Abstract
This cohort study compares the use of adjuvant radiotherapy following surgery with surgery alone in patients treated for cutaneous squamous cell carcinoma with perineural invasion.
Perineural invasion in cutaneous squamous cell carcinoma (cSCC) is associated with an increased risk of local recurrence, nodal metastases, and disease-specific death. Adjuvant radiotherapy has been suggested to mitigate aggressive behavior of cSCC with perineural invasion.1,2,3 To clarify the utility of adjuvant radiotherapy for cSCC with perineural invasion, we investigated the outcomes of local recurrence, nodal metastases, distant metastases, and disease-free survival of patients treated surgically with or without adjuvant radiotherapy.
Methods
Patients with histologically confirmed cSCC with perineural invasion at New York University from January 1, 2005, to December 31, 2014, were included. All patients were referred to radiation oncology services for consideration of adjuvant radiotherapy based on the presence of large-caliber perineural invasion (≥0.1 mm in diameter) or small-caliber perineural invasion (<0.1 mm) with additional high-risk features. High-risk features included tumor size greater than 2 cm, poor differentiation, invasion beyond subcutaneous fat, immunosuppression, and head or neck location.1,3,4 This study was approved by the institutional review board of NYU Langone Health. Written informed consent was obtained, and patients received no compensation. Data were analyzed from December 1, 2019, to March 1, 2020.
Results
All 31 patients (24 [77.4%] men; mean [SD] age, 70.8 [15.2] years) were treated with Mohs micrographic surgery, with negative surgical margins achieved. While all patients were recommended for adjuvant radiotherapy by radiation oncology services, only 15 individuals (48.4%) completed treatment. Adjuvant radiotherapy was directed to the local tumor site and consisted of 1500 to 6000 cGy over 10 to 30 treatment sessions. Reasons for refusal were patient specific, with concerns of adverse effects or time restraints most common. Adjuvant radiotherapy was well tolerated, with 6 patients developing mild dermatitis.
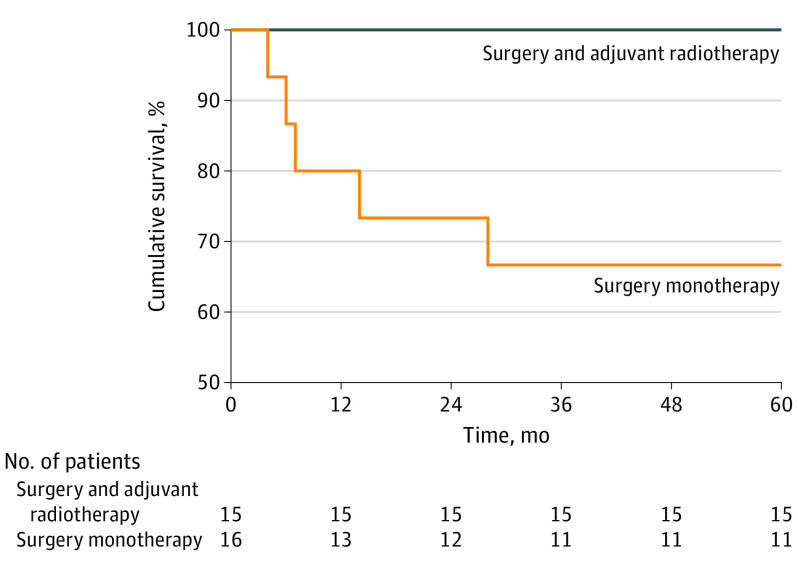
Patient age was the only significant difference between the 2 groups (surgery monotherapy vs surgery with adjuvant radiotherapy); patients who received surgery alone were older (age ≥70 years) than those who received surgery with adjuvant radiotherapy (66.7% vs 33.3%, P = .04) (Table). Regarding outcomes, there were no cases of local recurrence at 5 years of follow-up. There was a significant difference in the presence of nodal metastasis between the 2 groups, as all 5 patients who developed nodal metastases did not receive adjuvant radiotherapy and none of those in the combined therapy group developed nodal metastasis (P = .02) (Table). The estimated 5-year disease-free survival for patients treated with adjuvant radiotherapy was 100% (95% CI, 100%-100%) vs 68.8% (95% CI, 60.9%-76.7%) for those who did not receive adjuvant radiotherapy (P = .01) (Figure).
Table. Patient Characteristics, Tumor Features, and Outcome Data of 31 Patients With cSCC With Perineural Invasion.
| Variable | No. | Total No. | P value | |
|---|---|---|---|---|
| Surgery monotherapy (n = 16) | Surgery with adjuvant radiotherapy (n = 15) | |||
| Sex, No. (%) | ||||
| Women | 5 (31.2) | 2 (13.3) | 7 (22.6) | .23 |
| Men | 11 (68.8) | 13 (86.7) | 24 (77.4) | |
| Age, y | ||||
| 30-49 | 1 | 4 | 5 | .04 |
| 50-69 | 1 | 5 | 6 | |
| 70-89 | 12 | 6 | 18 | |
| ≥90 | 2 | 0 | 2 | |
| Fitzpatrick skin type | ||||
| I/II | 14 | 15 | 29 | .96 |
| III/IV | 1 | 1 | 2 | |
| V/VI | 0 | 0 | 0 | |
| Immunosuppression | ||||
| No | 13 | 12 | 25 | .93 |
| Yes | 3 | 3 | 6 | |
| Type of immunosuppression (n = 6) | ||||
| Chronic lymphocytic leukemia | 1 | 0 | 1 | .50 |
| Solid organ transplantation | 2 | 2 | 4 | |
| Systemic lupus erythematosus | 0 | 1 | 1 | |
| Anatomic site | ||||
| Head/neck | 12 | 14 | 26 | .21 |
| Extremities | 3 | 0 | 3 | |
| Trunk | 1 | 1 | 2 | |
| Primary vs recurrent | ||||
| Primary | 15 | 13 | 28 | .51 |
| Recurrent | 1 | 2 | 3 | |
| Tumor size, cm | ||||
| <2 | 7 | 10 | 17 | .20 |
| ≥2 | 9 | 5 | 14 | |
| Histologic differentiation | ||||
| Well | 2 | 2 | 4 | NA |
| Moderate | 10 | 10 | 20 | |
| Poor | 4 | 3 | 7 | |
| Invasion beyond subcutaneous fat | ||||
| Yes | 8 | 4 | 12 | .18 |
| No | 8 | 11 | 19 | |
| Diameter of involved nerve, mm | ||||
| <0.1 | 4 | 5 | 9 | .61 |
| ≥0.1 | 12 | 10 | 22 | |
| AJCC 7 (n = 5)a | ||||
| T1 | 1 | 0 | 1 | .58 |
| T2 | 3 | 1 | 4 | |
| AJCC 8 (n = 26)b | ||||
| T1 | 1 | 0 | 1 | .23 |
| T2 | 0 | 0 | 0 | |
| T3 | 10 | 15 | 25 | |
| BWH | ||||
| T1 | 1 | 0 | 1 | .55 |
| T2a | 3 | 4 | 7 | |
| T2b | 11 | 11 | 22 | |
| T3 | 1 | 0 | 1 | |
| Local recurrence | ||||
| No | 16 | 15 | 31 | NA |
| Nodal metastasis | ||||
| Yes | 5 | 0 | 5 | .02 |
| No | 11 | 15 | 26 | |
| Distant metastasis | ||||
| Yes | 2 | 0 | 2 | .16 |
| No | 14 | 15 | 29 | |
Abbreviations: AJCC 7, American Joint Committee on Cancer Staging Manual, 7th Edition; AJCC 8, American Joint Committee on Cancer Staging Manual, 8th Edition; BWH, Brigham & Women’s Hospital staging system; cSCC, cutaneous squamous cell carcinoma; NA, not applicable.
AJCC 7 staging performed for patients with tumors on the trunk and extremities.
AJCC 8 staging performed for patients with tumors on the head and neck.
Figure. Disease-Free Survival.
The estimated 5-year disease-free survival rate for patients treated with adjuvant radiotherapy was 100% (95% CI, 100%-100%) vs 68.8% (95% CI, 60.9%-76.7%) for those who did not receive adjuvant radiotherapy (P = .01).
Of the 5 patients who developed nodal metastasis, 4 patients (80.0%) had large-diameter perineural invasion, and all had at least 1 additional high-risk feature. Overall, 71.0% of patients in this cohort had large-caliber perineural invasion. The patient with small-diameter perineural invasion had additional high-risk features of tumor size greater than 2 cm, invasion beyond the subcutaneous fat, and location on the temple. Four of the 5 patients (80.0%) had T2b tumors according to the Brigham & Women’s Hospital (BWH) staging system, whereas 1 patient had a BWH T3 tumor. All head and neck tumors that metastasized were T3 per the American Joint Committee on Cancer Staging Manual, 8th Edition (AJCC 8). One truncal tumor that metastasized was considered T2 per AJCC 7, since these were not staged according to AJCC 8.
Discussion
In a recent study by Miller et al,2 91% of high-risk patients with cSCC had no evidence of disease after treatment with surgery and adjuvant radiotherapy. Similar to the study cohort, this series was dominated by T2b/T3 BWH tumors, most with perineural invasion. Our data support the use of adjuvant radiotherapy for cSCC with perineural invasion and highlight the need to stratify patients at highest risk in an effort to identify those who might benefit from adjuvant radiotherapy to mitigate the risk of metastasis.
Currently, adjuvant radiotherapy is recommended for cSCC with substantial perineural invasion.5 While it was previously thought that the extent of nerve caliber invasion may affect the outcome,3 small-caliber perineural invasion has been associated with a 4-fold increased risk of nodal metastasis when found in patients with 2 other risk factors and a 14-fold increased risk in patients with 3 other risk factors.6 Although 71.0% of patients in the study cohort had large-caliber perineural invasion, 1 patient who received surgical monotherapy and developed nodal metastasis had small-caliber perineural invasion. Thus, adjuvant radiotherapy may improve prognosis for patients with small-caliber perineural invasion in the setting of additional high-risk features and should be considered.
Limitations of this study include its retrospective design, cohort size, treatment bias, and potential referral bias, with our findings suggesting the need for prospective, multicenter controlled trials. Our data suggest favorable outcomes of cSCC with perineural invasion treated with surgery and adjuvant radiotherapy, highlighting the potential role of adjuvant radiotherapy for patients with large-caliber perineural invasion and those with small-caliber perineural invasion in the presence of other high-risk features.
References
- 1.Thompson AK, Kelley BF, Prokop LJ, Murad MH, Baum CL. Risk factors for cutaneous squamous cell carcinoma recurrence, metastasis, and disease-specific death: a systematic review and meta-analysis. JAMA Dermatol. 2016;152(4):419-428. doi: 10.1001/jamadermatol.2015.4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller J, Chang T, Schwartz D, Peters M, Baum C. Outcomes of adjuvant radiotherapy following negative surgical margins for cutaneous squamous cell carcinoma. Dermatol Surg. 2019;45(9):1111-1116. doi: 10.1097/DSS.0000000000001827 [DOI] [PubMed] [Google Scholar]
- 3.Jambusaria-Pahlajani A, Miller CJ, Quon H, Smith N, Klein RQ, Schmults CD. Surgical monotherapy versus surgery plus adjuvant radiotherapy in high-risk cutaneous squamous cell carcinoma: a systematic review of outcomes. Dermatol Surg. 2009;35(4):574-585. doi: 10.1111/j.1524-4725.2009.01095.x [DOI] [PubMed] [Google Scholar]
- 4.Veness MJ, Porceddu S, Palme CE, Morgan GJ. Cutaneous head and neck squamous cell carcinoma metastatic to parotid and cervical lymph nodes. Head Neck. 2007;29(7):621-631. doi: 10.1002/hed.20576 [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network Cutaneous squamous cell carcinoma. 2017.
- 6.Ross AS, Whalen FM, Elenitsas R, Xu X, Troxel AB, Schmults CD. Diameter of involved nerves predicts outcomes in cutaneous squamous cell carcinoma with perineural invasion: an investigator-blinded retrospective cohort study. Dermatol Surg. 2009;35(12):1859-1866. doi: 10.1111/j.1524-4725.2009.01354.x [DOI] [PubMed] [Google Scholar]