Abstract
Background
Lobectomy has long been regarded as the standard treatment for operable non-small cell lung cancer (NSCLC). Recent studies suggested that segmentectomy could achieve a good prognosis for early-stage NSCLC and might be an alternative to lobectomy in this cohort. Until now, on the issue of comparison between lobectomy and segmentectomy, there remains no published randomized controlled trial (RCT), and all existing evidence is low. Recently, a categorization of lower-level evidence has been proposed, namely, the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system. The aim of this meta-analysis is to compare the oncologic outcome between lobectomy and segmentectomy in NSCLC with the clinical T1N0M0 stage according to the GRADE system.
Methods
PubMed, the PMC database, EMBASE, Web of Science, and the Cochrane library were searched prior to May 2019 to identify studies that compared the prognosis between lobectomy and segmentectomy for clinical T1N0M0 NSCLC. The evidence level of the included studies was assessed according to the GRADE system, including level IIA, probably not confounded nonrandomized comparison; level IIB, possibly confounded nonrandomized comparison; and level IIC, probably confounded nonrandomized comparison. The predefined outcomes included overall survival (OS) and disease-free survival (DFS). Univariable and multivariable hazard ratios (HRs) with 95% confidence intervals (95% CI) were pooled using a random-effects model.
Results
Twelve nonrandomized studies involving 8,072 participants were included. Of these studies, two were classified as IIA level (16.7%), six as IIB level (50.0%), and four as IIC level (33.3%). When crude HRs were included, compared with lobectomy, segmentectomy was associated with shorter OS but comparable DFS in the entire cohort (OS, pooled HR =1.45, 95% CI, 1.23 to 1.67; DFS, pooled HR =1.03, 95% CI, 0.65 to 1.82) and in patients with nodules ≤2 cm (OS, pooled HR =1.55, 95% CI, 1.33 to 1.80; DFS, pooled HR =0.98, 95% CI, 0.55 to 1.77). When adjusted HRs were included, the impact of segmentectomy on OS and DFS was comparable to that of lobectomy in the entire cohort (OS, pooled HR =1.39, 95% CI, 0.92 to 2.10; DFS, pooled HR =0.83, 95% CI, 0.66 to 1.03) and in patients with nodules ≤2 cm (OS, pooled HR =1.61, 95% CI, 0.87 to 3.00; DFS, pooled HR =0.90, 95% CI, 0.63 to 1.27).
Conclusions
Based on our results, although shorter OS is observed in patients received segmentectomy, it is necessary to wait for more results from RCT to draw a valid conclusion.
Keywords: Non-small cell lung cancer (NSCLC), lobectomy, segmentectomy, clinical stage, IA stage, meta-analysis
Introduction
Despite the development of treatment, lung cancer remains the leading cause of cancer death worldwide and is characterized by early metastasis and dismal prognosis (1-3). In recent years, with the use of high-resolution computed tomography and low-dose helical computed tomography in lung cancer screening, more cases of non-small cell lung cancer (NSCLC) have been identified at an early stage, and the mainstay treatment is surgery (4).
For operable NSCLC, surgical resection includes lobectomy and sublobar resection (wedge resection and segmentectomy). Lobectomy has long been regarded as indispensable in the management of NSCLC, whereas sublobar resection is traditionally introduced when pulmonary function cannot tolerate lobectomy (5,6). However, recent studies indicate that performing segmentectomy in clinical T1N0M0 NSCLC might achieve a comparable prognosis to lobectomy (7-18). However, without a randomized controlled trial (RCT), all of these studies are low in evidence, with inevitable bias.
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system, which has been widely utilized for guideline recommendations, simply classifies available clinical evidence into two levels: a well-designed RCT is classified as strong evidence, whereas other studies are classified as weak evidence (19). However, the GRADE system can be inefficient in a setting where there is only lower-level evidence for most of the clinical decisions, including the weighing of risks and benefits between lobectomy and segmentectomy in early-stage NSCLC. Recently, a practical framework was proposed to categorize low-level evidence based on the degree of confounding in nonrandomized comparisons (20). It is believed that categorizing the available evidence in this framework can promote a better understanding of the strengths and limitations of these studies.
Therefore, we introduced the categorization of low-level evidence and performed a meta-analysis to compare the prognosis between lobectomy and segmentectomy in clinical T1N0M0 NSCLC.
Methods
Study eligibility and selection
The preferred Reporting Items for Systematic Reviews and Meta-Analysis statement was used as the basis for reporting the materials and methods of this study. A systematic literature search was conducted in electronic databases, including PubMed, the PMC database, EMBASE, Web of Science, and the Cochrane library. The search terms were “lung neoplasms”, “early stage/clinical stage IA/pulmonary nodule ≤3 cm/T1”, “lobectomy/sublobectomy/segmentectomy/limited resection” and “recurrence/prognosis/survival”. We also manually searched the reference lists of all the included studies as well as relevant review articles. Additionally, we reviewed conference abstracts for unpublished work but failed to identify any eligible studies for inclusion. The searches were limited to articles published in English prior to May 2019.
We included any retrospective or prospective studies (such as cohort studies, case-control studies, or RCTs) that compared the prognosis between lobectomy and segmentectomy in clinical T1N0M0 NSCLC. We combined the search results in a bibliographic management tool (EndNote) and eliminated duplicates. The primary endpoints include overall survival (OS) and disease-free survival (DFS). Only studies reporting at least one of the outcomes were included. When studies reported the comparison of limited resection and lobectomy, only those that separately provided segmentectomy were included. Finally, all the trials had to be published as full text. After preliminary screening of titles and abstracts, three independent authors (YZZ, WYZ, and HYL) assessed the full text for final selection.
Data extraction and quality assessment
Data extraction was performed using a predefined electronic database. Both OS and DFS were chosen as the outcomes for efficacy, and the HRs and their 95% confidence intervals (95% CIs) were our preferred outcome measure. If HRs or 95% CIs were not reported, we would first contact either the first or corresponding author by email for data support. In this study, the data from Tustani and Jiang were obtained in this way (9,14). For studies with no response, the Kaplan-Meier survival curves would be read by Engauge Digitizer version 2.11 (http://engauge-digitizer.updatestar.com/), and the HRs and 95% CIs of OS and DFS would be calculated (21). In addition, we also extracted other clinicopathological characteristics of each study, including general information (first author, publication year, and sample size), patient clinical characteristics and demographics, and histologic type of tumor.
According to the proposed Extended Evidence-Based Medicine Grading System, we stratified our included studies into three levels of evidence (LOE): level IIA, probably not confounded nonrandomized comparison; level IIB, possibly confounded nonrandomized comparison; and level IIC, probably confounded nonrandomized comparison (20). Data extraction and quality assessment were performed by RXL and independently confirmed by two other authors (WJG and SSF). Any discrepancies were resolved by discussion involving all three to achieve consensus.
Data synthesis and statistical analysis
Univariate and multivariate HRs and corresponding 95% CIs for the OS and DFS were pooled using a random-effects model, accounting for clinical heterogeneity. Heterogeneity across studies was assessed using the Q statistic with its P value and I2 statistic. The I2 statistic was used to quantify the proportion of total variation in the effect estimation that is due to between-study variation. An I2 value greater than 50% indicates significant heterogeneity.
The meta-analysis was conducted in accordance with recommendations of the Cochrane Collaboration and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement (PRISMA) using the standard software (Stata 12.0, Stage Corporation, College Station, TX) (22,23). A two-sided P value of <0.05 was considered significant.
Results
Literature search
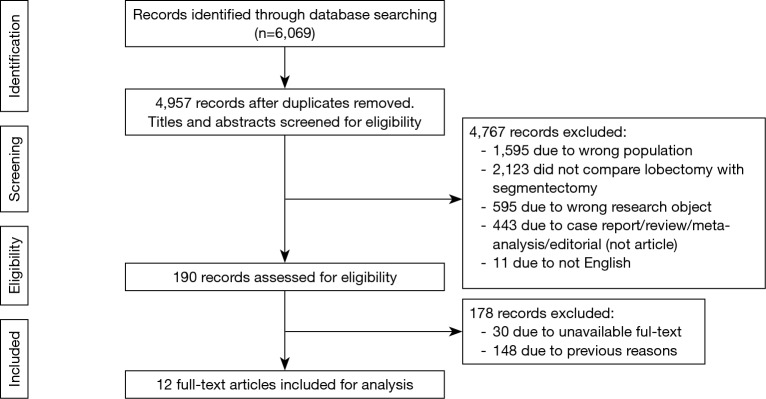
Figure 1 shows the study selection process. Our initial search yielded 6,069 records. After excluding duplicates and screening the titles and abstracts, we obtained 160 articles. After reviewing the full text, no RCTs were identified. Finally, 12 studies with lower-level evidence met the inclusion criteria and were included in the qualitative meta-analysis (7-18).
Figure 1.
Flow diagram of the study selection process.
Characteristics and evidence levels of the included studies
The characteristics of the included studies are shown in Table 1. These studies were published between 2001 and 2016. The number of participants in the studies ranged from 90 to 5,143. The meta-analysis consisted of 8,072 participants. Details of the quality assessment of the included studies are outlined in Table 2. Of the 12 nonrandomized studies, two studies were classified as IIA LOE (16.7%) and six as IIB LOE (50.0%). In addition, four studies (33.3%) were classified as IIC LOE due to defects such as the absence of clear treatment strategy selection (n=2) and the absence of multivariate or propensity-matched analysis (n=3).
Table 1. Characteristics of the included studies.
| Study | Country | Tumor diameter | Pathology | Imaging features | Study design | Number of participants | Propensity score match | Covariates in PSM analysis | Multivariate analysis | Covariates in adjusted model | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lob | Seg | ||||||||||
| Okada 2001 | Japan | ≤2 cm | NSCLC | Undefined | Retrospective cohort | 139 | 70 | No | – | No | – |
| Yamato 2008 | Japan | ≤2 cm | Adenocarcinoma | Undefined | Retrospective cohort | 277 | 153 | No | – | Yes | Age, sex, pT status, pN status, Noguchi classification |
| Zhong 2012 | China | ≤2 cm | NSCLC | Undefined | Retrospective cohort | 81 | 39 | No | – | Yes | Age, sex, tumor size, histology, location |
| Tsutani 2013 | Japan | ≤3 cm | Adenocarcinoma | Undefined | Retrospective cohort | 81 | 81 | Yes | Age, sex, sold tumor diameter, SUVmax, side, and lobe | No | – |
| Okada 2014 | Japan | ≤3 cm | Adenocarcinoma | Undefined | Retrospective cohort | 479 | 155 | No | – | Yes | Age, sex, tumor diameter, SUVmax |
| Jiang 2014 | China | ≤1 cm | NSCLC | Undefined | Retrospective cohort | 71 | 19 | No | – | Yes | Age, comorbidities, tumor diameter, serum CEA, pN status, differentiation, Visceral pleural involvement |
| Ogawa 2015 | Japan | 2–3 cm | NSCLC | Undefined | Retrospective cohort | 147 | 31 | No | - | No | - |
| Khullar 2015 | USA | ≤2 cm | NSCLC | Undefined | Retrospective cohort | 4857 | 286 | No | - | Yes | Age, sex, pathology, insurance, income, Charlson/Deyo score, differentiation, tumor diameter |
| Kodama 2016 | Japan | ≤2 cm | NSCLC | Non-GGN | Retrospective cohort | 232 | 80 | Yes | Age, sex, tumor diameter, CT feature, GGN ratio, preoperative diagnosis | Yes | CT feature, histology, lymphatic invasion, vascular invasion |
| Nishio 2016 | Japan | ≤2 cm | NSCLC | Undefined | Retrospective cohort | 72 | 118 | Yes | Age, sex, tumor diameter, GGN ratio, location | Yes | Age, tumor diameter, GGN ratio, location |
| Koike 2016 | Japan | ≤2 cm | NSCLC | Pure solid | Retrospective cohort | 151 | 100 | Yes | Age, sex, smoking status, percent predicted VC, FEV1/FVC, Serum CEA, tumor diameter, histology, lymphadenectomy extent, number of node examined | Yes | Age, smoking status, FEV1/FVC, serum CEA |
| Hattori 2017 | Japan | ≤2 cm | NSCLC | Non-GGN | Retrospective cohort | 270 | 83 | No | – | No | – |
Lob, lobectomy; Seg, segmentectomy; PSM, propensity score match; SUV, standard uptake volume; GGN, ground glass nodule; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; NSCLC, non-small cell lung cancer.
Table 2. Methodological Quality Assessment of Included studies According to the categorization of lower-level evidence.
| Study | Comparable cohort | Clear treatment strategy selection and detailed methodologies | Multivariate analysis or matching analysis | Covariates included | Bias | Evidence level |
|---|---|---|---|---|---|---|
| Okada 2001 | Yes | Yes | No | – | Probably confounded | IIC |
| Yamato 2008 | Yes | Yes | Multivariate analysis | Most relevant factors | Possibly confounded | IIB |
| Zhong 2012 | Yes | Yes | Multivariate analysis | Most relevant factors | Possibly confounded | IIB |
| Tsutani 2013 | Yes | Yes | Both | All known factors | Probably not confounded | IIA |
| Okada 2014 | Yes | Yes | Multivariate analysis | Most relevant factors | Possibly confounded | IIB |
| Jiang 2014 | Yes | Yes | Multivariate analysis | Most relevant factors | Possibly confounded | IIB |
| Ogawa 2015 | Yes | Yes | No | – | Probably confounded | IIC |
| Khullar 2015 | Yes | No | Multivariate analysis | Most relevant factors | Probably confounded | IIC |
| Kodama 2016 | Yes | Yes | Both | Most relevant factors | Possibly confounded | IIB |
| Nishio 2016 | Yes | Yes | Both | Most relevant factors | Possibly confounded | IIB |
| Koike 2016 | Yes | Yes | Both | All known factors | Probably not confounded | IIA |
| Hattori 2017 | Yes | Yes | No | – | Probably confounded | IIC |
Survival analysis
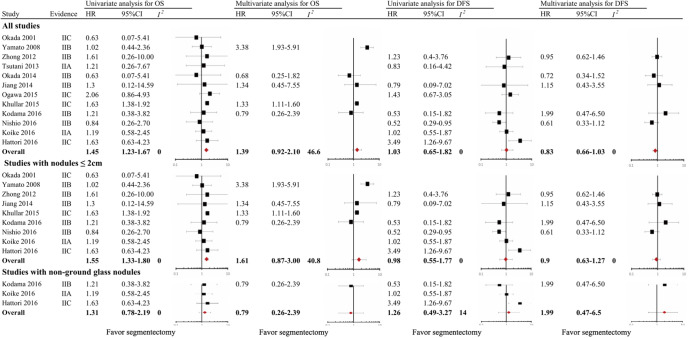
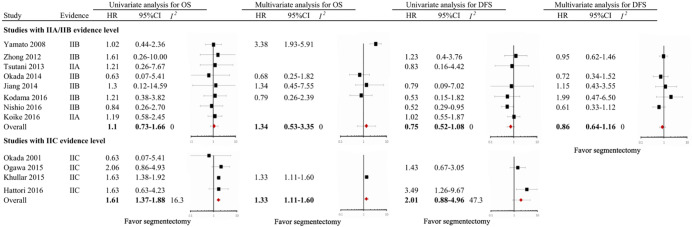
In the overall analysis of the entire cohort, univariate HRs for OS were obtained in 12 studies (100%); meta-analysis of the data showed significantly shorter OS in the segmentectomy group, with a pooled HR of 1.45 (95% CI, 1.23–1.67; I2=0.0%). Multivariate HRs for OS were obtained in five studies (38.5%); meta-analysis of the data showed comparable OS in the segmentectomy group, with a pooled HR of 1.39 (95% CI, 0.92–2.10; I2=46.6%). Univariate HRs for DFS were obtained in 8 studies (61.5%); meta-analysis of the data showed comparable DFS between the two groups, with a pooled HR of 1.03 (95% CI, 0.65–1.82; I2=0.0%). Multivariate HRs for DFS were obtained in 5 studies (38.5%); meta-analysis of the data showed comparable DFS in the segmentectomy group, with a pooled HR of 0.83 (95% CI, 0.66–1.03; I2=0.0%) (Figure 2). As shown in Figure S1, for studies with IIA/IIB evidence level, the prognosis of segmentectomy is comparable to lobectomy, with a pooled HR of 1.1 (95% CI, 0.73–1.66; I2=0.0%); for studies with IIC evidence level, the prognosis of segmentectomy is inferior to lobectomy, with a pooled HR of 1.61 (95% CI, 1.37–1.88; I2=16.3%).
Figure 2.
Forest plot of the relationship between surgical procedure (lobectomy versus segmentectomy) and mortality among patients with clinical T1N0M0 non-small cell lung cancer (stratified by clinicopathological parameters).
Figure S1.
Forest plot of the relationship between surgical procedure (lobectomy versus segmentectomy) and mortality among patients with clinical T1N0M0 non-small cell lung cancer (stratified by evidence level).
Nine studies focused on solitary nodules ≤2 cm. Univariate HRs for OS were obtained in nine studies (100%); meta-analysis of the data showed significantly shorter OS in the segmentectomy group, with a pooled HR of 1.55 (95% CI, 1.33–1.80; I2=0.0%). Multivariate HRs for OS were obtained in four studies (44.4%); meta-analysis of the data showed comparable OS in the segmentectomy group, with a pooled HR of 1.61 (95% CI, 0.87–3.00; I2=40.8%). Univariate HRs for DFS were obtained in 6 studies (66.7%); meta-analysis of the data showed comparable DFS in the segmentectomy group, with a pooled HR of 0.98 (95% CI, 0.55–1.77; I2=0.0%). Multivariate HRs for DFS were obtained in four studies (44.4%); meta-analysis of the data showed comparable DFS in the segmentectomy group, with a pooled HR of 0.90 (95% CI, 0.63–1.27; I2=0.0%) (Figure 2).
Three studies focused on non-GGN. Univariate HRs for OS were obtained in three studies (100%); meta-analysis of the data showed comparable OS in the segmentectomy group, with a pooled HR of 1.31 (95% CI, 0.78–2.19; I2=0.0%). Multivariate HRs for OS were obtained in only one study, with an adjusted HR of 0.79 (95% CI, 0.26–2.39). Univariate HRs for DFS were obtained in 3 studies (60.0%); meta-analysis of the data showed comparable DFS in the segmentectomy group, with a pooled HR of 1.26 (95% CI, 0.49–3.27; I2=14.0%). Multivariate HRs for DFS were obtained in only one study, with an adjusted HR of 1.99 (95% CI, 0.47–6.50) (Figure 2).
Publication bias
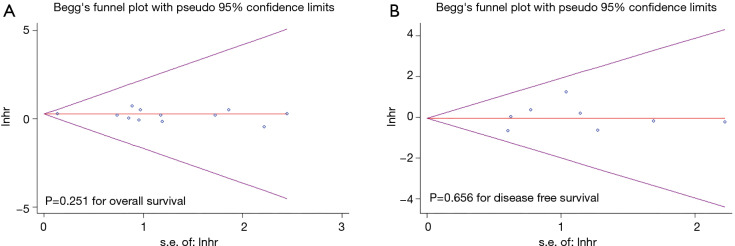
Although the Egger test suggests that there was no evidence of publication bias (OS, P=0.251, Figure 3A; DFS, P=0.656, Figure 3B), it is difficult to rule out the existence of publication bias by visual inspection of the funnel plot since only thirteen studies were included in the quantitative meta-analysis of OS and only eight studies were included in the quantitative meta-analysis of DFS.
Figure 3.
Funnel plot for publication bias of the relationship between surgical procedure and overall survival (A) and disease-free survival (B) among patients with clinical T1N0M0 non-small cell lung cancer. HR, hazard ratio.
Discussion
Lobectomy has long been regarded as the standard treatment for operable NSCLC. However, recent studies proved that the prognosis for NSCLC with T1N0M0 status is fairly good even after sublobar resection (24). Segmentectomy is an anatomical sublobar resection that is able to achieve a better prognosis than wedge resection (25). It is indicated that segmentectomy might be an alternative to lobectomy in NSCLC with T1N0M0 status. In this study, we carried out a meta-analysis to compare lobectomy and segmentectomy in NSCLC patients with clinical T1N0M0 status and observed longer OS in the lobectomy group.
In the literature, there has been some meta-analysis comparing the oncologic outcomes between lobectomy and sublobar resection in early-stage NSCLC (26-29). In some of these studies, patients with the same pathological stage and clinical stage were included together (26,29). As is well known, pathological stage and clinical stage are two disparate concepts. Although the pathological stage holds accurate prognostic information, clinical stage remains one of the most critical decision-making factors for treatment. In addition, comparisons were commonly performed between lobectomy and sublobar resection, and the effectiveness of segmentectomy was not evaluated (26,27). In addition, in these meta-analyses, the inclusion criteria were set as stage I NSCLC, including nodules with diameters ≥3 cm, which could not represent the real situation of patients with T1N0M0 status (26-29). As indicated by a recent study, a reason to rationalize segmentectomy utilization instead of lobectomy is the naturally good prognosis, which is commonly observed in T1N0M0 status (24). Therefore, based on NSCLC with clinical T1N0M0 status, our study would have special significance, would play a supplementary role to existing evidence, and should be considered as a highlight of this study.
Twelve eligible studies involving 8,072 participants were included in this meta-analysis. Based on our results, segmentectomy was associated with a shorter OS than lobectomy, with a pooled HR of 1.45 (95% CI, 1.23–1.67) (univariate analysis for all included studies). A similar result was also observed in patients with nodules ≤2 cm, with a pooled HR of 1.55 (95% CI, 1.33–1.80) (univariate analysis for all included studies). However, when the evaluation was performed for adjusted HR, the survival difference was no longer significant, regardless of whether the entire cohort was included (pooled HR =1.39; 95% CI, 0.92–2.10) or only those with nodules ≤2 cm (pooled HR =1.61; 95% CI, 0.87–3.00). A potential explanation is that the effectiveness of segmentectomy in NSCLC with T1N0M0 status might be greatly influenced by several factors, such as histological subtype and imaging manifestations. It was reported that sublobar resection could achieve a fairly good prognosis in patients with adenocarcinoma in situ (AIS) or minimally invasive adenocarcinoma (MIA) (24,30). However, for patients with invasive histology, the effectiveness of sublobar resection would diminish. Nitadori et al. enrolled 734 patients with resected small adenocarcinoma of the lung from the Memorial Sloan-Kettering Cancer Center. Based on their results, the micropapillary component is an important prognostic factor, and sublobar resection leads to a worse prognosis than lobectomy when the micropapillary component is 5% or greater (31). In addition, ground-glass appearance might be another decision-making factor for treatment. It is widely accepted that for nodules with ≥50% ground-glass appearance, the prognosis is fairly good, and sublobar resection should be acceptable (32,33). However, for radiologically solid nodules, the risk is elevated and sublobar resection might be inferior to lobectomy (15,34). Some studies emphasized the importance of a PET/CT scan before performing sublobar resection for small-sized lung cancers. Hattori et al. enrolled 200 patients with clinical IA NSCLC who received a preoperative PET/CT scan and found that the prognosis of sublobar resection is similar to lobectomy when the maximum standardized uptake value (SUVmax) is 3.3 or smaller but inferior to lobectomy when the SUVmax is larger than 3.3 (35). Recently, Tsutani et al. proposed criteria based on a solid component size of less than 0.8 cm on high-resolution computed tomography (HRCT) or SUVmax of less than 1.5 on PET/CT. Based on their results, nodules that met these criteria were significantly associated with a low-grade adenocarcinoma subtype that had an excellent prognosis after sublobar resection that was similar to that for lobectomy (36). The other explanation is that patients with declared clinical IA stage may include some who actually have more advanced stages of the disease, and lobectomy offers the best chance of a cure. Based on these considerations, we recommend regularly performing HRCT, PET/CT, and intraoperative frozen sectioning before intentional segmentectomy in small NSCLC.
Until now, there has been no published randomized control trial (RCT) on the comparison between lobectomy and sublobar resection in early-stage NSCLC. In this meta-analysis, all included studies were retrospective in nature and provided low-level evidence. Therefore, we adopted the latest proposed categorization of low-level evidence (20). According to this categorization, we found that the I2 value of the studies with IIA/IIB evidence level is commonly lower than the entire cohort or those with IIC evidence level, which suggests a good bias assessment capacity of this categorization, especially in clinically relevant areas that are devoid of high-level evidence.
In nowadays, the standard to perform intentional segmentectomy has not yet been well established, therefore, a lot of segmentectomy were performed for patients with compromised pulmonary function or severe comorbidity. In the literature, most studies on segmentectomy were retrospective in nature, and the evaluations were mostly performed without distinguishing the attitude (compromised or intentional) (26-29). Therefore, one major limitation of this meta-analysis is the underlying selection bias among segmentectomy group, which is indicated by our results. In this study, compared with lobectomy, segmentectomy is associated with reduced OS but comparable disease free survival. It is plausible that, patients undergoing segmentectomy might have more comorbidity and thus would suffer more non-cancer related deaths. Therefore, although we have observed shorter OS in segmentectomy group, it is still unable to conclude that lobectomy is superior to segmentectomy for NSCLC with clinical T1N0M0 status. It is necessary to wait for more results from RCT to draw a valid conclusion.
Based on our results, although shorter OS is observed in patients received segmentectomy, it is necessary to wait for more results from RCT to draw a valid conclusion.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by grants from the Natural Science Foundation of Guangdong Province of China (Grant No. 2017A030310641), Medical Scientific Research Foundation of Guangdong Province of China (Grant No. A2018301), and Medical Scientific Research Foundation of Guangdong Province of China (Grant No. A2020150). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-19-3802). The authors have no conflicts of interest to declare.
References
- 1.Liang W, He J, Shen Y, et al. Impact of Examined Lymph Node Count on Precise Staging and Long-Term Survival of Resected Non-Small-Cell Lung Cancer: A Population Study of the US SEER Database and a Chinese Multi-Institutional Registry. J Clin Oncol 2017;35:1162-70. 10.1200/JCO.2016.67.5140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shao W, Xiong X, Chen H, et al. Long-term survival outcomes of video-assisted thoracic surgery for patients with non-small cell lung cancer. Chin J Cancer Res 2014;26:391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sekihara K, Hishida T, Yoshida J, et al. Long-term survival outcome after postoperative recurrence of non-small-cell lung cancer: who is 'cured' from postoperative recurrence? Eur J Cardiothorac Surg 2017;52:522-8. 10.1093/ejcts/ezx127 [DOI] [PubMed] [Google Scholar]
- 4.Li J, Yang X, Xia T, et al. Stage I synchronous multiple primary non-small cell lung cancer: CT findings and the effect of TNM staging with the 7th and 8th editions on prognosis. J Thorac Dis 2017;9:5335-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai C, Shen J, Ren Y, et al. Choice of Surgical Procedure for Patients With Non-Small-Cell Lung Cancer </= 1 cm or > 1 to 2 cm Among Lobectomy, Segmentectomy, and Wedge Resection: A Population-Based Study. J Clin Oncol 2016;34:3175-82. 10.1200/JCO.2015.64.6729 [DOI] [PubMed] [Google Scholar]
- 6.Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol 2015;33:861-9. 10.1200/JCO.2014.56.6661 [DOI] [PubMed] [Google Scholar]
- 7.Yamato Y, Koike T, Yoshiya K, et al. Results of surgical treatment for small (2 cm or under) adenocarcinomas of the lung. Surg Today 2008;38:109-14. 10.1007/s00595-007-3594-5 [DOI] [PubMed] [Google Scholar]
- 8.Zhong C, Fang W, Mao T, et al. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy for small-sized stage IA lung cancer. Ann Thorac Surg 2012;94:362-7. 10.1016/j.athoracsur.2012.04.047 [DOI] [PubMed] [Google Scholar]
- 9.Tsutani Y, Miyata Y, Nakayama H, et al. Oncologic outcomes of segmentectomy compared with lobectomy for clinical stage IA lung adenocarcinoma: propensity score-matched analysis in a multicenter study. J Thorac Cardiovasc Surg 2013;146:358-64. 10.1016/j.jtcvs.2013.02.008 [DOI] [PubMed] [Google Scholar]
- 10.Kodama K, Higashiyama M, Okami J, et al. Oncologic Outcomes of Segmentectomy Versus Lobectomy for Clinical T1a N0 M0 Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:504-11. 10.1016/j.athoracsur.2015.08.063 [DOI] [PubMed] [Google Scholar]
- 11.Koike T, Kitahara A, Sato S, et al. Lobectomy Versus Segmentectomy in Radiologically Pure Solid Small-Sized Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:1354-60. 10.1016/j.athoracsur.2015.10.048 [DOI] [PubMed] [Google Scholar]
- 12.Nishio W, Yoshimura M, Maniwa Y, et al. Re-Assessment of Intentional Extended Segmentectomy for Clinical T1aN0 Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;102:1702-10. 10.1016/j.athoracsur.2016.05.071 [DOI] [PubMed] [Google Scholar]
- 13.Okada M, Yoshikawa K, Hatta T, et al. Is segmentectomy with lymph node assessment an alternative to lobectomy for non-small cell lung cancer of 2 cm or smaller? Ann Thorac Surg 2001;71:956-60; discussion 961. 10.1016/S0003-4975(00)02223-2 [DOI] [PubMed] [Google Scholar]
- 14.Jiang W, Pang X, Xi J, et al. Clinical outcome of subcentimeter non-small cell lung cancer after surgical resection: single institution experience of 105 patients. J Surg Oncol 2014;110:233-8. 10.1002/jso.23647 [DOI] [PubMed] [Google Scholar]
- 15.Hattori A, Matsunaga T, Takamochi K, et al. Locoregional recurrence after segmentectomy for clinical-T1aN0M0 radiologically solid non-small-cell lung carcinoma. Eur J Cardiothorac Surg 2017;51:518-25. [DOI] [PubMed] [Google Scholar]
- 16.Okada M, Mimae T, Tsutani Y, et al. Segmentectomy versus lobectomy for clinical stage IA lung adenocarcinoma. Ann Cardiothorac Surg 2014;3:153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogawa H, Uchino K, Tanaka Y, et al. Outcomes of segmentectomy for cT1bN0M0 lung adenocarcinoma and squamous cell carcinoma: a possible association with pathological invasion. Eur J Cardiothorac Surg 2015;48:77-82. 10.1093/ejcts/ezu429 [DOI] [PubMed] [Google Scholar]
- 18.Khullar OV, Liu Y, Gillespie T, et al. Survival After Sublobar Resection versus Lobectomy for Clinical Stage IA Lung Cancer: An Analysis from the National Cancer Data Base. J Thorac Oncol 2015;10:1625-33. 10.1097/JTO.0000000000000664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diekemper RL, Patel S, Mette SA, et al. Making the GRADE: CHEST Updates Its Methodology. Chest 2018;153:756-9. 10.1016/j.chest.2016.04.018 [DOI] [PubMed] [Google Scholar]
- 20.Detterbeck FC, Gould MK, Lewis SZ, et al. Extending the Reach of Evidence-Based Medicine: A Proposed Categorization of Lower-Level Evidence. Chest 2018;153:498-506. 10.1016/j.chest.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 21.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. [DOI] [PubMed] [Google Scholar]
- 22.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647. 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 23.Page MJ, Moher D. Evaluations of the uptake and impact of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) Statement and extensions: a scoping review. Syst Rev 2017;6:263. 10.1186/s13643-017-0663-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sagawa M, Oizumi H, Suzuki H, et al. A prospective 5-year follow-up study after limited resection for lung cancer with ground-glass opacity. Eur J Cardiothorac Surg 2018;53:849-56. 10.1093/ejcts/ezx418 [DOI] [PubMed] [Google Scholar]
- 25.Hou B, Deng XF, Zhou D, et al. Segmentectomy versus wedge resection for the treatment of high-risk operable patients with stage I non-small cell lung cancer: a meta-analysis. Therapeutic advances in respiratory disease 2016;10:435-43. 10.1177/1753465816667121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Sun Y, Wang R, et al. Meta-analysis of lobectomy, segmentectomy, and wedge resection for stage I non-small cell lung cancer. J Surg Oncol 2015;111:334-40. 10.1002/jso.23800 [DOI] [PubMed] [Google Scholar]
- 27.Fan J, Wang L, Jiang GN, et al. Sublobectomy versus lobectomy for stage I non-small-cell lung cancer, a meta-analysis of published studies. Ann Surg Oncol 2012;19:661-8. 10.1245/s10434-011-1931-9 [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Huang C, Liu H, et al. Sublobectomy versus lobectomy for stage IA (T1a) non-small-cell lung cancer: a meta-analysis study. World J Surg Oncol 2014;12:138. 10.1186/1477-7819-12-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bedetti B, Bertolaccini L, Rocco R, et al. Segmentectomy versus lobectomy for stage I non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Dis 2017;9:1615-23. 10.21037/jtd.2017.05.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu S, Wang R, Zhang Y, et al. Precise Diagnosis of Intraoperative Frozen Section Is an Effective Method to Guide Resection Strategy for Peripheral Small-Sized Lung Adenocarcinoma. J Clin Oncol 2016;34:307-13. 10.1200/JCO.2015.63.4907 [DOI] [PubMed] [Google Scholar]
- 31.Nitadori J, Bograd AJ, Kadota K, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Inst 2013;105:1212-20. 10.1093/jnci/djt166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsutani Y, Miyata Y, Nakayama H, et al. Appropriate sublobar resection choice for ground glass opacity-dominant clinical stage IA lung adenocarcinoma: wedge resection or segmentectomy. Chest 2014;145:66-71. 10.1378/chest.13-1094 [DOI] [PubMed] [Google Scholar]
- 33.Sim HJ, Choi SH, Chae EJ, et al. Surgical management of pulmonary adenocarcinoma presenting as a pure ground-glass nodule. Eur J Cardiothorac Surg 2014;46:632-6; discussion 636. 10.1093/ejcts/ezu007 [DOI] [PubMed] [Google Scholar]
- 34.Hattori A, Suzuki K, Matsunaga T, et al. Is limited resection appropriate for radiologically "solid" tumors in small lung cancers? Ann Thorac Surg 2012;94:212-5. 10.1016/j.athoracsur.2012.03.033 [DOI] [PubMed] [Google Scholar]
- 35.Hattori A, Matsunaga T, Takamochi K, et al. Indications for sublobar resection of clinical stage IA radiologic pure-solid lung adenocarcinoma. J Thorac Cardiovasc Surg 2017;154:1100-8. 10.1016/j.jtcvs.2017.03.153 [DOI] [PubMed] [Google Scholar]
- 36.Tsutani Y, Nakayama H, Tasaki T, et al. Long-term outcomes after sublobar resection for clinical stage IA lung adenocarcinoma meeting node-negative criteria defined by HRCT and FDG-PET/CT. J Clin Oncol 2018;36:abstr 8554.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as