Introduction
Coronavirus disease 2019 (COVID-19) has resulted in more than 8.7 million laboratory-confirmed cases and 0.46 million deaths globally (1). Few therapies, if any, have been shown to rapidly ameliorate the respiratory symptoms and prevent against the disease progression. An important mechanism contributing to dyspnea and disease progression in patients with COVID-19 might be the increased work of breathing because of the heightened airway resistance (2). Inhalation of hydrogen/oxygen mixed gas (H2-O2) might have a role in the treatment of COVID-19 given the decreased resistance compared with room air when passing through the airways.
Methods
Recently, we conducted an open-label multicenter clinical trial, between January 21st and March 23rd, 2020, among patients with laboratory-confirmed COVID-19 from seven hospitals in China. The patients were aged 18–85 years, and had dyspnea both on hospital admission and at enrollment [See Online Supplement (http://dx.doi.org/10.21037/jtd-2020-057) for patient sources, inclusion/exclusion criteria and outcome measures].
Trial Registration: www.clinicaltrials.gov, No. NCT04378712.
Randomization was not applied because of the urgency to deal with the outbreak. Patients were assigned to treatment group and control group at the discretion of attending clinicians. On the basis of standard-of-care (3), patients in treatment group inhaled H2-O2 (66% hydrogen; 33% oxygen) at 6 L/min via nasal cannula by using the Hydrogen/Oxygen Generator (model AMS-H-03, Shanghai Asclepius Meditec Co., Ltd., China) daily until discharge [see Figure E1 in Online Supplement (http://dx.doi.org/10.21037/jtd-2020-057)]. Patients in control group received standard-of-care (with oxygen therapy each day) alone until discharge. Clinical assessments included the five-category ordinal scale [see Panel 1 in Online Supplement (http://dx.doi.org/10.21037/jtd-2020-057)], four-category ordinary scale of dyspnea, coughing, chest distress and chest pain (0: None; 1: Mild; 2: Moderate; 3: Severe; 4: Very severe) and adverse events, performed on admission, at enrollment, at days 2 and 3, and the day before discharge (end-of-treatment). The primary endpoint was the proportion of patients with improved disease severity (by at least one scale). Secondary endpoints comprised the change from baseline in oxygen saturation and symptom scales.
Analyses of the full-analysis set were performed with R software version 3.5.1. Count (percentage) was adopted for summarizing categorical variables, and compared with Chi-square tests or Fisher’s exact test. The relative risk (RR) along with the 95% confidence interval (95% CI) were calculated to reflect the likelihood of the event in treatment group. Continuous variables were presented with mean ± standard deviation, and compared with independent t-test or Wilcoxon rank-sum test. All testing was two-sided, with P<0.05 being statistically significant.
Results
Of 633 patients being screened, 215 and 328 were excluded from treatment and control group [see Figure E2 in Online Supplement (http://dx.doi.org/10.21037/jtd-2020-057)], respectively, because of the lack of dyspnea at enrollment. Finally, 44 patients were included in treatment group, and 46 in control group. The median duration of H2-O2 and oxygen inhalation was 7.7 (interquartile range, 6.0–18.3) h and 24 (interquartile range, 22.6–24.0) h per day, respectively. The demographics and disease severity were comparable at baseline [see Table E1,E2 in Online Supplement (http://dx.doi.org/10.21037/jtd-2020-057)].
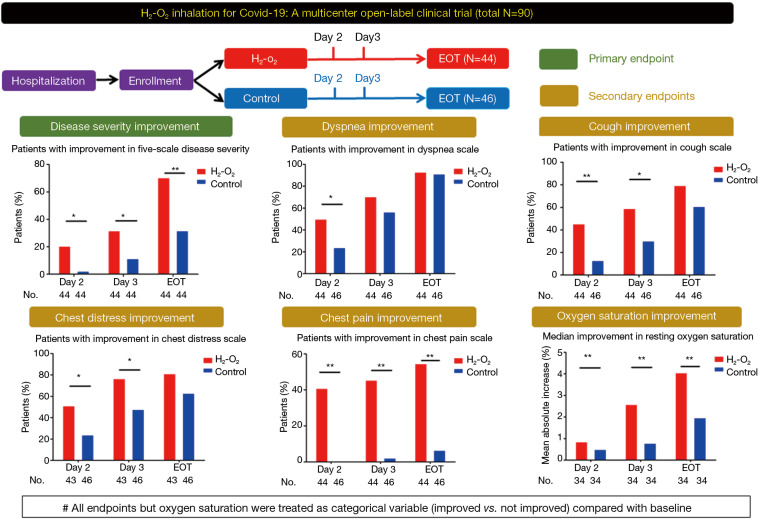
H2-O2 inhalation resulted in significantly more patients with improved disease severity at day 2 (20.5% vs. 2.3%, P=0.019; RR: 9.0, 95% CI: 1.2–68.1) and 3 (31.8% vs. 11.5%, P=0.038; RR: 2.8, 95% CI: 1.1–7.1) and end-of-treatment (70.5% vs. 31.8%, P<0.001; RR: 2.2, 95% CI: 1.4–3.6) (Figure 1). The improvement of dyspnea scale (50.0% vs. 23.9%, P=0.019; RR: 2.1, 95% CI: 1.2–3.8) was greater in H2-O2 treatment group at day 2. H2-O2 inhalation improved chest distress and chest pain (all P<0.05). The improvement in cough scale was greater in treatment group at days 2 and 3 (both P<0.05). Furthermore, the improvement in resting oxygen saturation was greater after H2-O2 inhalation (all P<0.05, Table 1).
Figure 1.
Study design and the main treatment effects of H2-O2 inhalation in patients with coronavirus disease 2019 who had dyspnea at enrollment. All endpoints but resting oxygen saturation were treated as categorical variables (improved vs. not improved) compared with the baseline levels. *, P<0.05; **, P<0.01. EOT, end-of-treatment, which was the day before hospital discharge.
Table 1. Treatment effects in terms of the primary and secondary endpoints at different time points.
| Variables | Day 2 | Day 3 | End-of-treatment | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment group | Control group | P value | RR | 95%CI | Treatment group | Control group | P value | RR | 95%CI | Treatment group | Control group | P value | RR | 95%CI | |||
| n* | 44 | 44 | – | – | – | 44 | 44 | – | – | – | 44 | 44 | – | – | – | ||
| Patients with improvement in five-scale disease severity (%) | 20.5 | 2.3 | 0.019 | 9 | (1.2, 68.1) | 31.8 | 11.4 | 0.038 | 2.8 | (1.1, 7.1) | 70.5 | 31.8 | <0.001 | 2.2 | (1.4, 3.6) | ||
| n* | 44 | 46 | – | – | – | 44 | 46 | – | – | – | 44 | 46 | – | – | – | ||
| Patients with improvement in dyspnea scale (%) | 50 | 23.9 | 0.019 | 2.1 | (1.2, 3.8) | 70.5 | 56.5 | 0.249 | 1.2 | (0.9, 1.7) | 93.1 | 91.3 | >0.999 | 1 | (0.9, 1.2) | ||
| n* | 43 | 46 | – | – | – | 43 | 46 | – | – | – | 43 | 46 | – | – | – | ||
| Patients with improvement in chest distress scale (%) | 51.2 | 23.9 | 0.015 | 2.1 | (1.2, 3.9) | 76.7 | 47.8 | 0.01 | 1.6 | (1.1, 2.3) | 81.4 | 63 | 0.063 | 1.3 | (1.0, 1.7) | ||
| n* | 44 | 46 | – | – | – | 44 | 46 | – | – | – | 44 | 46 | – | – | – | ||
| Patients with improvement in chest pain scale (%) | 40.9 | 0 | <0.001 | NA | NA | 45.5 | 2.2 | <0.001 | 20.9 | (2.9, 149.2) | 54.6 | 6.5 | <0.001 | 8.4 | (2.7, 25.8) | ||
| n* | 44 | 46 | – | – | – | 44 | 46 | – | – | – | 44 | 46 | – | – | – | ||
| Patients with improvement in cough scale (%) | 45.5 | 13 | 0.002 | 3.5 | (1.5, 7.9) | 59.1 | 30.4 | 0.012 | 1.9 | (1.2, 3.2) | 79.6 | 60.9 | 0.089 | 1.3 | (1.0, 1.7) | ||
| n* | 34 | 34 | – | – | – | 34 | 34 | – | – | – | 34 | 34 | – | – | – | ||
| Mean improvement in resting oxygen saturation** (%) | 1.6 | 0.5 | 0.003 | – | (0.4, 1.8) | 2.6 | 0.8 | <0.001 | – | (0.9, 2.7) | 4.1 | 2 | 0.001 | – | (0.9, 3.3) | ||
Count (percentage) was adopted for summarizing categorical variables, and compared with Chi-square tests or Fisher’s exact test. The relative risk (RR) and 95% confidence interval (95% CI) were calculated. Continuous variables were presented with mean ± standard deviation, and compared with independent t-test or Wilcoxon rank-sum test. The median duration of hydrogen inhalation was 64 h (interquartile range, 24–175) h in treatment group, corresponding to 7.7 (interquartile range, 6.0–18.3) h per day. Oxygen therapy in control group lasted for a median of 24 (interquartile range, 22.6–24.0) h per day. *, denotes number of patients evaluated; **, mean difference at Day 2: 1.1; at Day 3: 1.8; at the End of the Treatment: 2.1. NA, not applicable.
Similar findings were found when analyzing the outcome measures as the continuous variables [all P<0.05, Table E3 in Online Supplement (http://dx.doi.org/10.21037/jtd-2020-057)]. In the H2-O2 treatment group, the dyspnea scale improved more significantly at end-of-treatment regardless of baseline disease severity [Table E4 in Online Supplement (http://dx.doi.org/10.21037/jtd-2020-057)]. Patients who inhaled H2-O2 for less than the median duration (64 h) still presented with consistently significant improvements [Table E5 in Online Supplement (http://dx.doi.org/10.21037/jtd-2020-057)].
The most common adverse events were worsening of cough (6.8% in treatment group; 8.7% in control group) and chest distress (2.3% in treatment group; 21.7% in control group). Abnormal laboratory findings were rare (2.3% in treatment group; 13.0% in control group). No serious adverse events were reported [Table E6 in Online Supplement (http://dx.doi.org/10.21037/jtd-2020-057)].
Discussion
This is the first multicenter randomized clinical trial that verifies the efficacy and safety of H2-O2 inhalation in patients with COVID-19. The clinical benefits were likely to be attributable to the ability of H2-O2 to decrease the inspiratory efforts due to the significantly lower resistance when passing through the respiratory tract compared with room air (previously verified with impulse oscillometry) (4). Patients with COVID-19 frequently presented with dyspnea, coughing, chest pain and distress, and oxygen desaturation (5), which cannot be rapidly ameliorated with other existing therapies (including oxygen therapy). The therapeutic effects of H2-O2 became significant as early as days 2 and 3 and the amelioration of most respiratory symptoms persisted till the end-of-treatment, which again cannot be readily interpreted by miscellaneous supportive therapies including oxygen therapy.
Heliox inhalation reportedly resulted in amelioration of dyspnea and decreased respiratory tract resistance in adults and children (6,7). However, due to the lower cost-effectiveness, heliox has not been recommended for routine clinical use. H2-O2 could be generated via direct electrolysis of water using commercially available instrument which has made it possible for clinical application at home and in hospital settings (particularly in medical facilities critically lacking oxygen supplies). The safety profiles have rendered H2-O2 inhalation particularly suitable for relieving dyspnea and other respiratory symptoms in patients with COVID-19, regardless of the disease severity.
Our study was limited by the open-label design and variable duration of H2-O2 inhalation due to the urgency. We neither randomly assigned patients with COVID-19 due to the emergency nor matched the patients with propensity scores, which could have resulted in selection bias. The protocol for H2-O2 inhalation was established empirically and might warrant optimization. Nonetheless, H2-O2 inhalation might be considered useful to patients with dyspnea or those in facilities without sufficient oxygen supplies.
Supplementary
The article’s supplementary files as
Acknowledgement
We thank Drs. Chun-Liang Lei, Xiao-Dong Li for their participation in patient recruitment and Shanghai Asclepius Meditec Co., Ltd. for their provision of the Hydrogen/Oxygen Generator (model AMS-H-03).
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: Given the revisions and the wide concern and pressing importance of research relating to COVID-19, this short communication was managed via the rapid communication pathway and underwent internal review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-2020-057). Prof. Nanshan Zhong serves as the unpaid Editor-in-Chief of Journal of Thoracic Disease. Other authors have no conflicts of interest to declare.
References
- 1.World Health Organization Coronavirus disease 2019 (COVID-19) Situation Reports. (accessed on June 22nd, 2020). Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200621-covid-19-sitrep-153.pdf?sfvrsn=c896464d_2
- 2.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420-2. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Health Commission. The Diagnosis and Treatment Protocol for COVID-19 (Trial Version 5). (accessed on June 22nd, 2020). Available online: http://www.nhc.gov.cn/xcs/zhengcwj/202002/d4b895337e19445f8d728fcaf1e3e13a.shtml
- 4.Zhou ZQ, Zhong CH, Su ZQ, et al. Breathing Hydrogen-Oxygen Mixture Decreases Inspiratory Effort in Patients with Tracheal Stenosis. Respiration 2019;97:42-51. 10.1159/000492031 [DOI] [PubMed] [Google Scholar]
- 5.Wu C, Chen X, Cai Y, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 6.Morgan SE, Vukin K, Mosakowski S, et al. Use of heliox delivered via high-flow nasal cannula to treat an infant with coronavirus-related respiratory infection and severe acute air-flow obstruction. Respir Care 2014;59:e166-70. 10.4187/respcare.02728 [DOI] [PubMed] [Google Scholar]
- 7.Kneyber MC, van Heerde M, Markhorst DG, et al. Mechanical ventilation with heliox decreases respiratory system resistance and facilitates CO2 removal in obstructive airway disease. Intensive Care Med 2006;32:1676-7. 10.1007/s00134-006-0348-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as