Abstract
Low-level tragus stimulation (LLTS) is a non-invasive approach of transcutaneous vagus nerve stimulation. LLTS has applications in diseases of multiple systems, including epilepsy, depression, headache and potentially several cardiovascular diseases. LLTS has shown promising results in suppressing AF, alleviating post-MI ventricular arrhythmias and ischaemia-reperfusion injury along with improving diastolic parameters in heart failure with preserved left ventricular ejection fraction (HFpEF). Preliminary pilot clinical studies in patients with paroxysmal AF, HFpEF, heart failure with reduced ejection fraction and acute MI have demonstrated promising results. The beneficial effects are likely secondary to favourable alteration of the sympathovagal imbalance. On-going exploratory work focused on underlying mechanisms of LLTS in cardiovascular disease states and larger scale clinical trials will shed more light on the non-invasive modulation of the neuro-immune axis.
Keywords: Arrhythmia, autonomic nervous system, neuromodulation, tragus
Imbalance of the autonomic nervous system (sympathetic/ parasympathetic) is known to contribute to the pathophysiology of multiple cardiovascular diseases, including AF, MI (and related ventricular arrhythmias) and heart failure. The concept of neuroimmune axis has been proposed to tightly integrate the brain–heart– periphery axis, which is characterised by various pathways of the antiinflammatory properties of the vagus nerve.[1,2] For instance, immune responses can activate the hypothalamic–pituitary–adrenal axis through the vagal afferents and the cholinergic anti-inflammatory pathway through the vago-parasympathetic and sympathetic reflexes.[3]
Modulating this neuro-immune axis could play a key role in the alleviation of several cardiovascular diseases. Several modalities have been developed to modulate this axis and restore a favourable balance between the sympathetic and parasympathetic system.A few examples of this include direct vagus nerve stimulation (VNS), baroreceptor stimulation, renal artery sympathetic denervation, low-level tragus stimulation (LLTS), cardiac sympathetic denervation and spinal cord stimulation.[4] Among them, direct cervical VNS has been used in multiple diseases, including epilepsy, drug-resistant depression, migraine, angina pectoris, hypertension and impaired upper limb function after stroke.[5–11] In patients with heart failure with reduced ejection fraction (HFrEF), three major clinical trials of direct VNS have yielded mixed results.[12–14] One of the major drawbacks of cervical vagus stimulation is the invasive nature of this treatment with inherent surgical complications and poor patient tolerance.[15]
Designed to stimulate the auricular branch of the vagus nerve (ABVN), LLTS is a non-invasive transcutaneous technique of VNS that can modulate the autonomic function (Figure 1).[16] The aim of this current review is to summarise the evidence to date pertaining to the use of LLTS in various cardiovascular disease states.
Figure 1: Low-level Tragus Stimulation.
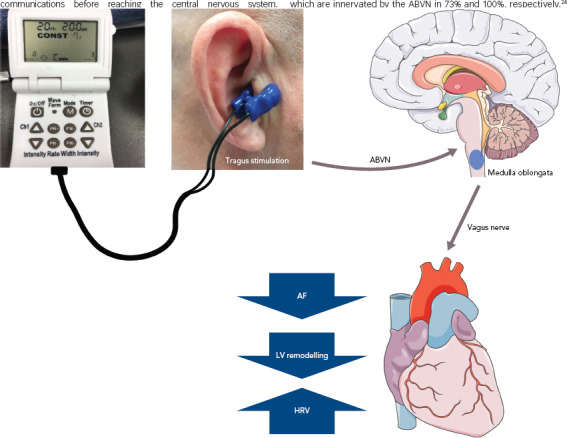
Electric impulses delivered to the tragus stimulate the auricular branch of the vagus nerve (afferent fibres). The excitation then enters the medulla oblongata at the brain stem, which excites the vagus efferent fibres to modulate cardiac function. Low-level tragus stimulation is reported to reduce AF burden, alleviate LV remodelling after MI and increase HRV. ABVN = auricular branch of the vagus nerve; HRV = heart rate variability; LV = left ventricular.
The Anatomical and Physiological Basis of Low-level Tragus Stimulation
Twenty per cent of the vagus nerve fibres are efferent fibres originating from the brainstem, providing parasympathetic control of the viscera, peripheral vasculature and heart. The remaining 80% of the nerve fibres are afferent fibres that relay sensory information from the periphery to the central nervous system. The afferent fibres distribute widely in the heart, lungs, liver, adrenal medulla and the gastrointestinal tract up to the splenic flexure of the colon.[17] The vagus nerve contains three fibre types: highly myelinated A fibres, which have low activation thresholds; lightly myelinated B fibres; and unmyelinated C fibres, which have high activation thresholds.[18,19] The ABVN is the cutaneous branch of the vagus nerve which innervates the antihelix, tragus and cavity of the concha.[20] The innervation of the auricle is shown in Figure 2. Currently, how the external auricle is innervated remains controversial. In an anatomical study, ABVN was able to be observed in 94% of cases (17/18). All of the ABVN were observed to distribute to the external acoustic meatus and auricle. Cutaneous branches from the facial nerve were observed to innervate the external acoustic meatus as well.[21] The nerve branches around the auricle and the acoustic meatus have various complex Nevertheless, ample evidence indicates that the ABVN remains the most important innervation of the external auricle and the cymba conchae.[22]
Figure 2: Innervation of the Auricular Skin.
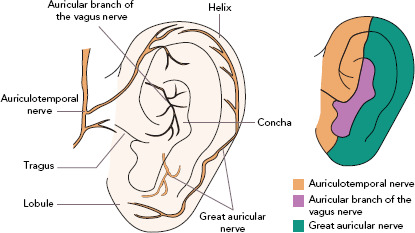
The anterior auricular branches of the auriculotemporal nerve innervates the skin overlying the tragus, as well as the adjacent part of the helix. The auricular branch of the vagus nerve innervates the ear canal, tragus and part of the auricle. The great auricular nerve innervates the skin of the lateral auricle and the skin over both the parotid gland and mastoid process. Source: Roberts 2017.[58] Reproduced with permission from Elsevier.
Brain activation patterns, based on functional MRI (fMRI) during LLTS, were associated with greater activation of the vagal centres (dorsal vagal complex) throughout cortical, subcortical and cerebellar brain regions compared to sham stimulation.[23] In one of the most cited anatomical studies, 14 ears from seven cadavers were examined. A total of 45% of the ears were shown to be innervated by the great auricular nerve (GAN), 9% by the auriculotemporal nerve (ATN) and 46% were innervated by both the GAN and ATN. However, there has been some debate on the optimal site of transcutaneous VNS (tVNS). An fMRI study recently showed that stimulation at the cymba conchae had a greater effect on the vagal pathways and the dorsal vagal complex compared to three other locations: the inner surface of the tragus, the posterior-inferior wall of the external acoustic meatus and the earlobe.[23] A recent study used the triangular fossa innervated by the GAN as the stimulation site, because it allowed stimulation for a longer period of time without interfering with patient comfort, daily hygiene requirements and sleep. Most importantly, it is located proximal to the antihelix and the concha, Stimulating the effective triangular fossa was effective in suppressing postoperative AF in this study.
Parameters of Low-level Tragus Stimulation
Currently, optimal settings of LLTS remain undetermined. Parameters of LLTS for both preclinical and clinical studies have generally been empirical (Tables 1 and 2). In a rat model of heart failure with preserved ejection fraction (HFpEF), LLTS below the threshold of heart rate reduction was applied by placing two oppositely charged magnetic electrodes over the auricular concha region at 20 Hz frequency, 0.2 ms pulse duration and 2 mA amplitude.[25] In a rat MI model, LLTS at 20 Hz, 1 ms pulse wide and 80% below the threshold of slowing the sinus heart rate was used.[26] The same settings were extended to a canine model of post-infarction ventricular arrhythmia.[27] The majority of LLTS studies were right-sided, while one canine model study demonstrated that left-sided LLTS is also effective in suppressing AF.[28]
Table 1: Summary of Preclinical Tragus Stimulation Studies.
| Publication | Subjects | n | Stimulation Parameters | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Frequency (Hz) | Pulse Width (ms) | Amplitude | On and Off Cycle | Side | Duration | Chronic/Acute | ||||
| Wang et al. 2014[59] | MI dogs | 30 | 20 | 1 | 80% below the heart rate slowing threshold | Yes, 5 s on and 5 s off | Bi | 4 h | Daily, 90 days | Attenuated LV remodelling in dogs with healed MI |
| Wang et al. 2015[26] | MI dogs | 22 | 20 | 1 | 80% below the heart rate slowing threshold | Yes, 5 s on and 5 s off | Bi | 4 h | Daily, 6 weeks | Improved cardiac function and attenuated cardiac remodelling in late stages after MI |
| Yu et al. 2016[27] | MI dogs | 22 | 20 | 1 | 80% below the heart rate slowing threshold | Yes, 5 s on and 5 s off | R | 2 h | Daily, 2 months | Reduced ventricular arrhythmia inducibility, LSG neural activity and sympathetic neural remodelling |
| Nasi-Er et al. 2019[41] | MI dogs | 12 | 20 | 1 | 50% below the heart rate slowing threshold | No | Bi | 1 h | Every other day, 4 weeks | Reduced spontaneous ventricular arrhythmias, increased ventricular electrical stability and alleviated ventricular interstitial fibrosis |
| Yu et al. 2017[38] | OSA dogs | 18 | 20 | 1 | 80% below the heart rate slowing threshold | Yes, 5 s on and 5 s off | Left | 1 h | Acute | LLTS suppressed shortening of atrial refractoriness and autonomic remodelling |
| Chen et al. 2015[28] | AF dogs | 32 | 20 | 1 | 80% below the heart rate slowing threshold | No | Left | 9 h | Acute | Left-sided LLTS exerts anti-AF effects as effectively as right-sided LLTS |
| Yu et al. 2013[37] | AF dogs | 16 | 20 | 1 | 80% below the heart rate slowing threshold | No | Right | 3 h | Acute | LLTS reversed RAP-induced atrial remodelling and inhibited AF inducibility |
| Zhou et al. 2019[25] | HFpEF rats | 48 | 20 | 0.2 | 2 mA | No | Bi | 30 min | Daily, 4 weeks | Chronic intermittent LLTS ameliorated diastolic dysfunction, and attenuated cardiac inflammation and fibrosis |
| Zhou et al. 2016[60] | IST dogs | 16 | 20 | 2 | 80% below the heart rate slowing threshold | No | Right | 3 h | Acute | LLTS suppressed RSG activity and inhibited sympathetically induced sinus node acceleration |
Bi = bilateral; HFpEF = heart failure with preserved ejection fraction; IST = inappropriate sinus tachycardia; LLTS = low-level tragus stimulation; LSG = left satellite ganglion; LV = left ventricular; OSA: obstructive sleep apnoea; R = right; RAP = rapid atrial pacing; RSG = right stellate ganglion.
Table 2: Summary of Human Tragus Stimulation Studies.
| Publication | Subjects | n | Stimulation Parameters | Follow- Outcome up period | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency (Hz) | Pulse Width (ms) | Amplitude | On and Off Cycle | Side | Duration | Chronic/Acute | |||||
| Stavrakis et al. 2015[30] | Paroxysmal AF | 40 | 20 | 1 | 50% below the heart rate slowing threshold | No | Right | 1 h | Acute | None | Suppressed AF and decreased inflammatory cytokines in patients with paroxysmal AF |
| Tran et al. 2018[47] | HFpEF | 24 | 20 | 0.2 | 1 mA below uncomfortable threshold | No | Right | 1 h | Acute | None | LLTS acutely ameliorated LV longitudinal mechanics and improved HRV |
| Dasari et al. 2018[48] | HFrEF | 20 | 20 | 0.2 | 5–40mA | No | Right | 1 h | Acute | None | Beneficial effect on micro-circulation and endothelial function |
| Yu et al. 2017[29] | STEMI | 95 | 20 | 1 | 50% below the heart rate slowing threshold | Yes, 5 s on and 5 s off | Right | 2 h | Acute | 7 days | Reduced myocardial ischaemia-reperfusion injury in patients with STEMI |
| Bretherton et al. 2019[50] | Age ≥55 years | Study 1: 14 | 30 | 0.2 | Just below the threshold of causing discomfort | No | NS | 15 min | Acute | None | TS increased vagal tone and was associated with greater increases in baroreflex sensitivity |
| Study 2: 51 | Acute | None | |||||||||
| Study 3: 29 | Chronic, daily for 2 weeks | 2 weeks | Improvements in HRV parameters, higher quality of life, better moods and sleep quality |
HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; HRV = heart rate variability; LLTS = low-level tragus stimulation; LV = left ventricular; NS = not specified; STEMI = ST-segment elevation MI.
In a clinical study in patients with ST-segment elevation MI (STEMI), LLTS was set at 20 Hz, 1 ms pulse wide and 50% below the heart rate-slowing threshold with a duty cycle of 5 seconds on and 5 seconds off.[29] The same parameters were used in an AF suppression study, but in a continuous manner without the on-and-off cycle.[30] To test if LLTS has a parameter-specific effect on heart rate, an exploratory study compared nine combinations of stimulating parameters (pulse width: 100 μs, 200 μs, 500 μs; frequency: 1 Hz, 10 Hz, 25 Hz) among 15 healthy volunteers.[31] Essentially, the setting of 500 μs and 10 Hz had the strongest effect on heart rate reduction (.2.40 ± 0.275 BPM) during the 60 seconds of stimulation. Recently, a large clinical trial using VNS in patients with HFrEF failed to show a beneficial effect while another trial in patients with HFrEF using different stimulation parameters demonstrated efficacy, highlighting the importance of optimising stimulation parameters.[13,32] Mechanistic studies have suggested that the optimal stimulation parameters for VNS are at the point at which the afferent and efferent fibres are activated in a well-balanced manner, i.e. the afferent-driven decreases in central parasympathetic drive are counteracted by direct activation of the cardiac parasympathetic efferent projections to the intrinsic cardiac autonomic nervous system, resulting in a relatively neutral heart rate response.[33] Consistent with this notion, a recent study of LLTS (30 Hz,10–50 mA, 200 ms, which was slightly below the sensory threshold) in healthy volunteers showed a significant decrease in muscle sympathetic nerve activity (MSNA) without a change in heart rate.[34] Recently, the works by Sclocco et al. suggested that tVNS is headed toward a more refined and sophisticated direction.[35,36] Through respiratory gating and EEG-informed fMRI, the efficacy and accuracy of this therapy can be further improved.
Results in Animal Models
AF
It has been established that the abnormal regulation of the cardiac autonomic nervous system contributes to the initiation and perpetuation of AF. High sympathetic/vagal tone contribute to the substrate of AF while sudden changes in autonomic balance lead to AF episodes. Theoretically, suppressing either the sympathetic or parasympathetic tone could have a moderating effect on AF. There have been at least two animal models testing the acute effect of LLTS. For example, in a canine AF model, right sided LLTS reduced AF inducibility and reversed acute atrial electrical remodelling. Its antiremodelling effect was indicated by a prolonged effective refractory period and a narrowed window of vulnerability (to measure the difficulty of AF induction).[37] In a canine model of obstructive sleep apnoea, LLTS suppressed shortening of atrial refractoriness and autonomic remodelling caused by obstructive sleep apnoea, thus reducing the vulnerability to AF.[38]
Post MI
The close relationship between ventricular arrhythmias after MI and sympathetic activation is well recognised.[39,40] Suppressing the sympathetic tone and increasing the parasympathetic tone protected against post-MI ventricular arrhythmias.
In a canine MI model, right-sided LLTS performed 2 hours a day for 2 months reduced left stellate ganglion activity in both frequency and amplitude.[27] Both of these parameters of neural activity were significantly lower in the MI plus LLTS group than the MI group alone. Ventricular arrhythmias inducibility evaluated by ventricular programed stimulation was also suppressed by LLTS. Besides electrical remodelling, in a canine model intermittent bilateral LLTS for 4 weeks alleviated left ventricular remodelling by reducing ventricular interstitial fibrosis post MI.[41] In another post-MI canine model, 6 weeks of bilateral LLTS (20 Hz, 1 ms pulse wide, 80% below the sinus slowing threshold) for 4 hours per day reduced left ventricle dilation and infarct size, improved both left ventricular contractility and diastolic function and alleviated myocardial fibrosis.[26]
Heart Failure with Preserved Ejection Fraction
HFpEF, another clinical syndrome associated with dysregulation of the autonomic nervous system and inflammation, comprises approximately 50% of all patients with heart failure.[42] Patients with HFpEF have been shown to have increases in plasma renin activity and arginine vasopressin compared with healthy controls.[43] In a rat model of HFpEF (Dahl salt-sensitive rats), LLTS (20 Hz, 2 mA, 0.2 ms) attenuated the elevation of both systolic and diastolic blood pressure. Echocardiography revealed that LLTS improved left ventricular hypertrophy, circumferential strain and diastolic function as measured by E/A ratio and E/e’ ratio.[25] Histological studies showed that LLTS attenuated ventricular inflammatory cell infiltration and fibrosis. Downregulation of the pro-inflammatory and pro-fibrotic genes (interleukin [IL]-11, IL18, IL23-A and osteopontin) was seen in the LLTS group as well.[25]
Mechanisms of Low-level Tragus Stimulation
Tragus stimulation exerts its modulating effects through an integrated nervous reflex system. The ABVN is the afferent nerve fibre while the medulla oblongata (nuclei including NTS, nucleus of the solitary tract; NSNT, nucleus spinalis of the trigeminal nerve; NA, nucleus ambiguous; DMN, dorsal motor nucleus) function as the reflex centre.[44] The efferent vagus nerve then arrives at the ganglionated plexi within the epicardial fat pads to achieve the related physiological effects. Though the neuropathway of tVNS is grossly understood, the exact mechanisms underlying the various beneficial effects of LLTS are still poorly understood. The inherent sympathovagal imbalance is different in various cardiovascular states. Modulation of this tone may have variable beneficial effects and is likely directly related to the underlying pathophysiology. For example, even in heart failure, acute and chronic states exhibit a wide spectrum of sympathovagal imbalance and there are likely differential effects of non-invasive vagal modulation. In common cardiovascular disease states, vagal stimulation probably suppress arrhythmias, ischaemia and heart failure through different mechanisms. Up-regulation of c-fos (a proto-oncogene expressed within some neurons following depolarisation) and nerve growth factor (NGF) expression in left superior ganglionated plexus and the left stellate ganglion is found to contribute to autonomic remodelling of AF. LLTS suppressed AF by down-regulating both c-fos and NGF.[38] Connexins (Cx) are an essential component of gap junctions and a key player in the formation of AF substrate. LLTS has been shown to suppress AF by preventing the loss of atrial Cxs (Cx40 and Cx43).[28]
At the cardiac remodelling and fibrosis level, downregulation of matrix metalloproteinase 9 and transforming growth factor-beta 1 was observed in LLTS treated post-MI dogs.[26] Small conductance calcium-activated potassium channel type 2 (SK2) is an ion channel molecule responsible for after-hyperpolarisation that suppresses nerve discharges that are crucial to regulating neuronal excitability. In a canine MI model, LLTS down-regulated the NGF protein and up-regulated the SK2 protein.[27] These studies indicate that LLTS affects the pathophysiological progress on multiple levels – from neural remodelling to myocardial remodelling.
Human Studies
AF
Reports of tragus stimulation on human subjects are still limited. LLTS for 1 hour during ablation procedures reduced the duration of induced AF, while levels of inflammatory markers (circulatory tumour necrosis factor [TNF] alpha and C-reactive protein) were reduced.[30] In patients with paroxysmal AF who received chronic LLTS for 1 h/day for 6 months, there was a 85% decrease in AF burden in the active stimulation group compared to the sham group (stimulating the earlobe).[45]
MI and Angina
In patients with STEMI undergoing primary percutaneous coronary intervention, 2 hours of right LLTS after reperfusion attenuated reperfusion-related ventricular arrhythmias during the first 24 hours, with smaller area under the curve of creatine kinase myocardial band, which theoretically indicated reduced infarct size.[29] In addition to reduced ventricular arrhythmias and cardiac biomarkers, significant improvement in left ventricular ejection fraction and N-terminal pro–Btype natriuretic peptide was observed in the LLTS group. The wall motion index, calculated using the 16-segment model, was used to evaluate the extent of left ventricular dysfunction. The percentage of left ventricular akinetic and dyskinetic segments was significantly lower in the LLTS group than the sham group. The level of inflammatory cytokines including IL-6, IL1-beta, TNF-alpha and HMGB1 were significantly reduced. However, MRI was not conducted to accurately evaluate the infarct size and their differences between groups. It remains to be proven if chronic LLTS would improve morbidity and mortality in patients with STEMI.
Alongside STEMI, the application of vagal neural stimulation for the treatment of coronary artery disease is also worth mentioning. Though conducted in a small population of patients, the clinical and histological messages in the article by Zamotrinsky et al. were impressive.[46] Vagal stimulation via trans-auricular electroacupuncture showed antianginal effect that could last for 2–3 weeks after the completion of the treatments. The effects of LLTS in refractory angina deserves further study.
Heart Failure
Human studies of LLTS on heart failure patients are also limited. In a prospective, randomised, double-blind, 2x2 cross-over study, 1 hour of LLTS acutely ameliorated left ventricular longitudinal mechanics and favourably altered heart rate variability (HRV) frequency domain components in patients with HFrEF.[47] In a pilot, sham controlled, randomised study in patients with HFrEF, 1 hour of LLTS (20 Hz frequency and 200 μs pulse width) led to improvements in microcirculation (as assessed by flow mediated vasodilatation) and nail bed microcirculation (assessed by laser speckle contrast imaging).[48]
Ageing
As people age, the contribution of the parasympathetic activity to the heart declines, whereas sympathetic tone increases, resulting in a less balanced autonomic function. Tragus stimulation was found to be beneficial in healthy young subjects as demonstrated by an increase in HRV.[49] After preliminary LLTS studies showed its safety and efficacy as an alternative to invasive stimulation of cervical vagus nerve, Bretherton et al. applied daily LLTS at a level just below the threshold of causing discomfort (usually 2–4 mA) to people ≥55 years of age.[50] Higher baroreflex sensitivity and HRV were observed by LLTS. Furthermore, it was reported in the same article that in 26 healthy adults with an average age of 64 years, daily sessions of LLTS for 14 days resulted in improvements in HRV parameters, higher quality of life questionnaire score (SF-36), better moods (measured by profile of mood states questionnaire) and better sleep quality (measured by sleep questionnaire), thus showing potential against aging.
Safety of Low-level Tragus Stimulation
The non-invasive nature of tVNS granted it an impressive safety record. Drop in blood pressure and/or heart rate during stimulation is rarely observed. Some mild skin lesions were observed in the study by Stavrakis et al. and they did not recur after adjusting the tension of the medal clips delivering the stimulation.[30]
A systematic review published by Redgrave et al. summarised all available literature on tVNS, not limited to cardiovascular diseases and found that local irritation (tingling/pain/redness/itching) is the most common discomfort (16.7%) that is attributable to stimulation, with headache being the second (3.3%) and nasopharyngitis (1.6%) the third.[51] Dizziness and syncope was found in 20 patients across eight studies, with a prevalence of 1.4%. However, only three severe adverse events were considered tVNS-related.
Conventional wisdom suggests that stimulation of the right vagus nerve exerts more prominent cardiovascular effects than stimulation of the left vagus nerve. Left vagal stimulation is therefore the preferred approach to treat epilepsy.[52] This conventional wisdom has been challenged by reported side effects of left VNS studies.[53,54] For noninvasive VNS, whether left and right tragus stimulation will affect the sinus node and atrioventricular node is still unknown.
Next Steps
There are some limitations associated with previous studies. These include lack of a well controlled sham group, absence of longitudinal data and small sample sizes. Optimal parameters for stimulation are yet to be determined. Furthermore, the lack of a uniform stimulation parameter makes results less generalisable. Some studies used active stimulation at another site while others used sham stimulation at the same site. All of the current clinical studies are acute or short-term studies except one recent study by Stavrakis et al. reporting that chronic LLTS suppressed paroxysmal AF.[45] Longitudinal data are warranted to evaluate the long-term benefit of LLTS.
Lastly, there is a lack of a reliable biomarker to indicate the effectiveness of LLTS. MSNA may serve as an attractive method to determine changes in sympathetic tone with LLTS. However, this technique is time-consuming and lack of widespread availability limits its usage. Serum/plasma markers of neuroendocrine activity (such as vasostatin and catestatin) could also potentially be used to gauge change in neuro endocrine function, pending future data. Stimulus-driven event-related potentials and vagus-sensory evoked potentials in electroencephalogram, fMRI and HRV are potential biomarkers as well.[34,55–57] In some clinical studies, a simple reduction in heart rate is considered a marker of parasympathetic activation.[29] Individual disease states may have respective biomarkers that would predict adequate response to LLTS. While HRV may sound attractive as a biomarker of response, it would be premature to state that this alone could direct this field forward. Searching for a reliable biomarker for effective LLTS should be a continued focus of future research. The aforementioned limitations show clear paths for future research studies.
Conclusion
Non-invasive autonomic modulation using LLTS appears promising in its early stages. The ability to stimulate the central vagal complexes at a level below the heart-rate-lowering threshold seems very attractive. LLTS has promising data in animal models of AF, post-acute MI and heart failure. Accumulating data in humans with paroxysmal AF, post-acute MI and both systolic and diastolic heart failure may pave the way for larger clinical trials focused on demonstrating improvement in morbidity and mortality associated with such disease states. Larger trials are needed to prove the safety and efficacy of tragus stimulation.
Clinical Perspective
Tragus stimulation is an emerging therapy for non-invasive modulation of the autonomic nervous system.
Pre-clinical and clinical studies proved the potential of tragus stimulation in AF, MI and heart failure.
Though the number of clinical trials is still limited, available data point to safety and efficacy of tragus stimulation. Large-scale trials are critically warranted.
References
- 1.Andersson U, Tracey KJ. Neural reflexes in inflammation and immunity. J Exp Med. 2012;209:1057–68. doi: 10.1084/jem.20120571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chavan SS, Tracey KJ. Essential neuroscience in immunology. J Immunol. 2017;198:3389–97. doi: 10.4049/jimmunol.1601613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonaz B, Sinniger V, Pellissier S. The vagus nerve in the neuro-immune axis: Implications in the pathology of the gastrointestinal tract. Front Immunol. 2017;8:1452. doi: 10.3389/fimmu.2017.01452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatterjee NA, Singh JP. Novel interventional therapies to modulate the autonomic tone in heart failure. JACC Heart Fail. 2015;3:786–802. doi: 10.1016/j.jchf.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Giordano F, Zicca A, Barba C et al. Vagus nerve stimulation: Surgical technique of implantation and revision and related morbidity. Epilepsia. 2017;58(Suppl 1):85–90. doi: 10.1111/epi.13678. [DOI] [PubMed] [Google Scholar]
- 6.Carreno FR, Frazer A. Vagal nerve stimulation for treatment-resistant depression. Neurotherapeutics. 2017;14:716–27. doi: 10.1007/s13311-017-0537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lendvai IS, Maier A, Scheele D et al. Spotlight on cervical vagus nerve stimulation for the treatment of primary headache disorders: a review. J Pain Res. 2018;11:1613–25. doi: 10.2147/JPR.S129202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braunwald E, Epstein SE, Glick G et al. Relief of angina pectoris by electrical stimulation of the carotid-sinus nerves. N Engl J Med. 1967;277:1278–83. doi: 10.1056/NEJM196712142772402. [DOI] [PubMed] [Google Scholar]
- 9.Annoni EM, Xie X, Lee SW et al. Intermittent electrical stimulation of the right cervical vagus nerve in saltsensitive hypertensive rats: effects on blood pressure, arrhythmias, and ventricular electrophysiology. Physiol Rep. 2015;3:e12476. doi: 10.14814/phy2.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyers EC, Solorzano BR, James J et al. Vagus nerve stimulation enhances stable plasticity and generalization of stroke recovery. Stroke. 2018;49:710–7. doi: 10.1161/STROKEAHA.117.019202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimberley TJ, Pierce D, Prudente CN et al. Vagus nerve stimulation paired with upper limb rehabilitation after chronic stroke. Stroke. 2018;49:2789–92. doi: 10.1161/STROKEAHA.118.022279. [DOI] [PubMed] [Google Scholar]
- 12.Dicarlo L, Libbus I, Amurthur B et al. Autonomic regulation therapy for the improvement of left ventricular function and heart failure symptoms: the ANTHEM-HF study. J Card Fail. 2013;19:655–60. doi: 10.1016/j.cardfail.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Gold MR, Van Veldhuisen DJ, Hauptman PJ et al. Vagus nerve stimulation for the treatment of heart failure: the INOVATE-HF trial. J Am Coll Cardiol. 2016;68:149–58. doi: 10.1016/j.jacc.2016.03.525. [DOI] [PubMed] [Google Scholar]
- 14.De Ferrari GM, Stolen C, Tuinenburg AE et al. Long-term vagal stimulation for heart failure: eighteen month results from the NEural Cardiac TherApy foR Heart Failure (NECTAR-HF) trial. Int J Cardiol. 2017;244:229–34. doi: 10.1016/j.ijcard.2017.06.036. [DOI] [PubMed] [Google Scholar]
- 15.Akdemir B, Benditt DG. Vagus nerve stimulation: An evolving adjunctive treatment for cardiac disease. Anatol J Cardiol. 2016;16:804–10. doi: 10.14744/AnatolJCardiol.2016.7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deuchars SA, Lall VK, Clancy J et al. Mechanisms underpinning sympathetic nervous activity and its modulation using transcutaneous vagus nerve stimulation. Exp Physiol. 2018;103:326–31. doi: 10.1113/EP086433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clancy JA, Deuchars SA, Deuchars J. The wonders of the wanderer. Exp Physiol. 2013;98:38–45. doi: 10.1113/expphysiol.2012.064543. [DOI] [PubMed] [Google Scholar]
- 18.Schwaber JS, Cohen DH. Electrophysiological and electron microscopic analysis of the vagus nerve of the pigeon, with particular reference to the cardiac innervation. Brain Res. 1978;147:65–78. doi: 10.1016/0006-8993(78)90772-2. [DOI] [PubMed] [Google Scholar]
- 19.Jones JF, Wang Y, Jordan D. Heart rate responses to selective stimulation of cardiac vagal C fibres in anaesthetized cats, rats and rabbits. J Physiol. 1995;489:203–14. doi: 10.1113/jphysiol.1995.sp021042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peuker ET, Filler TJ. The nerve supply of the human auricle. Clin Anat. 2002;15:35–7. doi: 10.1002/ca.1089. [DOI] [PubMed] [Google Scholar]
- 21.Eshraghi AA, Buchman CA, Telischi FF. Sensory auricular branch of the facial nerve. Otol Neurotol. 2002;23:393–6. doi: 10.1097/00129492-200205000-00028. [DOI] [PubMed] [Google Scholar]
- 22.Frangos E, Ellrich J, Komisaruk BR. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul. 2015;8:624–36. doi: 10.1016/j.brs.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Badran BW, Dowdle LT, Mithoefer OJ et al. Neurophysiologic effects of transcutaneous auricular vagus nerve stimulation (taVNS) via electrical stimulation of the tragus: a concurrent taVNS/fMRI study and review. Brain Stimul. 2018;11:492–500. doi: 10.1016/j.brs.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andreas M, Arzl P, Mitterbauer A et al. Electrical stimulation of the greater auricular nerve to reduce postoperative atrial fibrillation. Circ Arrhythm Electrophysiol. 2019;12:e007711. doi: 10.1161/CIRCEP.119.007711. [DOI] [PubMed] [Google Scholar]
- 25.Zhou L, Filiberti A, Humphrey MB, Po SS et al. Low-level transcutaneous vagus nerve stimulation attenuates cardiac remodelling in a rat model of heart failure with preserved ejection fraction. Exp Physiol. 2019;104:28–38. doi: 10.1113/EP087351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, Yu L, Huang B, Wang S et al. Low-level transcutaneous electrical stimulation of the auricular branch of vagus nerve ameliorates left ventricular remodeling and dysfunction by downregulation of matrix metalloproteinase 9 and transforming growth factor beta1. J Cardiovasc Pharmacol. 2015;65:342–8. doi: 10.1097/FJC.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 27.Yu L, Wang S, Zhou X et al. Chronic intermittent low-level stimulation of tragus reduces cardiac autonomic remodeling and ventricular arrhythmia inducibility in a post-infarction canine model. JACC Clin Electrophysiol. 2016;2:330–9. doi: 10.1016/j.jacep.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Chen M, Zhou X, Liu Q, Sheng X, Yu L, Wang Z et al. Left-sided noninvasive vagus nerve stimulation suppresses atrial fibrillation by upregulating atrial gap junctions in canines. J Cardiovasc Pharmacol. 2015;66:593–9. doi: 10.1097/FJC.0000000000000309. [DOI] [PubMed] [Google Scholar]
- 29.Yu L, Huang B, Po SS et al. Low-level tragus stimulation for the treatment of ischemia and reperfusion injury in patients with st-segment elevation myocardial infarction: A proof-ofconcept study. JACC Cardiovasc Interv. 2017;10:1511–20. doi: 10.1016/j.jcin.2017.04.036. [DOI] [PubMed] [Google Scholar]
- 30.Stavrakis S, Humphrey MB, Scherlag BJ et al. Low-level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J Am Coll Cardiol. 2015;65:867–75. doi: 10.1016/j.jacc.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Badran BW, Mithoefer OJ, Summer CE et al. Short trains of transcutaneous auricular vagus nerve stimulation (taVNS) have parameter-specific effects on heart rate. Brain Stimul. 2018;11:699–708. doi: 10.1016/j.brs.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Premchand RK, Sharma K, Mittal S et al. Autonomic regulation therapy via left or right cervical vagus nerve stimulation in patients with chronic heart failure: results of the ANTHEM-HF trial. J Card Fail. 2014;20:808–16. doi: 10.1016/j.cardfail.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Ardell JL, Nier H, Hammer M et al. Defining the neural fulcrum for chronic vagus nerve stimulation: implications for integrated cardiac control. J Physiol. 2017;595:6887–903. doi: 10.1113/JP274678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clancy JA, Mary DA, Witte KK et al. Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimul. 2014;7:871–7. doi: 10.1016/j.brs.2014.07.031. [DOI] [PubMed] [Google Scholar]
- 35.Sclocco R, Garcia RG, Kettner NW et al. The influence of respiration on brainstem and cardiovagal response to auricular vagus nerve stimulation: a multimodal ultrahigh-field (7T) fMRI study. Brain Stimul. 2019;12:911–21. doi: 10.1016/j.brs.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sclocco R, Tana MG, Visani E et al. EEG-informed fMRI analysis during a hand grip task: estimating the relationship between EEG rhythms and the BOLD signal. Front Hum Neurosci. 2014;8:186. doi: 10.3389/fnhum.2014.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu L, Scherlag BJ, Li S et al. Low-level transcutaneous electrical stimulation of the auricular branch of the vagus nerve: a noninvasive approach to treat the initial phase of atrial fibrillation. Heart Rhythm. 2013;10:428–35. doi: 10.1016/j.hrthm.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 38.Yu L, Li X, Huang B et al. Atrial fibrillation in acute obstructive sleep apnea: autonomic nervous mechanism and modulation. J Am Heart Assoc. 2017;6:e006264. doi: 10.1161/JAHA.117.006264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao JM, Chen LS, KenKnight BH et al. Nerve sprouting and sudden cardiac death. Circ Res. 2000;86:816–21. doi: 10.1161/01.res.86.7.816. [DOI] [PubMed] [Google Scholar]
- 40.Swissa M, Zhou S, Gonzalez-Gomez I et al. Long-term subthreshold electrical stimulation of the left stellate ganglion and a canine model of sudden cardiac death. J Am Coll Cardiol. 2004;43:858–64. doi: 10.1016/j.jacc.2003.07.053. [DOI] [PubMed] [Google Scholar]
- 41.Nasi-Er BG, Wenhui Z, HuaXin S et al. Vagus nerve stimulation reduces ventricular arrhythmias and increases ventricular electrical stability. Pacing Clin Electrophysiol. 2019;42:247–56. doi: 10.1111/pace.13585. [DOI] [PubMed] [Google Scholar]
- 42.Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14:591–602. doi: 10.1038/nrcardio.2017.65. [DOI] [PubMed] [Google Scholar]
- 43.Benedict CR, Weiner DH, Johnstone DE et al. Comparative neurohormonal responses in patients with preserved and impaired left ventricular ejection fraction: results of the Studies of Left Ventricular Dysfunction (SOLVD) Registry. J Am Coll Cardiol. 1993;22(4 Suppl A):146A–53A. doi: 10.1016/0735-1097(93)90480-o. [DOI] [PubMed] [Google Scholar]
- 44.Kaniusas E, Kampusch S, Tittgemeyer M et al. Current directions in the auricular vagus nerve stimulation ii -An engineering perspective. Front Neurosci. 2019;13:772. doi: 10.3389/fnins.2019.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stavrakis S, Stoner JA, Humphrey MB et al. TREAT AF (Transcutaneous Electrical Vagus Nerve Stimulation to Suppress Atrial Fibrillation): a randomized clinical trial. JACC Clin Electrophysiol. 2020;6:282–91. doi: 10.1016/j.jacep.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zamotrinsky A, Afanasiev S, Karpov RS, Cherniavsky A. Effects of electrostimulation of the vagus afferent endings in patients with coronary artery disease. Coron Artery Dis. 1997;8:551–7. [PubMed] [Google Scholar]
- 47.Tran N, Asad Z, Elkholey K et al. Autonomic neuromodulation acutely ameliorates left ventricular strain in humans. J Cardiovasc Transl Res. 2019;12:221–30. doi: 10.1007/s12265-018-9853-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dasari TW, Gabor F, Csipo T et al. Non-invasive neuromodulation of vagus activity improves endothelial function in patients with heart failure with reduced ejection fraction: a randomized study. J Card Fail. 2018;24(Suppl):S59–60. doi: 10.1016/j.cardfail.2018.07.266. [DOI] [Google Scholar]
- 49.Antonino D, Teixeira AL, Maia-Lopes PM et al. Non-invasive vagus nerve stimulation acutely improves spontaneous cardiac baroreflex sensitivity in healthy young men: a randomized placebo-controlled trial. Brain Stimul. 2017;10:875–81. doi: 10.1016/j.brs.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 50.Bretherton B, Atkinson L, Murray A et al. Effects of transcutaneous vagus nerve stimulation in individuals aged 55 years or above: potential benefits of daily stimulation. Aging. 2019;11:4836–57. doi: 10.18632/aging.102074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Redgrave J, Day D, Leung H et al. Safety and tolerability of transcutaneous vagus nerve stimulation in humans; a systematic review. Brain Stimul. 2018;11:1225–38. doi: 10.1016/j.brs.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 52.McGregor A, Wheless J, Baumgartner J, Bettis D. Right-sided vagus nerve stimulation as a treatment for refractory epilepsy in humans. Epilepsia. 2005;46:91–6. doi: 10.1111/j.0013-9580.2005.16404.x. [DOI] [PubMed] [Google Scholar]
- 53.Tatum WO 4th, Moore DB, Stecker MM et al. Ventricular asystole during vagus nerve stimulation for epilepsy in humans. Neurology. 1999;52:1267–9. doi: 10.1212/wnl.52.6.1267. [DOI] [PubMed] [Google Scholar]
- 54.Matheny RG, Shaar CJ. Vagus nerve stimulation as a method to temporarily slow or arrest the heart. Ann Thorac Surg. 1997;63(6 Suppl):S28–9. doi: 10.1016/s0003-4975(97)00423-2. [DOI] [PubMed] [Google Scholar]
- 55.Lewine JD, Paulson K, Bangera N, Simon BJ. Exploration of the impact of brief noninvasive vagal nerve stimulation on EEG and event-related potentials. Neuromodulation. 2019;22:564–72. doi: 10.1111/ner.12864. [DOI] [PubMed] [Google Scholar]
- 56.Hagen K, Ehlis AC, Schneider S et al. Influence of different stimulation parameters on the somatosensory evoked potentials of the nervus vagus--how varied stimulation parameters affect VSEP. J Clin Neurophysiol. 2014;31:143–8. doi: 10.1097/WNP.0000000000000038. [DOI] [PubMed] [Google Scholar]
- 57.Fang J, Egorova N, Rong P et al. Early cortical biomarkers of longitudinal transcutaneous vagus nerve stimulation treatment success in depression. Neuroimage Clin. 2017;14:105–11. doi: 10.1016/j.nicl.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roberts J. Chapter 63: Otolaryngologic procedures. In: Roberts and Hedges’ Clinical Procedures in Emergency Medicine and Acute Care Philadephia, PA: Elsevier. 2017. pp. 1338–83.e2.
- 59.Wang Z, Yu L, Wang S et al. Chronic intermittent low-level transcutaneous electrical stimulation of auricular branch of vagus nerve improves left ventricular remodeling in conscious dogs with healed myocardial infarction. Circ Heart Fail. 2014;7:1014–21. doi: 10.1161/CIRCHEARTFAILURE.114.001564. [DOI] [PubMed] [Google Scholar]
- 60.Zhou X, Zhou L, Wang S et al. The use of noninvasive vagal nerve stimulation to inhibit sympathetically induced sinus node acceleration: A potential therapeutic approach for inappropriate sinus tachycardia. J Cardiovasc Electrophysiol. 2016;27((2)):217–23. doi: 10.1111/jce.12859. [DOI] [PubMed] [Google Scholar]


