This cross-sectional study assesses national 30-day and 1-year outcomes of mitral valve surgery and defines the hospital- and surgeon-level volume-outcome associations for mitral valve repair or replacement in patients with primary mitral regurgitation.
Key Points
Question
What is the national hospital- and surgeon-level volume-outcome association of mitral valve surgery for 30-day and 1-year outcomes of isolated mitral valve repair or replacement for primary mitral regurgitation?
Findings
In this cross-sectional study of 55 311 patients, 1094 hospitals, and 2410 surgeons, increasing hospital and surgeon volumes were associated with lower risk-adjusted 30-day mortality, lower 30-day composite mortality plus morbidity, and higher rate of successful mitral valve repair. The lowest vs highest hospital volume quartile had higher 1-year risk-adjusted mortality, but not mitral reoperation or hospitalization for heart failure; the surgeon-level 1-year volume-outcome associations were similar for mortality but not significant for mitral reoperation or hospitalization for heart failure.
Meaning
National hospital and surgeon-level inverse volume-outcome associations were observed for 30-day and 1-year mortality after mitral valve surgery for primary mitral regurgitation.
Abstract
Importance
Early surgery for severe primary degenerative mitral regurgitation is recommended, provided optimal outcomes are achievable. Contemporary national data defining mitral valve surgery volume and outcomes are lacking.
Objective
To assess national 30-day and 1-year outcomes of mitral valve surgery and define the hospital- and surgeon-level volume-outcome association with mitral valve repair or replacement (MVRR) in patients with primary mitral regurgitation.
Design, Setting, and Participants
This multicenter cross-sectional observational study used the Society of Thoracic Surgeons Adult Cardiac Surgery Database to identify patients undergoing isolated MVRR for primary mitral regurgitation in the United States. Operative data were collected from July 1, 2011, to December 31, 2016, and analyzed from March 1 to July 1, 2019, with data linked to the Centers for Medicare and Medicaid Services.
Main Outcomes and Measures
The primary outcome was 30-day in-hospital operative mortality after isolated MVRR for primary mitral regurgitation. Secondary outcomes were 30-day composite mortality plus morbidity (any occurrence of bleeding, stroke, prolonged ventilation, renal failure, or deep wound infection), rate of successful mitral valve repair of primary mitral regurgitation (residual mitral regurgitation of mild [1+] or better), and 1-year mortality, reoperation, and rehospitalization for heart failure.
Results
A total of 55 311 patients, 1094 hospitals, and 2410 surgeons were identified. Increasing hospital and surgeon volumes were associated with lower risk-adjusted 30-day mortality, lower 30-day composite mortality plus morbidity, and higher rate of successful repair. The lowest vs highest hospital volume quartile had higher 1-year risk-adjusted mortality (hazard ratio [HR], 1.61, 95% CI, 1.31-1.98), but not mitral reoperation (odds ratio [OR], 1.51; 95% CI, 0.81-2.78) or hospitalization for heart failure (HR, 1.25; 95% CI, 0.96-1.64). The surgeon-level 1-year volume-outcome associations were similar for mortality (HR, 1.60; 95% CI, 1.32-1.94) but not significant for mitral reoperation (HR, 1.14; 95% CI, 0.60-2.18) or hospitalization for heart failure (HR, 1.17; 95% CI, 0.91-1.50).
Conclusions and Relevance
National hospital- and surgeon-level inverse volume-outcome associations were observed for 30-day and 1-year mortality after mitral valve surgery for primary mitral regurgitation. These findings may help to define access to experienced centers and surgeons for the management of primary mitral regurgitation.
Introduction
An association between case volume and outcome has been documented for complex procedures,1 including major cancer surgery,2,3 ruptured abdominal aortic aneurysm repair,4 carotid endarterectomy,5 solid organ transplantation,6,7 coronary artery bypass grafting,8 surgical aortic valve replacement,9 pediatric cardiac surgery,10 and transcatheter aortic valve replacement.11 A recent analysis of administrative data from New York State12 and the updated valvular heart disease guidelines14 have suggested possible operator and institutional metrics, including volume thresholds, to promote high-quality outcomes after mitral valve surgery.
Increasing center and surgeon mitral valve operative volumes may be associated with increasing rates of successful mitral valve repair and decreasing rates of adverse events in patients with primary degenerative mitral valve disease.12,13,14,15,16 However, despite recent advances in surgical and transcatheter mitral therapy, the contemporary volume-outcome association in mitral valve surgery has not been assessed nationally, nor have rigorous data been provided to assist in defining a mitral reference center. This important information has the potential to shape quality improvement, patient-physician referral patterns, and resource allocation regarding the management of primary mitral regurgitation. The objective of this study was to assess national 30-day and 1-year outcomes of mitral valve surgery and define the hospital- and surgeon-level volume-outcome association for mitral valve repair or replacement (MVRR) in patients with primary mitral regurgitation using the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database (ACSD).
Methods
This analysis was approved by the STS Research Center and granted a waiver of informed consent by the Duke University institutional review board owing to the use of publicly available deidentified data. This cross-sectional observational study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Study Population
The STS ACSD includes 1111 participating hospitals with 3137 surgeons in 50 states, and current penetration is estimated to be more than 95% of adult cardiac operations performed annually in the United States.17 Data quality is ensured by routine internal validation as well as random annual third-party audits of 10% of hospitals.
All patients undergoing elective or urgent isolated mitral valve surgery for moderate to severe or severe primary mitral regurgitation from July 1, 2011, to December 31, 2016, were included in the analysis, thus representing contemporary surgical techniques in mitral valve surgery in the era of available minimally invasive surgical and transcatheter repair. Isolated mitral valve surgery was defined as MVRR with or without concomitant closure of a patent foramen ovale, surgical ablation of atrial fibrillation, or tricuspid valve repair.18 Primary mitral regurgitation was defined in the STS ACSD as degenerative or annular dilatation, per published algorithms.19,20 This analysis focused on the outcome of patients undergoing isolated mitral valve surgery for primary mitral regurgitation for the following reasons: (1) this patient population has the strongest guideline indication for mitral valve repair, and (2) the assessment of a volume-outcome association in this group may directly assist implementation of current and future guidelines.14,21
Site-reported patient and procedural characteristics were defined according to the STS ACSD.22 Annualized hospital or surgeon MVRR volume was defined as the number of MVRRs performed by a hospital or a surgeon from July 1, 2011, through December 31, 2016, divided by the number of months from the first to last cases by that hospital or surgeon during the study period, and then multiplied by 12. We chose this approach because any mitral valve operation contributes experience directly relevant to performing the procedure (eFigure 1 in the Supplement). Because it may be less likely for surgeons early in their career to receive referrals for isolated mitral valve surgery for primary mitral regurgitation, we performed a sensitivity analysis on surgeons with at least 3 years of practice.
Outcomes
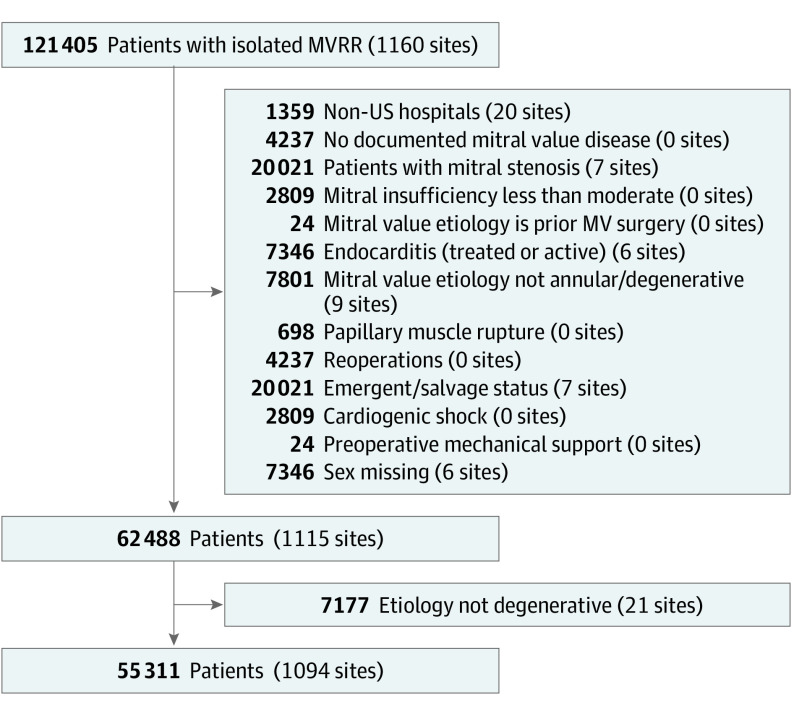
The primary outcome was risk-adjusted 30-day mortality. Secondary risk-adjusted outcomes included a 30-day weighted composite of mortality and/or major morbidity (occurrence of stroke, reoperation, prolonged ventilation, renal failure, and deep sternal wound infection)18 and rates of attempted and successful mitral valve repair. Attempted mitral valve repair was defined as having a site-reported original presurgical plan for mitral valve repair, regardless of whether a repair was performed. Successful mitral valve repair was defined by postrepair echocardiography of mitral regurgitation of mild (1+) or better. Additional secondary outcomes included 1-year outcomes of mortality, mitral reoperation, and hospitalization for heart failure, as defined by linkage to fee-for-service Centers for Medicare & Medicaid Services (CMS) claims data (eMethods 1 and 2 in the Supplement)23 among those patients 65 years or older (18 204 [74.2%] of CMS eligible records were successfully linked) (Figure 1).
Figure 1. CONSORT Flow Diagram.
MVRR indicates mitral valve repair or replacement.
Statistical Analysis
Data were analyzed from March 1 to July 1, 2019. Annualized hospital volume of mitral valve surgery was analyzed both as a continuous variable and as a categorical variable (in quartiles). Quartiles were chosen to ensure an adequate number of hospitals in each volume category and to protect hospital identity. Categorical variables were presented as frequencies and proportions. Continuous variables were summarized as medians with interquartile range (IQR). There was no prespecified plan to adjust for multiple comparisons. Except for the primary analyses, results are reported with 95% CIs without P values. For primary analyses, we performed χ2 and Kruskal-Wallis tests, and 2-sided P < .05 indicated significance.
The primary prespecified analysis examined the association between hospital MVRR volume as a continuous variable and risk-adjusted 30-day mortality. Generalized linear mixed models were developed to assess hospital MVRR volume-outcome associations. Restricted cubic splines were used to explore potential nonlinear associations between continuous case volume and outcomes, consistent with recently published methods11 (eMethods 1 in the Supplement). Associations were plotted as curves for annualized hospital procedural volume vs outcome. A 3-level (patient, surgeon, and hospital) hierarchical structure was adopted with the use of random intercepts with a covariance matrix that accounted for interhospital variability and intersurgeon variability nested within hospitals, to reflect clustering of mitral valve surgical outcomes. Analyses were repeated after adjustment of outcomes for relevant covariates (eMethods 3 in the Supplement). Covariates for adjusted models were derived from the covariates of the validated 2017 STS MVRR risk model limited to patients undergoing isolated mitral valve surgery for degenerative disease.17 An interaction term for the mitral valve intervention type (repair vs replacement) was included in the model to assess whether the association between volume and outcome differed by mitral valve intervention. All risk factors for each outcome were first combined into a single risk score before construction of the hierarchical model. To create this score, we performed an ordinary logistic-regression model and used estimated log odds of the outcome. The risk score was then added as a single independent variable in the subsequent hierarchical model. Missing covariate data were handled with single imputation (eMethods 1 in the Supplement). Data on mortality within 30 days were missing in 5610 patients, and data on the 30-day composite outcome were missing in 5172 patients. Missing outcome data were handled by multiple imputation (eMethods 1 in the Supplement).
Association between hospital MVRR volume and 1-year outcomes was modeled using Cox proportional hazards regression with a random statement to account for hospital clustering. For nonfatal outcomes, we used cause-specific Cox proportional hazards regression, with mortality as competing risk. Potential nonlinear associations were explored using restricted cubic splines for volume, and unadjusted and adjusted hazard ratios (HRs) were computed for volume quartiles. In addition, time-to-event analysis was used to compare long-term outcomes across volume quartiles. Univariate and multivariable adjusted failure curves and cumulative incidence function curves were generated by calculating the mean across patient-specific curves computed with Cox proportional hazards regression and cause-specific modified Cox proportional hazards regression for heart failure and mitral valve reoperation.
A prespecified analysis of the unadjusted and adjusted association between annualized surgeon procedural volume and outcomes was performed by means of the methods described above. All analyses were performed with the use of SAS software, version 9.4 (SAS Institute, Inc).
Results
Hospital and Surgeon Procedural Volumes
Within the study period, 121 405 isolated MVRR operations were performed at 1160 sites by 2856 surgeons. After incorporating inclusion and exclusion criteria, 55 311 patients undergoing isolated mitral valve operations for primary mitral regurgitation at 1094 hospitals by 2410 surgeons remained (Figure 1). Distributions of annualized hospital and surgeon volumes are shown in eFigure 1 in the Supplement. The median annual total hospital MVRR volume was 23 (IQR, 11-46) and the median hospital mitral valve repair volume was 11 (IQR, 5-25). The median annual surgeon MVRR volume was 12 (IQR, 6-22), and the median surgeon mitral volume repair volume was 5 (IQR, 2-11).
Patient and Procedural Characteristics According to Hospital Quartile
No clinically significant differences in age or sex were noted among quartiles of MVRR volume (Table 1). A higher overall proportion of black and Hispanic patients were treated at hospitals in the lowest compared with the highest volume quartile (220 [14.8%] vs 4012 [10.2%]; P < .001). However, of a total of 5783 black and Hispanic patients undergoing isolated MVRR for primary mitral regurgitation, 4012 (69.4%) were treated at hospitals in the 2 highest volume quartiles. Patients with no insurance made up a higher percentage of patients in the lowest quartile compared to the highest quartile of MVRR volume (60 [4.0%] vs 616 [1.6%]; P < .001). The median left ventricular end-systolic diameter of 34.0 (IQR, 30.0-39.5) mm, left ventricular end-diastolic diameter of 53.7 (IQR, 48.0-59.0) mm, and systolic pulmonary artery pressure of 38 (IQR, 30-49) mm Hg were similar across all volume quartiles. Compared with the lowest volume quartile, the highest quartile had fewer symptomatic patients with New York Heart Association class III to IV disease (9359 [23.8%] vs 743 [31.9%]; P < .001) and more patients with New York Heart Association class I disease (1610 [4.1%] vs 26 [1.8%]; P < .001).
Table 1. Patient Characteristics of Isolated MVRR for Primary Mitral Regurgitation by Quartiles of MVRR Volumea.
| Variable | Overall (N = 55 311) | Annual volume quartile | P value | |||
|---|---|---|---|---|---|---|
| Quartile 1 (0.80-10.80) (n = 1485) | Quartile 2 (10.88-23.27) (n = 4198) | Quartile 3 (23.45-46.36) (n = 10 247) | Quartile 4 (>46.55) (n = 39 381) | |||
| Age, median (IQR), y | 64 (56-73) | 65 (57-74) | 66 (57-74) | 66 (57-74) | 64 (55-73) | <.001 |
| Sex | ||||||
| Female | 23 764 (43.0) | 622 (41.9) | 1872 (44.6) | 4512 (44.0) | 16 758 (42.6) | .005 |
| Male | 31 547 (57.0) | 863 (58.1) | 2326 (55.4) | 5735 (56.0) | 22 623 (57.4) | |
| Race/ethnicity | ||||||
| Other | 808 (1.5) | 18 (1.2) | 46 (1.1) | 129 (1.3) | 615 (1.6) | <.001 |
| Native American | 131 (0.2) | 4 (0.3) | 14 (0.3) | 20 (0.2) | 93 (0.2) | |
| Asian | 1447 (2.6) | 39 (2.6) | 94 (2.2) | 253 (2.5) | 1061 (2.7) | |
| Hispanic | 1838 (3.3) | 75 (5.1) | 145 (3.5) | 372 (3.6) | 1246 (3.2) | |
| Black | 3945 (7.1) | 145 (9.8) | 319 (7.6) | 715 (7.0) | 2766 (7.0) | |
| White | 46 594 (84.2) | 1182 (79.6) | 3535 (84.2) | 8659 (84.5) | 33 218 (84.4) | |
| BMI, median (IQR)b | 26.3 (23.4-29.9) | 26.5 (23.5-30.3) | 27.0 (24.0-31.0) | 26.6 (23.6-30.5) | 26.2 (23.3-29.6) | <.001 |
| BSA, median (IQR),b m2 | 1.92 (1.74-2.09) | 1.92 (1.75-2.07) | 1.90 (1.80-2.10) | 1.93 (1.74-2.10) | 1.92 (1.74-2.09) | .008 |
| Diabetes | ||||||
| Insulin used | 1313 (2.4) | 43 (2.9) | 126 (3.0) | 291 (2.8) | 853 (2.2) | <.001 |
| No insulin used | 5231 (9.5) | 175 (11.8) | 467 (11.1) | 1105 (10.8) | 3484 (8.8) | |
| None | 48 743 (88.1) | 1267 (85.3) | 3603 (85.8) | 8849 (86.4) | 35 024 (88.9) | |
| Hypertension | ||||||
| Yes | 35 389 (64.0) | 1063 (71.6) | 2886 (68.7) | 6926 (67.6) | 24 514 (62.2) | <.001 |
| No | 19 899 (36.0) | 421 (28.4) | 1310 (31.2) | 3315 (32.4) | 14 853 (37.7) | |
| Chronic lung disease | ||||||
| Unknown severity | 757 (1.4) | 30 (2.0) | 68 (1.6) | 162 (1.6) | 497 (1.3) | <.001 |
| Severe | 1594 (2.9) | 65 (4.4) | 179 (4.3) | 353 (3.4) | 997 (2.5) | |
| Moderate | 2249 (4.1) | 100 (6.7) | 302 (7.2) | 495 (4.8) | 1352 (3.4) | |
| Mild | 5445 (9.8) | 197 (13.3) | 510 (12.1) | 1121 (10.9) | 3617 (9.2) | |
| None | 44 918 (81.2) | 1082 (72.9) | 3101 (73.9) | 8049 (78.5) | 32 686 (83.0) | |
| Dialysis | ||||||
| Yes | 662 (1.1) | 24 (1.6) | 70 (1.7) | 127 (1.2) | 441 (1.1) | .007 |
| No | 54 617 (98.7) | 1457 (98.1) | 4127 (98.3) | 10 106 (98.6) | 38 927 (98.8) | |
| Last creatinine level, median (IQR), mg/dLc | 0.9 (0.8-1.1) | 1.0 (0.8-1.1) | 1.0 (0.8-1.2) | 0.9 (0.8-1.1) | 0.9 (0.8-1.1) | <.001 |
| Immunocompromised | ||||||
| Yes | 1518 (2.7) | 34 (2.3) | 108 (2.6) | 237 (2.3) | 1139 (2.9) | .007 |
| No | 53 648 (97.0) | 1446 (97.4) | 4072 (97.0) | 9997 (97.6) | 38 133 (96.8) | |
| Peripheral vascular disease | ||||||
| Yes | 2028 (3.7) | 56 (3.8) | 180 (4.3) | 384 (3.7) | 1408 (3.6) | .12 |
| No | 53 213 (96.2) | 1426 (96.0) | 4003 (95.4) | 9846 (96.1) | 37 938 (96.3) | |
| CVD/CVA | ||||||
| CVD with no CVA | 2067 (3.7) | 54 (3.6) | 162 (3.9) | 439 (4.3) | 1412 (3.6) | .002 |
| CVD and CVA | 2043 (3.7) | 46 (3.1) | 182 (4.3) | 401 (3.9) | 1414 (3.6) | |
| No CVD | 51 104 (92.4) | 1379 (92.9) | 3845 (91.6) | 9378 (91.5) | 36 502 (92.7) | |
| Ejection fraction, median (IQR), %d | 60 (55-64) | 59 (50-63) | 59 (50-63) | 60 (53-63) | 60 (55-65) | <.001 |
| Atrial fibrillation type | ||||||
| Persistent | 9351 (16.9) | 269 (18.1) | 809 (19.3) | 2021 (19.7) | 6252 (15.9) | <.001 |
| Paroxysmal | 8272 (15.0) | 251 (16.9) | 671 (16.0) | 1723 (16.8) | 5627 (14.3) | |
| None | 37 529 (67.9) | 960 (64.6) | 2698 (64.3) | 6477 (63.2) | 27 394 (69.6) | |
| Congestive heart failure within 2 wk | ||||||
| Yes | 25 074 (45.3) | 668 (45.0) | 1786 (42.5) | 4463 (43.6) | 18 157 (46.1) | <.001 |
| No | 30 069 (54.4) | 814 (54.8) | 2393 (57.0) | 5760 (56.2) | 21 102 (53.6) | |
| NYHA classe | ||||||
| IV | 3766 (15.0) | 173 (25.9) | 379 (21.2) | 891 (20.0) | 2323 (12.8) | <.001 |
| III | 10 087 (40.2) | 300 (44.9) | 833 (46.6) | 1918 (43.0) | 7036 (38.8) | |
| II | 8775 (35.0) | 162 (24.3) | 444 (24.9) | 1285 (28.8) | 6884 (37.9) | |
| I | 1996 (8.0) | 26 (3.9) | 88 (4.9) | 272 (6.1) | 1610 (8.9) | |
| Mitral insufficiency | ||||||
| Severe | 51 756 (93.6) | 1403 (94.5) | 3965 (94.4) | 9642 (94.1) | 36 746 (93.3) | <.001 |
| Moderate to severe | 3555 (6.4) | 82 (5.5) | 233 (5.6) | 605 (5.9) | 2635 (6.7) | |
| Mitral valve procedure | ||||||
| Replacement | 10 619 (19.2) | 537 (36.2) | 1255 (29.9) | 2725 (26.6) | 6102 (15.5) | <.001 |
| Repair | 44 692 (80.8) | 948 (63.8) | 2943 (70.1) | 7522 (73.4) | 33 279 (84.5) | |
| Repair attempted before mitral valve replacementf | ||||||
| Yes | 2603 (24.5) | 140 (26.1) | 339 (27.0) | 648 (23.8) | 1476 (24.2) | .11 |
| No | 7940 (74.8) | 396 (73.7) | 907 (72.3) | 2070 (76.0) | 4567 (74.8) | |
| Operative approach | ||||||
| Minimally invasive thoracotomy | 16 199 (29.3) | 109 (7.3) | 539 (12.8) | 1965 (19.2) | 13 586 (34.5) | <.001 |
| Sternotomy | ||||||
| Partial | 1228 (2.2) | 8 (0.5) | 73 (1.7) | 216 (2.1) | 931 (2.4) | |
| Full | 37 804 (68.3) | 1366 (92.0) | 3580 (85.3) | 8054 (78.6) | 24 804 (63.0) | |
| Robotic technology assisted | ||||||
| Yes | 5756 (10.4) | 7 (0.5) | 96 (2.3) | 580 (5.7) | 5073 (12.9) | <.001 |
| No | 49 300 (89.1) | 1474 (99.3) | 4097 (97.6) | 9660 (94.3) | 34 069 (86.5) | |
| Cardiopulmonary bypass time, median (IQR), ming | 117 (90-151) | 126 (100-161) | 128 (100-162) | 125 (98-159) | 113 (87-147) | <.001 |
| Highest level of postoperative mitral insufficiencyh | ||||||
| Not documented | 1044 (2.2) | 38 (3.1) | 152 (4.4) | 266 (3.1) | 588 (1.7) | <.001 |
| Severe | 984 (2.1) | 67 (5.4) | 118 (3.4) | 292 (3.4) | 507 (1.5) | |
| Moderate | 602 (1.3) | 20 (1.6) | 75 (2.2) | 127 (1.5) | 380 (1.1) | |
| Mild | 3485 (7.4) | 113 (9.1) | 248 (7.1) | 696 (8.2) | 2428 (7.2) | |
| Trace/trivial | 13 657 (29.2) | 352 (28.5) | 915 (26.3) | 2563 (30.1) | 9827 (29.2) | |
| None | 26 157 (55.8) | 615 (49.8) | 1869 (53.6) | 4360 (51.2) | 19 313 (57.5) | |
| Mitral implant type | ||||||
| Annuloplasty | ||||||
| Band | 9764 (17.7) | 84 (5.7) | 301 (7.2) | 962 (9.4) | 8417 (21.4) | <.001 |
| Ring | 32 172 (58.2) | 782 (52.7) | 2434 (58.0) | 6113 (59.7) | 22 843 (58.0) | |
| Bioprosthesis | 8574 (15.5) | 397 (26.7) | 964 (23.0) | 2121 (20.7) | 5092 (12.9) | |
| Mechanical | 1842 (3.3) | 120 (8.1) | 256 (6.1) | 532 (5.2) | 934 (2.4) | |
| Atrial fibrillation surgical procedure | ||||||
| Yes | 16 406 (29.7) | 390 (26.3) | 1387 (33.0) | 3401 (33.2) | 11 228 (28.5) | <.001 |
| No | 38 795 (70.1) | 1093 (73.6) | 2805 (66.8) | 6838 (66.7) | 28 059 (71.3) | |
| STS estimated risk of mortality (IQR), % | 0.9 (0.5-2.0) | 1.2 (0.6-2.6) | 1.2 (0.6-2.5) | 1.1 (0.5-2.3) | 0.8 (0.4-1.9) | <.001 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by the square of height in meters); BSA, body surface area; CVA, cerebrovascular accident; CVD, cerebrovascular disease; IQR, interquartile range; MVRR, mitral valve repair or replacement; NYHA, New York Heart Association; STS, Society of Thoracic Surgeons.
SI conversion factor: To convert creatinine to μmol/L, multiply by 88.4.
Unless otherwise indicated, data are expressed as number (percentage) of patients. Owing to missing data, totals for characteristics may not sum to number in column head and percentages may not total 100.
Includes 55 247 patients (1482 in quartile 1, 4192 in quartile 2, 10 234 in quartile 3, and 39 339 in quartile 4).
Includes 55 213 patients (1474 in quartile 1, 4186 in quartile 2, 10 223 in quartile 3, and 39 330 in quartile 4).
Includes 54 296 patients (1443 in quartile 1, 4105 in quartile 2, 9996 in quartile 3, and 38 752 in quartile 4).
Includes those with congestive heart failure within 2 weeks.
Includes those undergoing replacement.
Includes 55 177 patients (1478 in quartile 1, 4183 in quartile 2, 10 230 in quartile 3, and 39 286 in quartile 4).
Includes 46 847 patients (1235 in quartile 1, 3485 in quartile 2, 8523 in quartile 3, and 33 604 in quartile 4).
The overall mitral valve repair rate for primary degenerative mitral regurgitation was 80.8% (44 692 of 55 311). The rate of mitral valve repair for primary mitral regurgitation was higher in the highest vs lowest volume quartile (33 279 [84.5%] vs 948 [63.8%]; P < .001). Cardiopulmonary bypass times were lower at higher vs lower volume quartile centers (median, 113 [IQR, 87-147] vs 126 [IQR, 100-161] minutes; P < .001), yet the frequency of mitral valve replacement after attempted mitral valve repair was similar across quartiles (lowest quartile, 140 of 536 [26.1%]; highest quartile, 1476 of 6043 [24.4%]; P = .11). Minimally invasive thoracotomy or robotic approach was more commonly used in the highest vs lowest hospital volume quartiles (14 045 [35.7%] vs 113 [7.6%]; P < .001).
Hospital Volume of Mitral Valve Surgery and 30-Day Outcomes
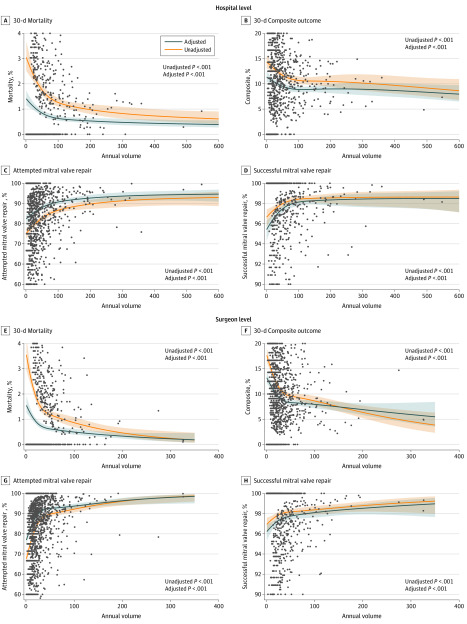
There was a significant, nonlinear association among 30-day mortality, 30-day composite outcome, and 30-day successful mitral valve repair (adjusted and unadjusted) and annualized hospital MVRR surgical volume (Table 2 and Figure 2A-D). Adjusted 30-day mortality after isolated MVRR for primary mitral regurgitation was increased in the lowest volume quartile (1.36%; 95% CI, 1.12%-1.66%) compared with the highest volume quartile (0.72%; 95% CI, 0.61%-0.84%) (adjusted odds ratio [OR], 2.06; 95% CI, 1.47-2.90) and showed a stepwise decrease with increasing volume quartile (eTable 1 in the Supplement). Adjusted 30-day composite outcome was increased in the lowest volume quartile (10.68%; 95% CI, 9.83%-11.59%) compared with the highest volume quartile (8.74%; 95% CI, 8.17%-9.33%) (adjusted OR, 1.35; 95% CI, 1.14-1.60). Attempted mitral valve repairs were less frequent in the lowest volume quartile (83.13%; 95% CI, 81.15%-84.94%) than in the highest volume quartile (89.78%; 95% CI, 88.45%-90.97%) (adjusted OR, 0.49; 95% CI 0.38-0.62). Rates of successful mitral valve repair were decreased in the lowest volume quartile (96.34%; 95% CI, 95.49%-97.03%) compared with the highest volume quartile (98.06%; 95% CI, 97.67%-98.38%) (adjusted OR, 0.50; 95% CI, 0.34-0.72). Only 148 of 1094 hospitals (13.5%) performed at least 75 MVRR procedures per year.
Table 2. Hospital- and Surgeon-Level Volume-Outcome Associations of Mitral Valve Surgery for Primary Mitral Regurgitationa.
| Outcome | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Hospital level | ||||
| Operative mortality | 2.21 (1.57-3.12) | <.001 | 2.06 (1.47-2.90) | <.001 |
| Composite | 1.40 (1.18-1.67) | <.001 | 1.35 (1.14-1.60) | <.001 |
| Repair success | 0.57 (0.38-0.87) | .004 | 0.50 (0.34-0.72) | <.001 |
| Surgeon level | ||||
| Operative mortality | 2.74 (2.05-3.67) | <.001 | 2.25 (1.68-3.01) | <.001 |
| Composite | 2.00 (1.73-2.30) | <.001 | 1.72 (1.50-1.98) | <.001 |
| Repair success | 0.59 (0.43-0.83) | .002 | 0.62 (0.45-0.85) | .003 |
Abbreviation: OR, odds ratio.
Comparisons are for the lowest vs highest quartiles of procedure volume.
Figure 2. Hospital- and Surgeon-Level Volume-Outcome Association.
Shaded areas represent 95% CIs. Circles represent X.
Hospital Volume of Mitral Valve Surgery and 1-Year Outcomes
Among the 55 311 patients undergoing isolated mitral valve operations for primary mitral regurgitation at 1094 hospitals by 2410 surgeons in the analytic population, 18 204 were linked to CMS claims data (Figure 1). Patient and procedural characteristics between those with and without linkable CMS claims were not clinically different (eTable 2 in the Supplement). At 1-year follow-up, the lowest volume quartile had increased risk-adjusted mortality (9.58%; 95% CI, 7.86%-11.27%) compared with the highest volume quartile (6.20%; 95% CI, 5.82%-6.58%) (HR, 1.61, 95% CI, 1.31-1.98) (eFigure 2 and eTable 3 in the Supplement). There was no significant difference in 1-year rates of hospitalization for heart failure between the lowest volume quartile (9.24%; 95% CI, 7.13%-11.30%) and the highest volume quartile (7.47%; 95% CI, 7.02%-7.92%) (HR, 1.25; 95% CI, 0.96-1.64) (eFigure 3 and eTable 4 in the Supplement). Mitral reoperation at 1 year was not significantly associated with volume by either a continuous or quartile approach, with an adjusted rate of mitral valve reoperation in the lowest volume quartile of 1.45% (95% CI, 0.60%-2.30%) vs 1.00% (95% CI, 0.08%-1.20%) in the highest quartile (OR, 1.51, 95% CI, 0.81-2.78) (eFigure 4 and eTable 5 in the Supplement).
Surgeon Volume for Mitral Valve Surgery and 30-Day Outcomes
There was a significant, nonlinear association among 30-day mortality, 30-day composite outcome, and 30-day successful mitral valve repair (adjusted and unadjusted) and annualized surgeon MVRR surgical volume (Table 2 and Figure 2E-H). Adjusted 30-day mortality was increased in the lowest volume quartile (1.53%; 95% CI, 1.24%-1.89%) compared with the highest volume quartile (0.99%; 95% CI, 0.87%-1.13%) (adjusted OR, 2.25; 95% CI, 1.68-3.01) and showed a stepwise decrease with increasing volume quartile (eTable 6 in the Supplement). Adjusted 30-day composite was increased in the lowest volume quartile (12.57%; 95% CI, 11.58%-13.65%) compared with the highest volume quartile (9.91%; 95% CI, 9.46%-10.38%) (adjusted OR, 1.72; 95% CI, 1.50-1.98). The rate of attempted mitral valve repair was decreased in the lowest volume quartile (77.67%; 95% CI, 75.49%-79.71%) compared with the highest volume quartile (87.24%; 95% CI, 86.32%-88.12%) (adjusted OR, 0.34; 95% CI, 0.29-0.41). The rate of successful mitral valve repair was decreased in the lowest volume quartile (96.75%; 95% CI, 95.94%-97.40%) compared with the highest volume quartile (97.34%; 95% CI, 96.97%-97.66%) (adjusted OR, 0.62; 95% CI, 0.45-0.85). Surgeons with 3 years of experience had outcomes similar to the overall surgeon cohort (eTables 6 and 7, eFigure 5, and eResults in the Supplement). Only 303 surgeons (12.6%) performed at least 35 MVRR cases per year.
Surgeon Volume for Mitral Valve Surgery and 1-Year Outcomes
At 1-year follow-up, the surgeons in the lowest volume quartile compared with the highest volume quartile had increased risk-adjusted mortality (9.37% [95% CI, 7.84%-10.88%] vs 6.11% [95% CI, 5.74%-6.48%]) (HR, 1.60; 95% CI, 1.32-1.93) (eFigure 6 and eTable 8 in the Supplement). There was no significant difference in 1-year rate of hospitalization for heart failure between the lowest volume quartile (8.73%; 95% CI, 6.88%-10.56%) and the highest volume quartile (7.48%; 95% CI, 7.03%-7.92%) (HR, 1.17; 95% CI, 0.91-1.50) (eFigure 7 and eTable 9 in the Supplement). Mitral reoperation at 1 year was not significantly associated with volume by either a continuous or quartile approach, with an adjusted rate of mitral valve reoperation in the lowest volume quartile of 1.07% (95% CI, 0.40%-1.75%) vs 0.95% (95% CI, 0.77%-1.12%) (HR, 1.14; 95% CI, 0.60-2.18) in the highest quartile (eFigure 8 and eTable 10 in the Supplement).
The interaction between mitral valve intervention type (repair vs replacement) and the association between hospital or surgeon MVRR volume and 30-day mortality, 30-day composite outcome, or 1-year outcome was not significant. A sensitivity analysis restricted to surgeons with at least 3 years of experience in the STS ACSD had results similar to those in the primary analysis of all surgeons (eTables 11-13, eFigures 9-11, and eResults in the Supplement).
Discussion
For the surgical management of primary mitral regurgitation, this national clinical registry analysis provides the following important contributions: (1) definition of a clear hospital volume-outcome association for 30-day mortality and 30-day major morbidity plus mortality; (2) definition of a clear surgeon volume-outcome association for the 30-day mortality and 30-day major morbidity plus mortality; and (3) documentation that hospital and surgeon volume-outcome associations exists for rates of successful mitral valve repair and 1-year mortality but not for 1-year mitral valve reoperation or 1-year hospitalization for heart failure. These findings may assist efforts to define access to experienced centers and surgeons for primary MR or complex primary MV disease.
Existing estimations of volume-outcome associations in mitral valve surgery are limited to single-state or administrative claims data,12,13 theoretical thresholds based on expert consensus only,15 or noncontemporary 30-day outcomes limited to volume assessment of surgery for primary mitral regurgitation.16 The present analysis is unique for several reasons. First, a large contemporary national surgical cohort was used from the current era of innovative surgical techniques and availability of transcatheter mitral valve therapy. Second, the volume assessment used in this analysis was all mitral valve surgery performed, because the cumulative experience of all mitral valve operations performed by a surgeon or a hospital was believed to be a more comprehensive measure of overall mitral valve surgical experience. Third, the outcome assessment was focused on 55 311 patients undergoing isolated mitral valve operations for primary mitral regurgitations with evaluable postoperative echocardiograms performed in 1094 hospitals with 2410 surgeons. Fourth, both hospital and surgeon volume-outcome associations were examined for 30-day mortality, 30-day composite of major morbidity plus mortality, and MV repair rate. Finally, the outcome assessment was linked to CMS claims data to estimate hospital and surgeon data for 1-year mortality, 1-year hospitalization for heart failure, and 1-year mitral valve reoperation rates. The result is the largest and most comprehensive volume-outcome analysis of mitral valve surgery for primary mitral regurgitation and the only US national assessment, to our knowledge, that has been performed to date.
We have several important observations. A significant hospital and surgeon volume-outcome association was identified for the primary outcome of operative mortality, as well as for the secondary outcomes of composite in-hospital morbidity plus operative mortality, successful mitral valve repair of primary mitral regurgitation, and 1-year mortality, but not for 1-year mitral valve reoperation or hospitalization for heart failure. Most interestingly, the inflection points of the significant volume-outcome associations noted in Figure 2 appear consistent for all hospital-level end points (estimated at 75 cases) and surgeon-level end points (estimated at 35 cases). We included 148 hospitals in this cohort (13.5%) performing at least 75 MVRRs per year and 303 surgeons (12.6%) performing at least 35 MVRRs per year.
The data generated from this analysis may provide further support for efforts to regionalize mitral valve surgery for primary mitral regurgitation, especially for patients who may be asymptomatic but meet guideline criteria for mitral valve repair,14 in whom the pathology may be deemed complex, or for patients seeking innovative mitral valve repair approaches, such as minimally invasive or robotic surgery.21 Such cases may be better suited to experienced mitral valve surgeons and centers based on volume. An unintended consequence of potential volume thresholds could be that such volume requirements might decrease access to mitral valve surgery by underserved populations. However, a recent STS ACSD analysis24 examined access to mitral valve surgery by hospital referral region, defined by the Dartmouth Atlas, and revealed that the proportion of the US population living within a hospital referral region performing at least 25 surgical MVRRs per year was 92.0%, and 81.7% lived within a hospital referral region of a center performing at least 40 surgical MVRRs per year. In addition, centers performing at least 40 surgical MVRRs annually, of which at least 10 were mitral valve repairs, were within the same hospital referral region as 81.6% of the population, and 78.7% of the population resided near centers performing at least 40 surgical MVRRs, of which at least 20 were mitral valve repairs. Thus, efforts to regionalize mitral valve surgery for primary mitral regurgitation may not place substantial limitations on access to care. Given that the present study revealed that the national repair rate for primary mitral regurgitation has increased to 81%, approaching 90% in the highest volume quartile, this information in the aggregate may aid efforts to define access and quality.
Limitations
This report has the inherent limitations of any analysis of a large clinical registry. One key limitation is incomplete data, particularly related to etiology. The ascertainment of etiology may be imprecise, and it was not core laboratory adjudicated. However, published algorithms19 were applied to increase the accuracy of documented etiology. All echocardiographic data were site reported and not core laboratory adjudicated. Although predischarge or 30-day transthoracic postoperative echocardiography was preferentially used, the echocardiographic data for some patients included only the intraoperative postrepair transesophageal echocardiography, which may be subject to loading conditions. In some centers, mitral valve surgeon specialization may currently exist where some may not perform any mitral valve surgery. The present analysis examined all surgeons in the STS ACSD and not just those performing MVRR regularly. Thus, the surgeon volume thresholds reported may be conservative. There may be unmeasured confounders that were not accounted for in the current risk-adjusted analysis of these observational data. Finally, because the STS ACSD is currently limited to 30-day or in-hospital outcomes, linkage to CMS claims files was necessary to assess 1-year outcomes, which is inherently limiting owing to the age limit of 65 years or older.
Conclusions
In this cross-sectional study, a hospital and surgeon volume-outcome association was found for 30-day and 1-year mortality in isolated mitral valve surgery for primary mitral regurgitation. These findings may help to define experienced centers and surgeons for the management of primary mitral regurgitation or complex disease.
eMethods 1. Data Linkage and Management of Missing Data
eMethods 2. ICD-9/10 Codes for 1-Year Outcomes
eMethods 3. Covariates for Model Adjustment
eTable 1. Hospital Mitral Valve Surgery Volume-Outcome Association Stratified by Volume Quartiles
eTable 2. Patient and Procedural Characteristics of Patients Linked and Not Linked to CMS Claims Data
eTable 3. Hospital 1-Year Death Time to Event Rates
eTable 4. Hospital 1-Year Heart Failure Hospitalization Time to Event Rates
eTable 5. Hospital 1-Year Mitral Valve Reoperation Time to Event Rates
eTable 6. All Surgeon Mitral Valve Surgery Volume-Outcome Association Stratified by Volume Quartiles
eTable 7. Experienced Surgeons Mitral Valve Surgery Volume-Outcome Association Stratified by Volume Quartiles
eTable 8. All Surgeon 1-Year Mortality Time to Event Rates
eTable 9. All Surgeon 1-Year HF Hospitalization Rates
eTable 10. All Surgeon 1-Year MV Reoperation Time to Event Rates
eTable 11. Experienced Surgeons 1-Year Mortality Time to Event Rates
eTable 12. Experienced Surgeon 1-Year HF Hospitalization Time to Event Rates
eTable 13. Experienced Surgeons 1-Year MV Reoperation Time to Event Rates
eFigure 1. Hospital- and Surgeon-Level Annualized Mitral Valve Repair and Replacement (MVRR) Volume
eFigure 2. Hospital-Level Linked CMS 1-Year Risk-Adjusted Mortality
eFigure 3. Hospital-Level Linked CMS 1-Year HF Hospitalization
eFigure 4. Hospital-Level Linked CMS 1-Year MV Reoperation
eFigure 5. Experienced Surgeon Sensitivity Analysis: 30-Day Volume-Outcome Association for Mortality, Composite Major Morbidity Plus Mortality, Attempted MV Repair Rate, Successful MV Repair Rate
eFigure 6. All Surgeon-Linked CMS 1-Year Risk-Adjusted Mortality
eFigure 7. All Surgeon-Linked CMS 1-Year HF Hospitalization
eFigure 8. All Surgeon-Linked CMS 1-Year MV Reoperation
eFigure 9. Experienced Surgeon-Linked CMS 1-Year Risk-Adjusted Mortality
eFigure 10. Experienced Surgeon-Linked CMS 1-Year HF Hospitalization
eFigure 11. Experienced Surgeon-Linked CMS 1-Year MV Reoperation
eResults. Surgeons With 3 Years of Experience
References
- 1.Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? the empirical relation between surgical volume and mortality. N Engl J Med. 1979;301(25):1364-1369. doi: 10.1056/NEJM197912203012503 [DOI] [PubMed] [Google Scholar]
- 2.Begg CB, Cramer LD, Hoskins WJ, Brennan MF. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280(20):1747-1751. doi: 10.1001/jama.280.20.1747 [DOI] [PubMed] [Google Scholar]
- 3.Hannan EL, Radzyner M, Rubin D, Dougherty J, Brennan MF. The influence of hospital and surgeon volume on in-hospital mortality for colectomy, gastrectomy, and lung lobectomy in patients with cancer. Surgery. 2002;131(1):6-15. doi: 10.1067/msy.2002.120238 [DOI] [PubMed] [Google Scholar]
- 4.Dardik A, Burleyson GP, Bowman H, et al. Surgical repair of ruptured abdominal aortic aneurysms in the state of Maryland: factors influencing outcome among 527 recent cases. J Vasc Surg. 1998;28(3):413-420. doi: 10.1016/S0741-5214(98)70126-0 [DOI] [PubMed] [Google Scholar]
- 5.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349(22):2117-2127. doi: 10.1056/NEJMsa035205 [DOI] [PubMed] [Google Scholar]
- 6.Edwards EB. Summary of 1994 report of center-specific graft and patient survival rates. Clin Transpl. 1994:541-554. [PubMed] [Google Scholar]
- 7.Weiss ES, Meguid RA, Patel ND, et al. Increased mortality at low-volume orthotopic heart transplantation centers: should current standards change? Ann Thorac Surg. 2008;86(4):1250-1259. doi: 10.1016/j.athoracsur.2008.06.071 [DOI] [PubMed] [Google Scholar]
- 8.Kim LK, Looser P, Swaminathan RV, et al. Outcomes in patients undergoing coronary artery bypass graft surgery in the United States based on hospital volume, 2007 to 2011. J Thorac Cardiovasc Surg. 2016;151(6):1686-1692. doi: 10.1016/j.jtcvs.2016.01.050 [DOI] [PubMed] [Google Scholar]
- 9.Patel HJ, Herbert MA, Drake DH, et al. Aortic valve replacement: using a statewide cardiac surgical database identifies a procedural volume hinge point. Ann Thorac Surg. 2013;96(5):1560-1565. doi: 10.1016/j.athoracsur.2013.05.103 [DOI] [PubMed] [Google Scholar]
- 10.Kansy A, Zu Eulenburg C, Sarris G, et al. Higher programmatic volume in neonatal heart surgery is associated with lower early mortality. Ann Thorac Surg. 2018;105(5):1436-1440. doi: 10.1016/j.athoracsur.2017.11.028 [DOI] [PubMed] [Google Scholar]
- 11.Vemulapalli S, Carroll JD, Mack MJ, et al. Procedural volume and outcomes for transcatheter aortic-valve replacement. N Engl J Med. 2019;380(26):2541-2550. doi: 10.1056/NEJMsa1901109 [DOI] [PubMed] [Google Scholar]
- 12.Chikwe J, Toyoda N, Anyanwu AC, et al. Relation of mitral valve surgery volume to repair rate, durability, and survival. J Am Coll Cardiol. 2017;S0735-1097(17)30677-0. Published online April 24, 2017. doi: 10.1016/j.jacc.2017.02.026 [DOI] [PubMed] [Google Scholar]
- 13.LaPar DJ, Ailawadi G, Isbell JM, et al. ; Virginia Cardiac Surgery Quality Initiative . Mitral valve repair rates correlate with surgeon and institutional experience. J Thorac Cardiovasc Surg. 2014;148(3):995-1003. doi: 10.1016/j.jtcvs.2014.06.039 [DOI] [PubMed] [Google Scholar]
- 14.Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70(2):252-289. doi: 10.1016/j.jacc.2017.03.011 [DOI] [PubMed] [Google Scholar]
- 15.Bridgewater B, Hooper T, Munsch C, et al. Mitral repair best practice: proposed standards. Heart. 2006;92(7):939-944. doi: 10.1136/hrt.2005.076109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolling SF, Li S, O’Brien SM, Brennan JM, Prager RL, Gammie JS. Predictors of mitral valve repair: clinical and surgeon factors. Ann Thorac Surg. 2010;90(6):1904-1911. doi: 10.1016/j.athoracsur.2010.07.062 [DOI] [PubMed] [Google Scholar]
- 17.Jacobs JP, Shahian DM, He X, et al. Penetration, completeness, and representativeness of the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg. 2016;101(1):33-41. doi: 10.1016/j.athoracsur.2015.08.055 [DOI] [PubMed] [Google Scholar]
- 18.Badhwar V, Rankin JS, He X, et al. The Society of Thoracic Surgeons Mitral Repair/Replacement Composite Score: a report of the Society of Thoracic Surgeons Quality Measurement Task Force. Ann Thorac Surg. 2016;101(6):2265-2271. doi: 10.1016/j.athoracsur.2015.11.049 [DOI] [PubMed] [Google Scholar]
- 19.Rankin JS, Grau-Sepulveda M, Shahian DM, et al. The impact of mitral disease etiology on operative mortality after mitral valve operations. Ann Thorac Surg. 2018;106(5):1406-1413. doi: 10.1016/j.athoracsur.2018.04.053 [DOI] [PubMed] [Google Scholar]
- 20.Gammie JS, Chikwe J, Badhwar V, et al. Isolated mitral valve surgery: the Society of Thoracic Surgeons Adult Cardiac Surgery Database analysis. Ann Thorac Surg. 2018;106(3):716-727. doi: 10.1016/j.athoracsur.2018.03.086 [DOI] [PubMed] [Google Scholar]
- 21.O’Gara PT, Grayburn PA, Badhwar V, et al. 2017 ACC expert consensus decision pathway on the management of mitral regurgitation: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70(19):2421-2449. doi: 10.1016/j.jacc.2017.09.019 [DOI] [PubMed] [Google Scholar]
- 22.Jacobs JP, Shahian DM, D’Agostino RS, et al. The Society of Thoracic Surgeons National Database 2018 annual report. Ann Thorac Surg. 2018;106(6):1603-1611. doi: 10.1016/j.athoracsur.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 23.Jacobs JP, Edwards FH, Shahian DM, et al. Successful linking of the Society of Thoracic Surgeons Adult Cardiac Surgery Database to Centers for Medicare and Medicaid Services Medicare data. Ann Thorac Surg. 2010;90(4):1150-1156. doi: 10.1016/j.athoracsur.2010.05.042 [DOI] [PubMed] [Google Scholar]
- 24.Vemulapalli S, Grau-Sepulveda M, Habib R, Thourani V, Bavaria J, Badhwar V. Mitral valve surgery in the United States: patient and hospital characteristics. JAMA Cardiol. Published online October 2, 2019. doi: 10.1001/jamacardio.2019.3659 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Data Linkage and Management of Missing Data
eMethods 2. ICD-9/10 Codes for 1-Year Outcomes
eMethods 3. Covariates for Model Adjustment
eTable 1. Hospital Mitral Valve Surgery Volume-Outcome Association Stratified by Volume Quartiles
eTable 2. Patient and Procedural Characteristics of Patients Linked and Not Linked to CMS Claims Data
eTable 3. Hospital 1-Year Death Time to Event Rates
eTable 4. Hospital 1-Year Heart Failure Hospitalization Time to Event Rates
eTable 5. Hospital 1-Year Mitral Valve Reoperation Time to Event Rates
eTable 6. All Surgeon Mitral Valve Surgery Volume-Outcome Association Stratified by Volume Quartiles
eTable 7. Experienced Surgeons Mitral Valve Surgery Volume-Outcome Association Stratified by Volume Quartiles
eTable 8. All Surgeon 1-Year Mortality Time to Event Rates
eTable 9. All Surgeon 1-Year HF Hospitalization Rates
eTable 10. All Surgeon 1-Year MV Reoperation Time to Event Rates
eTable 11. Experienced Surgeons 1-Year Mortality Time to Event Rates
eTable 12. Experienced Surgeon 1-Year HF Hospitalization Time to Event Rates
eTable 13. Experienced Surgeons 1-Year MV Reoperation Time to Event Rates
eFigure 1. Hospital- and Surgeon-Level Annualized Mitral Valve Repair and Replacement (MVRR) Volume
eFigure 2. Hospital-Level Linked CMS 1-Year Risk-Adjusted Mortality
eFigure 3. Hospital-Level Linked CMS 1-Year HF Hospitalization
eFigure 4. Hospital-Level Linked CMS 1-Year MV Reoperation
eFigure 5. Experienced Surgeon Sensitivity Analysis: 30-Day Volume-Outcome Association for Mortality, Composite Major Morbidity Plus Mortality, Attempted MV Repair Rate, Successful MV Repair Rate
eFigure 6. All Surgeon-Linked CMS 1-Year Risk-Adjusted Mortality
eFigure 7. All Surgeon-Linked CMS 1-Year HF Hospitalization
eFigure 8. All Surgeon-Linked CMS 1-Year MV Reoperation
eFigure 9. Experienced Surgeon-Linked CMS 1-Year Risk-Adjusted Mortality
eFigure 10. Experienced Surgeon-Linked CMS 1-Year HF Hospitalization
eFigure 11. Experienced Surgeon-Linked CMS 1-Year MV Reoperation
eResults. Surgeons With 3 Years of Experience