This analysis describes a potential strategy for improving the design of clinical trials of antipsychotic drugs for schizophrenia submitted for approval to the US Food and Drug Administration.
Key Points
Question
Can a shortened version of the Positive and Negative Syndrome Scale and shorter randomized clinical trial duration, in isolation or in combination, facilitate the development of new drugs to treat schizophrenia?
Findings
In this analysis of 32 randomized clinical trials involving 14 219 participants, the overall concordance rate between the change from baseline scores in the modified Positive and Negative Syndrome Scale and change from baseline scores in the total Positive and Negative Syndrome Scale was 93.0% at week 4 and 97.7% at week 6 of the trial. Analysis of the change in scores compared with baseline scores revealed that the earliest time point in which a treatment outcome could be observed was 1 week after initiation of medication compared with placebo.
Meaning
Results of this analysis suggest that the combination of the modified version of the Positive and Negative Syndrome Scale and a shortened drug trial duration may provide an alternative regulatory pathway to the development of new drugs for schizophrenia.
Abstract
Importance
Facilitating the development of safe and effective medications for schizophrenia is a public health imperative.
Objectives
To evaluate the association of shortening randomized clinical trial (RCT) duration with the modification of the Positive and Negative Syndrome Scale (PANSS) for the design of RCTs of medications for schizophrenia and to offer perspective on an alternative regulatory pathway to the historically accepted trial duration and response assessment.
Data Sources
A database was created consisting of clinical trial data from 32 placebo-controlled RCTs of 8 atypical antipsychotic drugs approved by the US Food and Drug Administration (FDA) between January 1, 2001, and December 31, 2015. The database included information on total and individual PANSS item ratings, demographic characteristics, disposition, and adverse events (AEs).
Study Selection
All clinical trials submitted to 8 new drug applications of atypical antipsychotic drugs were selected.
Data Extraction and Synthesis
Quality control checks were performed to ensure that the collected data were consistent with the reported results of each trial. Data were collected from March 15, 2015, to September 30, 2015. Data analysis was conducted from October 1, 2015, to June 20, 2016.
Main Outcomes and Measures
The following analyses were performed: (1) longitudinal assessment of mean change from baseline in total PANSS score, (2) correlation analyses between change from baseline in total PANSS score at week 6 and earlier time points, (3) concordance analyses of outcomes across trials between week 6 and earlier time points using total PANSS and modified PANSS, and (4) analyses of time course of treatment–emergent AEs.
Results
The final database contained data from 14 219 participants enrolled in 32 drug trials; 9805 of 14 219 participants (69.0%) were male and were either white (7183 [50.5%]) or black (4346 [30.6%]) individuals. The mean (SD) age during treatment was 38.9 (10.9) years, and the mean (SD) age at schizophrenia diagnosis was 25 (8.5) years. Statistically significant separation between treatment response and placebo response was observed after 1 week of treatment. The overall concordance rate across treatment groups steadily increased from week 1 to week 4 (68.0% for week 1, 74.0% for week 2, 83.0% for week 3, and 93.0% for week 4). Trends in AE occurrence were evident by week 1 and percentage of AEs were similar across weeks 3, 4, and 6. The overall concordance rate between change from baseline in the modified PANSS score and change from baseline in the total PANSS score was 93.0% (80 of 86 treatment groups) at week 4 and 97.7% (84 of 86 treatment groups) at week 6. Shortening the trial duration to 4 weeks increased the required sample size to 502 participants. Using the modified PANSS as the end point, the sample size for a 4-week trial was 402 participants and 296 participants for a 6-week trial.
Conclusions and Relevance
Findings from this analysis suggest that there is the potential to streamline the design of schizophrenia drug clinical trials. Trial sponsors may consider incorporating these strategies and are encouraged to consult with the FDA early in the drug development process.
Introduction
The availability of safe and effective drugs for schizophrenia treatment is a public health need. The US Food and Drug Administration (FDA) has been supportive of efficient randomized clinical trial designs that facilitate the approval of therapies for life-threatening diseases and unmet medical needs across multiple indications.1 Similar regulatory flexibility is needed to expand the pipeline of new schizophrenia drugs and to increase access to safe and effective medications. One approach for achieving this goal is to leverage the knowledge generated during the development of approved therapies to optimize clinical trial design elements and improve the drug development process. This analysis describes such an approach for the development of drugs to treat schizophrenia.
Schizophrenia is a disease with substantial economic, clinical, and societal implications worldwide.1,2,3,4 Pharmacotherapy has been a mainstay in the treatment of schizophrenia, although treatment response to antipsychotic agents is highly variable and drug development is challenging.
Development of drugs for schizophrenia has been particularly difficult because of a multitude of factors, including an incomplete understanding of the underlying disease biology and challenges in clinical trial design and conduct.5 Twenty-five percent of schizophrenia drug trials did not show the therapeutic benefit of the drug,6 and the treatment effect (ie, mean improvement from drug minus mean improvement from placebo) diminished over time.7 Demonstrating therapeutic benefit in clinical trials has been hindered by factors such as high placebo response rates, high participant dropout rates over time, and variable sensitivity and specificity of clinical trial end points to isolate the pharmacological effect for schizophrenia symptoms.8 As a result of these challenges, several large pharmaceutical companies have announced plans to abandon drug development programs in psychiatric diseases, including schizophrenia, despite unmet medical needs.9
There is a public health imperative to facilitate the development of new treatments for schizophrenia, and the FDA has recognized the need to improve the quality of clinical trials of these medications. Two potential strategies for improving clinical trials are as follows: (1) decrease the duration of clinical trials to the shortest time interval in which a treatment response can be demonstrated while still allowing for sufficient accrual of safety information, and (2) streamline the clinical assessment of treatment response through modification of the Positive and Negative Syndrome Scale (PANSS; score range: 30-210, with the highest score indicating more severe symptoms), an FDA-accepted clinical trial end point in schizophrenia drug trials. In this analysis, we evaluated the implication of shortened trial duration and the use of a modified, 19-item version of the PANSS for the overall design of clinical trials submitted to the FDA in the past 15 years. This analysis also offers perspective on an alternative regulatory pathway to the historically accepted duration and response assessment of clinical trials.
Methods
Data were collected from March 15, 2015, to September 30, 2015. Data analysis was conducted from October 1, 2015, to June 20, 2016.
Clinical Trial Database
We created a database consisting of clinical trial data (including efficacy and safety information) from 32 placebo-controlled randomized clinical trials that were at least 4 weeks in duration and were submitted with the new drug applications (NDAs) of 8 atypical antipsychotic drugs approved by the FDA between January 1, 2001, and December 31, 2015. The database included longitudinal, participant-level information of total and individual PANSS item ratings, demographic characteristics, disposition, and adverse events (AEs). The variables in the database were harmonized using the Study Data Tabulation Model format.10 Quality control checks were performed to ensure that the collected data were consistent with the reported results of each trial.
Implications of Shortened Trial Duration for Detection of Treatment Effects
To identify the implications of a shortened trial duration for detecting treatment outcomes, we performed the following analyses: (1) longitudinal mean change from baseline in total PANSS score, (2) correlation of change from baseline in total PANSS score between week 6 and earlier time points, (3) concordance of trial outcomes in week 6 with trial outcomes in earlier time points, and (4) time course of treatment–emergent AEs.
A statistically significant change in total PANSS score at week 6 is currently considered the regulatory standard for evidence of the efficacy of antipsychotic drugs. We assessed the various trial-level concordance rates when clinical trial outcomes were defined at earlier time points compared with trial outcomes observed at week 6.
The mean change from baseline in PANSS score by week for each treatment was estimated using the mixed-model repeated measure (MMRM) analysis for each trial in the database. The MMRM model included baseline PANSS score, treatment randomization, and time-by-treatment interaction, in which Dikt was the change from baseline in total PANSS score for the ith participant in treatment group k at week t: Dikt = weekt + treatmentk + weekt × treatmentk + error.
The indicator variables were treatment, denoting drug or placebo, and week, denoting week of follow-up. An unstructured covariance matrix was used to estimate the within-participant variation error. A treatment group was considered to have a favorable outcome at a given time point if a statistically significant difference from the placebo group was found, and it was considered to have an unfavorable outcome at a given time point if no such difference was observed. Concordance probability was calculated as follows: concordance probability = (number of favorable outcomes + number of unfavorable outcomes)/total number of treatment group comparisons.
A positive trial was defined as a trial in which a favorable outcome (ie, met significance criteria) was observed at both week X (some time point before week 6) and week 6. A negative trial was defined as a trial in which an unfavorable outcome (ie, did not meet significance criteria) was observed at both week X and week 6. Discordance probability was calculated as follows: discordance probability = 1 − concordance probability.
A false-positive outcome was defined as observing a favorable outcome at week X and an unfavorable outcome at week 6. A false-negative outcome was defined as observing an unfavorable outcome at week X and a unfavorable outcome at week 6.
Time to Response, AE, and Modified PANSS
A participant who had a clinically meaningful percentage reduction in total PANSS score from baseline of greater than 20% (the least response rate that is considered clinically meaningful in the literature) was considered a responder, and this occurrence was calculated at each week. Kaplan-Meier analysis was used to assess the cumulative distribution of time until participants first achieved responder status as defined by the total PANSS score. Data imputation was performed using the method of last observation carried forward.
The primary focus of this analysis was on drug efficacy, but drug safety was also important. Therefore, we conducted an AE analysis to explore the implication of shortened trial duration for AE detection, focusing on the proportion and time course of major AEs associated with antipsychotic medications. The method we used is described in eAppendix 1 in the Supplement.
We applied the item response theory method (eAppendix 1 in the Supplement) to assess the feasibility of deriving the modified PANSS with reduced subscore items to improve its sensitivity in detecting treatment outcomes. Baseline PANSS data were used to perform this analysis. Concordance analysis for modified PANSS was performed in a way that was similar to the analysis conducted for the total PANSS.
Sample Size Estimation
A power analysis was performed to assess the sample size required at each of the early time points, using total PANSS or modified PANSS as the primary end point. This estimation allowed the assessment of the implication of shortened clinical trial duration in isolation and in conjunction with the modified PANSS for sample size requirements of alternative trial designs. The estimated effect size, a measure of the magnitude of the treatment outcome, was calculated from the primary MMRM analysis, as previously described. A typical SD for total PANSS scores, as used in schizophrenia drug trial design, was considered to obtain the effect size at each time point.
In the case of the modified PANSS scores, the pooled SD was 11.83 at week 4 and and 12.6 at week 6. Therefore, an SD of 13 was assumed to estimate the sample size required to conduct a study with the modified PANSS as the variable of interest.
Double delta was calculated as follows: the change from baseline is treatment in treatment outcome (total PANSS or modified PANSS) in the drug group, and the change from baseline is treatment outcome (total PANSS or modified PANSS) in the placebo group.
Statistical Analysis
All statistical analyses were performed with SAS, version 9.4 (SAS Institute Inc). All graphs were created with R, version 3.6.1 (R Foundation for Statistical Computing), running under the RStudio interface (RStudio). A 2-sided statistical significance level of α = .05 was used.
Results
Data Characteristics
The final database contained data from 14 219 participants, of whom 3533 (24.8%) were randomized to receive placebo and 10 686 (75.2%) were randomized to receive the active treatment (or drug). Data were obtained from 32 trials. Most trials (28 [87.5%]) were 6 weeks long and 4 trials (12.5%) were 4 weeks long, with a total of 86 treatment groups (including active comparator and active treatment). Most trials had 2 to 3 weeks of mandatory in-house hospital stay, after which study participants were treated on an outpatient basis during the double-blind randomized phase. At least 1200 patients were enrolled in each of the 8 NDA submissions examined.
Most participants were male (9805 of 14 219 [69.0%]), and most participants were either white (7183 [50.5%) or black (4346 [30.6%]) individuals. The mean (SD) age during treatment was 38.9 (10.9) years, and the mean (SD) age at schizophrenia diagnosis was 25 (8.5) years. Most of the trials (28 of 32 [87.5%]) were multiregional, with predominantly North American and European sites. The overall mean (SD) baseline PANSS score was 94.4 (13.6), similar across all trials in the development programs and ranging from 90.6 to 96.3.
Longitudinal Change From Baseline in Total PANSS
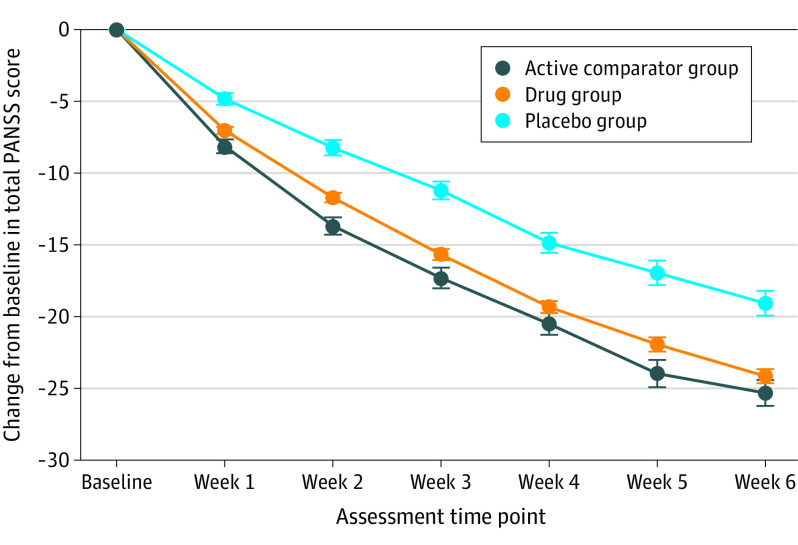
In general, statistically significant separation between the treatment and placebo groups was observed after 1 week of treatment based on longitudinal analysis of change from baseline in total PANSS score (Figure 1) for both active comparator and treatment groups, with greater separation from placebo observed at later time points. By week 4, treatment response (baseline-adjusted response minus placebo response) was 88% of that observed at week 6 (Figure 1).
Figure 1. Global Change From Baseline in Positive and Negative Syndrome Scale (PANSS) Score Across 32 Clinical Trials of 8 Drugs.
Circles represent means, and error bars represent SEs.
Concordance Analysis
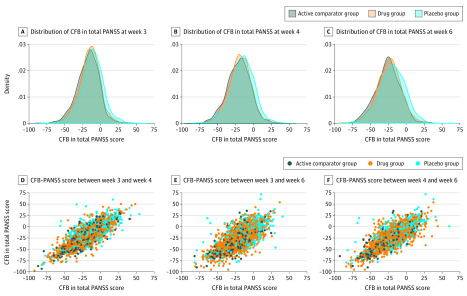
Figure 2 and eTable 1 in the Supplement present the correlation in change from baseline in total PANSS score between week 6 and week 3 or week 4. The concordance for each treatment group between response at week 6 and at earlier time points was calculated. The overall concordance rate across treatment groups steadily increased from week 1 to week 4 (68.0% for week 1, 74.0% for week 2, 83.0% for week 3, and 93.0% for week 4). At week 4, 80 of the 86 treatment groups (93.0%) produced outcomes similar to those observed in week 6. The 7% discordance rate was associated with the 6 treatment groups that did not demonstrate a statistically significant difference from the placebo group, whereas at week 6, the treatment group’s superiority over the placebo group was demonstrated. The 6 treatment groups demonstrating a false-negative outcome were spread among 4 NDAs, with 1 NDA having 3 groups with a false-negative outcome.
Figure 2. Correlation in Change From Baseline (CFB) in Total Positive and Negative Syndrome Scale (PANSS) Score Between Week 6 and Weeks 3 and 4 for Participants in 32 Clinical Trials.
The scatterplots show changes from baseline in total PANSS between week 3 and week 4, week 3 and week 6, and week 4 and week 6 for the 3 treatment groups.
Distribution of Time to Response
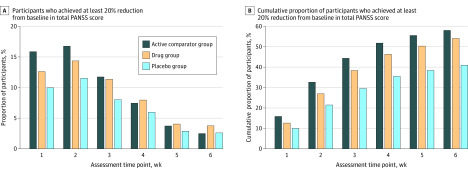
Across the 32 trials, 35.5% to 51.8% of participants by week 4 (placebo [1219 of 3435], drug [3970 of 8578], or active comparator [918 of 1772]) and 41.0% to 58.0% of participants by week 6 in all of the treatment groups (placebo [1407 of 3435], drug [4639 of 8578] or active comparator [1028 of 1772]) were classified as responders or achieved at least 20% reduction from baseline in total PANSS score. Comparative responder analyses among the 3 groups at the week 1 through week 6 time points are shown in Figure 3A. In week 1 of treatment, 10.0% to 15.9% of participants achieved at least a 20% response, with a cumulative proportion of 50% by week 4 (Figure 3B). Week 5 and week 6 response rates contributed an additional 7% of participants to the overall responder rate. Cumulative proportions of participants who achieved 30%, 40%, and 50% response by assessment week are shown in eFigures 5, 6, and 7, respectively.
Figure 3. Comparative Analysis of Treatment Response Among Participants From Week 1 to Week 6.
PANSS indicates Positive and Negative Syndrome Scale.
AE Data and Dropout Rates
The main AEs examined were akathisia, extrapyramidal symptoms, somnolence, dizziness, tachycardia, agitation, and headache (eAppendix 2 in the Supplement). The trends in the occurrence of AEs were evident by week 1, and trends appeared similar over the 6-week duration for akathisia, extrapyramidal symptoms, agitation, and tachycardia (eFigure 1 in the Supplement). Somnolence and dizziness rates were higher initially and then decreased over time (eFigure 1 in the Supplement). The percentages of AEs were similar across week 3, week 4, and week 6 (eFigure 2 in the Supplement). The total number of AEs was higher in week 6 than in week 3 and week 4. The time to first occurrence of AE curves for extrapyramidal symptoms, akathisia, and somnolence was well separated between the placebo and the active treatment group by week 4, in contrast to agitation, tachycardia, and headache, in which the curves were not separated at week 6 (eFigure 3 in the Supplement). Dropout from trials started as early as week 1 and continued throughout the trial in all treatment groups. At week 6, the overall percentage of participants remaining in the trial was 49.1% in the placebo group, 51.2% in the drug group, and 57.5% in the active comparator group (eFigure 4 in the Supplement).
Performance of Modified PANSS as the Clinical End Point
The analysis identified 19 PANSS items as informative, the items that can accurately associate the observed responses with the underlying symptom severity of schizophrenia (eAppendix 1 and eTable 2 in the Supplement). These items included 5 (71.4%) of the 7 Positive subscale (delusions, hallucinatory behavior, excitement, suspiciousness, and hostility), 6 (85.7%) of the 7 Negative subscale (blunted effect, emotional withdrawal, poor rapport, passive or apathetic social withdrawal, difficulty in abstract thinking, and lack of spontaneity and flow of conversation), and 8 (50.0%) of the 16 General Psychopathology subscale (tension, motor retardation, uncooperativeness, unusual thought content, poor attention, lack of judgment and insight, disturbance of volition, and preoccupation).
The overall concordance rate between change from baseline in modified PANSS score and change from baseline in total PANSS score was 93.0% (80 of 86) at week 4 and 97.7% (84 of 86) at week 6. At week 4, the concordance rate was 100% in 4 NDAs. One false-positive and 5 false-negative outcomes were observed at week 4. At week 6, the concordance rate was 100% in 6 NDAs. One false-positive and 1 false-negative outcome at week 6 were observed.
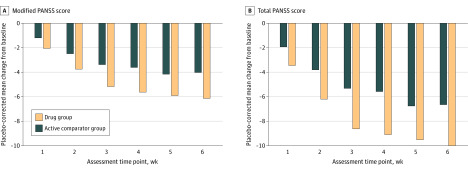
Jointly Considering Treatment Duration and End Point
Figure 4 and eTable 3 in the Supplement display the MMRM model–estimated, placebo-corrected mean change from baseline in total PANSS score (double Δ) by study week for participants in the drug and active comparator groups across all 32 trials. The Table displays the sample size estimates at week 4 and week 6 to detect a difference in change from baseline between total PANSS score and modified PANSS scores with 90% power and a 2-sided α = .05. The sample size for a 6-week trial using total PANSS score as the end point was 380 participants. Shortening the trial duration to 4 weeks increased the sample size to 502 participants. The sample size for a 6-week trial using modified PANSS as the end point was 296 participants, and the sample size for a 4-week trial using modified PANSS as the end point was 402.
Figure 4. Mixed-Model, Repeated Measure–Estimated, Placebo-Corrected Change From Baseline in Modified and Total Positive and Negative Syndrome Scale (PANSS) Score by Treatment Week.
Table. Estimated Sample Size Required for Detecting a Difference in Total and Modified Positive and Negative Syndrome Scale.
| Week | ∆∆ Total PANSS (SD = 20) | Sample size (1:1) | ∆∆ Modified PANSS (SD = 13) | Sample size (1:1) |
|---|---|---|---|---|
| 4 | 5.80 | 502 | 4.22 | 402 |
| 6 | 6.70 | 380 | 4.92 | 296 |
Abbreviations: ∆∆, double delta; PANSS, Positive and Negative Syndrome Scale.
Discussion
Results of the analyses support the feasibility of shortening the schizophrenia drug trial duration to 4 weeks for medications with a reasonably short half-life (approximately 1-2 days). In addition, the results suggest that, for drugs with a longer half-life (2-4 days), the efficacy evaluation may be conducted after 4 weeks of treatment. Several points support these assertions. First, drug discrimination of the antipsychotic properties was observed within 2 weeks of treatment, and it was consistently observed across sex, geographic region, and type of dose (flexible or fixed). Second, the concordance rate for outcomes across treatment groups between week 4 and week 6 was 93.0%. The discordance rate of 7.0% was associated with 6 of the treatment groups demonstrating a false-negative outcome at week 4 compared with week 6. From a regulatory perspective, a false-negative outcome may not be concerning because it happens at a small rate across applications. From a trial sponsor’s perspective, the observed rate of a false-negative outcome may be of concern. Third, at least 80% of participants achieved a clinically significant response (≥20% reduction from baseline in total PANSS score) by week 4, and few required 6 weeks to respond. Fourth, treatment-emergent AEs were detected in sufficient numbers if the trial duration were shortened to 4 weeks. Major AEs associated with antipsychotic therapy were evident as early as week 1, and capturing adequate counts of the AEs by week 4 is possible. The trial may be extended to assess late-occurring AEs. Fifth, a 32% increase in the sample size was required to detect evidence of efficacy if the duration of the placebo-controlled trial were shortened to 4 weeks. This increase in sample size was partially offset by the relatively low dropout rate at week 4, indicating that the patient population would be more representative when assessing treatment outcomes.
Shorter trial duration is further supported by published reports. A large part of interparticipant variability in a schizophrenia trial’s placebo group and dropout rate was explained by study duration.11 Patients who showed little to no response to antipsychotic treatments at week 2 were not expected to show improved response at week 4 and week 6.12,13,14,15 Kinon et al14 proposed that reducing the duration of schizophrenia placebo-controlled trials from 6 weeks to 2 to 4 weeks may minimize dropouts, leading to a more representative group of patients completing the study and more robust conclusions given that the data analysis will depend on observed data rather than statistically imputed data. In addition, shortened schizophrenia drug trial duration will reduce individual patient exposure to experimental medication, minimize dropouts, and allow exploration of more doses in a trial as well as potentially reduce the cost of the drug development program. Shortening the trial duration while using change from baseline in total PANSS score as the primary end point will require a relatively larger sample size, which will increase the number of participants exposed to experimental medications.
Results of these analyses support the use of the modified PANSS in several ways. First, the retrospective application of the modified PANSS increased the overall effect size because of decreased variability (eTable 3 in the Supplement), thus enhancing the signal-to-noise ratio. Second, the PANSS items identified as being less informative were judged as not clinically important because they measure either primary symptoms of diseases other than schizophrenia (eg, grandiosity, anxiety, depression, and poor impulse control) or advanced forms of motor illness seen in some patients with schizophrenia not likely to be enrolled in clinical trials (eg, mannerisms and posturing and motor retardation). Exclusion of less informative items of PANSS that do not adequately assess the underlying symptom severity might not add any additional information with respect to disease status. Third, the concordance rate at week 6 between modified PANSS and total PANSS scores was 97.7%. The discordance rate of less than 3% was associated with 2 of the treatment groups, 1 demonstrating a false-negative and the other demonstrating a false-positive outcome at week 4. Fourth, the 6-week trial using the modified PANSS had a 32% decrease in sample size compared with the 6-week trial using the total PANSS. These findings are consistent with literature reports suggesting that not all items of the PANSS are sufficiently informative to detect a true treatment outcome and that the modified PANSS may be more reliable with insight from future research.16,17
An obstacle to using the modified PANSS instead of the total PANSS is the need to validate it before it can be fully implemented in clinical trials. In the meantime, investigators may consider conducting the full PANSS interview; however, the modified PANSS can be used in the statistical analysis to determine sample size requirements and trial outcomes.
Combining the modified PANSS and shorter trial duration is perhaps the most practical option for designing future drug trials in patients with schizophrenia. The concordance rate between modified PANSS and total PANSS at week 4 was 93.0%. The discordance was associated with 5 of the treatment groups demonstrating a false-negative outcome and 1 treatment group demonstrating a false-positive outcome at week 4. The sample size needed using this approach was 6% higher than the sample size needed to conduct a 6-week trial using total PANSS. Exposing patients to fewer weeks of placebo was directly advantageous for the population under study and reduced dropouts in all groups of the trial.
This analysis identifies various options for modifying the traditional design of schizophrenia drug clinical trials. Shortening the trial duration can be helpful in accelerating drug development, limiting placebo exposure time, and potentially allowing the exploration of more doses in phase 2 trials. Although the data generally support a 4-week trial duration, 3 weeks may be a more feasible option for some drugs that demonstrate substantial early separation between treatment response and placebo response in a phase 2a trial. The modified PANSS is a more informative instrument for assessing a drug’s outcome with considerable reduction in sample size. Although combining the shorter duration option and the modified PANSS option does not appear to decrease sample size, this option can provide a substantial reduction in sample size and trial cost for some drugs that are expected to yield high treatment outcomes.
Limitations
This analysis has limitations. The analysis used data from clinical trials of 8 second generation atypical antipsychotics with a similar mechanism of action because no other data are available. It is assumed that these findings are generalizable regardless of the medication mechanism of action; however, the validity of this assumption could not be assessed in this analysis.
Conclusions
We believe that this analysis provides insights into improving the design of schizophrenia drug trials. Drug manufacturers and other developers may consider incorporating these strategies in their development programs and are encouraged to consult the FDA early when planning such trials.
eAppendix 1. Derivation of mPANSS
eAppendix 2. Adverse Event Data Analysis and Discussion
eFigure 1. Proportion of Adverse Events Over the 6-week Duration Pooled Across All Drugs
eFigure 2. Percent of Adverse Events by Trial Week
eFigure 3. Time to First Occurrence Adverse Event in Schizophrenia Trials
eFigure 4. Overall Dropout Rates by Study Week
eFigure 5. Proportion of Subjects Achieving 30% Response Rate (Responders) by Trial Week
eFigure 6. Proportion of Subjects Achieving 40% Response Rate (Responders) by Trial Week
eFigure 7. Proportion of Subjects Achieving 50% Response Rate (Responders) by Trial Week
eTable 1. Pearson Correlation Coefficient Between Different Weeks for All Treatment Groups
eTable 2. List of PANSS Items To Be Included In or Excluded From mPANSS
eTable 3. MMRM Estimated Mean Placebo Corrected Week 6 CFB-PANSS (Total PANSS and mPANSS) by Type of Treatment Group
References
- 1.Downing NS, Aminawung JA, Shah ND, Krumholz HM, Ross JS. Clinical trial evidence supporting FDA approval of novel therapeutic agents, 2005-2012. JAMA. 2014;311(4):368-377. doi: 10.1001/jama.2013.282034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mueser KT, McGurk SR. Schizophrenia. Lancet. 2004;363(9426):2063-2072. doi: 10.1016/S0140-6736(04)16458-1 [DOI] [PubMed] [Google Scholar]
- 3.Cloutier M, Aigbogun MS, Guerin A, et al. The economic burden of schizophrenia in the United States in 2013. J Clin Psychiatry. 2016;77(6):764-771. doi: 10.4088/JCP.15m10278 [DOI] [PubMed] [Google Scholar]
- 4.Wu EQ, Birnbaum HG, Shi L, et al. The economic burden of schizophrenia in the United States in 2002. J Clin Psychiatry. 2005;66(9):1122-1129. doi: 10.4088/JCP.v66n0906 [DOI] [PubMed] [Google Scholar]
- 5.Keshavan MS, Lawler AN, Nasrallah HA, Tandon R. New drug developments in psychosis: Challenges, opportunities and strategies. Prog Neurobiol. 2017;152:3-20. doi: 10.1016/j.pneurobio.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laughren TP. The scientific and ethical basis for placebo-controlled trials in depression and schizophrenia: an FDA perspective. Eur Psychiatry. 2001;16(7):418-423. doi: 10.1016/S0924-9338(01)00600-9 [DOI] [PubMed] [Google Scholar]
- 7.Leucht S, Arbter D, Engel RR, Kissling W, Davis JM. How effective are second-generation antipsychotic drugs? a meta-analysis of placebo-controlled trials. Mol Psychiatry. 2009;14(4):429-447. doi: 10.1038/sj.mp.4002136 [DOI] [PubMed] [Google Scholar]
- 8.Kemp AS, Schooler NR, Kalali AH, et al. What is causing the reduced drug-placebo difference in recent schizophrenia clinical trials and what can be done about it? Schizophr Bull. 2010;36(3):504-509. doi: 10.1093/schbul/sbn110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbott A. Novartis to shut brain research facility. Nature. 2011;480(7376):161-162. doi: 10.1038/480161a [DOI] [PubMed] [Google Scholar]
- 10.CDISC SDTM. Accessed May 26, 2020. https://www.cdisc.org/standards/foundational/sdtm
- 11.Pilla Reddy V, Kozielska M, Johnson M, et al. Modelling and simulation of the Positive and Negative Syndrome Scale (PANSS) time course and dropout hazard in placebo arms of schizophrenia clinical trials. Clin Pharmacokinet. 2012;51(4):261-275. doi: 10.2165/11598460-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 12.Agid O, Kapur S, Arenovich T, Zipursky RB. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry. 2003;60(12):1228-1235. doi: 10.1001/archpsyc.60.12.1228 [DOI] [PubMed] [Google Scholar]
- 13.Chang YC, Lane HY, Yang KH, Huang CL. Optimizing early prediction for antipsychotic response in schizophrenia. J Clin Psychopharmacol. 2006;26(6):554-559. doi: 10.1097/01.jcp.0000246211.95905.8c [DOI] [PubMed] [Google Scholar]
- 14.Kinon BJ, Chen L, Stauffer VL, et al. Early onset of antipsychotic action in schizophrenia: evaluating the possibility of shorter acute efficacy trials. J Clin Psychopharmacol. 2010;30(3):286-289. doi: 10.1097/JCP.0b013e3181dcb7c3 [DOI] [PubMed] [Google Scholar]
- 15.Leucht S, Shamsi SA, Busch R, Kissling W, Kane JM. Predicting antipsychotic drug response - replication and extension to six weeks in an international olanzapine study. Schizophr Res. 2008;101(1-3):312-319. doi: 10.1016/j.schres.2008.01.018 [DOI] [PubMed] [Google Scholar]
- 16.Khan A, Lewis C, Lindenmayer JP. Use of non-parametric item response theory to develop a shortened version of the Positive and Negative Syndrome Scale (PANSS). BMC Psychiatry. 2011;11:178. doi: 10.1186/1471-244X-11-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santor DA, Ascher-Svanum H, Lindenmayer JP, Obenchain RL. Item response analysis of the Positive and Negative Syndrome Scale. BMC Psychiatry. 2007;7:66. doi: 10.1186/1471-244X-7-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Derivation of mPANSS
eAppendix 2. Adverse Event Data Analysis and Discussion
eFigure 1. Proportion of Adverse Events Over the 6-week Duration Pooled Across All Drugs
eFigure 2. Percent of Adverse Events by Trial Week
eFigure 3. Time to First Occurrence Adverse Event in Schizophrenia Trials
eFigure 4. Overall Dropout Rates by Study Week
eFigure 5. Proportion of Subjects Achieving 30% Response Rate (Responders) by Trial Week
eFigure 6. Proportion of Subjects Achieving 40% Response Rate (Responders) by Trial Week
eFigure 7. Proportion of Subjects Achieving 50% Response Rate (Responders) by Trial Week
eTable 1. Pearson Correlation Coefficient Between Different Weeks for All Treatment Groups
eTable 2. List of PANSS Items To Be Included In or Excluded From mPANSS
eTable 3. MMRM Estimated Mean Placebo Corrected Week 6 CFB-PANSS (Total PANSS and mPANSS) by Type of Treatment Group