Key Points
Question
What are the incidence rates of histopathologically confirmed cutaneous squamous cell carcinoma (cSCC) in situ in the Netherlands, and what is the risk of developing invasive cSCC among patients with cutaneous squamous cell carcinoma in situ compared with the general population?
Findings
In this cohort study of 88 754 patients with cSCC in situ, the age-standardized incidence rates for cSCC in situ increased over the study period to 72 cases per 100 000 person-years in women and 68 cases per 100 000 person-years in men. Patients with cSCC in situ had a 16-fold greater risk of developing invasive cutaneous squamous cell carcinoma in the year after receiving a diagnosis of cSCC in situ compared with the general population.
Meaning
The study’s findings highlight the burden of disease associated with precursors of keratinocyte cancer and the need to incorporate cSCC in situ into future health policies for skin cancer.
Abstract
Importance
The incidence rates of keratinocyte cancer are increasing globally; however, the incidence rates of cutaneous squamous cell carcinoma (cSCC) in situ and the risk of developing subsequent invasive cSCC remain unknown.
Objective
To estimate annual population-based age-standardized incidence rates of histopathologically confirmed cSCC in situ stratified by sex, age, and body site and to assess the risk of developing invasive cSCC among patients with cSCC in situ compared with the general population.
Design, Setting, and Participants
This nationwide epidemiological population-based cohort study used cancer registry data to identify all patients with a first incident of histopathologically confirmed cSCC in situ between January 1, 1989, and December 31, 2017. In addition, all patients with cSCC in situ who subsequently had a first incident of invasive cSCC were identified up to June 11, 2019. Data were analyzed between March 18 and November 12, 2019.
Main Outcomes and Measures
Age-standardized incidence rates per year for cSCC in situ, standardized to the 2013 edition of the European Standard Population, were calculated by sex, age, and body site. Cumulative risks, standardized incidence ratios, and absolute excess risks were calculated to assess the risk of invasive cSCC in patients with cSCC in situ compared with the general population.
Results
In this population-based cohort study of 88 754 patients with a first incident of cSCC in situ between January 1, 1989, and December 31, 2017, 58.8% were women; the median age was 75 years (interquartile range [IQR], 67-82 years) for women and 73 years (IQR, 65-80 years) for men. Increasing incidence rates were observed, with the highest incidence rates in 2017 among women in general (71.7 cases per 100 000 person-years) and among men 80 years and older (540.9 cases per 100 000 person-years). The most common body site among women was the face (15.9 cases per 100 000 person-years) and among men was the scalp and/or neck (12.3 cases per 100 000 person-years). After 5 years of follow-up, among patients with cSCC in situ, the cumulative risk of developing an invasive cSCC at any anatomic location was 11.7% (95% CI, 11.6%-11.9%) in men and 6.9% (95% CI, 6.8%-7.0%) in women (P < .001). The standardized incidence ratio was highest in the first year of follow-up among both men (16.6; 95% CI, 15.7-17.5) and women (15.1; 95% CI, 14.2-16.1).
Conclusions and Relevance
This study reports the first nationwide incidence rates of cSCC in situ to date. The increasing incidence rates of cSCC in situ and the high risk of developing invasive cSCC among patients with cSCC in situ may increase the health care burden associated with precursors of keratinocyte cancer and highlight the need to include cutaneous skin cancer precursor lesions when exploring policies to address skin cancer care.
This cohort study uses data from the Netherlands Cancer Registry to assess the incidence rates of cutaneous squamous cell carcinoma in situ and the risk of developing invasive cutaneous squamous cell carcinoma among patients compared with the general population in the Netherlands from 1989 to 2017.
Introduction
Cutaneous squamous cell carcinoma (cSCC) in situ, also known as Bowen disease, presents as a gradually growing erythematous or scaly patch on the skin.1 Although usually harmless in itself, cSCC in situ has a 3% to 5% risk of progressing to invasive cSCC when left untreated.1,2 The main risk factors for cSCC in situ include cumulative exposure to UV radiation, radiotherapy, and immunosuppression.3,4,5,6 Literature regarding the epidemiology of cSCC in situ is scarce, indicating the limited attention given to the in situ form of cSCC. This limited attention is indicative of a worldwide lack of registration of keratinocyte cancers in general, as keratinocyte cancers are often excluded from cancer registries owing to their high incidence and the difficulty of correctly registering multiple tumors per patient.6,7,8
Given the increasing incidence rates of invasive cSCC along with the associated costs and health care burden, it is important to have reliable numbers for the incidence rates of cSCC in situ and the cumulative incidence of the subsequent development of invasive cSCC.8,9,10,11 To date, the few available studies on the epidemiology of cSCC in situ reported varying incidence rates. Furthermore, the studies were conducted in small populations, and most of these studies date from the previous century.12,13,14,15 Therefore, current representative incidence rates from population-based cohorts are needed. The Netherlands Cancer Registry (NCR) registers all cases of histopathologically confirmed skin cancers, including cases of cSCC in situ. The primary objective of this study was to investigate annual age-standardized incidence rates for the first incident of primary cSCC in situ in the Netherlands between 1989 and 2017 and to assess the risk of developing invasive cSCC among patients with cSCC in situ compared with the general population.
Methods
Design, Setting, and Population
Data on all patients with a first incident of histopathologically confirmed cSCC in situ between January 1, 1989, and December 31, 2017, were retrieved from the nationwide NCR.16 Data included in the NCR are based on notification of all histopathologically confirmed cancers from the nationwide network and registry of histopathology and cytopathology (PALGA) in the Netherlands.17 Until September 1, 2016, patients’ demographic characteristics, tumor characteristics, and treating medical specialists were obtained manually from their secondary care medical records by trained NCR personnel. Because of the variation in registration procedures over time and between regional registries, any patients with cSCC in situ who received treatment solely from a general practitioner or pathologist were excluded to ensure reliable trend analyses. In response to the high incidence rates of skin cancer, the NCR replaced manual registration with automatic linkage to the PALGA network beginning on September 1, 2016. This change in registration method enabled reliable estimations by including patients with cSCC in situ who received diagnoses from general practitioners, pathologists, or private clinics without further hospital referral. In addition, after optimization of the automatic linkage with the PALGA network, patients with cSCC in situ with a previous or simultaneous invasive cSCC, who had not been registered under the former procedure, were also included. The patients’ date of death was retrieved from municipal registries up to December 31, 1995, and from the nationwide population registries thereafter. Information on the historical composition of the Dutch population was retrieved from Statistics Netherlands.18 This study followed the code of conduct of the Foundation Federation of Dutch Medical Scientific Societies, which states that informed consent is not needed for studies in which all data are anonymized and not reducible to the patient level.
Patients with cSCC in situ were defined as those with a topography code for skin (C44) and morphology codes for Bowen disease (8081/2) or carcinoma in situ (8010/2) in the International Classification of Diseases for Oncology, Third Edition (ICD-O-3).19 Follow-up duration was calculated as the time from diagnosis of cSCC in situ until death or the end of the study period (June 11, 2019).
To examine trends, the study was divided into 6 periods (1989-1993, 1994-1998, 1999-2003, 2004-2008, 2009-2013, and 2014-2015). Given the change in registration method, the years 2016 and 2017 were evaluated separately. Patient age at diagnosis of cSCC in situ was divided into 3 categories (<70 years, 70-79 years, and ≥80 years), and the anatomical subsite was categorized as follows: lips (ICD-O-3 code C44.0), eyelid (ICD-O-3 code C44.1), ear (ICD-O-3 code C44.2), face (ICD-O-3 code C44.3), scalp and/or neck (ICD-O-3 code C44.4), trunk (ICD-O-3 code C44.5), arms (ICD-O-3 code C44.6), legs (ICD-O-3 code C44.7), and unknown or overlapping (ICD-O-3 codes C44.8 and C44.9, respectively).
Statistical Analysis
All analyses were stratified by sex. Annual crude and age-standardized incidence rates per 100 000 person-years were calculated using the population size (determined on January 1 of each year) obtained from Statistics Netherlands. For the main analyses, the European Standard Population, 2013 edition (ESP 2013),20 was used in the direct standardization method to calculate European standardized rates, as this standard population best reflected the current aging population. European standardized rates were calculated by sex, age group, and body site. Age standardization to other standard populations was also performed (eTable 1 in the Supplement).
To evaluate trends in incidence rates for cSCC in situ over time, we calculated the estimated annual percentage change with corresponding 95% CIs using joinpoint regression analyses, with year of diagnosis as the independent variable in the regression models.21 To prevent inaccurate estimation of an increasing trend associated with automatic linkage to the PALGA network beginning in mid-2016, joinpoint regression analyses were restricted to the years up to 2015 in our main analysis. In a revised analysis, we included the years 2016 and 2017 and omitted the period from 2013 to 2015, as a flawed trend was expected because of the insufficient registration of patients with cSCC in situ associated with nonregistration of private practices during this period.
The risk of developing invasive cSCC during follow-up was estimated by calculating the cumulative incidence curve after diagnosis of the first incident of cSCC in situ among patients without a cSCC history. All first incidents of invasive cSCCs were included, regardless of the anatomical location of the first incident of cSCC in situ. Thus, the cumulative incidence represents the general risk of developing subsequent invasive cSCC rather than the risk of progression of a specific lesion. The difference in the risk of developing invasive cSCC between men and women was assessed using the Gray test for equality of cumulative incidence functions.22,23 Standardized incidence ratios (SIRs) and absolute excess risks (AERs) were calculated for the follow-up periods of 0 to 1 year, 2 to 5 years, 6 to 10 years, and 11 to 29 years after diagnosis of cSCC in situ. The SIR and AER were calculated as the ratio (SIR) and the difference per 10 000 person-years (AER) between the observed number of invasive cSCCs among patients with cSCC in situ and the expected number of invasive cSCCs among the general population. Explanations for all outcome measure acronyms are provided in eTable 2 in the Supplement.
Proportions of medical specialties providing treatment for patients who were newly diagnosed with cSCC in situ were calculated for the period of 2005 to 2015, as the registration method was uniform only for this period. All tests were 2-sided, with a significance level of P < .05. Statistical analyses were performed using the following software: IBM SPSS Statistics, version 25.0 (IBM); R, version 3.4.1, cmprsk package (R Project for Statistical Computing); SAS, version 9.4 (SAS Institute); Stata, version 14, strs-macro (StataCorp)24; and Joinpoint, version 4.7.0.0 (National Cancer Institute).21 Data were analyzed between March 18 and November 12, 2019.
Results
Overall Population
We identified 88 754 patients with a first incident of cSCC in situ between January 1, 1989, and December 31, 2017. Of those, 52 161 patients (58.8%) were female. The median age at cSCC in situ diagnosis was 73 years (interquartile range [IQR], 65-80 years) for men and 75 years (IQR, 67-82 years) for women.
Approximately one-third of all cases of cSCC in situ were located in the facial area for both women (n = 18 005 [34.5%]) and men (n = 12 565 [34.3%]). Although the ears were a common location in men (n = 3849 [10.5%]), only 520 women (1.0%) had periauricular cSCC in situ. The second most common location in men was the arms (n = 7147 [19.5%]) and in women was the legs (n = 13 957 [26.8%]).
Incidence Rates
The number of histopathologically confirmed cSCC in situ diagnoses increased from 447 men and 531 women in 1989 to 4719 men and 6335 women in 2017 within a population of approximately 16 million inhabitants. This increase corresponded with an increase in the crude incidence rate among men, from 6.1 cases per 100 000 inhabitants in 1989 to 55.7 cases per 100 000 inhabitants in 2017. The crude incidence rate in women increased 10-fold, from 7.1 cases per 100 000 inhabitants in 1989 to 73.6 cases per 100 000 inhabitants in 2017. After adjusting to the ESP 2013, the European standardized rate in men increased 6-fold between 1989 and 2017, from 11.1 cases to 67.8 cases per 100 000 person-years. The European standardized rate in 1989 was lower for women than men, but the increase in the European standardized rate during the same period was greater for women than men, from 9.3 cases to 71.7 cases of cSCC in situ per 100 000 person-years. In 2017, the highest European standardized rates of cSCC in situ were found in women, with a female to male ratio of 1.4 to 1.0. Table 1 shows the European standardized rates for cSCC in situ by period of diagnosis, age group, and body site for men and women. The European standardized rates of cSCC in situ for each year independently as well as age-standardized to standard populations other than the ESP 2013 population can be found in eTable 1 in the Supplement.
Table 1. European Standardized Rates of Histopathologically Confirmed Cases of Cutaneous Squamous Cell Carcinoma In situ From 1989 to 2017.
| Variable | European standardized ratea | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | |||||||||||||||||
| Cases, No. | Period | Cases, No. | Period | |||||||||||||||
| 1989-1993 | 1994-1998 | 1999-2003 | 2004-2008 | 2009-2013 | 2014-2015 | 2016 | 2017 | 1989-1993 | 1994-1998 | 1999-2003 | 2004-2008 | 2009-2013 | 2014-2015 | 2016 | 2017 | |||
| Total | 36 593 | 12.2 | 16.0 | 17.1 | 22.2 | 29.5 | 30.1 | 48.9 | 67.8 | 52 161 | 10.4 | 13.9 | 17.1 | 24.5 | 33.2 | 35.0 | 53.1 | 71.7 |
| Age group, y | ||||||||||||||||||
| <70 | 13 818 | 4.8 | 5.5 | 5.7 | 7.7 | 9.4 | 9.0 | 14.3 | 18.8 | 16 878 | 4.1 | 5.3 | 6.6 | 9.6 | 12.3 | 11.8 | 17.5 | 23.9 |
| 70-79 | 13 246 | 47.2 | 63.7 | 67.9 | 88.2 | 125.5 | 129.8 | 200.0 | 273 | 17 215 | 38.4 | 55.1 | 65.5 | 94.5 | 136.6 | 152.5 | 232.0 | 299.9 |
| ≥80 | 9529 | 77.5 | 110.8 | 122.5 | 153.3 | 202.3 | 213.8 | 373.6 | 540.9 | 18 068 | 68.5 | 87.8 | 110.4 | 154.0 | 207.2 | 223.5 | 341.9 | 482.7 |
| Body site | ||||||||||||||||||
| Lips | 188 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 212 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.3 |
| Eyelid | 376 | 0.2 | 0.2 | 0.2 | 0.2 | 0.3 | 0.2 | 0.3 | 0.5 | 351 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.2 | 0.4 | 0.5 |
| Ear | 3849 | 2.4 | 2.5 | 2.7 | 2.6 | 3.0 | 3.3 | 4.1 | 4.7 | 520 | 0.2 | 0.1 | 0.2 | 0.3 | 0.3 | 0.2 | 0.4 | 0.6 |
| Face | 8152 | 3.1 | 4.2 | 4.2 | 5.4 | 7.1 | 7.0 | 10.7 | 11.2 | 16 922 | 3.9 | 5.2 | 5.6 | 8.1 | 11.1 | 12.4 | 15.6 | 15.9 |
| Scalp and/or neck | 4983 | 1.3 | 2.0 | 2.0 | 3.2 | 4.3 | 4.7 | 7.6 | 12.3 | 3444 | 0.6 | 0.8 | 0.9 | 1.4 | 2.0 | 2.4 | 3.9 | 7.5 |
| Trunk | 5967 | 1.2 | 1.7 | 2.3 | 3.5 | 5.1 | 5.7 | 8.4 | 9.5 | 5895 | 1.0 | 1.3 | 1.6 | 2.5 | 4.0 | 4.6 | 6.9 | 8.0 |
| Arms | 7147 | 2.7 | 3.3 | 3.4 | 4.5 | 5.8 | 5.3 | 8.2 | 9.5 | 8732 | 1.9 | 2.3 | 2.9 | 4.3 | 5.7 | 5.6 | 8.7 | 10.8 |
| Legs | 4401 | 1.0 | 1.7 | 2.0 | 2.5 | 3.5 | 3.6 | 5.2 | 7.0 | 13 957 | 2.5 | 3.9 | 5.4 | 7.3 | 9.3 | 9.1 | 12.2 | 14.8 |
| Unknown | 1530 | 0.2 | 0.1 | 0.2 | 0.3 | 0.3 | 0.4 | 4.4 | 12.9 | 2128 | 0.2 | 0.1 | 0.2 | 0.3 | 0.4 | 0.4 | 5.0 | 13.3 |
Standardized rate per 100 000 person-years.
The European standardized rates differed substantially between the 3 age groups. The highest rates for both sexes were found in 2017 among the oldest age group (≥80 years), with a rate of 482.7 cases per 100 000 person-years for women. Although the absolute number of cases of cSCC in situ for men was highest in the subgroup younger than 70 years, after adjusting to the ESP 2013, which takes into account the population’s age distribution, the rate in men 80 years and older was even higher (540.9 cases per 100 000 person-years) than the rate in women.
Regarding body site, the sun-exposed locations in women (ie, the face [15.9 cases per 100 000 person-years] and the legs [14.8 cases per 100 000 person-years]) had the highest incidence rates compared with the sun-exposed locations in men (ie, the face [11.2 cases per 100 000 person-years] and the scalp and/or neck [12.3 cases per 100 000 person-years]) in 2017 (Table 1).
Incidence Rate Trends
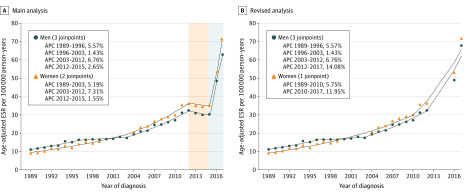
Joinpoint regression analyses for the years before the change in registration method (1989-2015) revealed 2 periods with a statistically significant increase in incidence rates for both sexes. In men, the estimated annual percentage change was 5.6% (95% CI, 4.2%-6.9%) for the period of 1989 to 1996 and 6.8% (95% CI, 5.7%-7.9%) for the period of 2003 to 2012. In women, the incidence rates increased by 5.2% (95% CI, 4.6%-5.8%) for the period of 1989 to 2003 and 7.3% (95% CI, 5.9%-8.8%) for the period of 2003 to 2012, outpacing the European standardized rate in men (Figure 1A). The revised analysis indicated an additional increase in European standardized rates in the period of 2012 to 2017 for men (estimated annual percentage change, 14.1%; 95% CI, 3.4%-25.9%) and the period of 2010 to 2017 for women (estimated annual percentage change, 12.0%; 95% CI, 9.2%-14.8%) (Figure 1B).
Figure 1. European Standardized Rates and Joinpoint Analyses of Cutaneous Squamous Cell Carcinoma In situ .
A, The red shading for the period of 2013 to 2015 indicates a potentially inaccurate decrease associated with the nonregistration of patients with cSCC in situ who were increasingly treated in private practices during this period. The gray shading for the years 2016 and 2017 indicates the improved registration method used for patients with cSCC in situ during these years. B, The period of 2013 to 2015 was excluded. This curve more accurately reflects the burden of cSCC in situ. APC indicates annual percentage change; cSCC, cutaneous squamous cell carcinoma; and ESR, European standardized rate.
With regard to body location–specific increases in incidence rates, the European standardized rates of cSCC in situ on the scalp and/or neck and cSCC in situ on the trunk indicated the highest increases in both men and women over the 29-year study period. The European standardized rate of cSCC in situ on the scalp and/or neck increased 12-fold in men and 15-fold in women, while the increase in the rate of cSCC in situ on the trunk increased 8-fold in both sexes. Although the facial area had the highest rates of cSCC in situ from 1989 to 2016, the relative 5-fold increase in rates over the same study period was not substantial compared with other body sites (Table 1).
Risk of Invasive cSCC
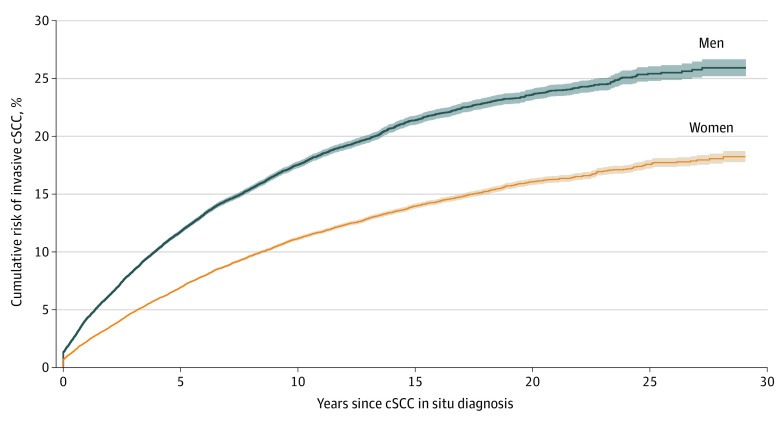
Of the 88 754 patients with a histopathologically confirmed case of cSCC in situ, 5066 patients (5.7%) had an invasive cSCC in their medical history. Among the remaining 83 688 patients with cSCC in situ who did not have a history of cSCC, 9363 patients (11.2%) developed an invasive cSCC at any anatomic location during follow-up. After accounting for the competing risk of death, the cumulative risk of developing invasive cSCC within 5 years of a diagnosis of cSCC in situ was significantly higher in men (11.7%; 95% CI, 11.6%-11.9%) compared with women (6.9%; 95% CI, 6.8%-7.0%; P < .001). The 10-year cumulative risk was 17.5% (95% CI, 17.2%-17.8%) for men and 11.1% (95% CI, 11.0%-11.3%) for women; the 20-year cumulative risk was 23.6% (95% CI, 23.1%-24.1%) for men and 16.1% (95% CI, 15.8%-16.4%) for women; and the 29-year cumulative risk was 25.9% (95% CI, 25.2%-26.7%) for men and 18.2% (95% CI, 17.8%-18.7%) for women (Figure 2).
Figure 2. Cumulative Incidence Curve .
Cumulative risk of developing invasive cSCC after the first incident of cSCC in situ among patients without a history of cSCC. cSCC indicates cutaneous squamous cell carcinoma.
The SIRs of invasive cSCC were highest in the first year after diagnosis of cSCC in situ for both men (16.6; 95% CI, 15.7-17.5) and women (15.1; 95% CI, 14.2-16.1). The invasive cSCC incidence rates among patients with a diagnosis of cSCC in situ remained at least 6 times higher than those of the general population for up to 29 years (Table 2). The AERs were also highest in the first year of follow-up (in men, 404 cases per 10 000 person-years; 95% CI, 381-427 cases per 10 000 person years; in women, 211 cases per 10 000 person-years; 95% CI, 198-224 cases per 10 000 person-years) and remained high up to 29 years of follow-up, with at least 123 additional diagnoses of invasive cSCC per 10 000 person-years among patients with cSCC in situ compared with the general population (Table 2).
Table 2. First Incident of Invasive Cutaneous Squamous Cell Carcinoma by Sex and Duration of Follow-up.
| Duration of follow-up, y | Cases, No. | SIR (95% CI) | Person-years at risk | AER (95% CI) | |
|---|---|---|---|---|---|
| Observed | Expected | ||||
| Men | |||||
| 2-5 | 1861 | 210 | 8.8 (8.4-9.3) | 77 222 | 214 (203-224) |
| 6-10 | 962 | 133 | 7.3 (6.8-7.7) | 44 031 | 188 (176-201) |
| 11-29 | 564 | 94 | 6.0 (5.5-6.5) | 25 556 | 184 (167-201) |
| Total | 4673 | 515 | 9.1 (8.8-9.3) | 176 705 | 235 (228-242) |
| Women | |||||
| 0-1 | 1030 | 68 | 15.1 (14.2-16.1) | 45 613 | 211 (198-224) |
| 2-5 | 1736 | 197 | 8.8 (8.4-9.2) | 124 669 | 123 (117-130) |
| 6-10 | 1062 | 135 | 7.9 (7.4-8.4) | 75 621 | 123 (115-130) |
| 11-29 | 653 | 98 | 6.7 (6.2-7.2) | 44 931 | 124 (113-134) |
| Total | 4481 | 498 | 9.0 (8.7-9.3) | 290 834 | 137 (133-141) |
Abbreviations: AER, absolute excess risk per 10 000 person-years; SIR, standardized incidence ratio.
Treating Medical Specialties
For the period of 2005 to 2015, we analyzed all treating medical specialties that provided secondary care for patients who were newly diagnosed with cSCC in situ. Most patients (82.0%) with cSCC in situ received treatment from a dermatologist, with 7.4% and 3.3% of patients receiving treatment from a plastic surgeon or a general surgeon, respectively. The proportions of all treating specialties and combined treatment trajectories are presented in Table 3. The eFigure in the Supplement shows the share of each medical specialty in the treatment of patients with cSCC in situ over time.
Table 3. Medical Specialties Treating Patients With a First Incident of Cutaneous Squamous Cell Carcinoma In situ From 2005 to 2015.
| Specialty | Patients treated, No. (%) (N = 44 186) |
|---|---|
| Dermatology | 36 025 (81.5) |
| Plastic surgery | 3260 (7.4) |
| General surgery | 1444 (3.3) |
| Specialty outside hospital | 324 (0.7) |
| Dermatology and plastic surgery | 212 (0.5) |
| Ear, nose, and throat | 209 (0.5) |
| Ophthalmology | 77 (0.2) |
| Gynecology | 38 (0.1) |
| Unknowna | 2346 (5.3) |
| Otherb | 251 (0.6) |
Includes incidents in which the treating medical specialty was not reported in the medical records.
Includes medical specialties treating less than 100 patients with cutaneous squamous cell carcinoma in situ.
An increasing trend was observed in treatment provided by dermatologists (105.6%), with a smaller increase observed in treatment provided by plastic surgeons (77.5%). In contrast, the number of patients with cSCC in situ who received treatment from general surgeons decreased by 53.5% over the 10-year period.
Discussion
To our knowledge, this is the first study to assess population-based incidence rates of cSCC in situ based on a comprehensive nationwide cancer registry. After further optimization of the skin cancer registration method in the second half of 2016, we were able to obtain incidence rates of histopathologically confirmed cases of cSCC in situ that better reflected the population-based incidence rates in a northern European population. We observed that the incidence rates of cSCC in situ are increasing, especially in women, and we found a substantially higher risk of developing invasive cSCC among patients with cSCC in situ compared with the general population.
In 2017, the highest European standardized rates of cSCC in situ were found in women, with a female to male ratio of 1.4 to 1.0. This finding is consistent with the preponderance of women with cSCC in situ reported in other studies.5,12,14,25,26,27,28 For invasive cSCC, opposite results were found in the same population from an earlier study period, with almost equal European standardized rates for cSCC in situ and invasive cSCC in women, while the rate of cSCC in situ was no more than half the rate of invasive cSCC in men.10 This finding could suggest an underdetection of cSCC in situ among men, while underdetection of invasive cSCC is less likely because this form of cSCC may generate more clinical concern among patients.
The body site–specific incidence rates support the important role of cumulative UV radiation exposure in the pathogenesis of cSCC in situ, as the highest European standardized rates were found in the most sun-exposed body sites for both sexes. These sites were the face and scalp and/or neck for men and the face and legs for women, which is consistent with findings from previous studies.5,13,25,26,27,29 An increasing trend of cSCC in situ localized to the trunk over time most likely reflects the increase in UV radiation exposure during recreational activities, such as holiday visits to sunny countries and tanning bed use.
Previous studies on the incidence rates of cSCC in situ are limited and were performed in small, often selected study populations with limited trend data (<5 years).12,13,15,29 The highest incidence rates were reported in the Hawaiian population, but no trend was observed during the period of 1983 to 1987.13 The incidence rates among populations in the United States (northern states),12 Canada,15 and the Netherlands29 were approximately 10 times lower than those of the Hawaiian population, which may be associated with differences in geographic locations, as the highest numbers were found in the sunniest climates.13 Although the study by Hansen et al29 was also conducted in the Netherlands, data from a tertiary referral center were used, which hindered comparison with our population-based data.
In this study, we found that patients with cSCC in situ had a higher risk of developing invasive cSCC compared with patients who did not have a history of cSCC in situ. Few studies have investigated this risk, leaving the treating physician without clear guidance about the prognosis of a patient with cSCC in situ. Jaeger et al30 used data from the Danish Cancer Registry in 1999 to identify an invasive cSCC risk of 4% at 3 years after diagnosis of cSCC in situ, but this study did not account for the competing risk of death, which could have produced an overestimation of the risk.31 However, the corresponding risk (5.5%) was higher in our study. This discrepancy might be associated with the more recent study period that we included, given the increase in incidence rates of both cSCC in situ and invasive cSCC in the past decades, the improvement in registration methods, and the higher awareness of the importance of skin cancer detection in general.8,9,10
A previous study among patients with at least 2 keratinocyte cancers reported that patients who had a previous cSCC in situ had a 2-fold higher risk of developing additional invasive cSCCs.32 In our study, we found a lifelong increased risk of developing invasive cSCC among patients with cSCC in situ compared with the general population, as both the SIRs (ie, the relative excess risk of developing invasive cSCC) and AERs (ie, the absolute excess risk of developing invasive cSCC) remained elevated for 29 years. Given the SIR, AER, and cumulative risk of developing a subsequent cSCC, additional surveillance of patients with cSCC in situ, especially in the first 5 years after diagnosis, might be valuable to detect subsequent cSCCs at an early stage. The role of precursor lesions as factors associated with future invasive disease has also been reported for other cutaneous cancers, such as the associations between melanoma in situ and melanoma,33,34 lentigo maligna and lentigo maligna melanoma,35 and actinic keratosis and cSCC,36 further highlighting the importance of identifying precursor lesions. When interpreting our results, one has to keep in mind that we did not investigate the risk of progression of a single cSCC in situ lesion; rather, we looked at the overall risk of developing invasive cSCC in patients with (mostly treated) cSCC in situ without a history of cSCC.
Strengths and Limitations
A strength of our study is the use of nationwide population-based data comprising all patients with histopathologically confirmed cSCC in situ lesions in the Netherlands who had high-quality data available over a long follow-up period.37 This strength allowed for accurate estimations of the number of cSCC in situ lesions, enabling us to draw reliable conclusions on the incidence rates of cSCC in situ as well as the subsequent development of invasive cSCC.
Nevertheless, the assessment of incidence rates of cSCC in situ based on cancer registration data also had several limitations that need to be considered when interpreting our findings. First, changes in the NCR registration method prevented us from performing regression analyses over the total study period, as these data would have produced incomparable trends over time. To overcome this problem, we also performed a revised analysis that included the 2 most recent years (2016 and 2017) with complete registration rates and excluded the period from 2013 to 2015, which reflected a potentially inaccurate decrease in European standardized rates for both sexes. This decrease may have been associated with the nonregistration of a large number of patients with cSCC in situ who received treatment in private practices during this period.38 By performing 2 analyses that each accounted for the respective limitations, we aimed to identify accurate trends in the incidence rates of cSCC in situ over a 29-year follow-up period.
Second, before implementation of the automatic linkage procedure, patients with cSCC in situ who had a previous or simultaneous invasive cSCC were not registered by NCR personnel. We could not assess the number of patients with cSCC in situ who had a previous cSCC and were therefore excluded from the NCR; thus, we likely missed patients with a diagnosis of cSCC in situ before September 2016 owing to the former registration procedure. In addition, a tumor was only registered in the NCR if it was histopathologically confirmed. Therefore, we missed patients with cSCC in situ who received a clinical diagnosis without histopathological confirmation or who did not seek any medical care. This limitation may have produced an underestimation of the burden of cSCC in situ among the Dutch population, suggesting that incidence rates are likely higher than we reported.
Conclusions
Nationwide incidence rates of cSCC in situ are increasing in the Netherlands and have consequences for the dermatological burden in secondary care. Patients with cSCC in situ have a significantly increased risk of developing invasive cSCC during their lifetimes compared with the general population, which may further increase the burden of disease associated with precursors of keratinocyte cancer. We also found that the number of patients with cSCC in situ who received treatment from a dermatologist doubled in the period of 2005 to 2015, and we expect that this increase has continued.
In times of increasing health care and skin cancer–associated expenditures, policies that address skin cancer care are and will continue to be regularly revisited.9,10,39,40,41,42 Our findings highlight the need to include cutaneous skin cancer precursor lesions, such as cSCC in situ, in these new policies because of their considerable burden and their association with increased risk of invasive skin cancer among patients who do not have a history of skin cancer.
eTable 1. Incidence Rates of Cutaneous Squamous Cell Carcinoma In Situ Among Men and Women in the Netherlands From 1989 to 2017
eTable 2. Overview and Explanation of All Outcome Measure Acronyms
eFigure. Numbers of Treating Medical Specialties for Patients With Cutaneous Squamous Cell Carcinoma In Situ Over Time (2005-2015)
References
- 1.Bowen JT. Centennial paper. May 1912 (J Cutan Dis Syph 1912;30:241-255). Precancerous dermatoses: a study of two cases of chronic atypical epithelial proliferation. by John T. Bowen, M.D., Boston. Arch Dermatol. 1983;119(3):243-260. doi: 10.1001/archderm.1983.01650270061020 [DOI] [PubMed] [Google Scholar]
- 2.Kao GF. Carcinoma arising in Bowen’s disease. Arch Dermatol. 1986;122(10):1124-1126. doi: 10.1001/archderm.1986.01660220042010 [DOI] [PubMed] [Google Scholar]
- 3.Cox NH, Eedy DJ, Morton CA. Guidelines for management of Bowen’s disease. British Association of Dermatologists. Br J Dermatol. 1999;141(4):633-641. doi: 10.1046/j.1365-2133.1999.03100.x [DOI] [PubMed] [Google Scholar]
- 4.Bordea C, Wojnarowska F, Millard PR, Doll H, Welsh K, Morris PJ. Skin cancers in renal-transplant recipients occur more frequently than previously recognized in a temperate climate. Transplantation. 2004;77(4):574-579. doi: 10.1097/01.TP.0000108491.62935.DF [DOI] [PubMed] [Google Scholar]
- 5.Kossard S, Rosen R. Cutaneous Bowen’s disease. an analysis of 1001 cases according to age, sex, and site. J Am Acad Dermatol. 1992;27(3):406-410. doi: 10.1016/0190-9622(92)70208-W [DOI] [PubMed] [Google Scholar]
- 6.Trakatelli M, Ulrich C, del Marmol V, Euvrard S, Stockfleth E, Abeni D. Epidemiology of nonmelanoma skin cancer (NMSC) in Europe: accurate and comparable data are needed for effective public health monitoring and interventions. Br J Dermatol. 2007;156(suppl 3):1-7. doi: 10.1111/j.1365-2133.2007.07861.x [DOI] [PubMed] [Google Scholar]
- 7.Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146(suppl 61):1-6. doi: 10.1046/j.1365-2133.146.s61.2.x [DOI] [PubMed] [Google Scholar]
- 8.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166(5):1069-1080. doi: 10.1111/j.1365-2133.2012.10830.x [DOI] [PubMed] [Google Scholar]
- 9.Hollestein LM, de Vries E, Aarts MJ, Schroten C, Nijsten TE. Burden of disease caused by keratinocyte cancer has increased in the Netherlands since 1989. J Am Acad Dermatol. 2014;71(5):896-903. doi: 10.1016/j.jaad.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 10.Hollestein LM, de Vries E, Nijsten T. Trends of cutaneous squamous cell carcinoma in the Netherlands: increased incidence rates, but stable relative survival and mortality 1989-2008. Eur J Cancer. 2012;48(13):2046-2053. doi: 10.1016/j.ejca.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 11.Housman TS, Feldman SR, Williford PM, et al. Skin cancer is among the most costly of all cancers to treat for the Medicare population. J Am Acad Dermatol. 2003;48(3):425-429. doi: 10.1067/mjd.2003.186 [DOI] [PubMed] [Google Scholar]
- 12.Chute CG, Chuang TY, Bergstralh EJ, Su WP. The subsequent risk of internal cancer with Bowen’s disease. a population-based study. JAMA. 1991;266(6):816-819. doi: 10.1001/jama.1991.03470060078030 [DOI] [PubMed] [Google Scholar]
- 13.Reizner GT, Chuang TY, Elpern DJ, Stone JL, Farmer ER. Bowen’s disease (squamous cell carcinoma in situ) in Kauai, Hawaii. a population-based incidence report. J Am Acad Dermatol. 1994;31(4):596-600. doi: 10.1016/S0190-9622(94)70222-5 [DOI] [PubMed] [Google Scholar]
- 14.Jansen MHE, Ozhan-Hasan H, Nelemans PJ, Winnepenninckx VJ, Mosterd K. Trends in the incidence of Bowen disease based on a single-center study in the Netherlands. Dermatol Surg. 2019;45(11):1353-1358. doi: 10.1097/DSS.0000000000001980 [DOI] [PubMed] [Google Scholar]
- 15.Arlette JP, Trotter MJ. Squamous cell carcinoma in situ of the skin: history, presentation, biology and treatment. Australas J Dermatol. 2004;45(1):1-9. doi: 10.1111/j.1440-0960.2004.00025.x [DOI] [PubMed] [Google Scholar]
- 16.Over werkgroepen. Integraal Kankercentrum Nederland. https://www.werkgroepeniknl.nl/.
- 17.Casparie M, Tiebosch AT, Burger G, et al. Pathology databanking and biobanking in the Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol. 2007;29(1):19-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Central Bureau of Statistics website. Accessed April 15, 2019. https://www.cbs.nl/.
- 19.Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology. 3rd ed World Health Organization; 2002. [Google Scholar]
- 20.Eurostat European Commission Revision of the European Standard Population: Report of Eurostat’s Task Force. 2013 ed. Publications Office of the European Union; 2013. [Google Scholar]
- 21.Joinpoint trend analysis software Version 4.7.0.0. National Cancer Institute Division of Cancer Control and Population Sciences; 2019. https://surveillance.cancer.gov/joinpoint.
- 22.Kim HT. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res. 2007;13(2 Pt 1):559-565. doi: 10.1158/1078-0432.CCR-06-1210 [DOI] [PubMed] [Google Scholar]
- 23.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141-1154. doi: 10.1214/aos/1176350951 [DOI] [Google Scholar]
- 24.Dickman PW, Coviello E. Estimating and modeling relative survival. Stata J. 2015;15(1):186-215. doi: 10.1177/1536867X1501500112 [DOI] [Google Scholar]
- 25.Eedy DJ, Gavin AT. Thirteen-year retrospective study of Bowen’s disease in Northern Ireland. Br J Dermatol. 1987;117(6):715-720. doi: 10.1111/j.1365-2133.1987.tb07351.x [DOI] [PubMed] [Google Scholar]
- 26.Cox NH. Body site distribution of Bowen’s disease. Br J Dermatol. 1994;130(6):714-716. doi: 10.1111/j.1365-2133.1994.tb03407.x [DOI] [PubMed] [Google Scholar]
- 27.Thestrup-Pedersen K, Ravnborg L, Reymann F. Morbus Bowen. a description of the disease in 617 patients. Acta Derm Venereol. 1988;68(3):236-239. [PubMed] [Google Scholar]
- 28.Kovacs A, Yonemoto K, Katsuoka K, Nishiyama S, Harhai I. Bowen’s disease: statistical study of a 10 year period. J Dermatol. 1996;23(4):267-274. doi: 10.1111/j.1346-8138.1996.tb04011.x [DOI] [PubMed] [Google Scholar]
- 29.Hansen JP, Drake AL, Walling HW. Bowen’s disease: a four-year retrospective review of epidemiology and treatment at a university center. Dermatol Surg. 2008;34(7):878-883. doi: 10.1111/j.1524-4725.2008.34172.x [DOI] [PubMed] [Google Scholar]
- 30.Jaeger AB, Gramkow A, Hjalgrim H, Melbye M, Frisch M. Bowen disease and risk of subsequent malignant neoplasms: a population-based cohort study of 1147 patients. Arch Dermatol. 1999;135(7):790-793. [DOI] [PubMed] [Google Scholar]
- 31.Verkouteren JA, Nijsten T, Hollestein LM. Competing risk of death in Kaplan-Meier curves when analyzing subsequent keratinocyte cancer. JAMA Dermatol. 2016;152(4):493-494. doi: 10.1001/jamadermatol.2015.5152 [DOI] [PubMed] [Google Scholar]
- 32.Xiong MY, Rizzo AE, Cohen TS, et al. ; Veterans Affairs Topical Tretinoin Chemoprevention (VATTC) Trial Group . Predictors of squamous cell carcinoma in high-risk patients in the VATTC trial. J Invest Dermatol. 2013;133(6):1521-1532. doi: 10.1038/jid.2013.35 [DOI] [PubMed] [Google Scholar]
- 33.van der Leest RJ, Liu L, Coebergh JW, et al. Risk of second primary in situ and invasive melanoma in a Dutch population-based cohort: 1989-2008. Br J Dermatol. 2012;167(6):1321-1330. doi: 10.1111/j.1365-2133.2012.11123.x [DOI] [PubMed] [Google Scholar]
- 34.Wassberg C, Thorn M, Yuen J, Hakulinen T, Ringborg U. Cancer risk in patients with earlier diagnosis of cutaneous melanoma in situ. Int J Cancer. 1999;83(3):314-317. doi: [DOI] [PubMed] [Google Scholar]
- 35.Greveling K, Wakkee M, Nijsten T, van den Bos RR, Hollestein LM. Epidemiology of lentigo maligna and lentigo maligna melanoma in the Netherlands, 1989-2013. J Invest Dermatol. 2016;136(10):1955-1960. doi: 10.1016/j.jid.2016.06.014 [DOI] [PubMed] [Google Scholar]
- 36.Tokez S, Alblas M, Nijsten T, Pardo LM, Wakkee M. Predicting keratinocyte carcinoma in patients with actinic keratosis: development and internal validation of a multivariable risk-prediction model. Br J Dermatol. Published online December 19, 2019. doi: 10.1111/bjd.18810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schouten LJ, Straatman H, Kiemeney LA, Gimbrere CH, Verbeek AL. The capture-recapture method for estimation of cancer registry completeness: a useful tool? Int J Epidemiol. 1994;23(6):1111-1116. doi: 10.1093/ije/23.6.1111 [DOI] [PubMed] [Google Scholar]
- 38.Share of independent treatment centers continues to increase. Vektis website. Accessed December 12, 2019. https://www.vektis.nl/nieuws/aandeel-zelfstandige-behandelcentra-blijft-toenemen.
- 39.Mackenbach JP. What are the health benefits of the most expensive healthcare system in Europe? Ned Tijdschr Geneeskd. 2015;159:A9433. [PubMed] [Google Scholar]
- 40.Noels E, Hollestein L, Luijkx K, et al. Increasing costs of skin cancer due to increasing incidence and introduction of pharmaceuticals, 2007-2017. Acta Derm Venereol. Published online March 19, 2020. doi: 10.2340/00015555-3463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morton CA, Birnie AJ, Eedy DJ. British Association of Dermatologists’ guidelines for the management of squamous cell carcinoma in situ (Bowen’s disease) 2014. Br J Dermatol. 2014;170(2):245-260. doi: 10.1111/bjd.12766 [DOI] [PubMed] [Google Scholar]
- 42.Damen-van Beek Z, Opstelten W. The Dutch College of General Practitioners practice guideline ‘suspicious skin lesions’. Ned Tijdschr Geneeskd. 2017;161:D1897. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Incidence Rates of Cutaneous Squamous Cell Carcinoma In Situ Among Men and Women in the Netherlands From 1989 to 2017
eTable 2. Overview and Explanation of All Outcome Measure Acronyms
eFigure. Numbers of Treating Medical Specialties for Patients With Cutaneous Squamous Cell Carcinoma In Situ Over Time (2005-2015)