Abstract
Desmosomes are cadherin-based adhesion structures that mechanically couple the intermediate filament cytoskeleton of adjacent cells to confer mechanical stress resistance to tissues. We have recently described desmosomes as mesoscale lipid raft membrane domains that depend on raft dynamics for assembly, function, and disassembly. Lipid raft microdomains are regions of the plasma membrane enriched in sphingolipids and cholesterol. These domains participate in membrane domain heterogeneity, signaling and membrane trafficking. Cellular structures known to be dependent on raft dynamics include the post-synaptic density in neurons, the immunological synapse, and intercellular junctions, including desmosomes. In this review, we discuss the current state of the desmosome field and put forward new hypotheses for the role of lipid rafts in desmosome adhesion, signaling and epidermal homeostasis. Furthermore, we propose that differential lipid raft affinity of intercellular junction proteins is a central driving force in the organization of the epithelial apical junctional complex.
Introduction: Plasma membrane organization and intercellular junctions
The establishment of plasma membrane heterogeneity represents a fundamental mechanism by which various cellular activities are compartmentalized at the cell surface. This organization is achieved through the formation of membrane domains where specific sets of proteins, lipids, and carbohydrates coalesce to carry out distinct functions such as signaling, transport, and adhesion [1]. Prominent examples of these domains include the post-synaptic density in neurons, the immunological synapse, and intercellular junction complexes [2–5]. It is widely appreciated that key aspects of plasma membrane heterogeneity are driven by protein-protein interactions that mediate the formation of macromolecular complexes. It is also clear that the lipid composition of the plasma membrane is heterogeneous and that protein-lipid and lipid-lipid associations are central factors in establishing membrane domain specialization.
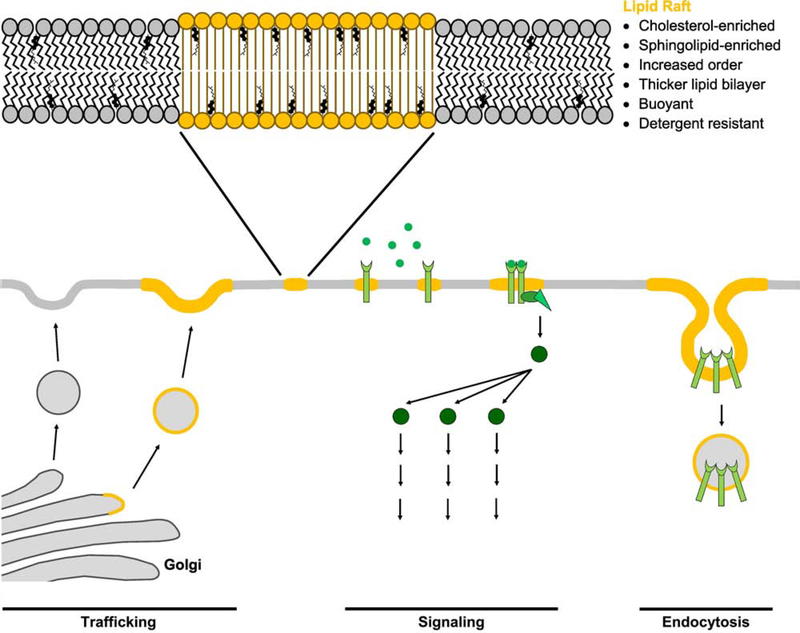
We recently reported that the desmosome is a specialized membrane domain with properties of a mesoscale (intermediately-sized, 10–1000nm) lipid raft [5]. Lipid rafts have emerged as domains essential for membrane organization and specialization (Figure 1). Lipid rafts are transient, 10–200nm clusters of protein and lipid nanodomains that can further assemble into larger, more stable microdomains through protein-protein and protein-lipid interactions [1]. Lipid rafts are enriched in sphingolipids and cholesterol, are detergent-resistant, and are more ordered than surrounding membrane regions. Importantly, only a specific subset of proteins associate with lipid rafts, thus providing a mechanism for collecting particular proteins into functional scaffolds while selectively excluding non-raft proteins. In cells, lipid rafts have been shown to be essential for numerous processes, including the polarization of the epithelial apical membrane, immunological signaling, and host-pathogen interactions [1, 3, 6, 7]. In recent years, evidence for the involvement of lipid rafts in intercellular junctions, particularly desmosomes, has emerged [5, 8].
Figure 1: Lipid raft composition and function.
Lipid rafts are membrane microdomains enriched for cholesterol and sphingolipids which cluster proteins for cell functions including trafficking, signaling, and endocytosis. Raft membranes have higher degrees of order and thicker lipid bilayers than surrounding membrane. They are experimentally characterized by buoyancy on sucrose gradients and detergent resistance.
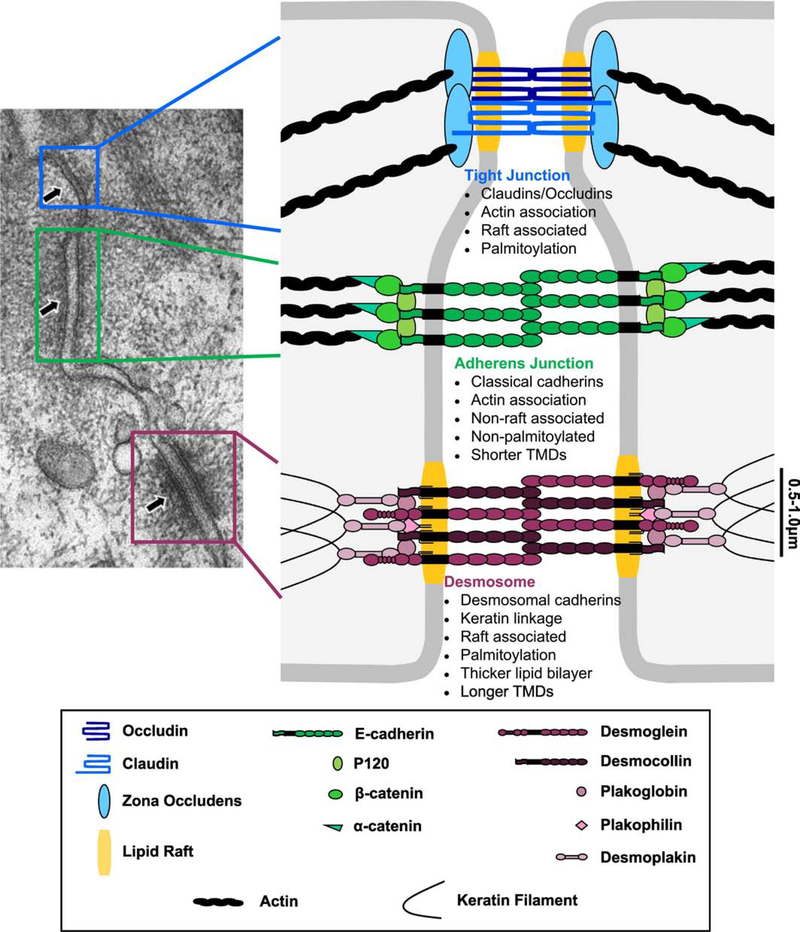
Intercellular junction complexes, including tight junctions (TJ), gap junctions, adherens junctions (AJ), and desmosomes, form at sites of cell-cell contact to mediate cell-cell adhesion and communication (Figure 2). These junctions exhibit different molecular features that contribute to their differential functions in epithelial biology. TJs are continuous, anastomosing strands of membrane contact that form barriers to establish tissue compartmentalization and to regulate paracellular solute flow [9]. Multipass transmembrane proteins, including claudins and occludin, associate with the actin cytoskeleton through adaptor proteins, including zona occludens proteins (e.g., ZO-1, ZO-2), cingulin, and others [10, 11]. Gap junctions allow solutes to pass between adjacent cells by forming pores composed of two connexons, one in each cell membrane, which are complexes of six single-pass transmembrane proteins called connexins [12]. Though functionally and morphologically distinct, AJs and desmosomes are both anchoring junctions that mediate adhesion at sites of cell-cell contact. AJs are composed of calcium-dependent classical cadherins and intracellular adaptor proteins, β-catenin and α-catenin, that link the cadherins to the actin cytoskeleton [13]. Desmosomes are composed of calcium-dependent desmosomal cadherins and intracellular adaptor proteins, plakoglobin, plakophilin, and desmoplakin, that connect the cadherins to the intermediate filament cytoskeleton [14]. Thus, cells assemble a variety of specialized intercellular contacts required for the complex processes that occur during development and adult tissue homeostasis.
Figure 2: Intercellular junction structure, composition and key characteristics.
Depicted in the electron microscopy image of polarized rat intestinal mucosa [modified image from [135]], intercellular junctions are arranged characteristically with TJs (blue) at the most apical side followed by AJs (green) and then desmosomes (purple). TJs maintain polarity and regulate paracellular ion flow and are composed of the membrane proteins claudins and occludin which interact with intracellular zona occludens proteins and additional adaptors to link to the actin cytoskeleton. AJs mediate calcium-dependent adhesion by anchoring classical cadherin transmembrane proteins to the actin cytoskeleton through intracellular adaptor proteins. Desmosomes also mediate calcium-dependent adhesion but can attain a stronger, calcium-independent state, allowing tissues to resist mechanical stress. Desmosomal cadherin transmembrane proteins are anchored to the intermediate filaments through intracellular adaptor proteins to mechanically couple adjacent cells.
This review focuses on desmosomes with an emphasis on the role of lipid rafts in the formation and function of this unique and important cell junction. We will summarize the current understanding of desmosomal components and how disruption of desmosome function leads to human skin and heart disorders. We then put forward new hypotheses that frame the desmosome as a specialized lipid raft-like membrane domain harboring proteins, lipids, and biophysical features that contribute to both the formation of the desmosome and the exclusion of non-desmosomal proteins.
Skin and heart require desmosomes to resist mechanical stress
Desmosomes are 0.5μm-1.0μm long protein complexes that anchor keratin filaments to the plasma membrane through a series of protein-protein interactions that mediate robust cell-cell adhesion, thus allowing tissues to resist mechanical stress. The desmosomal cadherins, including desmogleins (DSG) and desmocollins (DSC), are single pass transmembrane proteins that mediate adhesion through homophilic and heterophilic extracellular interactions between adjacent cells [15–18]. The desmosomal cadherins also interact with intracellular armadillo proteins, including plakoglobin (PG) and plakophilins (PKP), which also bind to desmoplakin (DP). DP binds to intermediate filaments, thereby coupling the cytoskeletal elements of adjacent cells. In this manner, desmosomes integrate cytoskeletal networks with adhesive complexes to provide mechanical strength throughout a tissue.
Desmosomes form in all epithelial tissues but are most abundant in the epidermis and heart where their function is crucial. Desmosomal protein composition depends on tissue- and differentiation-specific gene expression [14, 19]. In humans, four genes encode DSGs (DSG1–4) and three genes encode DSCs (DSC1–3); expression of at least one DSG and one DSC is necessary for normal desmosome formation [15]. DSGs and DSCs each contain five extracellular cadherin repeats, a transmembrane domain, and several intracellular domains, including an intracellular anchor and a cadherin-like sequence where plakoglobin binds [20–22]. DSGs also have a proline-rich domain, repeat unit domain, and a DSG terminal domain [23]. The function of these unique intracellular domains in DSG is not well understood but may aid in desmosomal cadherin clustering [24, 25].
Desmosomes are essential for epidermal differentiation and barrier formation [26]. As a barrier to the external environment, the epidermis is a constantly renewing stratified epithelium composed of proliferating keratinocytes within the basal layer that migrate suprabasally as they differentiate [27]. EGFR signaling partly maintains keratinocytes in the basal layer in a proliferative state, where DSG3 and DSC3 are predominantly expressed [28, 29]. At the interface between the basal layer and suprabasal layers, DSG1 initiates the differentiation process by interacting with Erbin to inhibit EGFR signaling [30, 31]. As keratinocytes differentiate and move suprabasally, DSG3 and DSC3 expression decreases while DSG1 continues to increase, also driving DSC1 expression. Keratin expression switches from keratin-5 and keratin-14 to keratin-1 and keratin-10 and additional epidermal differentiation markers are expressed, such as loricrin and filaggrin [27]. These findings suggest that the differential expression pattern of desmosomal cadherin genes is a key driver of the epidermal differentiation process.
Mutations in nearly all desmosomal genes are linked to numerous diseases of the heart and skin. In the heart, desmosomes provide mechanical integrity and cardiomyocyte connectivity in conjunction with AJs and gap junctions in the intercalated discs [32, 33]. DSG2 and PKP2 mutations cause heart-specific diseases such as arrythmogenic right ventricular cardiomyopathy/dysplasia and other congenital heart problems while mutations in DP, DSC2, PG, and most other desmosomal genes can cause heart and/or skin disorders. [34] Among the many skin-centric desmosomal diseases, common symptoms include woolly hair, hypotrichosis (hair formation defects), keratoderma (epidermal thickening), and skin fragility due to loss of epidermal integrity. The desmosome is also the target of autoimmune responses and bacterial toxins. Pemphigus vulgaris and pemphigus foliaceus are severe autoimmune epidermal blistering diseases resulting from autoantibodies (IgG) targeting DSG3 and DSG1, respectively [35]. These autoantibodies compromise desmosomal adhesion, leading to epidermal fragility and skin blistering. DSG1 can be cleaved by bacterially-produced toxins to cause bullous impetigo and staphylococcal scalded skin syndrome [35], resulting in epidermal blisters that are histologically indistinguishable from pemphigus foliaceus. Collectively, these clinical findings underscore the important role for desmosomes in resisting mechanical stress.
Insight into the importance of individual desmosomal components in epidermal differentiation and homeostasis is also evident from mouse genetic models. Full knockout, conditional knockout, and misexpression of various desmosomal proteins have revealed important roles in both heart and skin function as well as development and differentiation (Table 1). Such findings underscore the importance of desmosomal protein expression patterns in driving tissue specific functions and differentiation programs. Thus, many of the desmosomal components are essential not only for epidermal integrity but also for normal development and differentiation.
Table 1:
Mouse genetic studies reveal important roles for desmosomal proteins in development and homeostasis.
| Gene | Expression | Lethality | Observed Defects | References |
|---|---|---|---|---|
| Dsg1 | Knockout | Perinatal Lethal | epidermal water loss, severe blistering | [124] |
| Dsg2 | Knockout | Embryonic Lethal | Pre-implantation lethality, potentially non-desmosomal role | [125] |
| Suprabasal Epidermal Misexpression | Not Lethal | Hyperproliferation, abnormal differentiation, barrier defects | [28] | |
| Dsg3 | Knockout | Not Lethal | Separated keratinocytes, weakened desmosomal adhesion in oral mucosa | [126] |
| Suprabasal Epidermal Misexpression | Not Lethal | Hyperproliferation, abnormal differentiation, barrier defects | [127] | |
| Dsc1 | Knockout | Not Lethal | Epidermal hyperproliferation, loss of cell-cell adhesion | [128] |
| Dsc3 | Knockout | Embryonic Lethal | Post-implantation lethality, potentially non-desmosomal role | [129] |
| Epidermal Conditional | Not Lethal | Epidermal fragility, hair loss | [130] | |
| Suprabasal Epidermal Misexpression | Not Lethal | Hyperproliferation, abnormal differentiation, barrier defects | [123] | |
| PG | Knockout | Embryonic Lethal | Heart defects, skin fragility | [131] |
| Pkp1 | Knockout | Postnatal Lethality | Epidermal fragility, tight junction defects, growth defects | [132] |
| DP | Knockout | Embryonic Lethal | Die at E6.5 | [133] |
| Epidermal Conditional | Perinatal Lethal | Severe skin fragility, disrupted barrier function | [134] |
The desmosome has features characteristic of a lipid raft-like membrane domain
The molecular mechanisms of desmosome formation are not fully understood. Desmosomal cadherin adhesion is necessary for the assembly process, but the mechanisms by which desmosomal cadherins and plaque components coalesce into a densely-packed membrane domain are not clear. A number of studies have now shown that desmosomal proteins associate with lipid rafts and that desmosome assembly, cell-cell adhesion, and desmosome disassembly are all raft-dependent processes [36, 37]. Several previous studies have examined the role of raft domains in desmosome assembly [37, 38]; this work is reviewed elsewhere [35] and will not be discussed here. Early studies of desmosomes showed that cholesterol and sphingolipids, both of which are enriched in lipid raft membrane domains, are enriched in desmosomes isolated from bovine snout [39, 40]. More recently, evidence for lipid raft association has come from sucrose gradient fractionations in which desmosomal components were identified in detergent-resistant membrane (DRM) fractions, starting with the identification of DSG2 [41]. Later studies revealed that DSG1, DSG3, DSC2, PG, PKP2, DP, and keratins are all present in DRM fractions [5, 36, 37]. These studies also found that depleting cholesterol in cells with methyl-β-cyclodextrin (mβCD) redistributed desmosomal cadherins along cell borders, reduced adhesion strength, and prevented assembly of desmosomal components without disrupting AJ formation [36, 37, 42]. These studies also found that desmosomal components colocalized with certain raft markers, including ostreolysin, CD59, and caveolin but not clathrin [36, 37]. For ostreolysin, transmission immunoelectron microscopy was used to further show an association with desmosomes which was reduced when cells were treated with mβCD [42]. Furthermore, siRNA knockdown of the raft marker, flotillin-2, reduced cell-cell adhesion [43]. Collectively, these studies link desmosome assembly and function with lipid rafts.
Studies in model membranes have shown that lipid bilayers composed of longer and saturated acyl chains such as those found in rafts are thicker than those with unsaturated or shorter acyl chains found in non-raft membrane domains [44]. In addition, the presence of higher levels of cholesterol in raft domains thickens the bilayer, increases order, and stiffens the membrane [45–48]. Using cryo-electron tomography, we found that the lipid bilayer of the plasma membrane within the desmosome is thicker than non-desmosome and desmosome-adjacent bilayers [5]. These findings represent the first evidence that a plasma membrane domain known to contain lipid raft associating proteins is thicker than other regions of the plasma membrane. Based on the fact that desmosomal proteins associate with lipid rafts, disruption of rafts prevents desmosome assembly, and that the desmosomal lipid bilayer is thicker than surrounding membrane, we concluded that desmosomes represent a mesoscale, or intermediately-sized, raft-like plasma membrane domain.
Mechanism of desmosomal protein association with lipid raft membrane microdomains
Association with raft or non-raft lipid microdomains occurs by incompletely understood mechanisms but has been proposed to involve protein-lipid [49] and/or protein-cholesterol [50] interactions mediated by the transmembrane domain (TMD) of integral membrane proteins [51]. Recently, three physical properties of TMDs have been shown to dictate the raft affinity of single pass transmembrane proteins. Collectively termed physiochemical properties, these include TMD length, TMD surface area, and palmitoylation [51–53]. Lorent et al. [52] combined these properties into a model that can predict the free energy required for a single pass transmembrane protein to associate with rafts. This model for raft affinity is highly predictive across a wide range of single pass transmembrane proteins and incorporates TMD length, surface area and palmitoylation as key driving factors in lipid raft association.
TMD Length
Cholesterol and sphingolipid content increases as membranes are modified along the secretory pathway, leading to thicker lipid bilayers at the plasma membrane compared to the ER and Golgi [54]. Similarly, the amino acid (AA) length of TMDs of single-pass transmembrane proteins increases through the secretory pathway such that proteins localized to the ER have the shortest TMDs at about 16AA while those that localize to the plasma membrane are longer at about 21AA [55]. Consistent with the predicted increased thickness of raft bilayers in the plasma membrane, those proteins that partition into lipid rafts possess TMDs that are even longer (about 24AA or more) than a typical non-raft associated plasma membrane protein [51, 52]. This feature allows the extended hydrophobic TMD α-helix of raft proteins to associate with extended acyl chains and cholesterol present in lipid raft domains while excluding proteins with shorter TMDs due to hydrophobic mismatch [56, 57]. Interestingly, the DSG TMDs are all 24AA in length whereas the DSC TMDs are only 21AA in length even though DSCs are associated with raft domains. These observations highlight the importance of other protein features in regulating raft association, including TMD surface area and palmitoylation.
TMD Surface Area
The exposed surface area of a TMD refers to the area of the collective amino-acid residue side chains [58]. Single pass transmembrane proteins bearing TMDs with smaller surface areas can partition into rafts to a greater degree due to a smaller energy barrier; these proteins pack into the more ordered environment of the lipid raft more readily than those with larger surface areas. DSG TMDs contain a number of bulky leucine residues such that the surface area may be larger than anticipated for a raft-associated protein. The E-cadherin TMD also contains numerous leucines. These residues have been shown to mediate TMD-TMD dimerization via a leucine zipper-like motif which is important for cell adhesion through mechanisms that are not fully understood [59]. Because oligomerization via TMD-TMD dimerization can also increase raft affinity [53], leucine residues in Dsgs could be important for Dsg dimerization or oligomerization, as well as raft association. This possibility remains to be tested. Likewise, leucine residues are also present in the DSC TMD. These observations raise the possibility of heterodimerization between DSG and DSC, which, when coupled with the longer DSG TMD, could support raft association of Dscs as well as segregation of nascent AJs and desmosomes. Additional experiments are needed to fully understand how the leucine rich TMDs of these various cadherins contribute to dimerization, raft association and overall cadherin function for both classical and desmosomal cadherins.
Palmitoylation
The presence of a palmitoyl group on cysteine residues adjacent to TMDs in single pass transmembrane proteins has also been shown to increase raft association. Palmitoylation is a reversible post-translational modification that adds a 16C saturated acyl chain to cysteine residues [60]. For soluble cytoplasmic proteins, palmitoylation localizes a protein to the plasma membrane and is important in regulation, stability, and function [61, 62]. Integral membrane proteins are commonly palmitoylated on cysteine residues on the cytoplasmic face of the TMD [60] and this posttranslational modification is recognized as a key raft protein modification [53]. All desmosomal cadherins possess membrane proximal cysteines which are well conserved between species. Interestingly, palmitoylation of DSG2 regulates trafficking as well as stability of the protein but is not necessary for raft association [63]. DSG3, also, does not require palmitoylation for raft association [5]. Thus, palmitoylation appears to be important in regulating desmosomal cadherin dynamics, but it does not appear to be necessary for raft association.
Proteins are palmitoylated by palmitoyl acyl transferases (PATs), of which there are 23 in humans [64]. Named for their conserved DHHC (Asp-His-His-Cys) motif, the DHHC proteins are multimeric transmembrane proteins with differential tissue expression that can localize to ER, Golgi, or plasma membrane to palmitoylate targets [65]. While the PAT(s) responsible for palmitoylating desmosomal proteins remain to be identified, DHHC13 and DHHC21 have been shown to be important in keratinocyte proliferation and hair follicle differentiation in the epidermis [66–68]. There is some promiscuity among DHHC targets as many proteins have been shown to be palmitoylated by multiple DHHC forms in cultured cells, raising the possibility that multiple DHHC proteins are capable of palmitoylating desmosomal proteins.
In contrast to the desmosomal cadherins, palmitoylation of the cytoplasmic PKPs is necessary for both raft association and for desmosome assembly. Loss of membrane association and reduced Triton insolubility were seen in palmitoylation-deficient PKP2 and PKP3 mutants. Furthermore, expression of these mutants resulted in the loss of desmosome assembly and adhesion in a dominant-negative fashion. PKPs are thought to function by recruiting and clustering other desmosomal proteins. This is accomplished through actively guiding DP and intermediate filaments to sites of cell-cell contact and clustering desmosomal cadherins at the cell surface [69–71]. Though PKP1 is also palmitoylated, no studies have been done to address the impact of the modification on PKP1 function. Though similar, the PKP proteins serve different purposes. For example, PKP1 but not PKP3 regulates desmoglein clustering [72]. Disease-causing truncation mutations in PKP1 lead to reduced size and number of desmosomes in patients [73, 74] while overexpression of PKP1 can increase desmosome length and adhesion strength [75]. PKP3 overexpression also increases desmosome size and stability by upregulating the expression of other desmosomal proteins [76]. PKP palmitoylation likely enhances membrane association during clustering of desmosomal cytoplasmic plaque proteins, thereby promoting the lateral packing of desmosomal proteins along the two-dimensional plane of the plasma membrane.
Role of raft association in disease
Lipid raft associated proteins have been implicated in a variety of human diseases. Well-studied examples of diseases in which raft protein function is disrupted include Alzheimer’s Disease and Prion Diseases [77]. Various pathogens also have been shown to utilize rafts for various steps in their life cycles [77]. A possible link between lipid raft disruption and atopic dermatitis has been identified as genes involved in lipid biosynthesis were found to be downregulated in patient skin relative to healthy skin [78]. Furthermore, expression profiles were similar between patient skin and cultured keratinocytes in which lipid rafts were disrupted by mβCD treatment, including the same lipid biosynthesis genes downregulated in patient skin [79]. These findings raise the possibility that altered raft function or the inability of proteins to associate with rafts could represent an underlying disease pathomechanism.
Recently, we identified a dominantly-inherited disease-causing mutation in the DSG1 gene that causes a glycine-to-arginine substitution in the transmembrane spanning region of the DSG1 protein [5]. The individuals carrying this mutation were diagnosed with severe dermatitis, multiple allergies, and metabolic wasting (SAM) syndrome. SAM syndrome is characterized by epidermal thickening, fragility, and barrier defects [26]. Studies of this TMD mutation revealed that the mutant DSG1 protein was excluded from lipid rafts and failed to assemble into desmosomes both in patient skin and in cell culture models [5]. Molecular modeling predicts that the glycine-to-arginine substitution in the DSG1 TMD shortens the run of hydrophobic amino acids, resulting in hydrophobic mismatch with the thicker lipid bilayer present in the raft-like desmosomal membrane domain. This mutation appears to be the first example of a mutation that compromises raft association as part of the disease pathomechanism.
The mutation in the DSG1 TMD that causes SAM syndrome may represent a newly appreciated class of mutations that disrupt raft association and cause human disease, particularly among proteins bearing mutations in the TMD. Disease-causing glycine-to-arginine substitutions have been identified in the TMDs of other single pass transmembrane proteins including myelin protein zero (MPZ) and FGFR3 which cause Marie-Charcot-Tooth Syndrome [80, 81] and Achondroplasia [82], respectively. Both proteins have been identified in proteomic screens assaying raft association [83, 84]. Though raft association of FGFR3 has not been further verified, MPZ has been identified in DRMs from adult peripheral nerve myelin [85, 86]. In both examples, the mutation was found to disrupt TMD-TMD mediated interactions. As oligomerization is known to increase raft affinity [87], loss of lipid raft association could be central to the disease mechanisms of these mutations. Further investigation is warranted for these and other mutations that might involve loss of raft association as part of an underlying disease pathomechanism.
A new model for epithelial intercellular junction organization: lipid rafts as a driving force for the assembly and segregation of junctional complexes
The association of desmosomes with lipid rafts is emerging as a mechanism fundamental to the organization of the desmosomal membrane domain. A key question emerging from this work is how raft association of desmosomal components integrates with the assembly mechanisms of other junctional complexes, including AJs. In contrast to desmosomes, AJ components do not associate with lipid rafts biochemically [4, 37]. However, desmosome assembly requires E-cadherin-based adhesion [88–91]. The mechanisms by which AJs regulate desmosome assembly are not fully understood, although AJs and desmosomal proteins engage in a number of overlapping protein-protein interactions [92]. Recently, Shafraz et al. [93] showed that the requirement of AJs for desmosome assembly may be driven by a direct interaction between E-cadherin and desmosomal cadherins. Using a combination of atomic force microscopy and cell biological approaches, E-cadherin trans-homodimerization was found to initiate both AJ and desmosome assembly by allowing for the brief cisheterodimerization of E-cadherin and DSG2 [93]. Concurrently, short-lived DSC2 homodimers give way to DSG2:DSC2 heterodimers when the E-cadherin:DSG heterodimers disengage. In line with these findings, others have shown that the relative cell surface levels of DSGs and DSCs regulate the adhesion process [15], but the recruitment of desmosomal cadherins begins with DSC clustering at the cell surface [94, 95]. DSG and associated armadillo proteins then stabilize the DSC clusters [94, 95].
If AJ and desmosomal proteins can associate, how do these structures resolve into distinct membrane domains? One explanation is differential protein affinities. Both PG and PKPs are required for the formation of distinct, non-continuous desmosomes [69]. Mixed adherens/desmosome junctions were identified in cardiac tissue from mice lacking PG [96]. PG is unstable in the absence of a desmosomal cadherin [97], yet is also capable of binding E-cadherin and α-catenin in place of β-catenin. However, due to overlapping binding sites, PG cannot bind α-catenin if it is bound to a desmosomal cadherin, thus excluding the desmosomal cadherin:PG complex and likely contributing to segregation of the two junctions [92]. These examples highlight how differential protein affinities contribute to the assembly of different junctions.
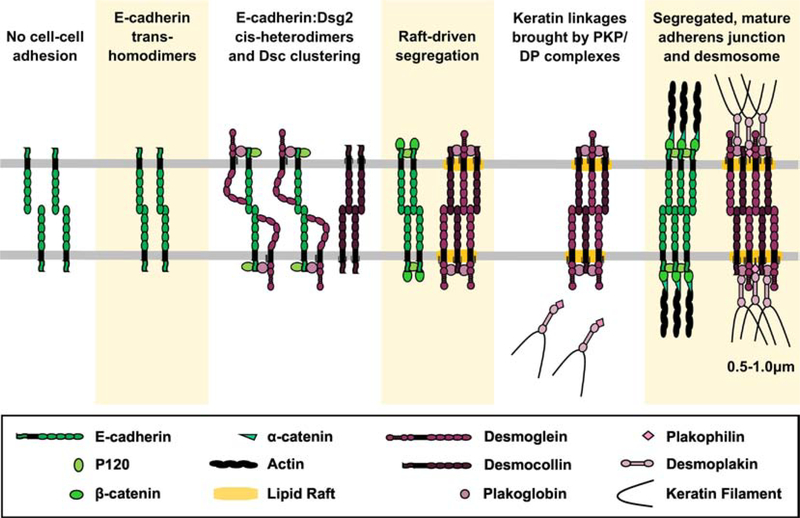
We propose that a second driving force to segregate AJs and desmosomes is differential affinity for lipid rafts (Figure 3). In contrast to desmosomal proteins, AJ components show poor affinity for raft fractions [4, 5, 37]. For example, when the desmoglein TMD is replaced with the E-cadherin TMD, the chimeric cadherin fails to associate with lipid rafts [5]. In addition, unlike desmosomal cadherins, E-cadherin is not palmitoylated [98], further reducing raft affinity. As discussed above, E-cadherin and desmoglein associate at the plasma membrane during initial cell-cell contact formation. Super-resolution imaging shows that these nascent complexes then resolve into separate membrane domains as junction formation progresses [93]. It is likely that desmosomal cadherin association with raft lipids harboring longer acyl chains and with higher levels of cholesterol leads to thickening of the lipid bilayer and the formation of a membrane environment energetically unfavorable for the shorter E-cadherin TMD. Through this mechanism, E-cadherin and cytoplasmic plaque proteins associated with E-cadherin would be excluded from the desmosome as junction formation proceeds. Similarly, the palmitoylated desmosomal plaque proteins, particularly PKPs, would further drive coalescence into a raft domain and further exclude AJ components. Such a mechanism would allow for coordinated assembly of the two junctions followed by subsequent resolution into distinct membrane domains.
Figure 3: Lipid rafts drive segregation of adherens junctions and desmosomes during junction assembly.
Transient interactions between desmosomal and classical cadherins coordinates cadherin clustering at the cell surface. Adaptor proteins stabilize these nascent cadherin clusters. Interplay between cadherins and membrane lipids leads to accumulation of cholesterol and raft lipids specifically around desmosomal cadherin clusters and drives segregation of AJs and desmosome components. Plakophilins further enhance desmosome formation and segregation from AJs by promoting raft-associated clustering and by promoting desmoplakin and keratin linkages.
It is possible that similar principles apply to TJ assembly. TJ components are also raft associated [4] and also require AJs to drive assembly [99, 100]. Like the desmogleins and desmocollins, the claudins are also palmitoylated [101–103]. Furthermore, there are overlapping protein-protein interactions between TJ and AJ components [104, 105]. In simple polarized epithelial cells, such as those lining the intestines, mature junctions are arranged along the basolateral sides of adjacent cells such that TJs are most apical followed by AJs and then desmosomes. An attractive model is that E-cadherin associates with TJ and desmosomal components to initiate the formation of the apical junctional complex, but that this process is followed by the recruitment of raft lipids and cholesterol into TJ and desmosome domains to drive the segregation of the proteins into distinct membrane domains. In this manner, differential raft association would be a key driver of the overall organization of the apical junctional complex.
Conclusions and Future Directions
Desmosomes represent an important intercellular junction that also offers unique properties for the broader study of raft-like membrane domains. Desmosomes are mesoscale domains that can be identified by both super-resolution optical imaging as well as electron microscopy. At the same time, desmosomal proteins have been identified as verified lipid raft targeting molecules. Desmosomes can also be identified within tissues and isolated from tissue homogenates, making these structures ideal for analysis of raft targeting properties of desmosomal proteins using both imaging and biochemical approaches. Ongoing studies to understand how raft association of desmosomal components impacts the ability of desmosomal proteins to cluster and mediate adhesion will be critical in defining how raft association contributes to the densely packed and strongly adhesive nature of the desmosomal junction. Similarly, although keratin linkages are essential for strong desmosomal adhesion [106–108] we do not yet know how these linkages influence raft association, or alternatively, how raft association of desmosomal proteins influences keratin filament organization.
Desmosomes are both morphologically and functionally distinct from AJs. One key difference is that desmosomes are tightly packed and are able to achieve a calcium-independent, hyperadhesive state [109–111]. This state is characterized by the appearance of an electron-dense midline in electron microscopy images and stronger adhesion [110]. Though the predominant state in tissues, the hyperadhesive state is reversible; desmosomes can return to a state of calcium-dependence for the purpose of wound healing or tissue remodeling [110]. Hyperadhesion is regulated by phosphorylation of DP by PKCα [112, 113]. PKC inhibition can even initiate desmosome assembly in the absence of AJs or calcium [114, 115]. Recent work has shown that PKC inhibition limits desmosomal plaque protein diffusion out of the desmosome, thereby conferring hyperadhesion and calcium independence [Bartle et al, in press, DOI10.1083/jcb.201906153]. Lastly, desmosomal cadherins have recently been shown by fluorescence polarization to arrange into highly ordered configurations [116] that presumably contribute to desmosomal cadherin clustering and adhesion, but we do not yet know how raft association impacts desmosomal cadherin organization within desmosomes.
In addition to the assembly of the desmosomal membrane domain, it is important to consider that the relationship between lipid rafts and desmosomes may extend beyond desmosome dynamics and adhesive functions. Lipid rafts have been shown to impact the processes of proliferation, migration, apoptosis, and differentiation in keratinocytes [117–121]. Desmosomal components have also been shown to play a role in each of these processes. The signaling molecules p38 mitogen-activated protein kinase, Akt, ERK1/2, and EGFR have been found to be abnormally activated when lipid rafts are disrupted in keratinocytes by methyl-β-cyclodextrin (mβCD) treatment [79, 118, 121, 122]. Similarly, many of the knockout mouse models described above have revealed that desmosomal components are involved in regulating important signaling pathways, including the Wnt [123], EGFR, PI3-kinase/AKT, and NF-κB signaling pathways [28]. Future studies will be needed to understand how the integration of desmosomal cadherin adhesion and signaling is achieved and how the association of signaling molecules and desmosomal components with raft domains impacts this adhesion and signaling network.
Acknowledgements
The authors would like to thank members of the Kowalczyk lab as well as Drs. Kathleen J. Green and Sara N. Stahley for helpful suggestions in the preparation of this manuscript. Work in the Kowalczyk lab is supported by grants R01AR050501 and R01AR048266 from the National Institutes of Health. SEZ was supported by a training grant from the National Institutes of Health T32GM008367.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Sezgin E, Levental I, Mayor S, Eggeling C, The mystery of membrane organization: composition, regulation and roles of lipid rafts, Nat Rev Mol Cell Biol, 18 (2017) 361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Suzuki T, Zhang J, Miyazawa S, Liu Q, Farzan MR, Yao WD, Association of membrane rafts and postsynaptic density: proteomics, biochemical, and ultrastructural analyses, J Neurochem, 119 (2011) 64–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Field KA, Holowka D, Baird B, Fc epsilon RI-mediated recruitment of p53_56lyn to detergent-resistant membrane domains accompanies cellular signaling, Proc Natl Acad Sci USA, 92 (1995) 9201–9205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nusrat A, Parkos CA, Verkade P, Foley CS, Liang TW, Innis-Whitehouse W, Eastburn KK, Madara JL, Tight junctions are membrane microdomains, Journal of Cell Science, 113 (2000) 1771–1781. [DOI] [PubMed] [Google Scholar]
- [5].Lewis JD, Caldara AL, Zimmer SE, Stahley SN, Seybold A, Strong NL, Frangakis AS, Levental I, Wahl JK 3rd, Mattheyses AL, Sasaki T, Nakabayashi K, Hata K, Matsubara Y, Ishida-Yamamoto A, Amagai M, Kubo A, Kowalczyk AP, The desmosome is a mesoscale lipid raft-like membrane domain, Mol Biol Cell, 30 (2019) 1390–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fiedler K, Kobayashi T, Kurzchalia TV, Simons K, Glycosphingolipid-enriched, detergent insoluble complexes in protein sorting in epithelial cells, Biochemistry, 32 (1993) 6365–6373. [DOI] [PubMed] [Google Scholar]
- [7].Scheiffele P, Rietveld A, Wilk T, Simons K, Influenza viruses select ordered lipid domains during budding from the plasma membrane, The Journal of Biological Chemistry, 274 (1999) 2038–2044. [DOI] [PubMed] [Google Scholar]
- [8].Head BP, Patel HH, Insel PA, Interaction of membrane/lipid rafts with the cytoskeleton: impact on signaling and function: membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling, Biochim Biophys Acta, 1838 (2014) 532–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tsukita S, Furuse M, Itoh M, Multifunctional strands in tight junctions, Nature Reviews, 2 (2001) 285–293. [DOI] [PubMed] [Google Scholar]
- [10].Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, Matsui T, Tsukita S, Furuse M, Tsukita S, ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation, Cell, 126 (2006) 741–754. [DOI] [PubMed] [Google Scholar]
- [11].Garcia MA, Nelson WJ, Chavez N, Cell-Cell Junctions Organize Structural and Signaling Networks, Cold Spring Harb Perspect Biol, 10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Goodenough DA, Connexins, connexons, and intercellular communication, Annu. Rev. Biochem, 65 (1996). [DOI] [PubMed] [Google Scholar]
- [13].Buckley CD, Tan J, Anderson KL, Hanein D, Volkmann N, Weis WI, Nelson WJ, Dunn AR, Cell adhesion. The minimal cadherin-catenin complex binds to actin filaments under force, Science, 346 (2014) 1254211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kowalczyk AP, Green KJ, Structure, function, and regulation of desmosomes, Prog Mol Biol Transl Sci, 116 (2013) 95–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Getsios S, Amargo EV, Dusek RL, Ishii K, Sheu L, Godsel LM, Green KJ, Coordinated expression of desmoglein 1 and desmocollin 1 regulates intercellular adhesion, Differentiation, 72 (2004) 419–433. [DOI] [PubMed] [Google Scholar]
- [16].Nie Z, Merritt A, Rouhi-Parkouhi M, Tabernero L, Garrod D, Membrane-impermeable cross-linking provides evidence for homophilic, isoform-specific binding of desmosomal cadherins in epithelial cells, J Biol Chem, 286 (2011) 2143–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Harrison OJ, Brasch J, Lasso G, Katsamba PS, Ahlsen G, Honig B, Shapiro L, Structural basis of adhesive binding by desmocollins and desmogleins, Proc Natl Acad Sci U S A, 113 (2016) 7160–7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chitaev NA, Troyanovsky SM, Direct Ca2+-dependent heterophilic interaction between desmosomal cadherins, desmoglein and desmocollin, contributes to cell-cell adhesion, The Journal of Cell Biology, 138 (1997) 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schafer S, Koch PJ, Frank WW, Identification of the ubiquitous human desmoglein, Dsg2, and the expression catalogue of the dsg subfamily of desmosomal cadherins, Experimental Cell Research, 211 (1994) 391–399. [DOI] [PubMed] [Google Scholar]
- [20].Troyanovsky SM, Troyanovsky RB, Eshkind LG, Leube RE, Franke WW, Identification of amino acid sequence motifs in desmocollin, a desmosomal glycoprotein, that are required for plakoglobin binding and plaque formation, Proc Natl Acad Sci U S A, 91 (1994) 10790–10794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Troyanovsky SM, Troyanovsky RB, Eshkind LG, Krutovskikh VA, Leube RE, Franke WW, Identification of the plakoglobin-binding domain in desmoglein and its role in plaque assembly and intermediate filament anchorage, J Cell Biol, 127 (1994) 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mathur M, Goodwin L, Cowin P, Interactions of the cytoplasmic domain of the desmosomal cadherin Dsg1 with plakoglobin, The Journal of Biological Chemistry, 269 (1994) 14075–14080. [PubMed] [Google Scholar]
- [23].Nilles LA, Parry DAD, Powers EE, Angst BD, Wagner RM, Green KJ, Structural analysis and expression of human desmoglein: a cadherin-like component of the desmosome, Journal of Cell Science, 99 (1991) 809–821. [DOI] [PubMed] [Google Scholar]
- [24].Chen J, Nekrasova OE, Patel DM, Klessner JL, Godsel LM, Koetsier JL, Amargo EV, Desai BV, Green KJ, The C-terminal unique region of desmoglein 2 inhibits its internalization via tail-tail interactions, J Cell Biol, 199 (2012) 699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rutman AJ, Buxton RS, Burdett IDJ, Visualisation by electron microscopy of the unique part of the cytoplasmic domain of desmoglein, a cadherin-like protein of the desmosome type of cell junction, FEBS Letters, 353 (1994) 194–196. [DOI] [PubMed] [Google Scholar]
- [26].Samuelov L, Sarig O, Harmon RM, Rapaport D, Ishida-Yamamoto A, Isakov O, Koetsier JL, Gat A, Goldberg I, Bergman R, Spiegel R, Eytan O, Geller S, Peleg S, Shomron N, Goh CSM, Wilson NJ, Smith FJD, Pohler E, Simpson MA, McLean WHI, Irvine AD, Horowitz M, McGrath JA, Green KJ, Sprecher E, Desmoglein 1 deficiency results in severe dermatitis, multiple allergies and metabolic wasting, Nat Genet, 45 (2013) 1244–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Elsholz F, Harteneck C, Muller W, Friedland K, Calcium--a central regulator of keratinocyte differentiation in health and disease, Eur J Dermatol, 24 (2014) 650–661. [DOI] [PubMed] [Google Scholar]
- [28].Brennan D, Hu Y, Joubeh S, Choi YW, Whitaker-Menezes D, O’Brien T, Uitto J, Rodeck U, Mahoney MG, Suprabasal Dsg2 expression in transgenic mouse skin confers a hyperproliferative and apoptosis-resistant phenotype to keratinocytes, J Cell Sci, 120 (2007) 758–771. [DOI] [PubMed] [Google Scholar]
- [29].Delva E, Tucker DK, Kowalczyk AP, The desmosome, Cold Spring Harb Perspect Biol, 1 (2009) a002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Getsios S, Simpson CL, Kojima S, Harmon R, Sheu LJ, Dusek RL, Cornwell M, Green KJ, Desmoglein 1-dependent suppression of EGFR signaling promotes epidermal differentiation and morphogenesis, J Cell Biol, 185 (2009) 1243–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Harmon RM, Simpson CL, Johnson JL, Koetsier JL, Dubash AD, Najor NA, Sarig O, Sprecher E, Green KJ, Desmoglein-1/Erbin interaction suppresses ERK activation to support epidermal differentiation, J Clin Invest, 123 (2013) 1556–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Franke WW, Borrmann CM, Grund C, Pieperhoff S, The area composita of adhering junctions connecting heart muscle cells of vertebrates. I. Molecular definition in intercalated disks of cardiomyocytes by immunoelectron microscopy of desmosomal proteins, Eur J Cell Biol, 85 (2006) 69–82. [DOI] [PubMed] [Google Scholar]
- [33].Vermij SH, Abriel H, van Veen TA, Refining the molecular organization of the cardiac intercalated disc, Cardiovasc Res, 113 (2017) 259–275. [DOI] [PubMed] [Google Scholar]
- [34].Ohno S, The genetic background of arrhythmogenic right ventricular cardiomyopathy, J Arrhythm, 32 (2016) 398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Stahley SN, Kowalczyk AP, Desmosomes in acquired disease, Cell Tissue Res, 360 (2015) 439–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Resnik N, Sepcic K, Plemenitas A, Windoffer R, Leube R, Veranic P, Desmosome assembly and cell-cell adhesion are membrane raft-dependent processes, The Journal of biological chemistry, 286 (2011) 1499–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Stahley SN, Saito M, Faundez V, Koval M, Mattheyses AL, Kowalczyk AP, Desmosome assembly and disassembly are membrane raft-dependent, PLoS One, 9 (2014) e87809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Vielmuth F, Waschke J, Spindler V, Loss of Desmoglein Binding Is Not Sufficient for Keratinocyte Dissociation in Pemphigus, J Invest Dermatol, 135 (2015) 3068–3077. [DOI] [PubMed] [Google Scholar]
- [39].Skerrow CJ, Matoltsy AG, Chemical characterization of isolated epidermal desmosomes, Journal of Cell Biology, 63 (1974) 524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Drochmans P, Freudenstein C, Wanson J-C, Laurent L, Keenan TW, Stadler J, Leloup R, Franke WW, Structure and biochemical composition of desmosomes and tonofilaments isolated from calf muzzle epidermis, Journal of Cell Biology, 79 (1978) 427–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nava P, Laukoetter MG, Hopkins AM, Laur O, Gerner-Smidt K, Green KJ, Parkos CA, Nusrat A, Desmoglein-2: a novel regulator of apoptosis in the intestinal epithelium, Mol Biol Cell, 18 (2007) 4565–4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Resnik N, de Luca GMR, Sepcic K, Romih R, Manders E, Veranic P, Depletion of the cellular cholesterol content reduces the dynamics of desmosomal cadherins and interferes with desmosomal strength, Histochem Cell Biol, (2019). [DOI] [PubMed] [Google Scholar]
- [43].Vollner F, Ali J, Kurrle N, Exner Y, Eming R, Hertl M, Banning A, Tikkanen R, Loss of flotillin expression results in weakened desmosomal adhesion and Pemphigus vulgaris-like localisation of desmoglein-3 in human keratinocytes, Sci Rep, 6 (2016) 28820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lewis BA, Engelman DM, Lipid bilayer thickness varies linearly with acyl chain length in fluid phosphatidylcholine vesicles, Journal of Molecular Biology, 166 (1983) 211–217. [DOI] [PubMed] [Google Scholar]
- [45].Nezil FA, Bloom M, Combined influence of cholesterol and synthetic amphiphillic peptides upon bilayer thickness in model membranes, Biophys. J, 61 (1992) 1176–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kucerka N, Perlmutter JD, Pan J, Tristram-Nagle S, Katsaras J, Sachs JN, The effect of cholesterol on short- and long-chain monounsaturated lipid bilayers as determined by molecular dynamics simulations and X-ray scattering, Biophys J, 95 (2008) 2792–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Niemela PS, Ollila S, Hyvönen MT, Karttunen M, Vattulainen I, Assessing the Nature of Lipid Raft Membranes, PLoS Computational Biology, preprint (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Molugu TR, Brown MF, Cholesterol-induced suppression of membrane elastic fluctuations at the atomistic level, Chem Phys Lipids, 199 (2016) 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hedger G, Sansom MSP, Lipid interaction sites on channels, transporters and receptors: Recent insights from molecular dynamics simulations, Biochim Biophys Acta, 1858 (2016) 2390–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hulce JJ, Cognetta AB, Niphakis MJ, Tully SE, Cravatt BF, Proteome-wide mapping of cholesterol-interacting proteins in mammalian cells, Nat Methods, 10 (2013) 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Diaz-Rohrer BB, Levental KR, Simons K, Levental I, Membrane raft association is a determinant of plasma membrane localization, Proc Natl Acad Sci U S A, 111 (2014) 8500–8505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lorent JH, Diaz-Rohrer B, Lin X, Spring K, Gorfe AA, Levental KR, Levental I, Structural determinants and functional consequences of protein affinity for membrane rafts, Nat Commun, 8 (2017) 1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Levental I, Lingwood D, Grzybek M, Coskun U, Simons K, Palmitoylation regulates raft affinity for the majority of integral raft proteins, Proc Natl Acad Sci U S A, 107 (2010) 22050–22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bretscher MS, Munro S, Cholesterol and the Golgi apparatus, Science, 261 (1993) 1280–1281. [DOI] [PubMed] [Google Scholar]
- [55].Sharpe HJ, Stevens TJ, Munro S, A comprehensive comparison of transmembrane domains reveals organelle-specific properties, Cell, 142 (2010) 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Mouritsen OG, Bloom M, Mattress model of lipid-protein interactions in membranes, Biophys J, 46 (1984) 141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lin Q, London E, Altering hydrophobic sequence lengths shows that hydrophobic mismatch controls affinity for ordered lipid domains (rafts) in the multitransmembrane strand protein perfringolysin O, J Biol Chem, 288 (2013) 1340–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Yuan Z, Zhang F, Davis MJ, Boden M, Teasdale RD, Predicting the solvent accessibility of transmembrane residues from protein sequence, Journal of Proteome Research, 5 (2006) 1063–1070. [DOI] [PubMed] [Google Scholar]
- [59].Xu L, Hu T-T, Luo S-Z, Leucine Zipper Motif Drives the Transmembrane Domain Dimerization of E-cadherin, International Journal of Peptide Research and Therapeutics, 20 (2013) 95–102. [Google Scholar]
- [60].Blaskovic S, Blanc M, van der Goot FG, What does S-palmitoylation do to membrane proteins?, FEBS Journal, 280 (2013) 2766–2774. [DOI] [PubMed] [Google Scholar]
- [61].Rocks O, Peyker A, Kahm M, Verveer PJ, Koerner C, Lumbierres M, Kuhlmann J, Waldmann H, Wittinghofer A, Bastiaens PIH, An acylation cycle regulates localization and activity of palmitoylated Ras forms, Science, 307 (2005) 1746–1752. [DOI] [PubMed] [Google Scholar]
- [62].El-Husseini AE-D, Schnell E, Dakoji S, Sweeney N, Zhou Q, Prange O, Gauthier-Campbell C, Aguilera-Moreno A, Nicoll RA, Bredt DS, Synaptic strength regulated by palmitate cycling on PSD-95, Cell, 108 (2002) 849–863. [DOI] [PubMed] [Google Scholar]
- [63].Roberts BJ, Svoboda RA, Overmiller AM, Lewis JD, Kowalczyk AP, Mahoney MG, Johnson KR, Wahl JK 3rd, Palmitoylation of Desmoglein 2 Is a Regulator of Assembly Dynamics and Protein Turnover, J Biol Chem, 291 (2016) 24857–24865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Fukata M, Fukata Y, Adesnik H, Nicoll RA, Bredt DS, Identification of PSD-95 palmitoylating enzymes, Neuron, 44 (2004) 987–996. [DOI] [PubMed] [Google Scholar]
- [65].Ohno Y, Kihara A, Sano T, Igarashi Y, Intracellular localization and tissue-specific distribution of human and yeast DHHC cysteine-rich domain-containing proteins, Biochim Biophys Acta, 1761 (2006) 474–483. [DOI] [PubMed] [Google Scholar]
- [66].Mill P, Lee AW, Fukata Y, Tsutsumi R, Fukata M, Keighren M, Porter RM, McKie L, Smyth I, Jackson IJ, Palmitoylation regulates epidermal homeostasis and hair follicle differentiation, PLoS Genet, 5 (2009) e1000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Perez CJ, Mecklenburg L, Jaubert J, Martinez-Santamaria L, Iritani BM, Espejo A, Napoli E, Song G, Del Rio M, DiGiovanni J, Giulivi C, Bedford MT, Dent SYR, Wood RD, Kusewitt DF, Guenet JL, Conti CJ, Benavides F, Increased Susceptibility to Skin Carcinogenesis Associated with a Spontaneous Mouse Mutation in the Palmitoyl Transferase Zdhhc13 Gene, J Invest Dermatol, 135 (2015) 3133–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Chen LY, Lin KR, Chen YJ, Chiang YJ, Ho KC, Shen LF, Song IW, Liu KM, Yang-Yen HF, Chen YJ, Chen YT, Liu FT, Yen JJY, Palmitoyl acyltransferase activity of ZDHHC13 regulates skin barrier development partly by controlling PADi3 and TGM1 protein stability, J Invest Dermatol, (2019). [DOI] [PubMed] [Google Scholar]
- [69].Bornslaeger EA, Godsel LM, Corcoran CM, Park JK, Hatzfeld M, Kowalczyk AP, Green KJ, Plakophilin-1 interferes with plakoglobin binding to desmoplakin, yet together with plakoglobin promotes clustering of desmosomal plaque complexes at cell-cell borders, Journal of Cell Science, 114 (2001) 727–738. [DOI] [PubMed] [Google Scholar]
- [70].Sobolik-Delmaire T, Katafiasz D, Wahl JK 3rd, Carboxyl terminus of Plakophilin-1 recruits it to plasma membrane, whereas amino terminus recruits desmoplakin and promotes desmosome assembly, J Biol Chem, 281 (2006) 16962–16970. [DOI] [PubMed] [Google Scholar]
- [71].Godsel LM, Hsieh SN, Amargo EV, Bass AE, Pascoe-McGillicuddy LT, Huen AC, Thorne ME, Gaudry CA, Park JK, Myung K, Goldman RD, Chew TL, Green KJ, Desmoplakin assembly dynamics in four dimensions: multiple phases differentially regulated by intermediate filaments and actin, J Cell Biol, 171 (2005) 1045–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Fuchs M, Foresti M, Radeva MY, Kugelmann D, Keil R, Hatzfeld M, Spindler V, Waschke J, Vielmuth F, Plakophilin 1 but not plakophilin 3 regulates desmoglein clustering, Cell Mol Life Sci, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].McGrath JA, McMillan JR, Shemanko CS, Runswick SK, Leigh IM, Lane EB, Garrod DR, Eady RAJ, Mutations in the plakophilin 1 gene result in ectodermal dysplasia/skin fragility syndrome, Nature Genetics, 17 (1997) 240–244. [DOI] [PubMed] [Google Scholar]
- [74].South AP, Wan H, Stone MG, Dopping-Hepenstal PJ, Purkis PE, Marshall JF, Leigh IM, Eady RA, Hart IR, McGrath JA, Lack of plakophilin 1 increases keratinocyte migration and reduces desmosome stability, J Cell Sci, 116 (2003) 3303–3314. [DOI] [PubMed] [Google Scholar]
- [75].Tucker DK, Stahley SN, Kowalczyk AP, Plakophilin-1 protects keratinocytes from pemphigus vulgaris IgG by forming calcium-independent desmosomes, J Invest Dermatol, 134 (2014) 1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Gurjar M, Raychaudhuri K, Mahadik S, Reddy D, Atak A, Shetty T, Rao K, Karkhanis MS, Gosavi P, Sehgal L, Gupta S, Dalal SN, Plakophilin3 increases desmosome assembly, size and stability by increasing expression of desmocollin2, Biochem Biophys Res Commun, 495 (2018) 768–774. [DOI] [PubMed] [Google Scholar]
- [77].Simons K, Ehehalt R, Cholesterol, lipid rafts, and disease, Journal of Clinical Investigation, 110 (2002) 597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Saaf AM, Tengvall-Linder M, Chang HY, Adler AS, Wahlgren CF, Scheynius A, Nordenskjold M, Bradley M, Global expression profiling in atopic eczema reveals reciprocal expression of inflammatory and lipid genes, PLoS One, 3 (2008) e4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Mathay C, Pierre M, Pittelkow MR, Depiereux E, Nikkels AF, Colige A, Poumay Y, Transcriptional Profiling after Lipid Raft Disruption in Keratinocytes Identifies Critical Mediators of Atopic Dermatitis Pathways, Journal of Investigative Dermatology, 131 (2011) 46–58. [DOI] [PubMed] [Google Scholar]
- [80].Eggers SD, Keswani SC, Melli G, Cornblath DR, Clinical and genetic description of a family with Charcot-Marie-Tooth disease type 1B from a transmembrane MPZ mutation, Muscle Nerve, 29 (2004) 867–869. [DOI] [PubMed] [Google Scholar]
- [81].Plotkowski ML KS, Phillips ML, Partridge AW, Deber CM, Bowie JU, Transmembrane domain of myelin protein zero can form dimers- possible implications for myelin construction, Biochemistry, 46 (2007) 12164–12173. [DOI] [PubMed] [Google Scholar]
- [82].Han X, Mihailescu M, Hristova K, Neutron diffraction studies of fluid bilayers with transmembrane proteins: structural consequences of the achondroplasia mutation, Biophys J, 91 (2006) 3736–3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Arielly SS, Ariel M, Yehuda R, Scigelova M, Yehezkel G, Khalaila I, Quantitative analysis of caveolin-rich lipid raft proteins from primary and metastatic colorectal cancer clones, J Proteomics, 75 (2012) 2629–2637. [DOI] [PubMed] [Google Scholar]
- [84].Staubach S, Muller S, Pekmez M, Hanisch FG, Classical Galactosemia: Insight into Molecular Pathomechanisms by Differential Membrane Proteomics of Fibroblasts under Galactose Stress, J Proteome Res, 16 (2017) 516–527. [DOI] [PubMed] [Google Scholar]
- [85].Erne B, Sansano S, Frank M, Schaeren-Wiemers N, Rafts in adult peripheral nerve myelin contain major structural myelin proteins and myelin and lymphocyte protein (MAL) and CD59 as specific markers, Journal of Neurochemistry, 82 (2002) 550–562. [DOI] [PubMed] [Google Scholar]
- [86].Fasano A, Amoresano A, Rossano R, Carlone G, Carpentieri A, Liuzzi GM, Pucci P, Riccio P, The different forms of PNS myelin P0 protein within and outside lipid rafts, J Neurochem, 107 (2008) 291–301. [DOI] [PubMed] [Google Scholar]
- [87].Harder T, Scheiffele P, Verkade P, Simons K, Lipid domain structure of the plasma membrane revealed by patching of membrane components, The Journal of Cell Biology, 141 (1998) 929–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Gumbiner B, Stevenson B, Grimaldi A, The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex, The Journal of Cell Biology, 107 (1988) 1575–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Watabe-Uchida M, Uchida N, Imamura Y, Nagafuchi A, Fujimoto K, Uemura T, Vermeulen S, F.v. Roy, E.D. Adamson, M. Takeichi, alpha-catenin-vinculin interaction functions to organize the apical junctional complex in epithelial cells, The Journal of Cell Biology, 142 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Lewis JE, Jensen PJ, Wheelock MJ, Cadherin Function Is Required for Human Keratinocytes to Assemble Desmosomes and Stratify in Response to Calcium, Journal of Investigative Dermatology, 102 (1994) 870–877. [DOI] [PubMed] [Google Scholar]
- [91].Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E, Hyperproliferation and defects in epithelial polarity upon conditional ablation of a-catenin in skin, Cell, 104 (2001) 605–617. [DOI] [PubMed] [Google Scholar]
- [92].Choi HJ, Gross JC, Pokutta S, Weis WI, Interactions of plakoglobin and beta-catenin with desmosomal cadherins: basis of selective exclusion of alpha- and beta-catenin from desmosomes, J Biol Chem, 284 (2009) 31776–31788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Shafraz O, Rubsam M, Stahley SN, Caldara AL, Kowalczyk AP, Niessen CM, Sivasankar S, E-cadherin binds to desmoglein to facilitate desmosome assembly, Elife, 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Lowndes M, Rakshit S, Shafraz O, Borghi N, Harmon RM, Green KJ, Sivasankar S, Nelson WJ, Different roles of cadherins in the assembly and structural integrity of the desmosome complex, J Cell Sci, 127 (2014) 2339–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Burdett ID, Sullivan KH, Desmosome assembly in MDCK cells: transport of precursors to the cell surface occurs by two phases of vesicular traffic and involves major changes in centrosome and Golgi location during a Ca(2+) shift, Exp Cell Res, 276 (2002) 296–309. [DOI] [PubMed] [Google Scholar]
- [96].Ruiz P, Brinkmann V, Ledermann B, Behrend M, Grund C, Thalhammer C, Vogel F, Birchmeier C, Gunthert U, Franke WW, Birchmeier W, Targeted mutation of PG in mice reveals essential functions of desmosomes in the embryonic heart, The Journal of Cell Biology, 135 (1996) 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kowalczyk AP, Palka HL, Luu HH, Nilles LA, Anderson JE, Wheelock MJ, Green KJ, Posttranslational regulation of plakoglobin expression: influence of the desmosomal cadherins on plakoglobin metabolic stability, The Journal of Biological Chemistry, 269 (1994) 31214–31223. [PubMed] [Google Scholar]
- [98].Roberts BJ, Johnson KE, McGuinn KP, Saowapa J, Svoboda RA, Mahoney MG, Johnson KR, Wahl JK 3rd, Palmitoylation of plakophilin is required for desmosome assembly, J Cell Sci, 127 (2014) 3782–3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Michels C, Aghdam SY, Niessen CM, Cadherin-mediated regulation of tight junctions in stratifying epithelia, Ann N Y Acad Sci, 1165 (2009) 163–168. [DOI] [PubMed] [Google Scholar]
- [100].Shigetomi K, Ono Y, Inai T, Ikenouchi J, Adherens junctions influence tight junction formation via changes in membrane lipid composition, J Cell Biol, 217 (2018) 2373–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Van Itallie CM, Gambling TM, Carson JL, Anderson JM, Palmitoylation of claudins is required for efficient tight-junction localization, J Cell Sci, 118 (2005) 1427–1436. [DOI] [PubMed] [Google Scholar]
- [102].Heiler S, Mu W, Zoller M, Thuma F, The importance of claudin-7 palmitoylation on membrane subdomain localization and metastasis-promoting activities, Cell Commun Signal, 13 (2015) 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Rajagopal N, Irudayanathan FJ, Nangia S, Palmitoylation of Claudin-5 Proteins Influences Their Lipid Domain Affinity and Tight Junction Assembly at the Blood-Brain Barrier Interface, J Phys Chem B, 123 (2019) 983–993. [DOI] [PubMed] [Google Scholar]
- [104].Yokoyama S, Tachibana K, Nakanishi H, Yamamoto Y, Irie K, Mandai K, Nagafuchi A, Monden M, Takai Y, a-Catenin-indepedent recruitment of ZO-1 to nectin-based cell-cell adhesion sites through afadin, Molecular Biology of the Cell, 12 (2001) 1595–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Ooshio T, Kobayashi R, Ikeda W, Miyata M, Fukumoto Y, Matsuzawa N, Ogita H, Takai Y, Involvement of the interaction of afadin with ZO-1 in the formation of tight junctions in Madin-Darby canine kidney cells, J Biol Chem, 285 (2010) 5003–5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Loranger A, Gilbert S, Brouard JS, Magin TM, Marceau N, Keratin 8 modulation of desmoplakin deposition at desmosomes in hepatocytes, Exp Cell Res, 312 (2006) 4108–4119. [DOI] [PubMed] [Google Scholar]
- [107].Kroger C, Loschke F, Schwarz N, Windoffer R, Leube RE, Magin TM, Keratins control intercellular adhesion involving PKC-alpha-mediated desmoplakin phosphorylation, J Cell Biol, 201 (2013) 681–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Bar J, Kumar V, Roth W, Schwarz N, Richter M, Leube RE, Magin TM, Skin fragility and impaired desmosomal adhesion in mice lacking all keratins, J Invest Dermatol, 134 (2014) 1012–1022. [DOI] [PubMed] [Google Scholar]
- [109].Watt FM, Mattey DL, Garrod DR, Calcium-induced reorganization of desmosomal components in cultured human keratinocytes, The Journal of Cell Biology, 99 (1984) 2211–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Garrod DR, Berika MY, Bardsley WF, Holmes D, Tabernero L, Hyper-adhesion in desmosomes: its regulation in wound healing and possible relationship to cadherin crystal structure, J Cell Sci, 118 (2005) 5743–5754. [DOI] [PubMed] [Google Scholar]
- [111].Kimura TE, Merritt AJ, Garrod DR, Calcium-independent desmosomes of keratinocytes are hyper-adhesive, J Invest Dermatol, 127 (2007) 775–781. [DOI] [PubMed] [Google Scholar]
- [112].Hobbs RP, Green KJ, Desmoplakin regulates desmosome hyperadhesion, J Invest Dermatol, 132 (2012) 482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Wallis S, Lloyd S, Wise I, Ireland G, Fleming TP, Garrod D, The alpha isoform of protein kinase C is involved in signaling the response of desmosomes to wounding in cultured epithelial cells, Molecular Biology of the Cell, 11 (2000) 1077–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Sheu H-M, Kitajima Y, Yaoita H, Involvement of protein kinase C in translocation of desmoplakins from cytosol to plasma membrane during desmosome formation in human squamous cell carcinoma cells grown in low to normal calcium concentration, Experimental Cell Research, 185 (1989) 176–190. [DOI] [PubMed] [Google Scholar]
- [115].Hengel J.v., Gohon L, Bruyneel E, Vermeulen S, Cornelissen M, Mareel M, Roy F.v., Protein kinase C activation upregulates intercellular adhesion of alpha-catenin-negative human colon cancer cell variants via induction of desmosomes, The Journal of Cell Biology, 137 (1997) 1103–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Bartle EI, Urner TM, Raju SS, Mattheyses AL, Desmoglein 3 Order and Dynamics in Desmosomes Determined by Fluorescence Polarization Microscopy, Biophys J, 113 (2017) 2519–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Calay D, Vind-Kezunovic D, Frankart A, Lambert S, Poumay Y, Gniadecki R, Inhibition of Akt signaling by exclusion from lipid rafts in normal and transformed epidermal keratinocytes, J Invest Dermatol, 130 (2010) 1136–1145. [DOI] [PubMed] [Google Scholar]
- [118].Jans R, Atanasova G, Jadot M, Poumay Y, Cholesterol depletion upregulates involucrin expression in epidermal keratinocytes through activation of p38, J Invest Dermatol, 123 (2004) 564–573. [DOI] [PubMed] [Google Scholar]
- [119].Lambert S, Ameels H, Gniadecki R, Herin M, Poumay Y, Internalization of EGF receptor following lipid rafts disruption in keratinocytes is delayed and dependent on p38 MAPK activation, J Cell Physiol, 217 (2008) 834–845. [DOI] [PubMed] [Google Scholar]
- [120].Giltaire S, Lambert S, Poumay Y, HB-EGF synthesis and release induced by cholesterol depletion of human epidermal keratinocytes is controlled by extracellular ATP and involves both p38 and ERK½ signaling pathways, J Cell Physiol, 226 (2011) 1651–1659. [DOI] [PubMed] [Google Scholar]
- [121].Mathay C, Giltaire S, Minner F, Bera E, Herin M, Poumay Y, Heparin-binding EGF-like growth factor is induced by disruption of lipid rafts and oxidative stress in keratinocytes and participates in the epidermal response to cutaneous wounds, J Invest Dermatol, 128 (2008) 717–727. [DOI] [PubMed] [Google Scholar]
- [122].Lambert S, Vind-Kezunovic D, Karvinen S, Gniadecki R, Ligand-independent activation of the EGFR by lipid raft disruption, J Invest Dermatol, 126 (2006) 954–962. [DOI] [PubMed] [Google Scholar]
- [123].Hardman MJ, Liu K, Avilion AA, Merritt A, Brennan K, Garrod DR, Byrne C, Desmosomal cadherin misexpression alters beta-catenin stability and epidermal differentiation, Mol Cell Biol, 25 (2005) 969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Kugelmann D, Radeva MY, Spindler V, Waschke J, Desmoglein 1 Deficiency Causes Lethal Skin Blistering, J Invest Dermatol, 139 (2019) 1596–1599 e1592. [DOI] [PubMed] [Google Scholar]
- [125].Eshkind L, Tian Q, Schmidt A, Franke WW, Windoffer R, Leube RE, Loss of desmoglein 2 suggests essential functions for early embryonic development and proliferation of embryonal stem cells, Eur J Cell Biol, 81 (2002) 592–598. [DOI] [PubMed] [Google Scholar]
- [126].Koch PJ, Mahoney MG, Ishikawa H, Pulkkinen L, Uitto J, Shultz L, Murphy GF, Whitaker-Menezes D, Stanley JR, Targeted disruption of the Pemphigus Vulgaris antigen (desmoglein 3) gene in mice causes loss of keratinocyte cell adhesion with a phenotype similar to Pemphigus Vulgaris, The Journal of Cell Biology, 137 (1997) 1091–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Merritt AJ, Berika MY, Zhai W, Kirk SE, Ji B, Hardman MJ, Garrod DR, Suprabasal desmoglein 3 expression in the epidermis of transgenic mice results in hyperproliferation and abnormal differentiation, Mol Cell Biol, 22 (2002) 5846–5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Chidgey M, Brakebusch C, Gustafsson E, Cruchley A, Hail C, Kirk S, Merritt A, North A, Tselepis C, Hewitt J, Byrne C, Fassler R, Garrod D, Mice lacking desmocollin 1 show epidermal fragility accompanied by barrier defects and abnormal differentiation, J Cell Biol, 155 (2001) 821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Den Z, Cheng X, Merched-Sauvage M, Koch PJ, Desmocollin 3 is required for preimplantation development of the mouse embryo, J Cell Sci, 119 (2006) 482–489. [DOI] [PubMed] [Google Scholar]
- [130].Chen J, Den Z, Koch PJ, Loss of desmocollin 3 in mice leads to epidermal blistering, J Cell Sci, 121 (2008) 2844–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Bierkamp C, McLaughlin KJ, Schwarz H, Huber O, Kemler R, Embryonic heart and skin defects in mice lacking plakoglobin, Developmental Biology, 180 (1996) 780–785. [DOI] [PubMed] [Google Scholar]
- [132].Rietscher K, Wolf A, Hause G, Rother A, Keil R, Magin TM, Glass M, Niessen CM, Hatzfeld M, Growth Retardation, Loss of Desmosomal Adhesion, and Impaired Tight Junction Function Identify a Unique Role of Plakophilin 1 In Vivo, J Invest Dermatol, 136 (2016) 1471–1478. [DOI] [PubMed] [Google Scholar]
- [133].Gallicano GI, Kouklis P, Bauer C, Yin M, Vasioukhin V, Degenstein L, Fuchs E, Desmoplakin is required early in development for assembly of desmosomes and cytoskeletal linkage, The Journal of Cell Biology, 143 (1998) 2009–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Vasioukhin V, Bowers E, Bauer C, Degenstein L, Fuchs E, Desmoplakin is essential in epidermal sheet formation, Nature Cell Biology, 3 (2001) 1076–1085. [DOI] [PubMed] [Google Scholar]
- [135].Farquhar MG, Palade GE, Junctional complexes in various epithelia, J Cell Biol, 17 (1963) 375–412. [DOI] [PMC free article] [PubMed] [Google Scholar]