Key Points
Question
What are the neurological manifestations of coronavirus disease 2019 (COVID-19) in children?
Findings
In a case series of 4 children with COVID-19 and neurological symptoms, all 4 patients had signal changes in the splenium of the corpus callosum on neuroimaging and required intensive care admission for the treatment of COVID-19 pediatric multisystem inflammatory syndrome.
Meaning
Children with COVID-19 may present with new neurological symptoms involving both the central and peripheral nervous system and splenial changes on imaging, in the absence of respiratory symptoms; this diagnosis should be considered within the differential diagnosis of splenial lesions.
This case series presents the clinical findings of 4 children who experienced neurological manifestations of coronavirus disease 2019.
Abstract
Importance
Neurological manifestations have been reported in adults with coronavirus disease 2019 (COVID-19), which is caused by the highly pathogenic virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Objective
To report the neurological manifestations of children with COVID-19.
Design, Setting, and Participants
In this case-series study, patients younger than 18 years who presented with SARS-CoV-2 infection and neurological symptoms to Great Ormond Street Hospital for Children (London, UK) between March 1, 2020, and May 8, 2020, were included after infection was confirmed by either a quantitative reverse transcription–polymerase chain reaction assay by nasopharyngeal swab or a positive test result for IgG antibodies against SARS-CoV-2 in serum.
Main Outcomes and Measures
Clinical and paraclinical features were retrieved from electronic patient records.
Results
Of the 27 children with COVID-19 pediatric multisystem inflammatory syndrome, 4 patients (14.8%) who were previously healthy had new-onset neurological symptoms. Symptoms included encephalopathy, headaches, brainstem and cerebellar signs, muscle weakness, and reduced reflexes. All 4 patients required intensive care unit admission for the treatment of COVID-19 pediatric multisystem inflammatory syndrome. Splenium signal changes were seen in all 4 patients on magnetic resonance imaging of the brain. In the 2 patients whose cerebrospinal fluid was tested, samples were acellular, with no evidence of infection on polymerase chain reaction or culture (including negative SARS-CoV-2 polymerase chain reaction results) and negative oligoclonal band test results. In all 3 patients who underwent electroencephalography, a mild excess of slow activity was found. Tests for N-methyl-d-aspartate receptor, myelin oligodendrocyte glycoprotein, and aquaporin-4 autoantibodies had negative results in all patients. In all 3 patients who underwent nerve conduction studies and electromyography, mild myopathic and neuropathic changes were seen. Neurological improvement was seen in all patients, with 2 making a complete recovery by the end of the study.
Conclusions and Relevance
In this case-series study, children with COVID-19 presented with new neurological symptoms involving both the central and peripheral nervous systems and splenial changes on imaging, in the absence of respiratory symptoms. Additional research is needed to assess the association of neurological symptoms with immune-mediated changes among children with COVID-19.
Introduction
Coronavirus disease 2019 (COVID-19), which is caused by the highly pathogenic virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first detected in Wuhan, China, in December 2019 and has since become a worldwide pandemic infecting more than 9 million people (as of mid-June 2020). In adults, COVID-19 ranges from an asymptomatic infection to severe respiratory failure. Data so far suggest that children and young adults are less likely to become severely ill than older adults.1 Increasing reports of children developing systemic inflammatory response requiring intensive care (labeled pediatric multisystem inflammatory syndrome temporally associated with COVID-192) and a further group of children with a far less severe, Kawasaki-like disease, who respond to a variety of immunomodulatory treatments,3 suggest that despite the typically mild acute infection, children may be at high risk of a secondary inflammatory syndrome.
Laboratory studies have revealed that the main host-cell receptor of SARS-CoV-2 is angiotensin-converting enzyme 2 (ACE2)4; given that ACE2 is expressed in both neurons and glial cells, direct viral invasion of the central nervous system (CNS) is a possible mechanism for neurological manifestations of COVID-19. More recently, an immune-mediated neurological syndrome was proposed in adult patients presenting with Miller-Fisher syndrome and polyneuritis cranialis5 or steroid-responsive encephalitis.6 Here, we report a case series of 4 children who presented with new-onset neurological symptoms in association with SARS-CoV-2.
Methods
Patients younger than 18 years who presented with new-onset neurological symptoms to Great Ormond Street Hospital for Children (London, UK) between March 1, 2020, and May 8, 2020, were included from a cohort of children with SARS-CoV-2 infection (confirmed by either quantitative reverse transcription–polymerase chain reaction [PCR] assay by nasopharyngeal swab or a positive SARS-CoV-2 IgG test result in serum). Data on demographics, comorbidities, neurological symptoms, relevant investigations (of cerebrospinal fluid, neuroimaging, and neurophysiology), treatments, and outcomes were retrieved from the electronic patient records. Because the data analysis was retrospective and no additional data were collected beyond those required for standard medical care, a full ethics review under the terms of the Governance Arrangements of Research Ethics Committees in the UK was not required. Written informed consent was obtained from the parents of all of the patients.
Results
Fifty children presented with SARS-CoV-2 infection during the study time frame. Of these, 27 had features consistent with COVID-19 pediatric multisystem inflammatory syndrome. A total of 4 patients (14.8%) with multisystem inflammatory syndrome had neurological involvement. These 4 patients have been previously reported in a UK cohort study of 58 children with pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2.7 The median age at onset of symptoms was 12 (range, 8-15) years. A summary of the clinical and paraclinical features is provided in Table 1, Table 2, and the Figure. Neurological symptoms included encephalopathy (n = 4), headache (n = 3), brainstem signs with dysarthria or dysphagia (n = 2), meningism (n = 1), and cerebellar ataxia (n = 1). Peripheral nervous system involvement was seen in all patients, with global proximal muscle weakness (n = 4) and reduced reflexes (n = 2). Neurological symptoms were part of the initial presentation in 2 patients.
Table 1. Patient Demographics and Neurological Characteristics.
| Patient No./ sex/ age, y | Ethnicity | Central nervous system manifestations | Peripheral nervous system manifestations | Cerebrospinal fluid findings | EEG | EMG | Immune therapy | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1/M/8 | South Asian | Encephalopathy (confused and agitated); meningism; headache | Generalized proximal weakness (MRC 3/5) on day 7; normal reflexes | White blood cell count, 8000 cells/μL; protein, 2.0 g/dL; negative culture and virology results (including SARS-CoV-2); negative oligoclonal band test results | Mild diffuse slowing | Patchy myopathic changes | Intravenous immunoglobulin (1 g/kg, 1 dose); dexamethasone (10 mg/m2, 7 d); IVMP (2 mg/kg, ongoing); Anakinra (2 mg/kg, 7 d) | Day 17: still inpatient; encephalopathy resolved, wheelchair bound |
| 2/M/9 | Afro- Caribbean |
Encephalopathy (confused); ataxia; dysarthria; headache | Bilateral proximal leg weakness (MRC 3/5) on day 2; normal reflexes; urinary retention | White blood cell count, 2000 cells/μL; protein, 1.9 g/dL; negative culture and virology results (including SARS-CoV-2); negative oligoclonal band test results | Diffuse slow activity | Not performed | None | Discharged after 11 d; encephalopathy resolved, fully ambulant |
| 3/F/15 | South Asian | Encephalopathy (confused); dysarthria; dysphagia | Global flaccid weakness (MRC 3/5), day 8; reduced reflexes | Not performed | Mild excess of slow activity over the anterior regions | Mild myopathic or neuropathic changes | Anakinra (2 mg/kg, 7 d); IVMP (10 mg/kg, 3 d); dexamethasone (10 mg/m2, 7 d); rituximab (375 mg/m2, 2 doses) | Day 32: still inpatient; encephalopathy resolved, wheelchair bound |
| 4/F/15 | Afro- Caribbean |
Encephalopathy (confused and disoriented); headache | Global proximal weakness (MRC 4/5) on day 4; reduced reflexes | Not performed | Not performed | Mild myopathic changes | Intravenous immunoglobulin (1 g/kg, 1 dose) | Discharged after 18 d; encephalopathy resolved, fully ambulant |
Abbreviations: EEG, electroencephalography; EMG, electromyography; IVMP, intravenous methylprednisolone; MRC, Medical Research Council power scale; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
SI conversion factors: To convert white blood cell count to cells × 109 per liter, multiply by 0.001; protein to g/L, multiply by 10.0.
Table 2. Comorbidities and Systemic Involvements.
| Patient No./ Comorbidities | Associated systemic symptoms | ICU stay, d (days ventilated) | Positive SARS-CoV-2 test type | Results of other virus testsa | Inflammatory markers | Vitamin D, ng/mL | Electrolyte, metabolic, and toxic screen results | Echocardiogram | ECG |
|---|---|---|---|---|---|---|---|---|---|
| 1/None | At onset: fever, abdominal pain, palmar rash, vomiting, and circulatory shock | 9 (6) | Respiratory PCR | All negative | CRP, 44.8 mg/dL; ferritin, 1414 ng/mL; D-dimer, 1625.4 μg/mL; LDH, 1016 U/L | 6.41 | Normal | Mild to moderate left ventricular impairment with no coronary arteriopathy | Changes consistent with pericarditis |
| 2/None | At onset: fever, palmar rash, vomiting, hyponatremia, and circulatory shock | 2 (1) | Respiratory PCR | All negative | CRP, 31.3 mg/dL; ferritin, 1192 ng/mL; D-dimer, 494.5 μg/mL; LDH, 900 U/L | 4.41 | Hyponatremia: sodium, 129 mEq/L; normal metabolic and toxic screen results | Normal structure and biventricular function with mitral regurgitation | Supraventricular ectopics |
| 3/None | 2 wk Prior to neurological symptoms: fever, dyspnea, vomiting, rash, and circulatory shock | 14 (7) | Respiratory PCR and serum IgG | Positive for sapovirus | CRP, 29.0 mg/dL; ferritin, 48 142 ng/mL; D-dimer, 1479.8 μg/mL; LDH, 4331 U/L | 4.00 | Normal | Mild pericardial effusion | Normal |
| 4/Obesity (BMI, 32) | 5 d Prior to neurological symptoms: fever, dyspnea, vomiting, rash, and circulatory shock | 4 (4) | Respiratory PCR and serum IgG | All negative | CRP, 32.8 mg/dL; ferritin, 1218 ng/mL; D-dimer, 1248.6 μg/mL; LDH, 1168 U/L | Not performed | Normal | Normal | Normal |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CRP, C-reactive protein; ECG, electrocardiogram; ICU, intensive care unit; LDH, lactate dehydrogenase; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
SI conversion factors: To convert C-reaction protein to mg/L, multiply by 10.0; D-dimer to nmol/L, multiply by 5.476; ferritin to μmol/L, multiply by 1.0; lactate dehydrogenase to μkat/L, multiply by 0.0167; sodium to mmol/L, multiply by 1.0; vitamin D to nmol/L, multiply by 2.496.
Stool enteric virus panel and other respiratory viruses.
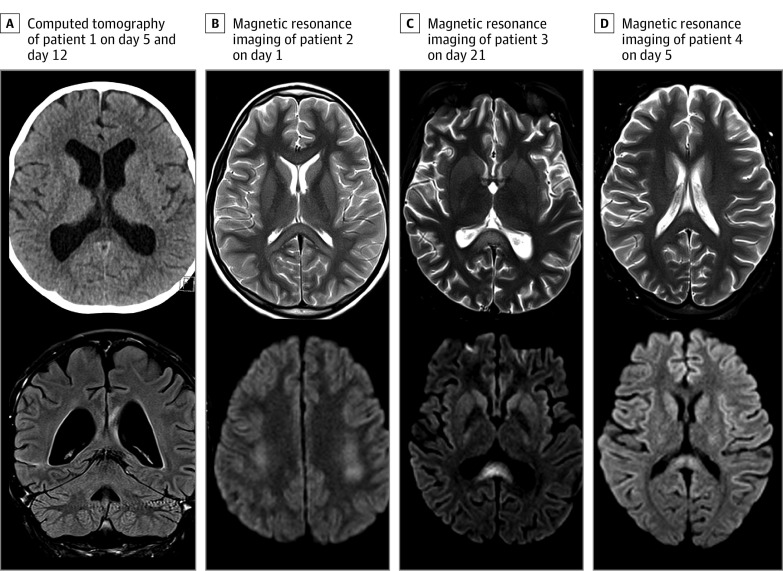
Figure. Neuroimaging Findings in Association With Coronavirus Disease 2019 in Children.
A, Computed tomography image of patient 1 on day 5 (top), during intensive care admission, showing hypodensity of the splenium of the corpus collosum (SCC). Coronal fluid-attenuated inversion recovery performed on day 12 (bottom) shows resolution of the changes previously seen on computed tomography, with persistent signal changes in the genu and SCC without restricted diffusion (not shown). B, Axial T2 magnetic resonance image of patient 2 on day 1, showing signal changes of the genu and SCC (top) and bilateral centrum semiovale with restricted diffusion (bottom). Repeated imaging on day 6 (not shown) demonstrated resolution of the restricted diffusion, with minimal signal changes remaining on T2-weighted imaging. C, Axial T2 magnetic resonance imaging of patient 3 on day 21, showing hyperintensities (top) with restricted diffusion (bottom) in the SCC and bilateral centrum semiovale (not shown). D, Axial T2 magnetic resonance imaging of patient 4 on day 5 (top), showing signal change in the SCC with mild restricted diffusion (bottom).
Systemic manifestations included fever (n = 4), cardiovascular shock (n = 4), rash (n = 4), and dyspnea (n = 2). All patients required mechanical ventilation and intensive care admission for cardiovascular shock (n = 4) and/or respiratory decompensation (n = 1). The intensive care unit stay was for a median 6.5 (range, 2-14) days, and mechanical ventilation duration was for a median of 5 (range, 1-7) days.
A comprehensive screening of all 4 patients for other infective causative mechanisms had negative results. In the 2 patients who had lumbar punctures, cerebrospinal fluid samples were acellular with normal protein and glucose levels, negative results for oligoclonal bands, and negative results for bacterial cultures and viral and bacterial PCR (including negative SARS-CoV-2 PCR). Tests for N-methyl-d-aspartate receptor, myelin oligodendrocyte glycoprotein, and aquaporin-4 autoantibodies had negative results in all patients.
Signal changes in the splenium of the corpus callosum (SCC) were seen in all 4 patients; T2-hyperintense lesions associated with restricted diffusion were seen in 3 children. The fourth patient presented with a splenial lesion on computed tomography, but on subsequent magnetic resonance imaging, no restricted diffusion was evident, although the signal change remained. The genu was involved in 2 patients and the bilateral centrum semiovale in 2 patients (Figure). No spinal cord involvement or pathological enhancement was observed. Patient 2 had a repeated magnetic resonance image on day 5 that showed resolution of diffusion restriction in the SCC and centrum semiovale.
Electroencephalography showed a mild excess of slow activity in the 3 patients tested. Nerve conduction studies and electromyography showed mild myopathic and neuropathic changes in all 3 patients tested.
Three patients received immunomodulatory therapies as part of COVID-19 pediatric multisystem inflammatory syndrome management (Table 1); these were intravenous methylprednisolone (n = 2), dexamethasone (n = 2), intravenous immunoglobulin (n = 2), anakinra (n = 2), and rituximab (n = 1). No patients required antiviral treatment. After a median follow-up of 18 (range, 11-32) days, patients 2 and 4 fully recovered and were discharged from hospital after 11 and 18 days respectively, fully ambulating. The remaining 2 patients have been discharged from the intensive care unit and remain inpatients. Both are improving clinically but currently wheelchair bound (as a result of proximal lower-limb muscle weakness).
Discussion
In this case series, we describe 4 children with confirmed COVID-19 who presented with a distinct neurological syndrome associated with lesions of the SCC on neuroimaging. In an adult cohort8 in Wuhan, China, 78 of 214 patients (36.4%) had neurological manifestations, which included dizziness (n = 36), headache (n = 28), impaired consciousness (n = 16), acute cerebrovascular disease (n = 6), ataxia (n = 1), and seizures (n = 1). In comparison with patients with nonsevere infection, those with severe infection had more neurological presentations, including acute cerebrovascular diseases (5 [5.7%] vs 1 [0.8%]) and impaired consciousness (13 [14.8%] vs 3 [2.4%]). Neuroimaging, cerebrospinal fluid, or neurophysiology tests were not performed in this cohort8 to reduce the risk of cross-infection.
A key observation in this cohort was the acute splenial lesions seen on neuroimaging in all 4 patients. Reversible lesions of the SCC are rare but have been previously reported in patients with encephalopathies and are thought to represent focal intramyelin edema secondary to inflammation. In a multicenter study from Japan of 15 adult patients, a variety of viral prodromes were reported in 5 patients; these were influenza A (n = 1), mumps (n = 2), adenovirus (n = 1), and varicella-zoster virus (n = 1).9 Other differential diagnoses of splenial lesions include ischemia, posterior reversible encephalopathy syndrome, severe electrolyte disturbances, and lymphoma.10 Interestingly, a typical, transient, oval-shaped lesion in the median aspect of the SCC, either in isolation or with more extensive brain involvement, has also been reported11 in children with Kawasaki disease.
Similar to the previous 2009 influenza A (H1N1) virus pandemic, the neurological symptom findings have not demonstrated neurotropism, and the pathobiology has been considered secondary to an immune-mediated causative mechanisms.12 A number of neuroimmune disorders are known to occur in close timing to viral infection; examples are in children who develop anti–N-methyl-d-aspartate receptor encephalitis after recovery from herpes simplex virus encephalitis and those who develop a primary CNS vasculitis after varicella-zoster virus infection.13 The phenotype of our cohort raises the possibility of a virus-specific immunological syndrome. A plausible mechanism would be exposure of the immune system to new CNS antigens as a result of blood-brain barrier damage from SARS-CoV-2, which causes endotheliopathy14 and leads to an immune-directed attack on the CNS.
Alternatively, the neurological symptoms may be part of the systemic autoinflammatory disease in keeping with the raised systemic inflammatory markers seen in our cohort (Table 1). The combinations of both CNS and peripheral nervous system symptom profiles are rare in pediatrics but can be seen in children with hemophagocytic lymphohistiocytosis.15 This condition, which can be either genetic or acquired, is traditionally characterized by a cytokine storm with multiorgan dysfunction. More recently, isolated CNS presentations have also been reported.16 Similarly, neurological symptoms secondary to cytokines storms were reported in 23 of 51 pediatric and young adult patients (45.1%) receiving chimeric antigen receptor–modified T-cell therapy.17
Limitations
The key limitation of this study is the small sample size. Further studies are now required to confirm our observation and evaluate the mechanism of disease in this distinct syndrome.
Conclusions
In conclusion, we describe 4 children with COVID-19 who have a clinical phenotype involving both the CNS and the peripheral nervous system and lesions of the SCC. The negative cerebrospinal fluid results, the response to immunosuppression, and the clinical overlap with hemophagocytic lymphohistiocytosis suggest that this is likely to be immune mediated. Although the imaging finding is not specific to SARS-CoV-2, in that it has been previously seen with other viral infections, clinicians should be adding SARS-CoV-2 to their differential diagnosis for children presenting with new neurologic symptoms and this imaging finding while still exploring other possible causes. Furthermore, because respiratory symptoms were uncommon in this cohort and, when present, were mild and easily missed, and because reports are growing of children carrying COVID-19 infection without symptoms (with this condition likely presenting late), SARS-CoV-2 should also be considered in pediatric patients presenting with primary neurologic symptoms without systemic involvement. Close neurodevelopmental surveillance is required to assess the neurological and cognitive outcomes in these patients.
References
- 1.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088-1095. doi: 10.1111/apa.15270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607-1608. doi: 10.1016/S0140-6736(20)31094-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771-1778. doi: 10.1016/S0140-6736(20)31103-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995-998. doi: 10.1021/acschemneuro.0c00122 [DOI] [PubMed] [Google Scholar]
- 5.Gutiérrez-Ortiz C, Méndez A, Rodrigo-Rey S, et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;10.1212/WNL.0000000000009619. doi: 10.1212/WNL.0000000000009619 [DOI] [PubMed] [Google Scholar]
- 6.Pilotto A, Odolini S, Masciocchi S, et al. Steroid-responsive encephalitis in COVID-19 disease. Ann Neurol. 2020. doi: 10.1002/ana.25783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whittaker E, Bamford A, Kenny J, et al. ; PIMS-TS Study Group and EUCLIDS and PERFORM Consortia . Clinical Characteristics of 58 Children With a Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2. JAMA. 2020;324(3):259-269. doi: 10.1001/jama.2020.10369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020. doi: 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tada H, Takanashi J, Barkovich AJ, et al. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Neurology. 2004;63(10):1854-1858. doi: 10.1212/01.WNL.0000144274.12174.CB [DOI] [PubMed] [Google Scholar]
- 10.Doherty MJ, Jayadev S, Watson NF, Konchada RS, Hallam DK. Clinical implications of splenium magnetic resonance imaging signal changes. Arch Neurol. 2005;62(3):433-437. doi: 10.1001/archneur.62.3.433 [DOI] [PubMed] [Google Scholar]
- 11.Kontzialis M, Soares BP, Huisman TAGM. Lesions in the splenium of the corpus callosum on MRI in children: a review. J Neuroimaging. 2017;27(6):549-561. doi: 10.1111/jon.12455 [DOI] [PubMed] [Google Scholar]
- 12.Sejvar JJ, Uyeki TM. Neurologic complications of 2009 influenza A (H1N1): heightened attention on an ongoing question. Neurology. 2010;74(13):1020-1021. doi: 10.1212/WNL.0b013e3181d6b869 [DOI] [PubMed] [Google Scholar]
- 13.Wells E, Hacohen Y, Waldman A, et al. ; attendees of the International Neuroimmune Meeting . Neuroimmune disorders of the central nervous system in children in the molecular era. Nat Rev Neurol. 2018;14(7):433-445. doi: 10.1038/s41582-018-0024-9 [DOI] [PubMed] [Google Scholar]
- 14.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417-1418. doi: 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horne A, Trottestam H, Aricò M, et al. ; Histiocyte Society . Frequency and spectrum of central nervous system involvement in 193 children with haemophagocytic lymphohistiocytosis. Br J Haematol. 2008;140(3):327-335. doi: 10.1111/j.1365-2141.2007.06922.x [DOI] [PubMed] [Google Scholar]
- 16.Benson LA, Li H, Henderson LA, et al. Pediatric CNS-isolated hemophagocytic lymphohistiocytosis. Neurol Neuroimmunol Neuroinflamm. 2019;6(3):e560. doi: 10.1212/NXI.0000000000000560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gofshteyn JS, Shaw PA, Teachey DT, et al. Neurotoxicity after CTL019 in a pediatric and young adult cohort. Ann Neurol. 2018;84(4):537-546. doi: 10.1002/ana.25315 [DOI] [PMC free article] [PubMed] [Google Scholar]