Highlights
-
•
We study frontal theta ERS and posterior theta lateralization in ADHD children.
-
•
ADHD children show increased frontal theta ERS and posterior theta lateralization.
-
•
Midfrontal theta ERS connects with right posterior theta modulation in ADHD children.
-
•
Right posterior theta modulation is linked with RT variability in ADHD children.
Keywords: ADHD, Children, Attention, Lateralization, Electroencephalography, Theta oscillations
Abstract
Previous studies have found that theta activities exhibit posterior lateralized modulation as well as midfrontal event-related synchronization (ERS) during covert visual attention in adults. The present study investigated whether these theta modulations existed in children and whether they were associated with attentional problems in attention-deficit/hyperactivity disorder (ADHD). Electroencephalography signals were recorded from typically developing (TD) children and children with ADHD (TD: n = 24; ADHD: n = 22) while they performed a cued covert visual attention task. The participants responded to a target following a cue designed as human eyes that gazed to the left or right visual field (70% validity). Compared with the TD children, the children with ADHD showed increased midfrontal theta ERS and significant posterior theta lateralization in response to the cues. More importantly, we found that the stronger posterior theta lateralization in the right hemisphere exhibited a positive trial-based correlation with the larger midfrontal theta ERS and predicted lower RT variability at the trial level in the children with ADHD. We suggest that ADHD may be associated with some enhanced systems in the frontal and posterior areas via theta oscillations, which may be involved in the compensatory maturation for their attention deficits in childhood, thereby promoting the stability of behavioral responses.
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD), a prevalent neurodevelopmental disorder in childhood, is characterized by an inappropriate pattern of inattentiveness, hyperactivity and/or impulsivity (American Psychiatric Association, 2013). The problems in directing and sustaining attention are among the most marked symptoms of ADHD. A recent electroencephalography (EEG) study indicated that the event-related potential (ERP) component related to target selection was delayed in adults with ADHD (Cross-Villasana et al., 2015). In children with ADHD, the ERP components related to target selection and distractor suppression were inhibited in a visual search task (Wang et al., 2016). In addition, the posterior alpha modulation was attenuated in covert spatial attention in both adults (ter Huurne et al., 2013) and children (Vollebregt et al., 2016) with ADHD. When attention was directed by social cues, the weakened alpha modulation in children with ADHD was mainly manifested in the left hemisphere and that was correlated with inattentive symptoms (Guo et al., 2019). Moreover, ADHD adults have been reported to have an attenuated modulation in the mu rhythm while engaged in the task response (ter Huurne et al., 2017). All of these EEG/ERP studies imply the occurrence of spatial attention impairments in ADHD.
In the spectrum studies, the theta oscillations are acknowledged to be associated with wide span of cognitive functions and visual attention. The theta activities in the midfrontal area reflect a neural mechanism of cognitive control (Cavanagh and Frank, 2014) and have been closely related to conflict monitoring (Nigbur et al., 2011, Töllner et al., 2017), error detection (Luu et al., 2004, van Driel et al., 2012) and mental vigilance (Wascher et al., 2014, Arnau et al., 2017) in the attention process. They also play a crucial role in cognitive processes, such as visual working memory (Onton et al., 2005, de Vries et al., 2018). The midfrontal theta activity is considered to reflect the role of prefrontal control networks involved in flexible adaptation of behavioral performance (Cohen and van Gaal, 2012, Jiang et al., 2018). In the ADHD domain, children with ADHD showed a greater elevated theta power difference between the task state and the resting state than typically developing (TD) children (Mann et al., 1992). In addition, the theta event-related synchronization (ERS) in children with ADHD was more increased than that in TD children during visual working memory tasks (Lenartowicz et al., 2014).
In addition to the frontal theta power, the theta power in the posterior area also increased after target presentation (Bastiaansen et al., 2002, Yamagishi et al., 2003). During covert visual attention, the posterior theta power is more elevated over the contralateral hemisphere to the attended hemifield than that of the ipsilateral hemisphere in normal adults (Kawasaki and Yamaguchi, 2012, Diao et al., 2017). Moreover, this type of theta lateralization could be affected by several emotional factors, such as subjective preference (Kawasaki and Yamaguchi, 2012) and valence of emotional stimuli (Diao et al., 2017). With regard to goal-directed attention, a recent study has reported similar theta lateralization in response to a nonpredictive exogenous cue, in which the theta power contralateral to the cue-directed hemifield was more elevated than the other hemisphere, and the theta lateralization was modulated by features of the cues related to the visual task in normal adults (Harris et al., 2017).
In the field of covert visual attention, the human eye gaze is a special type of cue stimuli. Some studies have indicated that eye gaze cues triggered more strongly reflexive attention than arrow cues and generated later inhibition of return than exogenous cues (Friesen et al., 2004, Frischen et al., 2007). In addition, humans can generate sensitivity to the direction of human eye gaze in the early infant years (Farroni et al., 2002, Vernetti et al., 2018). The effect of human eye gaze on infant attention can be effectively reflected by EEG/ERP responses (Farroni et al., 2004, Hoehl et al., 2008, Hoehl et al., 2014, Michel et al., 2015). Behavioral studies have indicated that children with ADHD showed significant differences in the orienting effects directed by human eye gaze cues from TD children, but this effect was absent for arrow and peripheral cues (Marotta et al., 2014, Marotta et al., 2017). These results indicated that human eye gaze cue is more suitable for the research on child visual attention. It has stronger indicative effect on children with ADHD than arrow and peripheral cues. Therefore, we selected human eye gaze as the cue stimulus in the present study.
Although the frontal theta activity and posterior theta lateralization modulation have been proven to be robust phenomena, the occurrence of posterior theta modulation in children and the relationship between the frontal theta activity and posterior theta modulation have yet to be determined. By using a covert visual attention task directed by a cue of human eye gaze, the present study aimed to investigate (1) whether midfrontal theta ERS and posterior theta lateralization were significantly modulated in children and (2) whether a significant difference in midfrontal theta ERS or posterior theta lateralization was found between TD children and children with ADHD. If so, we would explore (3) whether there is a relationship between the midfrontal theta ERS and the posterior theta modulation. We performed the trial-based correlation analyses to verify the relationship because the trial-level correlation reflects the dynamic functional connection between two features and is not affected by the distribution of these features in the population.
2. Material and methods
Some data used for analysis have been previously published, in which alpha oscillation in children with ADHD was reported (Guo et al., 2019). The study was approved by the ethics committee of Beijing Normal University in accordance with the Declaration of Helsinki. Written consent and verbal assent were obtained from all of the children’s parents.
2.1. Participants
Forty-six children, 8–13 years of age, were enrolled in the study (TD: n = 24; ADHD: n = 22). The children with ADHD were drug-naive and assessed by professional psychologists based on the DSM-IV criteria; TD children were determined to be free from ADHD using the ADHD DSM-IV Rating Scale completed by their parents. We used the Wechsler Intelligence Scale (WISC-III) or the Raven Standard Reasoning Test (RSRT) to estimate the intelligence quotient (IQ). All children were right-handed, had normal or corrected-to-normal vision, normal IQ (>80 for WISC-III, > 25% for RSRT), no history of head trauma with a loss of consciousness, no history of neurological illness, and no current diagnosis of schizophrenia, severe major depression, clinically significant panic disorder, bipolar disorder, or pervasive developmental disorders. The TD and ADHD groups were matched by age, sex ratio, and IQ (Table 1). Data from 18 additional participants (TD: n = 8; ADHD: n = 10) were excluded from analysis due to poor behavioral performance (accuracy < 75%) or excessive artifacts (rejected trials > 60%).
Table 1.
Demographic information of subjects in the final sample.
| TD | ADHD | Statistics | |
|---|---|---|---|
| Age (years) | 10.10 ± 1.03 | 10.18 ± 1.34 | t = 0.814, ns |
| Sex (boys, girls) | 15, 9 | 18, 4 | χ2 = 1.267, ns |
| WISC-III (n) | 107.14 ± 14.52 (7) | 103.55 ± 14.77 (11) | t = 0.507, ns |
| RSRT percentiles (n) | 85.06 ± 10.13 (17) | 78.55 ± 15.78 (11) | t = 1.335, ns |
| Inattention Score | 16.63 ± 3.35 | 27.09 ± 3.80 | t = -9.926, p < .001 |
| Hyperactivity Score | 14.33 ± 4.08 | 21.27 ± 6.36 | t = -4.444, p < .001 |
Values are the mean ± SD, unless otherwise indicated; the value of χ2 is corrected.
ADHD, attention-deficit/hyperactivity disorder; TD, typically developing; ns, not significant.
2.2. Attention task
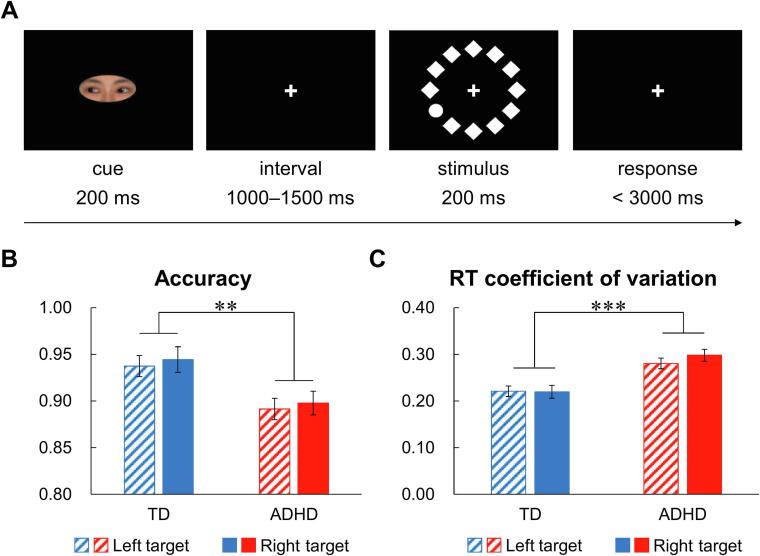
We used a modified Posner’s cueing paradigm (Posner, 1980) in the task. The presentation of each trial was shown as Fig. 1A. The trial was started with a cue presented for 200 ms, which was designed as a pair of human eyes that could gaze towards the left or right hemifield with equal probabilities (with a width of 2° visual angle). The direction of the gaze was predictive for the side where the following target would appear (with 70% validity). After a jittered interstimulus interval from 1000 to 1500 ms, a visual search array was presented for 200 ms, which was surrounded by a circle target and 11 diamonds with a visual angle of 5° from the center. The target might appear at one of four locations, i.e., in the 2, 4, 8 or 10 o’clock position. Participants were instructed to maintain their gaze at the center of the screen and indicate whether the target appeared in the upper or lower visual field. They made the response by pressing one of two buttons arranged at 90° to the screen with right middle or index finger. The intertrial intervals were jittered from 1000 ms to 1200 ms. The experiment consisted of 12 blocks of 30 trials.
Fig. 1.
Experimental paradigm and behavioral results. A) Experimental paradigm. B) Statistical analysis results of accuracy. C) Statistical analysis results of the RT coefficient of variation. **p < .01; ***p < .001.
2.3. EEG recording
A 128-channel EEG system (HydroCel Geodesic Sensor Net, Electrical Geodesics, Inc., Eugene, OR) was used to record the brain activities with Cz as the online reference. During data acquisition, the impedance of all electrodes was kept below 50 kΩ. The EEG data were digitized at 1000 Hz and bandpass filtered at 0.01–400 Hz.
2.4. Data preprocessing
We used the EEGLAB software package in the MATLAB environment for EEG processing (Delorme and Makeig, 2004). Because signals from the boundary electrodes were heavily susceptible to face and head movements, data from the 34 outermost electrodes were excluded from the analysis (Supplementary Fig. S1). This method was also used in one recent study (Debnath et al., 2019). The EEG data were resampled to 200 Hz and bandpass filtered at 1–30 Hz. After interpolating the bad electrodes (<10% for each subject), EEG data were referenced to the average of all electrodes.
For each trial, epochs from −200–1200 ms around the cue onset were extracted. Epochs containing excessive eye movements were removed using the step function in the ERPLAB toolbox (Lopez-Calderon and Luck, 2014). The horizontal gaze shifts were detected from the horizontal electrooculogram signal (difference between F9 and F10) from −50 to 750 ms with a window of 100 ms, a step of 50 ms, and a threshold of 50 μV. Eye blinks were detected from the vertical electrooculogram signal (average of Fp1 and Fp2) from −100 to 300 ms with a window of 200 ms, a step of 100 ms and a threshold of 75 μV. Then, components with electrooculographic origins were removed after an independent component analysis (Jung et al., 2000, Delorme and Makeig, 2004). We rejected epochs with overt artifacts with a semiautomatic method. A voltage threshold function was used to examine the absolute voltage values in all channels from −300 to 700 ms with a threshold of 100 μV.
2.5. EEG spectral analysis
We used custom-written MATLAB scripts and the FieldTrip software package to perform the spectral analysis (Oostenveld et al., 2011). To exclude the influence of phase-locked activities on the oscillatory analysis, the trial-averaged activities were subtracted from the signals in each trial (Yeung et al., 2007, van Driel et al., 2017). The time–frequency analysis was performed using the continuous wavelet transformation. The data in each epoch were convolved with a set of Morlet wavelets using linearly spaced 3–5 cycles of 3–8 Hz windows with a step of 0.5 Hz and a time resolution of 10 ms. The time interval was from −300 to 700 ms around the cue onset.
For the frontal area, data from the left and right cue epochs were averaged for analysis. We selected two electrodes (Fz and Afz) for spectral analysis because they showed the strongest theta power in the frontal area (Supplementary Fig. S2). For each frequency interval, the baseline power was averaged in from −300 to −100 ms relative to the cue onset. ERS was calculated by subtracting the baseline power from the power in each time–frequency interval. The subtraction was subsequently normalized by dividing the baseline power (Pfurtscheller and da Silva, 1999). We used cluster-based permutation tests (2000 iterations) to identify the time–frequency clusters in which the theta ERS was significantly different from zero within the time–frequency range of −300–700 ms and 3–8 Hz (with a threshold of p < .025, two-tailed). This method controls for multiple comparisons by identifying significant time-frequency clusters rather than independent points (Maris and Oostenveld, 2007). The largest significant cluster was used for statistical analyses.
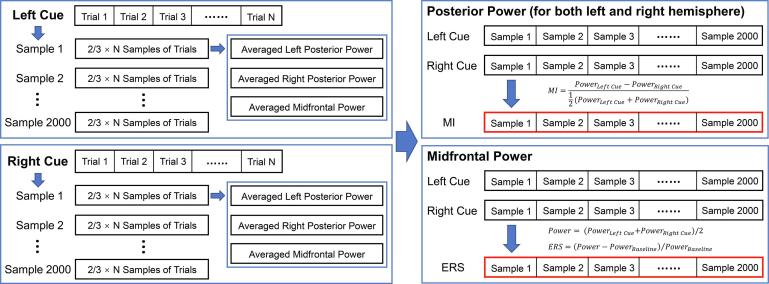
For the posterior area, we constructed the modulation index (MI) for each time–frequency interval to indicate the power change response to different cues. The formula is shown as follows:
In the above formula, Power Left Cue represents the time–frequency power when cue directs to the left visual field, Power Right Cue represents the time–frequency power when cue directs to the right visual field. We selected four electrodes (left hemisphere: 58 (P7) and 50; right hemisphere: 96 (P8) and 101) for analysis. For the left and right hemispheres, a cluster-based permutation test (2000 iterations) within the time–frequency range of −300–700 ms and 3–8 Hz was performed to identify the time–frequency clusters in which the theta MI was significantly different from zero (with a threshold of p < .025, two-tailed). The largest significant cluster was used for statistical analyses.
2.6. Trial-based correlations between midfrontal theta ERS and posterior theta MI
To examine whether a relationship exists between the midfrontal theta ERS and the posterior theta MI, we performed a trial-based correlation. For each participant, we calculated the time–frequency power of each trial for each condition and then performed a 2000-iteration process to construct the data for the correlation tests. In each iteration, the following steps were performed: (1) Two-thirds of the trials were randomly sampled for each condition. (2) The time–frequency power of sampled trials was averaged for each condition, the significant frequency ranges in the permutation tests in section 2.5 were selected for the calculation (midfrontal: 4.5–8 Hz; posterior: 3.5–6.5 Hz). (3) The midfrontal ERS and posterior MIs (left and right) were calculated using the averaged theta power from step 2. Therefore, 2000 iterations yielded 2000 sets of data containing the midfrontal ERS and posterior MIs (left and right). These steps are shown in Fig. 2. For each participant, the Pearson correlation coefficients (midfrontal ERS–left MI and midfrontal ERS–right MI) were computed using the midfrontal theta ERS and posterior theta MIs from the 2000 iterations for each pair of time intervals (midfrontal ERS: −100–700 ms; posterior MIs: −300–700 ms; time resolution: 10 ms). The correlation coefficients were then converted to z values using the Fisher r-z transformation to obtain a normally distributed variable. A cluster-based permutation test was used to detect the time-time clusters in which the correlation coefficients (z value) were significantly different from zero for each correlation in each group (threshold: p < .025, two-tailed). The largest significant cluster was used for the statistical analyses between the two groups.
Fig. 2.
Schematic diagram for calculating the midfrontal theta ERS and posterior theta MIs for trial-based correlation analysis. The data in red boxes indicates the parameters used for trial-based correlation analysis. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.7. Trial-based correlations between theta indexes and behavioral performance
To determine whether the theta indexes (posterior MI and midfrontal ERS) and the behavioral performance (reaction time (RT) and RT coefficient of variability (RTCV)) exhibited a relationship, we performed a trial-based correlation analysis involving only the trials with valid cues and correct responses. For each participant, we calculated the time–frequency power of each trial for each condition, and then performed a 2000-iteration process to construct the data for the calculation of correlation coefficient. In each iteration, the following steps were performed: (1) For each participant, two-thirds of the trials were randomly sampled for each condition. (2) The time–frequency-based theta indexes (posterior MI and midfrontal ERS) of sampled trials were calculated. (3) Behavioral indexes (mean RT and RTCV) of sampled trials were calculated. At each resampling, the RTCV reflects the normalized mean distance between RTs and mean RT in that sample. The Pearson correlation coefficients were then computed between the theta indexes and behavioral indexes from the 2000 iterations for each time–frequency interval. The correlation coefficients were converted to normally distributed values using the Fisher r-z transformation. A cluster-based permutation test was used to detect the time–frequency clusters in which the correlation coefficients (z value) were significantly different from zero for each correlation (threshold: p < .025, two-tailed). The largest significant cluster was used for the statistical analyses between the two groups.
2.8. Behavioral data analysis
Trials with incorrect-responses and trials with RTs longer than 1.5 s were rejected from the calculation of the individual’s mean RT and RTCV. Previous studies indicated that one of the most consistent findings in patients with ADHD is increased RT variability. This feature manifests that RTCV in patients with ADHD is significantly higher than that in normal controls (Vaurio et al., 2009, Antonini et al., 2013, Gonen-Yaacovi et al., 2016). Therefore, to characterize the behavioral variability of children with ADHD, we calculated the RTCV by dividing RT standard deviation by mean RT for each participant. We used RTCV as a measure of RT variability because it provides a normalized measure of intra-subject variability by removing the effect of response speed on the estimate of variability.
2.9. Statistical analysis
For the frontal theta ERS and trial-based correlation coefficients, we used the univariate covariance analysis (ANCOVA) to test the difference in theta ERS between the TD and ADHD groups, with age as a covariate. For the posterior theta MI, a repeated measures analysis of covariance (ANCOVA) was used to compare the theta MI between the two hemispheres (left and right) with the group (TD and ADHD) as a between-subject factor and age as a covariate. A simple effect analysis was performed if the results exhibited an interaction effect.
3. Results
3.1. Behavioral data
We used repeated measures ANCOVA to compare group differences with target location (left vs right) and cue validity (valid vs invalid) as within-subject factors and age as a covariate. The results indicated that the children with ADHD showed a significantly lower accuracy and a higher RTCV than the TD children (accuracy: F(1,43) = 9.062, p = .004, ηp2 = 0.174; RTCV: F(1,43) = 29.889, p < .001, ηp2 = 0.410). The results are shown in Fig. 1B and C. For the mean RT, no significant main effects or interactions were found in the analysis (p > .05).
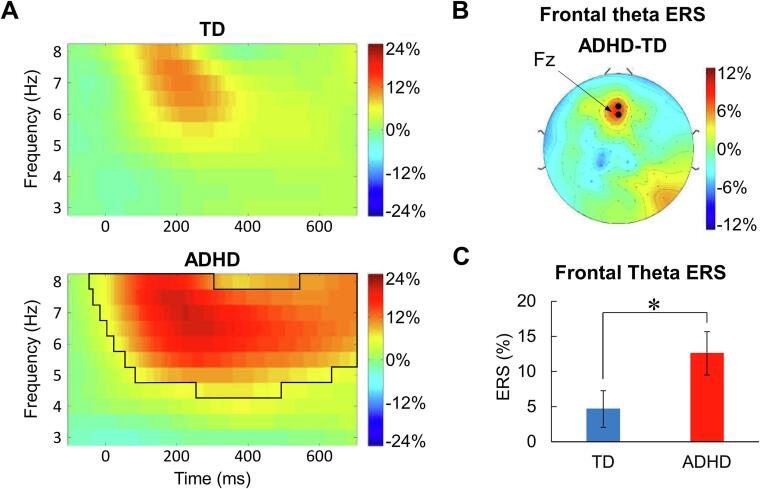
3.2. Theta synchronization in the midfrontal area
As shown in Fig. 3A, the cluster-based permutation test showed a significantly elevated ERS in 4.5–8 Hz in the children with ADHD (p = .001). The topographic map (Supplementary Fig. S2) revealed that two electrodes (11(Fz) and 16(Afz)) showed the strongest theta power in the midfrontal area. Thus, we selected them in the time–frequency cluster for the ANCOVA. The results showed that the theta ERS in the children with ADHD was significantly higher than that in the TD children (F(1,43) = 4.218, p = .046; ηp2 = 0.089), as shown in Fig. 3B and C. More importantly, ANCOVA of the logarithmic baseline theta power (unit: 10 × log10(μV2); −300–-100 ms; 4.5–8 Hz) did not show a significant group differences (F(1,43) = 0.301, p = .586; ηp2 = 0.007), indicating that the group difference in theta ERS could not be attributed to the baseline power difference.
Fig. 3.
The theta ERS in the midfrontal area. A) Time-frequency representation of the theta ERS in the midfrontal area. The black solid line indicates the significant cluster in which ERS is significantly different from zero (p = .001). B) Topographic representation of the difference in frontal theta ERS between TD and ADHD groups. The solid dots indicate the electrodes used for statistical analyses. C) Statistical analysis results of the theta ERS in the midfrontal area between TD and ADHD groups. ns: not significant; *p < .05.
Then, we used a one-sample t-test to compare the difference between the midfrontal theta ERS and zero for each group. Bonferroni correction was implemented to correct for multiple testing in 2 groups (two tailed; adjusted p = .025 = 0.05/2). The results indicated that the midfrontal theta ERS was significantly higher than zero in the children with ADHD but not in the TD children (TD: t = 1.816, p = .082, d = 0.371; ADHD: t = 4.189, p < .001, d = 0.893).
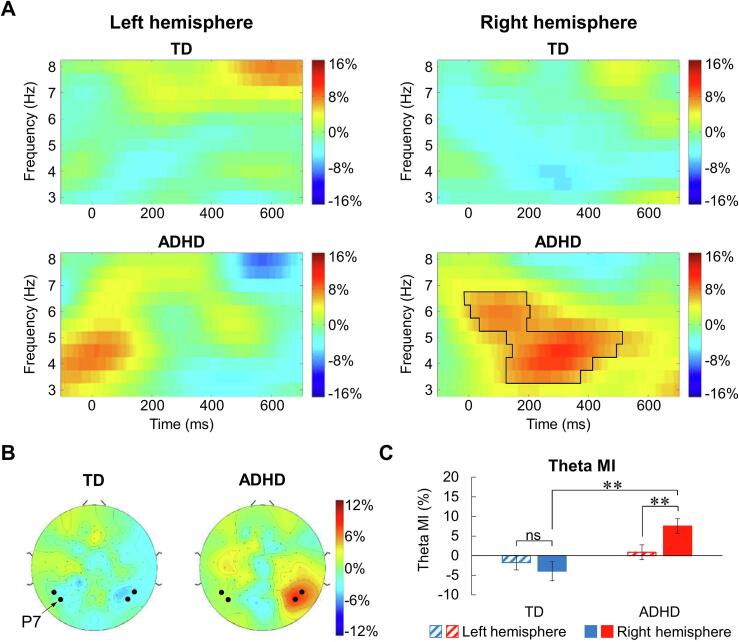
3.3. Theta modulation in the posterior area
As shown in Fig. 4A, the cluster-based permutation test showed a significant theta modulation in the right posterior area in 3.5–6.5 Hz in the children with ADHD (p = .008). The topographic map (Fig. 4B) revealed that the theta lateralization in the children with ADHD was focused on four electrodes (left hemisphere: 50 and 58 (P7); right hemisphere: 96 (P8) and 101). Thus, we selected them in the time–frequency cluster for repeated measures ANCOVA. The results showed a main effect of group (F(1,43) = 10.031, p = .003; ηp2 = 0.189) and a significant interaction of group × hemisphere (F(1,43) = 8.239, p = .006; ηp2 = 0.161). Simple effect analysis further showed that in the children with ADHD, the theta MI in the right hemisphere was significantly higher than that in the left hemisphere (p = .004), whereas this effect was absent in the TD children (p = .351). That is, in the right hemisphere, the theta MI in the children with ADHD was significantly higher than that in the TD children (p < .001). However, the theta MI in the two groups was similar in the left hemisphere (p < .235, Fig. 4C).
Fig. 4.
The theta MI in the posterior area. A) Time-frequency representation of the theta MI in the posterior area. The black solid line indicates the significant cluster in which MI is significantly different from zero (p = .008). B) Topographic representation of the posterior theta ERS. The solid dots indicate the electrodes used for statistical analyses. C) Statistical analysis results of the theta MI in the posterior area between TD and ADHD groups. ns: not significant; **p < .01.
Then, we used one-sample t-tests to compare the difference between the posterior theta MIs and zero for each group. Bonferroni correction was implemented to correct for multiple testing (two tailed; adjusted p = .0125 = 0.05/(2 × 2); repetitions: 2 hemispheres and 2 groups). The results indicated that in the right hemisphere, the posterior theta MI was significantly higher than zero in the children with ADHD but not in the TD children (TD: t = -1.644, p = .114, d = 0.336; ADHD: t = 4.188, p < .001, d = 0.893). In the left hemisphere, neither group of participants showed a significant difference between posterior theta MI and zero (TD: t = 0.971, p = .342, d = 0.198; ADHD: t = 0.466, p = .646, d = 0.099).
3.4. Relationship between midfrontal theta ERS and posterior theta MI
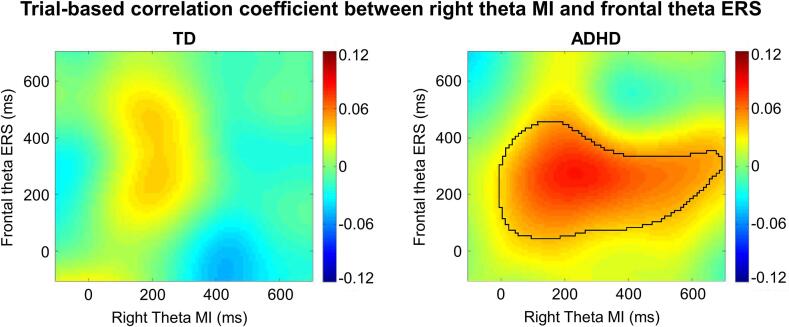
We investigated the association between the midfrontal theta ERS and the posterior theta MIs (left and right) using a trial-based correlation analysis (the waveforms are shown in Supplementary Fig. S3). The results of the permutation test showed a significant time cluster in the midfrontal ERS–right MI correlation (midfrontal ERS: 50–450 ms; right MI: 0–690 ms) in the children with ADHD (p = .010): trials with more elevated frontal theta ERS also showed stronger theta modulation in the right posterior area, but this correlation was absent in the TD children (Fig. 5). Thus, we selected z-values in this cluster for one-sample t-test and ANCOVA. For t-tests, Bonferroni correction was implemented to correct for multiple testing (two-tailed; adjusted p = .0125 = 0.05/(2 × 2); repetitions: 2 correlation coefficients and 2 groups). The results showed that the z-value of midfrontal ERS–right MI correlation was significantly greater than zero in the children with ADHD but not in the TD children (TD: t(44) = 0.722, p = .478, d = 0.147; ADHD: t(44) = 3.379, p = .003, d = 0.720). Compared with the TD children, the children with ADHD showed a significantly higher z-value between midfrontal ERS and right MI (F(1,43) = 6.253, p = .016; ηp2 = 0.127). No significant correlation was found between the midfrontal ERS and the left MI in either the TD or ADHD group (p > . 05).
Fig. 5.
Coefficient of trial-based correlation (Fisher z value) between the midfrontal theta ERS and the right posterior MI in TD (left picture) and ADHD (right picture) children. The black solid line indicates the significant cluster where the coefficient of correlation is significantly different from zero (p = .010).
3.5. Relationship between EEG indexes and behavioral performance
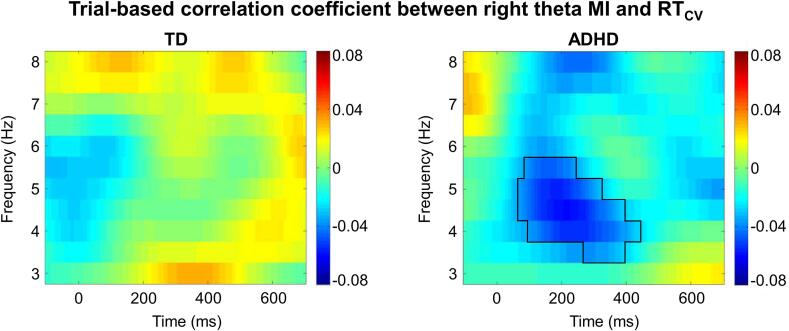
Compared with the TD children, the children with ADHD exhibited poorer behavioral performance and abnormal theta indexes in the midfrontal and right posterior areas. To examine whether a relationship exists between the theta indexes (right posterior MI and midfrontal ERS) and the behavioral performance (RT and RTCV), we performed a trial-based correlation analysis on trials with valid cues and correct responses. The results showed that the right posterior theta MI was negatively correlated with RTCV in a time–frequency cluster (time: 80–450 ms; frequency: 3.5–5.5 Hz) in the children with ADHD (p = .010): trials with stronger right posterior theta modulation showed a lower RT variability, but this correlation was absent in the TD children (Fig. 6). The one-sample t-test results (two-tailed; Bonferroni adjusted p = .0125 = 0.05/(2 × 2); repetitions: 2 correlation coefficients and 2 groups) showed that the z-value of the correlation between the right posterior theta MI and RTCV was significantly lower than zero in the children with ADHD, but not in the TD children (TD: t(44) = -0.257, p = .800, d = 0.053; ADHD: t(44) = -4.393, p < .001, d = 0.936). The ANCOVA results showed that compared with the TD children, the children with ADHD showed a significantly lower z-value between the right posterior theta MI and RTCV in this cluster (F(1,43) = 4.406, p = .042; ηp2 = 0.093).
Fig. 6.
Coefficient of trial-based correlation (Fisher z value) between the right posterior MI and RT variability (RTCV) in TD (left picture) and ADHD (right picture) children. The black solid line indicates the significant cluster where the coefficient of correlation is significantly different from zero (p = .010).
In addition, we also found a marginally negative correlation between the right posterior theta MI and RT in a time–frequency cluster (time: 60–290 ms; frequency: 3.5–7 Hz) in the children with ADHD (p < .05, Supplementary Fig. S4). In this cluster, the z-value of the right posterior theta MI–RT correlation was significantly lower than zero in the children with ADHD but not in the TD children (TD: t(44) = -1.382, p = .180, d = 0.282; ADHD: t(44) = -2.400, p = .026, d = 0.512, uncorrected). No significant difference in z-values was found between the TD and ADHD groups in this cluster (F(1,43) = 1.467, p = .232; ηp2 = 0.033). No other correlation was found between the theta indexes and behavioral performance.
3.6. Relationship between EEG indexes and ADHD symptoms
To verify whether the midfrontal theta ERS and the posterior theta modulation are related to ADHD symptoms in children with ADHD, we performed partial correlation analyses between the theta-band EEG indexes (midfrontal ERS, left posterior MI, right posterior MI and combined MI) and the scores of the ADHD rating scales (inattention subscale, hyperactivity/impulsivity subscale and full scale) with age controlled in the models. However, the results did not show any correlation between the EEG indexes and the symptom scores (ps > 0.297), these results are shown in Table S1 in Supplementary Material.
In addition, we used the partial correlation analyses to verify the relationship between trial-based EEG correlation coefficients (midfrontal ERS–left posterior MI, midfrontal ERS–right posterior MI) and the ADHD rating scores (inattention subscale, hyperactivity/impulsivity subscale and full scale). No significant correlation was found either (ps > 0.443), the results are shown in Table S2 in Supplementary Material.
3.7. Sensitivity analysis
To determine the reliability of the behavioral and EEG effects in this study, we performed power analyses (1-β) on the inter- and intra-group effects of the behavioral and EEG indicators at the level of α = 0.05 (α: probability of type 1 error, two-tailed). 1-β reflects the ability to find a difference in a test at the level of α = 0.05 when there is indeed a difference. The results of inter-group effects in ANCOVA between TD and ADHD groups are shown in Table S3 and the results of intra-group effects in one-sample t-test in TD and ADHD groups are shown in Table S4 in Supplementary Material. These results indicated that the behavioral and EEG effects found here are relatively reliable.
4. Discussion
In the present study, we investigated whether ADHD in 8- to 13-year-old children is related to theta oscillation dysfunction, as indexed by midfrontal theta ERS and posterior theta lateralization. Analyses of behavioral performance and simultaneous EEG recordings revealed remarkable differences between the ADHD and TD groups. The theta oscillations in the children with ADHD showed excessive elevated midfrontal ERS (4.5–8 Hz) and abnormal posterior modulation in the right hemisphere (3.5–6.5 Hz). More importantly, the larger right posterior theta MI exhibited a positive trial-based correlation with the stronger midfrontal theta ERS and predicted lower RTCV at the trial level in children with ADHD. Our data indicate that the poor covert visual spatial attention in ADHD is at least partly related to the dysfunction of brain theta oscillations.
Behaviorally, we found that 8- to 13-year-old children with ADHD responded with lower accuracy and higher RT variability than TD children. This finding is consistent with previous studies in which children with ADHD show poor behavioral performance in covert spatial attentional tasks (Wang et al., 2016, Wang et al., 2017). For EEGs, we found that the cues of human eyes elicited a more elevated midfrontal theta ERS in the children with ADHD than in the TD children. This pattern is similar to that in a recent study using a visual working memory task (Lenartowicz et al., 2014). We suggested that owing to a lower level of attention arousal and poorer response, the elevated midfrontal theta ERS in children with ADHD is potentially related to an increased effort to complete the task, as best as they possibly could, with better behavioral performance (Wascher et al., 2014). That is, the elevated theta ERS may reflect compensatory cognitive processing in children with ADHD. Research on concurrent EEG-fMRI in humans and invasive recordings in monkeys has indicated that frontal theta activities are generated by the midcingulate cortex (Debener et al., 2005, Womelsdorf et al., 2010), which plays a critical role in attention processing, such as error monitoring and conflict control (Braver et al., 2001, Margulies et al., 2007). The highly elevated frontal theta ERS may reflect hypofunction in the midcingulate cortex in those with ADHD (Bush et al., 1999, Bush, 2011).
Using a central-cue paradigm, we further found significant posterior theta lateralization in the children with ADHD during covert visual attention. That is, the posterior theta modulation in the children with ADHD revealed a stronger synchronization associated with the contralaterally directed cue in the right hemisphere. The pattern of theta lateralization was consistent with previous studies related to the orientation process in visual attention (Kawasaki and Yamaguchi, 2012, Diao et al., 2017, Harris et al., 2017). In the present study, the children with ADHD aged 8–13 years seemed to have developed “adult-like” posterior theta lateralization. Moreover, a negative trial-based correlation was found between right posterior theta modulation and behavioral RT variability in the children with ADHD. The excessive variability in behavior is known to be a typically stable feature in ADHD (Castellanos and Tannock, 2002, Kofler et al., 2013), and has been associated with both symptoms of inattention and hyperactivity/impulsivity (Gómez-Guerrero et al., 2011). We speculate that the generation of “adult-like” theta lateralization in children with ADHD may be closely related to neural maturation during childhood development. Moreover, children with ADHD may prematurely develop theta modulation to compensate for the attention deficits, which promote the stability of their behavioral performance. By using the same experimental task, a recent study indicated that alpha modulation in children with ADHD was heavily attenuated in the left but not in the right hemisphere (Guo et al., 2019). All of these results suggest that there might be an abnormal unilateral advantage in the parieto-occipital area in children with ADHD, which manifested hypoactivity in the left hemisphere as indexed by alpha modulation, as well as hyperactivity in the right hemisphere as indexed by theta modulation.
We found a positive trial-based correlation between midfrontal theta ERS and posterior theta modulation in the children with ADHD; however, this relationship was absent in the TD children. This finding was distinct from previous studies in which the frontal theta oscillations showed weaker functional connections with the posterior alpha oscillations in children and adolescents with ADHD (Mazaheri et al., 2010, Mazaheri et al., 2014). The current study revealed that the functional connection between midfrontal attention control and posterior perception regions could be enhanced in children with ADHD in some conditions and was modulated by theta oscillations. We speculated that the theta connection reflects a cooperation of multiple brain networks between frontal control and posterior sensory regions in the compensatory processes in children with ADHD. This finding implicated the presence of some excrescent neurological connections between these structures in ADHD, which should be verified by future research.
It is worth noting that the cues used in this paradigm were designed as human eye gaze. It contains social meaning in addition to the directional information. As it has been shown that the theta oscillations in the frontal and posterior areas can be regulated by some emotional factors (van Steenbergen et al., 2012, Diao et al., 2017), in addition to the findings that children with ADHD have lower abilities in modulating attention allocation and cognitive control in emotionally laden situations (Lugo-Candelas et al., 2017), further study is needed to distinguish whether these theta effects in the present study are influenced by the social meaning of the human eye gaze cues.
In summary, although we did not find a significant correlation between the theta-band EEG indexes and the clinical severity of ADHD in our research, these EEG indexes can still be used as potential features to distinguish children with ADHD from TD children. We are eager to investigate whether these theta oscillation features still exist in adolescents and adults with ADHD in further studies. Furthermore, it is also important to investigate the accurate brain regions involved in theta oscillation modulation. We hope that the present study may contribute to providing an integrative framework for the neural mechanism of ADHD and, prospectively, to developing neural markers for this heterogeneous psychiatric disease.
5. Conclusions
The present study provides a novel evidence that children with ADHD show overelevated midfrontal theta ERS and abnormal posterior theta modulation. The elevated theta ERS may reflect a lower level of attention arousal and compensatory cognitive processing related to the poorer behavioral performance in children with ADHD. The “adult-like” posterior theta modulation observed in children with ADHD may reflect compensatory maturation for attention deficits that promotes the stability of their behavioral performance. Excrescent neurological connections may be present in children with ADHD, as reflected by the significant connection between midfrontal theta ERS and right posterior theta modulations. Our finding sheds new light on a more comprehensive description of the developmental attentional deficits in ADHD that are related to the nature of brain theta oscillations.
Author contributions
Y. S. designed the experiment; J. G. programmed the experiment; J. G., X. L., B. L., Q. C. conducted the experiment; L. S. conducted subject evaluations; J. G. analyzed the data; J. G., Y. S. wrote the manuscript.
CRediT authorship contribution statement
Jialiang Guo: Methodology, Investigation, Software, Formal analysis, Writing - original draft, Visualization. Xiangsheng Luo: Investigation, Data curation. Bingkun Li: Investigation, Validation. Qinyuan Chang: Investigation, Validation. Li Sun: Project administration, Resources, Funding acquisition. Yan Song: Conceptualization, Supervision, Funding acquisition, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The present research was supported by the National Natural Science Foundation of China (No. 31871099, No. 61761166003, No. 81771479 and No. 81971284), the National Defense Basic Scientific Research Program of China (2018110B011) and the Beijing Brain Initiative of Beijing Municipal Science and Technology Commission (Z181100001518003).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102314.
Contributor Information
Li Sun, Email: sunlioh@bjmu.edu.cn.
Yan Song, Email: songyan@bnu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- American Psychiatric Association . fifth ed. American Psychiatric Publishing; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Antonini T.N., Narad M.E., Langberg J.M., Epstein J.N. Behavioral correlates of reaction time variability in children with and without ADHD. Neuropsychology. 2013;27(2):201–209. doi: 10.1037/a0032071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnau S., Möckel T., Rinkenauer G., Wascher E. The interconnection of mental fatigue and aging: an EEG study. Int. J. Psychophysiol. 2017;117:17–25. doi: 10.1016/j.ijpsycho.2017.04.003. [DOI] [PubMed] [Google Scholar]
- Bastiaansen M.C., Posthuma D., Groot P.F., de Geus E.J. Event-related alpha and theta responses in a visuo-spatial working memory task. Clin. Neurophysiol. 2002;113(12):1882–1893. doi: 10.1016/S1388-2457(02)00303-6. [DOI] [PubMed] [Google Scholar]
- Braver T.S., Barch D.M., Gray J.R., Molfese D.L., Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb. Cortex. 2001;11(9):825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Bush G. Cingulate, frontal, and parietal cortical dysfunction in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2011;69(12):1160–1167. doi: 10.1016/j.biopsych.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G., Frazier J.A., Rauch S.L., Seidman L.J., Whalen P.J., Jenike M.A., Rosen B.R., Biederman J. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the counting stroop. Biol. Psychiatry. 1999;45(12):1542–1552. doi: 10.1016/S0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- Castellanos F.X., Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat. Rev. Neurosci. 2002;3(8):617. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Cavanagh J.F., Frank M.J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 2014;18(8):414–421. doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M.X., van Gaal S. Dynamic interactions between large-scale brain networks predict behavioral adaptation after perceptual errors. Cereb. Cortex. 2012;23(5):1061–1072. doi: 10.1093/cercor/bhs069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross-Villasana F., Finke K., Hennig-Fast K., Kilian B., Wiegand I., Muller H.J., Tollner T. The speed of visual attention and motor-response decisions in adult attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2015;78(2):107–115. doi: 10.1016/j.biopsych.2015.01.016. [DOI] [PubMed] [Google Scholar]
- de Vries I.E., van Driel J., Karacaoglu M., Olivers C.N. Priority switches in visual working memory are supported by frontal delta and posterior alpha interactions. Cereb. Cortex. 2018;28(11):4090–4104. doi: 10.1093/cercor/bhy223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debener S., Ullsperger M., Siegel M., Fiehler K., Von Cramon D.Y., Engel A.K. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J. Neurosci. 2005;25(50):11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath R., Salo V.C., Buzzell G.A., Yoo K.H., Fox N.A. Mu rhythm desynchronization is specific to action execution and observation: Evidence from time-frequency and connectivity analysis. Neuroimage. 2019;184:496–507. doi: 10.1016/j.neuroimage.2018.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Diao L., Qi S., Xu M., Fan L., Yang D. Electroencephalographic theta oscillatory dynamics reveal attentional bias to angry faces. Neurosci. Lett. 2017;656:31–36. doi: 10.1016/j.neulet.2017.06.047. [DOI] [PubMed] [Google Scholar]
- Farroni T., Csibra G., Simion G., Johnson M.H. Eye contact detection in humans from birth. Proc. Natl. Acad. Sci. 2002;99(14):9602–9605. doi: 10.1073/pnas.152159999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farroni T., Johnson M.H., Csibra G. Mechanisms of eye gaze perception during infancy. J. Cognit. Neurosci. 2004;16(8):1320–1326. doi: 10.1162/0898929042304787. [DOI] [PubMed] [Google Scholar]
- Friesen C.K., Ristic J., Kingstone A. Attentional effects of counterpredictive gaze and arrow cues. J. Exp. Psychol. Hum. Percept. Perform. 2004;30(2):319–329. doi: 10.1037/0096-1523.30.2.319. [DOI] [PubMed] [Google Scholar]
- Frischen A., Smilek D., Eastwood J.D., Tipper S.P. Inhibition of return in response to gaze cues: the roles of time course and fixation cue. Visual Cogn. 2007;15:881–895. doi: 10.1080/13506280601112493. [DOI] [Google Scholar]
- Gómez-Guerrero L., Martín C.D., Mairena M.A., Di Martino A., Wang J., Mendelsohn A.L., Dreyer B.P., Isquith P.K., Gioia G., Petkova E., Castellanos F.X. Response-time variability is related to parent ratings of inattention, hyperactivity, and executive function. J. Attention Disord. 2011;15(7):572–582. doi: 10.1177/1087054709356379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonen-Yaacovi G., Arazi A., Shahar N., Karmon A., Haar S., Meiran N., Dinstein I. Increased ongoing neural variability in ADHD. Cortex. 2016;81:50–63. doi: 10.1016/j.cortex.2016.04.010. [DOI] [PubMed] [Google Scholar]
- Guo J.L., Luo X.S., Wang E.C., Li B.K., Chang Q.Y., Sun L., Song Y. Abnormal alpha modulation in response to human eye gaze predicts inattention severity in children with ADHD. Dev. Cogn. Neurosci. 2019;38:100671. doi: 10.1016/j.dcn.2019.100671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A.M., Dux P.E., Jones C.N., Mattingley J.B. Distinct roles of theta and alpha oscillations in the involuntary capture of goal-directed attention. Neuroimage. 2017;152:171–183. doi: 10.1016/j.neuroimage.2017.03.008. [DOI] [PubMed] [Google Scholar]
- Hoehl S., Michel C., Reid V.M., Parise E., Striano T. Eye contact during live social interaction modulates infants’ oscillatory brain activity. Soc. Neurosci. 2014;9(3):300–308. doi: 10.1080/17470919.2014.884982. [DOI] [PubMed] [Google Scholar]
- Hoehl S., Reid V., Mooney J., Striano T. What are you looking at? Infants’ neural processing of an adult’s object-directed eye gaze. Dev. Sci. 2008;11(1):10–16. doi: 10.1111/j.1467-7687.2007.00643.x. [DOI] [PubMed] [Google Scholar]
- Jiang J., Bailey K., Xiao X. Midfrontal theta and posterior parietal alpha band oscillations support conflict resolution in a masked affective priming task. Front. Hum. Neurosci. 2018;12:175. doi: 10.3389/fnhum.2018.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T.P., Makeig S., Humphries C., Lee T.W., McKeown M.J., Iragui V., Sejnowski T.J. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000;37(2):163–178. doi: 10.1111/1469-8986.3720163. [DOI] [PubMed] [Google Scholar]
- Kawasaki M., Yamaguchi Y. Effects of subjective preference of colors on attention-related occipital theta oscillations. Neuroimage. 2012;59(1):808–814. doi: 10.1016/j.neuroimage.2011.07.042. [DOI] [PubMed] [Google Scholar]
- Kofler M.J., Rapport M.D., Sarver D.E., Raiker J.S., Orban S.A., Friedman L.M., Kolomeyer E.G. Reaction time variability in ADHD: a meta-analytic review of 319 studies. Clin. Psychol. Rev. 2013;33(6):795–811. doi: 10.1016/j.cpr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Lenartowicz A., Delorme A., Walshaw P.D., Cho A.L., Bilder R.M., McGough J.J., McCracken J.T., Makeig S., Loo S.K. Electroencephalography correlates of spatial working memory deficits in attention-deficit/hyperactivity disorder: vigilance, encoding, and maintenance. J. Neurosci. 2014;34(4):1171–1182. doi: 10.1523/JNEUROSCI.1765-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Calderon J., Luck S.J. ERPLAB: an open-source toolbox for the analysis of event-related potentials. Front. Hum. Neurosci. 2014;8:213. doi: 10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo-Candelas C., Flegenheimer C., Harvey E., Mcdermott J.M. Neural correlates of emotion reactivity and regulation in young children with ADHD symptoms. J. Abnorm. Child Psychol. 2017;45(7):1311–1324. doi: 10.1007/s10802-017-0297-2. [DOI] [PubMed] [Google Scholar]
- Luu P., Tucker D.M., Makeig S. Frontal midline theta and the error-related negativity: neurophysiological mechanisms of action regulation. Clin. Neurophysiol. 2004;115(8):1821–1835. doi: 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Mann C.A., Lubar J.F., Zimmerman A.W., Miller C.A., Muenchen R.A. Quantitative analysis of EEG in boys with attention-deficit-hyperactivity disorder: controlled study with clinical implications. Pediatr. Neurol. 1992;8(1):30–36. doi: 10.1016/0887-8994(92)90049-5. [DOI] [PubMed] [Google Scholar]
- Margulies D.S., Kelly A.C., Uddin L.Q., Biswal B.B., Castellanos F.X., Milham M.P. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37(2):579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Maris E., Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods. 2007;164(1):177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Marotta A., Casagrande M., Rosa C., Maccari L., Berloco B., Pasini A. Impaired reflexive orienting to social cues in attention deficit hyperactivity disorder. Eur. Child Adolesc. Psychiatry. 2014;23(8):649–657. doi: 10.1007/s00787-013-0505-8. [DOI] [PubMed] [Google Scholar]
- Marotta A., Pasini A., Menotti E., Pasquini A., Pitzianti M.B., Casagrande M. Controlling attention to gaze and arrows in attention deficit hyperactivity disorder. Psychiatry Res. 2017;251:148–154. doi: 10.1016/j.psychres.2017.01.094. [DOI] [PubMed] [Google Scholar]
- Mazaheri A., Coffey-Corina S., Mangun G.R., Bekker E.M., Berry A.S., Corbett B.A. Functional disconnection of frontal cortex and visual cortex in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2010;67(7):617–623. doi: 10.1016/j.biopsych.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Mazaheri A., Fassbender C., Coffey-Corina S., Hartanto T.A., Schweitzer J.B., Mangun G.R. Differential oscillatory electroencephalogram between attention-deficit/hyperactivity disorder subtypes and typically developing adolescents. Biol. Psychiatry. 2014;76(5):422–429. doi: 10.1016/j.biopsych.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel C., Stets M., Parise E., Reid V.M., Striano T., Hoehl S. Theta- and alpha-band EEG activity in response to eye gaze cues in early infancy. Neuroimage. 2015;118:576–583. doi: 10.1016/j.neuroimage.2015.06.042. [DOI] [PubMed] [Google Scholar]
- Nigbur R., Ivanova G., Stürmer B. Theta power as a marker for cognitive interference. Clin. Neurophysiol. 2011;122(11):2185–2194. doi: 10.1016/j.clinph.2011.03.030. [DOI] [PubMed] [Google Scholar]
- Onton J., Delorme A., Makeig S. Frontal midline EEG dynamics during working memory. Neuroimage. 2005;27(2):341–356. doi: 10.1016/j.neuroimage.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Oostenveld R., Fries P., Maris E., Schoffelen J.M. FieldTrip: open source software for advanced analysis of meg, eeg, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011;2011:1–9. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G., da Silva F.L. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin. Neurophysiol. 1999;110(11):1842–1857. doi: 10.1016/S1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Posner M.I. Orienting of attention. Quart. J. Experiment. Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- ter Huurne N., Lozano-Soldevilla D., Onnink M., Kan C., Buitelaar J., Jensen O. Diminished modulation of preparatory sensorimotor mu rhythm predicts attention-deficit/hyperactivity disorder severity. Psychol. Med. 2017;47(11):1947–1956. doi: 10.1017/S0033291717000332. [DOI] [PubMed] [Google Scholar]
- ter Huurne N., Onnink M., Kan C., Franke B., Buitelaar J., Jensen O. Behavioral consequences of aberrant alpha lateralization in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2013;74(3):227–233. doi: 10.1016/j.biopsych.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Töllner T., Wang Y., Makeig S., Müller H.J., Jung T.P., Gramann K. Two independent frontal midline theta oscillations during conflict detection and adaptation in a Simon-type manual reaching task. J. Neurosci. 2017;37(9):2504–2515. doi: 10.1523/JNEUROSCI.1752-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Driel J., Gunseli E., Meeter M., Olivers C.N.L. Local and interregional alpha EEG dynamics dissociate between memory for search and memory for recognition. Neuroimage. 2017;149:114–128. doi: 10.1016/j.neuroimage.2017.01.031. [DOI] [PubMed] [Google Scholar]
- van Driel J., Ridderinkhof K.R., Cohen M.X. Not all errors are alike: theta and alpha EEG dynamics relate to differences in error-processing dynamics. J. Neurosci. 2012;32(47):16795–16806. doi: 10.1523/JNEUROSCI.0802-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steenbergen H., Band G.P., Hommel B. Reward valence modulates conflict-driven attentional adaptation: electrophysiological evidence. Biol. Psychol. 2012;90(3):234–241. doi: 10.1016/j.biopsycho.2012.03.018. [DOI] [PubMed] [Google Scholar]
- Vaurio R.G., Simmonds D.J., Mostofsky S.H. Increased intra-individual reaction time variability in attention-deficit/hyperactivity disorder across response inhibition tasks with different cognitive demands. Neuropsychologia. 2009;47(12):2389–2396. doi: 10.1016/j.neuropsychologia.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernetti A., Ganea N., Tucker L., Charman T., Johnson M.H., Senju A. Infant neural sensitivity to eye gaze depends on early experience of gaze communication. Dev. Cogn. Neurosci. 2018;34:1–6. doi: 10.1016/j.dcn.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollebregt M.A., Zumer J.M., ter Huurne N., Buitelaar J.K., Jensen O. Posterior alpha oscillations reflect attentional problems in boys with attention deficit hyperactivity disorder. Clin. Neurophysiol. 2016;127(5):2182–2191. doi: 10.1016/j.clinph.2016.01.021. [DOI] [PubMed] [Google Scholar]
- Wang E.C., Sun L., Sun M.R., Huang J., Tao Y., Zhao X.X., Wu Z.L., Ding Y.L., Newman D.P., Bellgrove M.A., Wang Y.F., Song Y. Attentional selection and suppression in children with attention-deficit/hyperactivity disorder. Biol. Psychiatry: Cogn. Neurosci. Neuroimag. 2016;1(4):372–380. doi: 10.1016/j.bpsc.2016.01.004. [DOI] [PubMed] [Google Scholar]
- Wang E.C., Sun M.R., Tao Y., Gao X.Y., Guo J.L., Zhao C.G., Li H., Qian Q.J., Wu Z.L., Wang Y.F., Sun L., Song Y. Attentional selection predicts rapid automatized naming ability in Chinese-speaking children with ADHD. Sci. Rep. 2017;7:939. doi: 10.1038/s41598-017-01075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wascher E., Rasch B., Sanger J., Hoffmann S., Schneider D., Rinkenauer G., Heuer H., Gutberlet I. Frontal theta activity reflects distinct aspects of mental fatigue. Biol. Psychol. 2014;96:57–65. doi: 10.1016/j.biopsycho.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T., Johnston K., Vinck M., Everling S. Theta-activity in anterior cingulate cortex predicts task rules and their adjustments following errors. Proc. Natl. Acad. Sci. 2010;107(11):5248–5253. doi: 10.1073/pnas.0906194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi N., Callan D.E., Goda N., Anderson S.J., Yoshida Y., Kawato M. Attentional modulation of oscillatory activity in human visual cortex. Neuroimage. 2003;20(1):98–113. doi: 10.1016/s1053-8119(03)00341-0. [DOI] [PubMed] [Google Scholar]
- Yeung N., Bogacz R., Holroyd C.B., Nieuwenhuis S., Cohen J.D. Theta phase resetting and the error-related negativity. Psychophysiology. 2007;44(1):39–49. doi: 10.1111/j.1469-8986.2006.00482.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.