNOPAR (Opinion 1,302,963) and followed the norms of Resolution 466/12 of the National Health Council on research involving humans.
Anthropometric measurements
Trained research assistants took all physical measurements. Body height was measured without shoes to the nearest 0.1 cm with a transportable stadiometer (Ottoboni HM-210D; Ottoboni). Bodyweight was measured to the nearest 0.1 kg with a calibrated beam balance scale (Toledo 2096 PP; Toledo). Waist circumference was measured midway between the lower rib margin and the iliac crest.
Motor tests of health-related physical fitness
The health-related physical fitness indicators were evaluated by applying the test battery proposed by the FitnessGram® administration manual.17
-
1.
Back-saver sit and reach: the purpose of the test was to evaluate flexibility by flexing the trunk forward with one leg extended and the other bent;
-
2.
Curl-up: to evaluate the strength/endurance of the muscles of the abdominal region through the flexion movement of the trunk;
-
3.
Push-up: to evaluate the strength/endurance of the upper limb muscles through flexion and extension of the elbows;
-
4.
Progressive aerobic cardiovascular endurance run: to evaluate cardiorespiratory fitness by running with changes of direction at progressive intensities. Each stage of the test lasts 1 min and the beep is emitted progressively faster at each stage, starting at 8.5 km/h in the first stage and increasing by 0.5 km/h per stage. The test was terminated when the adolescents voluntarily interrupted their displacement due to exhaustion, or because they could not maintain the pace proposed by the test, and the number of complete turns (i.e., laps) was noted. Maximum oxygen consumption (O2max) was estimated using the formula proposed by Burns et al.18
Physical activity
Physical activity level was evaluated by the Physical Activity Questionnaire (PAQ),19 adapted and validated for Brazilian adolescents by Guedes and Guedes.20 Each PAQ-A item presents a 5-point response scale, which allows the establishment of a score equivalent to the physical activity level.
Somatic maturation
Biological maturation was estimated through evaluation of somatic maturation by determining the distance in years of the individual from the baseline peak height velocity (PHV) using mathematical models based on measures of height, age, and sex.21
Blood pressure
Systolic and diastolic blood pressure were measured by the auscultatory method by using a mercury sphygmomanometer. After a 15-min rest period, blood pressure was measured in the left arm. Two measurements were performed, with the mean value retained.
Blood analysis
Blood was collected by trained nurses through the closed collection system (vacuum) in the school environment, from 07:30 to 09:30 in the morning, with the adolescents fasting for at least 10 h. After the blood samples were collected, all participants served breakfast, then continued with the physical and fitness measurements. The enzymatic colorimetric method (for plasma lipid analysis) and reference enzymatic UV-Hexokinase (glucose analysis) were performed using the cobas® 6000 analyzer (Roche Diagnostics, Basel, Switzerland) and corresponding kits.
Clustered cardiometabolic risk factors
For each risk factor, the z-score was calculated (individual value - sample mean/ sample standard deviation). For blood pressure, the mean of SBP and DBP (SBP + DBP/2) was used to calculate the z-score. For HDL-C, the score calculation was inverted (sample mean - individual value/sample standard deviation). The summed z-score across all risk factors represented the clustered cardiometabolic risk score (clustered cardiometabolic risk factor = waist circumference + blood pressure + HDL-C + triglycerides + glucose). This method for estimating clustered metabolic risk has been previously described in other studies with adolescents.22, 23, 24
Statistical analysis
The Kolmogorov-Smirnov test was used to verify the normality of the data. Paired t-tests (parametric data) or Wilcoxon signed-rank tests (non-parametric data) were used to compare variables between 2014 and 2017. Multiple regression analysis was used to examine the associations between health-related physical fitness indicators and clustered cardiometabolic risk factors. Cross-sectional association (2017): Model 1: adjusted by sex and age; Model 2: additional adjustment for somatic maturation; Model 3: additional adjustment for physical activity. Longitudinal association (2014–2017): Model 1: adjusted by sex and age; Model 2: additional adjustment for baseline somatic maturation and cardiometabolic risk factors; Model 3: additional adjustment for physical activity. Data were analyzed using SPSS 20.0 statistical software, with a significance level of p < 0.05.
Results
The participant’s characteristics are presented in Table 1. Significant increases were observed for flexibility, strength/endurance, and BMI, with a significant decrease for O2max between 2014 and 2017. Regarding cardiometabolic risk factors, a significant reduction in triglycerides dosages was found, while waist circumference, diastolic blood pressure, and HDL-C increased.
Table 1.
Characteristics of the study participants (n = 93).
| Variables | 2014 | 2017 | p-value | |
|---|---|---|---|---|
| Age (years)a | 13.5 ± 0.80 | 16.5 ± 0.80 | 22.3 | <0.001∗∗ |
| Weight (kg)a | 53.6 ± 12.5 | 60.3 ± 12.2 | 11.6 | <0.001∗∗ |
| Height (cm)a | 160.7 ± 8.4 | 166.4 ± 9.2 | 0.70 | <0.001∗∗ |
| Physical Fitness Measures | ||||
| Flexibility (cm)a | 17.7 ± 6.4 | 19.6 ± 7.5 | 10.3 | <0.05∗ |
| S/E abdomen (rpm)b | 16.5(9.0–27.7) | 23.0(14.0–36.5) | 102.1 | <0.001∗∗ |
| S/E upper limb (rpm)b | 0.00 (0.00–1,0) | 6.0 (0.00–12.0) | 0.00 | <0.001∗∗ |
| O2max (ml/kg/min)b | 37.6 (35.0–41.1) | 34.9 (31.8–39.9) | −7.5 | <0.001∗∗ |
| BMIb | 19.5 (18.0–23.1) | 21.0 (19.5–24.2) | 7.8 | <0.001∗∗ |
| Cardiometabolic Risk Factors | ||||
| WC (cm)a | 67.8 ± 10.4 | 71.9 ± 9.2 | 7.1 | <0.001∗∗ |
| SBP (mmHg)b | 100.0 (100.0–110.0) | 100.0 (100.0–110.0) | 0.00 | 0.36 |
| DBP (mmHg)b | 65.0 (60.0–70.0) | 80.0 (70.0–80.0) | 16.7 | <0.001∗∗ |
| Triglycerides (mg/dL)b | 81.7 (76.6–85.7) | 66.4 (51.4–93.6) | −14.1 | <0.05∗ |
| HDL-C (mg/dL)a | 52.8 ± 11.5 | 56.4 ± 10.7 | 9.6 | <0.001∗∗ |
| Blood glucose (mg/dL)b | 80.5 (77.1–85.1) | 80.8 (78.3–85.4) | 0.60 | 0.98 |
Abbreviations: BMI: body mass index; O2max: maximum oxygen consumption; S/E: strength and endurance; HDL: high density lipoprotein; DBP: diastolic blood pressure; SBP: systolic blood pressure; rpm: repetitions per minute; WC: waist circumference.
∗ p < 0,05; ∗∗p < 0,001.
Parametric data: mean ± standard deviation.
Non-parametric data: median (interquartile range).
Muscular fitness (curl-up: β = −0.37, p < 0.001; push-up: β = −0.38, p < 0.005) and cardiorespiratory fitness (β = −0.56, p < 0.001) were inversely associated with clustered cardiometabolic risk in the cross-sectional analysis, with BMI positively associated (β = 0.58, p < 0.001), after adjustment for somatic maturation and physical activity level (Table 2).
Table 2.
Cross-sectional association of health-related physical fitness indicators (2017) with the clustered cardiometabolic risk factors (2017).
| Variables |
Model 1 |
Model 2 |
Model 3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Beta | Beta adjust | p | Beta | Beta adjust | p | Beta | Beta adjust | p | |
| Flexibility | −0.07 | −0.17 | 0.09 | −0.05 | −0.13 | 0.19 | −0.08 | −0.20 | 0.05 |
| S/E abdomen | −0.22 | −0.23 | 0.04 | −0.05 | −0.33 | 0.002 | −0.05 | −0.37 | 0.001 |
| S/E upper limb | −0.14 | −0.32 | 0.01 | −0.14 | −0.30 | 0.02 | −0.17 | −0.38 | 0.005 |
| O2max | −0.27 | −0.47 | 0.001 | −0.25 | −0.44 | 0.002 | −0.32 | −0.56 | <0.001 |
| BMI | 0.52 | 0.58 | <0.001 | 0.51 | 0.57 | <0.001 | 0.52 | 0.58 | <0.001 |
Model 1: Regression model adjusted by sex and age.
Model 2: Additional adjustment for somatic maturation.
Model 3: Additional adjustment for physical activity level.
Abbreviations: BMI: body mass index (kg/m2); O2max: maximum oxygen consumption (ml/kg/min); S/E: strength/endurance (rpm); flexibility (cm).
Considering the longitudinal analysis, the cardiorespiratory fitness was inversely associated with clustered cardiometabolic risk (β = −0.33, p < 0.005), with BMI positively associated with clustered cardiometabolic risk (β = 0.46, p < 0.001). However, no significant associations between the health-related physical fitness and clustered cardiometabolic risk were observed after adjustment for baseline values (Table 3).
Table 3.
Longitudinal association of health-related physical fitness indicators (2014) with the clustered cardiometabolic risk factors (2017).
| Variables |
Model 1 |
Model 2 |
Model 3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Beta | Beta adjust | p | Beta | Beta adjust | p | Beta | Beta adjust | p | |
| Flexibility | −0.04 | −0.08 | 0.44 | 0.02 | 0.05 | 0.57 | 0.01 | 0.02 | 0.80 |
| S/E abdomen | 0.11 | 0.01 | 0.90 | 0.01 | 0.08 | 0.38 | 0.01 | 0.09 | 0.31 |
| S/E upper limb | −0.17 | −0.23 | 0.17 | −0.03 | −0.05 | 0.61 | −0.05 | −0.08 | 0.36 |
| O2max | −0.21 | −0.33 | 0.004 | −0.01 | −0.03 | 0.80 | −0.05 | −0.08 | 0.47 |
| BMI | 0.42 | 0.46 | <0.001 | 0.19 | 0.21 | 0.08 | 0.21 | 0.24 | 0.05 |
Model 1: Regression model adjusted by sex and age.
Model 2: Additional adjustment for baseline somatic maturation and cardiometabolic risk factors.
Model 3: Additional adjustment for physical activity level.
Abbreviations: BMI: body mass index (kg/m2); O2max: maximum oxygen consumption (ml/kg/min); S/E: strength/endurance (rpm); flexibility (cm).
Discussion
This longitudinal study examined the association between health-related physical fitness (cardiorespiratory, muscle strength/endurance, body composition, and flexibility) and clustered cardiometabolic risk (waist circumference, physical activity levels, HDL-C, triglycerides, and glucose) in Brazilian adolescents. Cross-sectionally, we have found significant associations between several health-related physical fitness components (e.g., muscular fitness, cardiorespiratory fitness, and BMI) and clustered cardiometabolic risk. However, in longitudinal analysis, no significant prospective associations were observed between health-related physical fitness and clustered cardiometabolic risk.
Previous studies have indicated that muscular fitness, cardiorespiratory fitness, and body fat are independently associated with clustered metabolic risk during adolescence.,9,25,26 Our findings increase the body of evidence from cross-sectional studies on the independent association between muscular fitness and cardiometabolic risk in adolescents.1,7,27,28 García-Hermoso et al.15 performed a systematic review and meta-analysis on the prospective associations between muscular fitness and future health status in childhood and adolescence. The authors observed significant, moderate-to-strong relationships between muscular fitness at baseline and body fat (i.e., BMI and skinfold thickness) and cardiometabolic parameters (i.e., HOMA-IR, triglycerides, and cardiovascular risk score) at follow-up. Such evidence may be explained by the fact that skeletal muscle is considered the main tissue for glucose and triglyceride metabolism, and is increasingly recognized in the prevention of chronic diseases.29
Significant inverse associations were found between CRF and clustered cardiometabolic risk in both the cross-sectional and longitudinal analyses. However, after adjustment for baseline values, CRF in 2014 was not significantly associated with clustered cardiometabolic risk in 2017. A meta-analysis30 of cross-sectional studies reported that low CRF is associated with an increased risk of cardiovascular disease, and decreased mental and skeletal health. Longitudinally, low CRF in childhood and adolescence has been shown to predict cardiovascular health later in life, with a risk of metabolic syndrome, arterial stiffness, and myocardial infarction.30
Another systematic review16 of prospective studies demonstrated that high CRF in childhood and adolescence was significantly associated with BMI, waist circumference, body fatness and the prevalence of metabolic syndrome later in life. Higher levels of CRF are related to decreased adiposity and improved glucose uptake by muscle tissue, further assisting in the imbalance between absorption and oxidation of fatty acids in skeletal muscle, and thus promoting cardiometabolic health benefits.31
Regarding body fat, our data are consistent with previous studies showing that excess weight increases cardiometabolic risk factors.4,32 Petkeviciene et al.33 observed an increased risk of metabolic syndrome, hyperglycemia, and type 2 diabetes in adulthood due to excess weight in youth, while the risk of high blood pressure, hypertriglyceridemia, and reduced HDL-C are associated with increased BMI over the years.
Data from four cohorts from childhood to adulthood identified that those with higher BMI from childhood to adulthood had an increased risk of type 2 diabetes (Relative Risk [RR] 5.4; 95%CI 3.4 to 8.5), hypertension (RR 2.7; 95%CI 2.2 to 3.3), elevated low-density lipoprotein cholesterol (LDL-C) (RR 1.8; 95%CI, 1.4 to 2.3) and triglyceride levels (RR 3.0; 95%CI, 2.4 to 3.8), reduced HDL-C levels (RR 2.1; CI%95, 1.8 to 2.5) compared to their peers with normal BMI.2
Excess body fat is a precursor to many health problems as hypertrophied adipose tissue is responsible for the production of inflammatory cytokines that play an important role in the atherosclerotic process, as well as being involved in the synthesis of various metabolic factors that contribute to insulin resistance, arterial hypertension, and dyslipidemia.34
Flexibility was not significantly associated with clustered risk factors in the cross-sectional or longitudinal analysis. This finding indicates that low levels of flexibility do not affect the outcome variables evaluated regarding the cardiometabolic profile. Furthermore, Nuzzo35 suggests the retired of flexibility as a major component of health-related physical fitness due to lacks predictive and concurrent validity value this component with meaningful health outcomes. However, it is known that flexibility in children and adolescents is associated with motor competence, which influences the practice of physical exercises and other fitness components.36,37
The relationship between health-related fitness and health consequences5,6 can be influenced by non-modifiable (age, sex, genetic) and modifiable (physical activity, diet, tobacco) factors.38 Physical activity is the main modifiable lifestyle factor with potentially large benefits to physical fitness, and this behavior is a promising focus for improving health-related fitness in adolescents.39,40 Research has consistently found that higher levels of vigorous physical activity (MVPA) are associated with better aerobic fitness,41 muscular fitness42 and lower body fat43 in adolescents. Therefore, there are plausible biological pathways through which MVPA affects both physical fitness and cardiometabolic risk and the corresponding associations.42,44 Nevertheless, when we adjusted for baseline physical activity in our regression models, the cross-sectional associations between physical fitness (except for flexibility) and clustered cardiometabolic risk remained statistically significant.
Another point that should be considered is the importance of statistically adjusting for baseline values of biological maturation and cardiometabolic risk.15 Maturational status could affect baseline physical fitness, and higher values of cardiometabolic risk at baseline could directly influence subsequent risk. In our study, no longitudinal associations were statistically significant when baseline values for somatic maturation and cardiometabolic risk were included in the regression models.
The findings of the present study support the cross-sectional evidence that higher muscular fitness and CRF, and lower body fat, are significantly related to clustered cardiometabolic health in adolescents. However, the longitudinal association between physical fitness and cardiometabolic risk in youth remain unclear. Some limitations need to be mentioned. Drop-outs were high because adolescents were lost to contact or refused to participate in the second stage of the research, resulting in a large sample loss. Regarding the fitness tests, all are indirect measures of underlying constructs, and may be influenced by practice and psychosocial aspects of performance (e.g., motivation), a problem common to all fitness testing studies, however, we do not consider this issue as a limitation, since the FitnessGram® test battery is widely used to evaluate physical fitness in adolescents.17
Conclusion
The present study demonstrated that several health-related physical fitness indicators are significantly associated with clustered cardiometabolic risk in adolescents. However, the causality between the baseline physical fitness and cardiometabolic health at follow-up appears to be influenced by baseline values of maturation, cardiometabolic risk and physical activity levels. In this regard, we reinforce the importance of monitoring the health-related physical fitness in adolescents, and suggest that interventions should be conducted to advance the understanding of how increases in physical fitness levels may attenuate and/or prevent the development of chronic diseases throughout life.
Practical applications
Good levels of muscular fitness, cardiorespiratory fitness, and body composition have been considered as a protective predictor against the development of cardiometabolic diseases. Thus, the periodic assessments of health-related physical fitness are an important strategy to the monitoring of health indicators, which must be taken into account for planning the sports program with the purpose to maintain physical fitness components within adequate levels in this population.
Declaration of competing interestCOI
None.
Funding/support statement
No financial or material support of any kind was received for the work described in this article.
CRediT authorship contribution statement
Paula Roldão da Silva: Conceptualization, Methodology, Writing - original draft. Géssika Castilho dos Santos: Methodology, Writing - original draft, Visualization. Jadson Marcio da Silva: Methodology, Writing - original draft. Waynne Ferreira de Faria: Formal analysis, Visualization. Raphael Gonçalves de Oliveira: Conceptualization, Investigation, Writing - review & editing. Antonio Stabelini Neto: Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing.
Contributor Information
Paula Roldão da Silva, Email: proldaosilva@gmail.com.
Géssika Castilho dos Santos, Email: gessika.castilho@gmail.com.
Jadson Marcio da Silva, Email: jadson_marcio@hotmail.com.
Waynne Ferreira de Faria, Email: fariawf@outlook.com.
Raphael Gonçalves de Oliveira, Email: rgoliveira@uenp.edu.br.
Antonio Stabelini Neto, Email: asneto@uenp.edu.br.
References
- 1.Buchan D.S., Boddy L.M., Young J.D. Relationships between cardiorespiratory and muscular fitness with cardiometabolic risk in adolescents. Res Sports Med. 2015;23(3):227–239. doi: 10.1080/15438627.2015.1040914. [DOI] [PubMed] [Google Scholar]
- 2.Juonala M., Magnussen C.G., Berenson G.S. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365(20):1876–1885. doi: 10.1056/NEJMoa1010112. [DOI] [PubMed] [Google Scholar]
- 3.Zaqout M., Michels N., Bammann K. Influence of physical fitness on cardio-metabolic risk factors in European children. The IDEFICS study. Int J Obes. 2016;40(7):1119–1125. doi: 10.1038/ijo.2016.22. [DOI] [PubMed] [Google Scholar]
- 4.Ortega F.B., Ruiz J.R., Castillo M.J. Physical fitness in childhood and adolescence: a powerful marker of health. Int J Obes. 2008;32(1):1–11. doi: 10.1038/sj.ijo.0803774. [DOI] [PubMed] [Google Scholar]
- 5.Blair S.N., Kohl H.W., Barlow C.E. Changes in physical fitness and all-cause mortality: a prospective study of healthy and unhealthy men. J Am Med Assoc. 1995;273(14):1093–1098. [PubMed] [Google Scholar]
- 6.Matos LS de, Zafra V.B., Elias R.M. Gênese da aterosclerose em crianças e adolescentes: artigo de revisão. Connect Line. 2012;14:27–35. [Google Scholar]
- 7.Artero E.G., Ruiz J.R., Ortega F.B. Muscular and cardiorespiratory fitness are independently associated with metabolic risk in adolescents: the HELENA study. Pediatr Diabetes. 2011;12(8):704–712. doi: 10.1111/j.1399-5448.2011.00769.x. [DOI] [PubMed] [Google Scholar]
- 8.Morikawa S.Y., Fujihara K., Hatta M. Relationships among cardiorespiratory fitness, muscular fitness, and cardiometabolic risk factors in Japanese adolescents: niigata screening for and preventing the development of non-communicable disease study-Agano (NICE EVIDENCE Study-Agano) 2. Pediatr Diabetes. 2018;19(4):593–602. doi: 10.1111/pedi.12623. [DOI] [PubMed] [Google Scholar]
- 9.Pérez-Bey A., Segura-Jiménez V., Fernández-Santos J. del R. The role of adiposity in the association between muscular fitness and cardiovascular disease. J Pediatr. 2018;199:178–185. doi: 10.1016/j.jpeds.2018.03.071. [DOI] [PubMed] [Google Scholar]
- 10.Grontved A., Ried-Larsen M., Moller N.C. Muscle strength in youth and cardiovascular risk in young adulthood (the European Youth Heart Study) Br J Sports Med. 2015;49(2):90–94. doi: 10.1136/bjsports-2012-091907. [DOI] [PubMed] [Google Scholar]
- 11.Steene-Johannessen J., Anderssen S.A., Kolle E. Low muscle fitness is associated with metabolic risk in youth. Med Sci Sports Exerc. 2009;41(7):1361–1367. doi: 10.1249/MSS.0b013e31819aaae5. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt M.D., Magnussen C.G., Rees E. Childhood fitness reduces the long-term cardiometabolic risks associated with childhood obesity. Int J Obes. 2016;40(7):1134–1140. doi: 10.1038/ijo.2016.61. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization . WHO Press; Geneva: 2010. Global Recommendations on Physical Activity for Health. [PubMed] [Google Scholar]
- 14.Department of Health & Human Services Physical activity guidelines advisory committee. Phys Act Guid Advis Comm Sci Rep. 2018:779. 2018. [Google Scholar]
- 15.García-Hermoso A., Ramírez-Campillo R., Izquierdo M. Is muscular fitness associated with future health benefits in children and adolescents? A systematic review and meta-analysis of longitudinal studies. Sports Med. 2019;49(7):1079–1094. doi: 10.1007/s40279-019-01098-6. [DOI] [PubMed] [Google Scholar]
- 16.Mintjens S., Menting M.D., Daams J.G. Cardiorespiratory fitness in childhood and adolescence affects future cardiovascular risk factors: a systematic review of longitudinal studies. Sports Med. 2018;48(11):2577–2605. doi: 10.1007/s40279-018-0974-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plowman S.A., Meredith M.D. fourth ed. The Cooper Institute; 2013. Fitnessgram/Activitygram Reference Guide. Dallas, TX. [Google Scholar]
- 18.Burns R.D., Hannon J.C., Brusseau T.A. Cross-validation of aerobic capacity prediction models in adolescents. Pediatr Exerc Sci. 2015;27(3):404–411. doi: 10.1123/pes.2014-0175. [DOI] [PubMed] [Google Scholar]
- 19.Kowalski K.C., Crocker P.R.E., Kowalski N.P. Convergent validity of the physical activity Questionnaire for adolescents. Pediatr Exerc Sci. 1997;9(4):342–352. [Google Scholar]
- 20.Guedes D.P., Guedes J.E.R.P. Medida da atividade física em jovens brasileiros: reprodutibilidade e validade do PAQ-C e do PAQ-A. Rev Bras Med do Esporte. 2015;21(6):425–432. [Google Scholar]
- 21.Moore S.A., McKay H.A., Macdonald H. Enhancing a somatic maturity prediction model. Med Sci Sports Exerc. 2015;47(8):1755–1764. doi: 10.1249/MSS.0000000000000588. [DOI] [PubMed] [Google Scholar]
- 22.Stabelini Neto A., de Campos W., dos Santos G.C. Metabolic syndrome risk score and time expended in moderate to vigorous physical activity in adolescents. BMC Pediatr. 2014;14(1):1–6. doi: 10.1186/1471-2431-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martínez-Vizcaino V., Ortega F.B., Solera-Martínez M. Stability of the factorial structure of metabolic syndrome from childhood to adolescence: a 6-year follow-up study. Cardiovasc Diabetol. 2011;10(1):81. doi: 10.1186/1475-2840-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castro-Piñero J., Laurson K.R., Artero E.G. Muscle strength field-based tests to identify European adolescents at risk of metabolic syndrome: the HELENA study. J Sci Med Sport. 2019;22(8):929–934. doi: 10.1016/j.jsams.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Artero E.G., Ruiz J.R., Ortega F.B. Muscular and cardiorespiratory fitness are independently associated with metabolic risk in adolescents: the HELENA study. Pediatr Diabetes. 2011;12(8):704–712. doi: 10.1111/j.1399-5448.2011.00769.x. [DOI] [PubMed] [Google Scholar]
- 26.Knaeps S., Bourgois J.G., Charlier R. Ten-year change in sedentary behaviour, moderate-To-vigorous physical activity, cardiorespiratory fitness and cardiometabolic risk: independent associations and mediation analysis. Br J Sports Med. 2018;52(16):1063–1068. doi: 10.1136/bjsports-2016-096083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen D.D., Gómez-Arbeláez D., Camacho P.A. Low muscle strength is associated with metabolic risk factors in Colombian children: the ACFIES study. PloS One. 2014;9(4):1–10. doi: 10.1371/journal.pone.0093150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson M.D., Saltarelli W.A., Visich P.S. Strenǵth capacity and cardiometabolic risk clustering in adolescents. Pediatrics. 2014;133(4):896–903. doi: 10.1542/peds.2013-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Artero E.G., Lee D., Lavie C.J. Effects of muscular strength on cardiovascular risk factors and prognosis. J Cardiopulm Rehabil. 2012;32(6):351–358. doi: 10.1097/HCR.0b013e3182642688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruiz J.R., Cavero-Redondo I., Ortega F.B. Cardiorespiratory fitness cut points to avoid cardiovascular disease risk in children and adolescents; What level of fitness should raise a red flag? A systematic review and meta-analysis. Br J Sports Med. 2016;50(23):1451–1458. doi: 10.1136/bjsports-2015-095903. [DOI] [PubMed] [Google Scholar]
- 31.Fraser B.J., Blizzard L., Schmidt M.D. Childhood cardiorespiratory fitness, muscular fitness and adult measures of glucose homeostasis. J Sci Med Sport. 2018;21(9):935–940. doi: 10.1016/j.jsams.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Buscot M.J., Thomson R.J., Juonala M. Distinct child-to-adult body mass index trajectories are associated with different levels of adult cardiometabolic risk. Eur Heart J. 2018;39(24):2263–2270. doi: 10.1093/eurheartj/ehy161. [DOI] [PubMed] [Google Scholar]
- 33.Petkeviciene J., Klumbiene J., Kriaucioniene V. Anthropometric measurements in childhood and prediction of cardiovascular risk factors in adulthood: kaunas cardiovascular risk cohort study. BMC Publ Health. 2015;15(1):1–8. doi: 10.1186/s12889-015-1528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.American College of Sports Medicine . third ed. 2011. Manual do ACSM para avaliação da aptidão física relacionada à saúde. Rio de Janeiro. [Google Scholar]
- 35.Nuzzo J.L. The case for retiring flexibility as a major component of physical fitness. Sports Med. 2020;50(5):853-870. doi: 10.1007/s40279-019-01248-w. [DOI] [PubMed] [Google Scholar]
- 36.Lima T.R., Silva D.A.S. Clusters of negative health-related physical fitness indicators and associated factors in adolescents. Rev Bras Cineantropometr Desempenho Hum. 2017;19(4):436–449. [Google Scholar]
- 37.Lopes L., Póvoas S., Mota J. Flexibility is associated with motor competence in schoolchildren. Scand J Med Sci Sports. 2017;27(12):1806–1813. doi: 10.1111/sms.12789. [DOI] [PubMed] [Google Scholar]
- 38.Bouchard C., Rankinen T. Individual differences in response to regular physical activity. Med Sci Sports Exerc. 2001;33(6 SUPPL):446–451. doi: 10.1097/00005768-200106001-00013. [DOI] [PubMed] [Google Scholar]
- 39.Marques A., Santos R., Ekelund U. Association between physical activity, sedentary time, and healthy fitness in youth. Med Sci Sports Exerc. 2014;47(3):575–580. doi: 10.1249/MSS.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 40.Morrow J.R., Tucker J.S., Jackson A.W. Meeting physical activity guidelines and health-related fitness in youth. Am J Prev Med. 2013;44(5):439–444. doi: 10.1016/j.amepre.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 41.Collings P.J., Westgate K., Väistö J. Cross-sectional associations of objectively-measured physical activity and sedentary time with body composition and cardiorespiratory fitness in mid-childhood: the PANIC Study. Sports Med. 2017;47(4):769–780. doi: 10.1007/s40279-016-0606-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith J.J., Eather N., Weaver R.G. Behavioral correlates of muscular fitness in children and adolescents: a systematic review. Sports Med. 2019;49(6):887–904. doi: 10.1007/s40279-019-01089-7. [DOI] [PubMed] [Google Scholar]
- 43.Ramires V.V., Dumith S.C., Gonçalves H. Longitudinal association between physical activity and body fat during adolescence: a systematic review. J Phys Activ Health. 2015;12(9):1344–1358. doi: 10.1123/jpah.2014-0222. [DOI] [PubMed] [Google Scholar]
- 44.van der Velde J.H.P.M., Schaper N.C., Stehouwer C.D.A. Which is more important for cardiometabolic health: sedentary time, higher intensity physical activity or cardiorespiratory fitness? The Maastricht Study. Diabetologia. 2018;61(12):2561–2569. doi: 10.1007/s00125-018-4719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]