Abstract
Purpose:
Positive affect has demonstrated unique benefits in the context of health-related stress and is emerging as an important target for psychosocial interventions. The primary objective of this meta-analysis was to determine whether psychosocial interventions increase positive affect in cancer survivors.
Methods:
We coded 28 randomized controlled trials of psychosocial interventions assessing 2,082 cancer survivors from six electronic databases. We calculated 76 effect sizes for positive affect and conducted synthesis using random effects models with robust variance estimation. Tests for moderation included demographic, clinical, and intervention characteristics.
Results:
Interventions had a modest effect on positive affect (g = 0.35, 95% CI [0.16, 0.54]) with substantial heterogeneity of effects across studies ( = 0.40; 𝐼2 = 78%). Three significant moderators were identified: in-person interventions outperformed remote interventions (P = .046), effects were larger when evaluated against standard care or wait-list control conditions versus attentional, educational, or component controls (P = .009), and trials with survivors of early-stage cancer diagnoses yielded larger effects than those with advanced-stage diagnoses (P = .046). We did not detect differential benefits of psychosocial interventions across samples varying in sex, age, on-treatment versus off-treatment status, or cancer type. Although no conclusive evidence suggested outcome-reporting biases (P = .370), effects were smaller in studies with lower risk of bias.
Conclusions:
In-person interventions with survivors of early stage cancers hold promise for enhancing positive affect, but more methodological rigor is needed.
Implications for Cancer Survivors:
Positive affect strategies can be an explicit target in evidence-based medicine and have a role in patient-centered survivorship care, providing tools to uniquely mobilize human strengths.
Keywords: meta-analysis, cancer, positive affect, interventions, randomized controlled trials
Introduction
Recent systematic reviews and meta-analyses have demonstrated the efficacy of psychosocial interventions to reduce prominent negative effects of cancer, including depression [1], pain [2], and fatigue [3]. The majority of psychosocial interventions for cancer survivors have been directed toward such outcomes [4]. However, empirical evidence suggests that increasing positive affect may be an important target for psychosocial interventions with cancer survivors. Positive affect refers to “feelings that reflect a level of pleasurable engagement with the environment such as happiness, joy, excitement, enthusiasm, and contentment” and contains both activated states such as excited and non-activated states such as peaceful [5].
Beyond being pleasurable and enjoyable itself, positive affect plays important roles that might lead to other benefits. Fredrickson theorizes that positive affect serves a “broaden and build” function; it broadens people’s momentary thought-action repertoires, which in turn serves to build their physical, intellectual, social, and psychological resources [6]. Positive affect has been associated with lower risk of morbidity and mortality in healthy and chronically ill samples, independent of the effects of negative affect[5, 7–14]. Although positive and negative are strongly inversely correlated in some instances, such as discrete periods of intense emotions, most often the relationship is quite small [15], underscoring the importance of assessing positive affect independent from negative affect. Similarly, interventions to decrease negative affects may not necessarily increase positive affects [16], highlighting that these outcomes need to be considered separately in intervention work.
Observational studies documenting the positive relationship between positive affect, overall health [5, 17–23], and health-related quality of life [24, 25], suggests that positive affect may be an important asset for cancer survivors. Indeed, higher positive affect has been related to shorter hospital stays for colorectal cancer surgery [26] and decreased likelihood of developing post-surgical pain for breast cancer [27]. Depression in cancer survivors may be more reflective of an absence of positive affect than an increase in negative affect [28, 29]. Within the context of cancer survivorship, emotional responses can be highly variable and the mutability and durability of positive affect, independent of negative affect (depression, anxiety), is important to understand. Indeed, longitudinal evidence suggests the relationship between physical symptom distress and depression and anxiety in cancer survivors may be mediated by changes in positive affect [30]. Thus, positive affect may indirectly impact important health and psychosocial outcomes among cancer survivors.
The importance of positive affect among cancer survivors extends beyond its effects on health and quality of life outcomes. Positive affect is valued by survivors in its own right and is inextricably linked with survivors’ conceptions of ‘living well.’ Positive affect is a component of psychological or emotional wellbeing which is critically important in the midst of objectively difficult and stressful experiences such as life-limiting illness [31–34]. Measures of positive affect frequently focus on high activated aspects of positive affect (e.g., excitement, joy))[35–37] with relatively less attention devoted to low activated forms of positive affect (e.g., peace, contentment).
Interventions to promote positive affect are being incorporated into routine psychological care [38] and may help advance psychosocial care for cancer survivors both by increasing positive affect among those experiencing distress as well as boosting positive affect to promote wellness [39]. These interventions have been tested in other chronic conditions including HIV [10], cardiopulmonary disease [11], hypertension [12], asthma [40], and Type 2 diabetes [41]. Meta-analyses suggest these interventions are efficacious in the general population and in those with clinically significant distress [42, 43], but a review of the effects of psychosocial interventions on positive affect among cancer survivors has yet to be conducted.
The overarching aim of this review was to provide a comprehensive summary of the efficacy of psychosocial interventions for increasing positive affect among cancer survivors by conducting a meta-analysis of randomized controlled trials (RCTs). Psychosocial interventions entail an array of theoretical approaches and therapeutic orientations such as cognitive-behavioral, non-behavioral counseling or psychotherapy, social support, complementary or alternative mind-body, and multicomponent (i.e., some combination of the above) approaches [44]. This distinction is critical because “one size” does not “fit all” and some interventions are likely more beneficial than others or with certain groups of participants. For example, one might expect that physical activity interventions among cancer survivors may be beneficial for promoting high activated positive affect whereas mindfulness or relaxation strategies might be particularly helpful in eliciting low activated positive affect for survivors with advanced cancer or functional limitations. Therefore, a second aim was to examine intervention (e.g., individual, group-delivered, whether positive affect was an explicit target) and sample characteristics (e.g., cancer stage, cancer treatment phase) that may be important moderators in explaining the size of the effect of psychosocial interventions on positive affect.
Method
Search Strategy
Studies were identified by searching electronic databases (Ovid MEDLINE, PsycINFO, CINAHL, EMBASE, Cochrane Central, and Web of Science), emailing professional listservs, and contacting study authors. Electronic searches were performed through September 27, 2018. For the MEDLINE search, we used the McMaster multi-term filters with the best balance of sensitivity and specificity for retrieving randomized controlled trials [45] and systematic reviews [46]. All of our search strategies are described in detail in the supplementary materials (online only). Unpublished studies were requested from professional LISTSERVs for the Society of Behavioral Medicine, American Psychosocial Oncology Society, and the American Psychological Association. We attempted to acquire missing information from recently published papers by contacting 32 study authors, 10 of whom responded; 5 authors were able to provide additional information.
Eligibility Criteria
Studies were included if they: (1) were randomized controlled trials (RCTs) of psychosocial interventions; (2) were written in English; (3) included an adult sample (≥18 years old) with a diagnosis of cancer (active treatment and post-treatment); and (4) reported a positive affect outcome. We included unpublished studies and dissertations. For positive affect outcomes, we included validated, self-report measures of “feelings that reflect a level of pleasurable engagement with the environment such as happiness, joy, excitement, enthusiasm, and contentment” [5] such as the positive affect subscale of the Positive and Negative Affect Schedule (PANAS)[35]. Excluded studies used quasi-experimental designs or comparative effectiveness designs with no control groups, focused on pediatric or caregiver samples, or employed pharmacological or medical interventions rather than psychosocial interventions.
Psychosocial interventions were defined as any non-pharmacologic, therapeutic approaches that purported to affect thoughts, feelings, or behavior, including cognitive-behavioral techniques, stress management, relaxation training, hypnosis, existential therapies or other experiential techniques [2]. We included interventions with and without an explicit focus on positive affect because interventions that are not designed to target positive affect may still impact positive affect as a byproduct of the therapeutic process. If generic or non-specific interventions can strengthen positive affect, that is worth noting.
Study Selection
The review team included 4 raters, each with doctoral degrees and expertise in positive affect and health. Using the Cochrane technology platform Covidence (www.covidence.org), reviewer pairs independently screened each title and abstract to determine which studies merited full review. Studies possibly meeting inclusion criteria underwent full-text review by a pair of raters, with each rater independently evaluating the study and abstracting key data elements for entry into Covidence. Discrepancies were resolved by consensus within rater pairs and discussed as a team, as needed, in order to standardize coding decisions.
Data Coding
Data extracted from the study included demographic and clinical information about the sample (average age, sex composition, education level, cancer type, cancer stage, and cancer treatment phase). We also coded RCTs based on several intervention characteristics: 1) intervention type, JMS, SMS, and LESM grouped interventions together in a face valid way based on type of positive affect potentially impacted (high activated positive affect: physical activity, interpersonal/supportive, cognitive-behavioral; low activated positive affect: mindfulness, music therapy, yoga, relaxation; both high and low activated positive affect: multicomponent; unclear: other); 2) intervention focus (individual, dyad, group); 3) delivery format (in-person, audiovisual, print, telephone, web/internet); 4) intervention sessions (total number); 5) intervention target (whether or not an intervention was designed to specifically “target” positive affect vs. simply including it as an outcome); and 6) type of control condition (standard care/wait-list control, attention/education/component control). For positive affect outcomes, we recorded measure names and further coded these as primary, secondary, or unspecified outcomes based on descriptions in the source article.
Assessment of Risk of Bias
To summarize the degree to which primary study findings may have under- or overestimated the true intervention effect, we assessed the potential for risk of bias (ROB) across studies. Rater pairs independently reviewed each article and coded for the following Cochrane risk of bias categories: randomization sequence generation, allocation concealment, attrition, and outcome reporting [47, 48]. Following the guidance of Higgins et al. [48], for each of these four categories, we made a categorical assessment of bias as either low, unclear, or high risk of bias. Blinding of participants was not considered because it is often not feasible for trials of psychosocial interventions, and blinding of outcome assessors was not considered because all studies used self-reported outcomes. Discrepancies were resolved by consensus within rater pairs.
Effect Size Calculations and Meta-Analytic Procedures
To quantify the effects of psychosocial interventions on positive affect outcomes, we used standardized mean differences between treatment and control groups, estimated using Hedge’s g correction [49]. For the numerator of the effect size, we used the estimated difference between treatment and control groups, adjusted for baseline differences (i.e., change-score adjustment or regression adjustment); for one study where baseline data were not available [50], we used unadjusted differences between groups at post-test. We estimated the denominator of the effect size using baseline (pre-intervention) SDs in the outcome, pooled across groups; if no baseline data were available, we standardized based on the pooled variance at post-test. We calculated effect size estimates from reported mean and SD estimates by group if available; otherwise, we used reported statistical tests (i.e., F or t statistics, p-values) to derive comparable effect size estimates.
An examination of the distribution of raw effect size estimates included in the meta-analysis revealed no outliers. We used Tukey’s [51] definition of outliers as values below the 1st quartile minus 3 times the inter-quartile range (g = −1.43) or above the 3rd quartile plus 3 times the interquartile range (g=1.84).
Many of the included studies reported intervention effects on multiple measures of positive affect and/or at multiple follow-up times. For purposes of synthesizing the effect size estimates across included studies, we used random effects meta-analysis in combination with robust variance estimation techniques [52] to account for potential dependencies among effect size estimates from common samples. Specifically, we used a “correlated effects” working model, assuming a correlation of 0.7, as well as small-sample corrections to standard errors, hypothesis tests, and confidence intervals. We used restricted maximum likelihood estimates of the between-study standard deviation, denoted as , to measure the extent of heterogeneity among the effect sizes. Additionally, we report the 𝐼2 statistic, a relative measure of the extent to which heterogeneity among true effect sizes contributes to observed variation in the effect size estimates [53, 54]. To examine differences in effect size across moderating variables, we used random effects meta-regression models that allowed for between-study variance components to differ across levels of the moderator.
To investigate possible risks of bias in the meta-analytic results due to small-study effects, we examined a funnel plot of effect size estimates and conducted a modified version of Egger’s regression test for funnel plot asymmetry.[55]. We again used robust variance estimation to account for dependence of effect size estimates nested within studies.
All analyses were conducted using the metafor package [56] and clubSandwich package [57] for the R statistical computing environment. The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) was used in reporting results [58]. The raw data (including effect size estimates, variance estimates, and moderator variables) and code for replicating all reported analyses are available in the supplementary materials accompanying this article.
Results
Study Selection
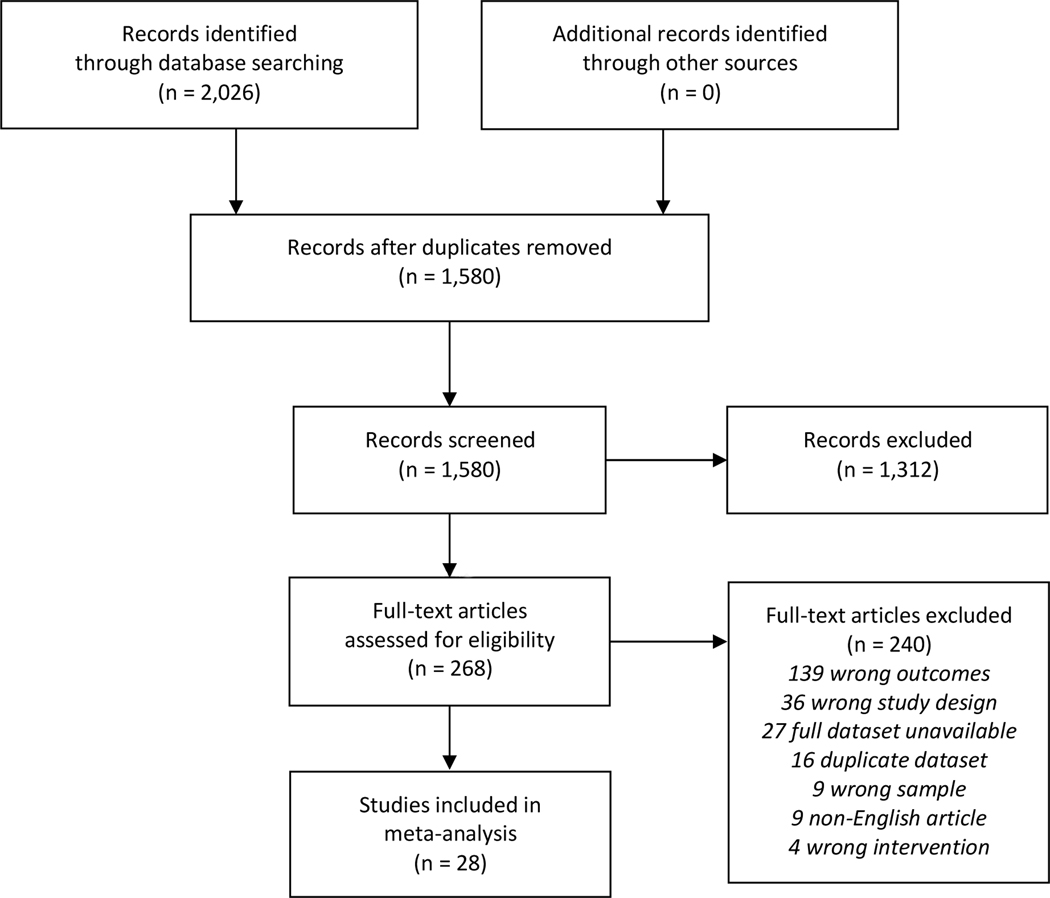
The search of the electronic databases retrieved 2,026 citations. After removal of duplicates, 1,580 remained and were evaluated on the basis of title and abstract. Of these, 1,312 were discarded because they clearly did not meet the inclusion criteria and were position or purely theoretical papers, review papers, descriptive or observational studies, or qualitative studies. Two hundred sixty-eight potentially relevant references were screened in more detail on the basis of the full texts. Of these, 28 met inclusion criteria (See Figure 1).)[50, 59–85]. Each study contributed between 1 and 8 effect size estimates, with a median of 2 effect sizes per study and a total of 76 effect size estimates. Studies contributing multiple effects involved multiple active intervention arms [68, 69, 82], used multiple measures of positive affect [59], or assessed positive affect at multiple follow-up times [50, 64, 81].
Figure 1.
PRISMA Flow Diagram
Overall Description of Studies and Effects
Table 1 provides a summary of the demographic, clinical, and intervention characteristics from the included studies. Across 28 RCTs, 76 effect sizes, and a combined sample size of 2,082 participants (M age=53.8, SD=4.8), the weighted average effect of positive affect outcomes was estimated as g = 0.35, 95% CI [0.16, 0.54], p < .001. The estimated between-study standard deviation was = 0.40 (𝐼2 = 78%), indicating substantial heterogeneity of effects across studies. One way of characterizing this degree of variability is to consider that, if the true effects are normally distributed, then about two thirds of effects will fall within one SD of the mean effect, or between −0.05 and 0.75.
Table 1.
Summary of Participant Demographic, Study, and Intervention Characteristics (N=2,082 participants)
| Demographic or characteristic | % | M | SD |
|---|---|---|---|
| Average age of patients | 53.8 | 4.8 | |
| White, % of patients | 82.6 | 11.9 | |
| Female, % of patients | 83.5 | 27.7 | |
| Cancer type, % of studies | |||
| Breast | 54 | ||
| Mixed | 29 | ||
| Cervical | 4 | ||
| Head/Neck | 4 | ||
| Prostate | 4 | ||
| Leukemia | 4 | ||
| Melanoma | 4 | ||
| Cancer stage, % of studies | |||
| Early | 39 | ||
| Mixed | 50 | ||
| Advanced | 11 | ||
| Treatment setting, % of studies | |||
| Inpatient care | 14 | ||
| Outpatient care | 86 | ||
| Type of intervention, % of studies | |||
| Cognitive behavioral, cardiovascular exercise, interpersonal, | 29 | ||
| or supportive | |||
| Mindfulness, music, yoga, or relaxation | 32 | ||
| Multicomponent | 29 | ||
| Other | 14 | ||
| Follow-up time, weeks from baseline | 14.2 | 18.1 | |
| Number of sessions | 8.6 | 7.1 | |
| Intervention delivery format, % of studies | |||
| In-person | 43 | ||
| Online | 4 | ||
| Telephone | 7 | ||
| 4 | |||
| Self-guided | 4 | ||
| Combined formats | 43 | ||
| Intervention focus, % of studies | |||
| Individual | 57 | ||
| Dyad | 7 | ||
| Group | 36 | ||
| Intervention provider, % of studies | |||
| Nurse | 11 | ||
| Psychologist | 32 | ||
| Social worker | 4 | ||
| Music therapist | 11 | ||
| Self-guided | 11 | ||
| Other | 25 | ||
| Not reported | 7 | ||
| Type of control group, % of studies | |||
| Standard care or wait-list | 71 | ||
| Attention control, education, or other component control | 32 | ||
| Outcome measure, % of studies | |||
| PANAS | 57 | ||
| Affect Balance Scale (positive affect subscale) | 4 | ||
| Affectivity Scale (positive affectivity subcale) | 4 | ||
| Differential Emotions Scale (enjoyment, interest, and positive affect subscales) | 7 | ||
| FACIT-Sp (peace subscale) | 14 | ||
| Fordyce Happiness Measure | 4 | ||
| Gratitude Questionnaire | 4 | ||
| Mood Report Form (positive affect subscale) | 4 | ||
| Positive States of Mind Scale | 7 | ||
NOTES:
Studies missing a participant characteristic are excluded from reported means and standard deviations.
Sum of percentages may exceed 100% because some studies included effects from multiple categories.
Leave-one-out sensitivity analyses indicated that the estimated effect size distribution was influenced by one study with an outlying effect size estimate. Excluding the single eligible effect size estimated in Victoria Cerezo [84] (g = 1.47, SE = 0.15) reduced the overall average effect estimate to g = 0.29, 95% CI [0.14, 0.45], p < .001 and the between-study heterogeneity estimate to = 0.29.
Risk of Bias
Table 2 reports the results of a sensitivity analysis examining how study risk-of-bias affected estimates of the overall average effect size and extent of heterogeneity with successively stronger inclusion criteria at each step. The first row reports the estimated distribution of effect sizes across all included studies. Subsequent rows report estimates for subsets of studies, illustrating how the overall average effect estimate is influenced by the stringency of inclusion criteria. Thus, including only the 18 studies (49 effects) that were at low risk-of-bias for outcome reporting, the overall average effect size estimate was g = 0.32, 95% CI [0.14, 0.50], = 0.25. Including only the 11 studies (28 effects) that were also at low risk-of-bias for randomization sequence generation resulted in a still smaller overall average effect (g = 0.27, 95% CI [−0.01, 0.56], = 0.33). Imposing further criteria related to allocation concealment and attrition (i.e., <10%) resulted in even fewer studies and further reductions in the average effect size estimate. As a whole, these analyses indicated that the risk-of-bias factors were associated with effect magnitude, such that studies with the least potential for risk of bias reported smaller effects on positive affect outcomes.
Table 2.
Risk-of-bias analysis using successive inclusion criteria
| Criteria | Studies (Effects) | Estimate [SE] | 95% CI | I2 | |
|---|---|---|---|---|---|
| All studies | 28 (76) | 0.35 [0.09] | [0.16, 0.54] | 0.40 | 78% |
| + Low ROB outcome reporting | 18 (49) | 0.32 [0.09] | [0.14, 0.50] | 0.25 | 59% |
| + Low ROB sequence generation | 11 (28) | 0.27 [0.13] | [−0.01, 0.56] | 0.33 | 65% |
| + Low ROB allocation concealment | 5 (15) | 0.10 [0.07] | [−0.12, 0.31] | 0.00 | 0% |
| + Overall attrition < 10% | 2 (3) | 0.16 [0.03] | [−0.19, 0.50] | 0.00 | 0% |
Note: ROB = risk-of-bias.
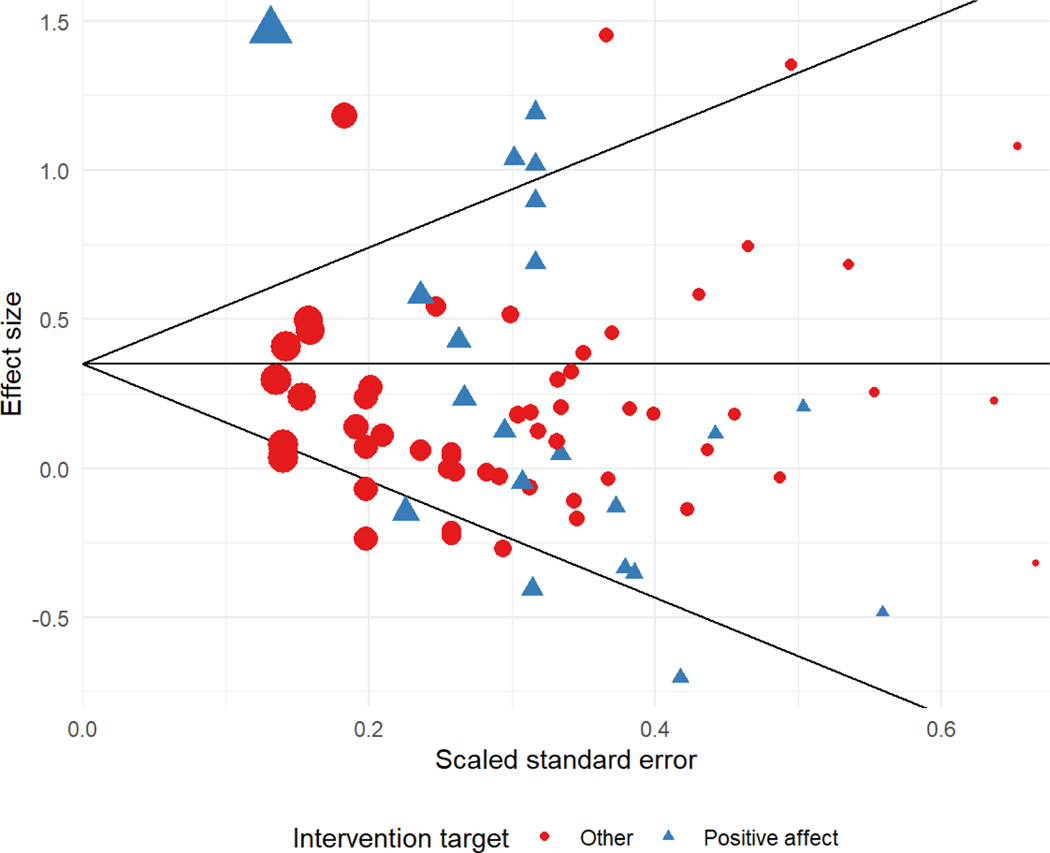
To investigate possible risks of bias due to small-study effects, we examined a funnel plot of effect size estimates and conducted modified versions of Egger’s regression test for funnel plot asymmetry. Figure 2 displays a funnel plot of effect size estimates versus scaled standard errors. Egger’s regression test was not significant at the 5% level and the estimated slope for the scaled standard error was = −0.81, 95% CI [−2.68, 1.05], P = .370, indicating that there was not conclusive evidence for small-study effects. Limiting the analytic sample to the 7 studies (21 effect size estimates) with post-test total sample sizes greater than 80 participants led to increased estimates of the overall average effect (g=0.44, 95% CI [−0.04, 0.91], P =.065) and degree of heterogeneity ( = 0.48, 𝐼2 = 90%). This was due to the increased weight given to the large effects from the Victoria Cerezo study [84], which was identified as a potential outlier based on leave-one-out sensitivity analysis. Excluding this study, the average effect estimate based on the 6 remaining large trials (20 effect size estimates) was g=0.26, 95% CI [0.04, 0.47], P =.030, very similar to the estimate based on all studies except Victoria Cerezo’s. The estimated degree of heterogeneity was substantially smaller ( = 0.13, 𝐼2 = 41%) among the large trials after excluding Victoria Cerezo.
Figure 2.
Funnel plot
Moderator analyses
Moderator analyses were conducted on demographic (age, sex), clinical variables (cancer type, stage, and phase of treatment) as well as characteristics of the interventions including delivery format (in person vs, other formats), focus of the intervention (individual, dyad, group), type of therapeutic approach (multicomponent vs., cognitive-behavioral/physical activity/interpersonal-supportive vs., mindfulness/music therapy/yoga/relaxation vs., other), control condition type (standard care/waitlist versus, attentional/education/component controls), and the extent to which interventions targeted positive affect outcomes (i.e., were designed to enhance or promote positive affect). Lastly, because the majority of studies (16 of 28) used the PANAS as the measure of positive affect, we examined differences by whether the positive affect outcome was assessed by the PANAS [35] or by a different self-report measure (e.g., Affect Balance Scale [36], Affectivity Scale [86], Differential Emotions Scale [37], Positive States of Mind Scale [87, 88], Peace subscale of the FACIT-Sp [89]). Measures could not be cleanly parsed into high vs., low activated positive affect as most scales relied on high-actived content or included aspects of both. Table 3 provides a summary of these results.
Table 3.
Moderator Analyses of Demographic, Clinical, and Intervention Characteristics
| Demographic or characteristic | Number of studies | Number of effect size estimates | Average ES [95% CI] | I2(%) | F (df1,df2) | p | |
|---|---|---|---|---|---|---|---|
| Average participant age | 26 | 73 | −0.02 [−0.06, 0.02] | 0.41 | 77 | 1.27 (1, 4.3) | .319 |
| Percent female | 26 | 73 | 0.00 [−0.01, 0.01] | 0.41 | 78 | 0.77 (1, 4.0) | .430 |
| Follow-up time (weeks from baseline) | 27 | 74 | −0.00 [−0.01, 0.00] | 0.40 | 78 | 1.81 (1, 1.1) | .393 |
| Number of sessions | 27 | 75 | 0.02 [−0.01, 0.04] | 0.41 | 76 | 2.02 (1, 5.6) | .208 |
| Cancer type | 73 | 0.44 (1, 24.8) | .514 | ||||
| Breast | 15 | 41 | 0.39 [0.11, 0.67] | 0.44 | |||
| Other1 | 13 | 35 | 0.27 [0.02, 0.53] | 0.30 | |||
| Cancer stage | 65 | 4.99 (2, 6.9) | .046 | ||||
| Early | 11 | 31 | 0.42 [0.10, 0.73] | 0.39 | |||
| Mixed | 14 | 35 | 0.38 [0.06, 0.69] | 0.45 | |||
| Advanced | 3 | 10 | −0.03 [−0.42, 0.36] | 0.00 | |||
| Cancer phase | 54 | 1.50 (2, 11.0) | .265 | ||||
| Pre-treatment | 1 | 2 | −0.08 [−0.62, 0.46] | 0.13 | |||
| Curative | 12 | 48 | 0.27 [−0.08, 0.61] | 0.48 | |||
| Mixed | 6 | 7 | 0.74 [0.11, 1.38] | 0.50 | |||
| Post-treatment | 8 | 13 | 0.29 [0.13, 0.46] | 0.00 | |||
| Palliative | 1 | 6 | 0.10 [−1.28, 1.47] | 0.13 | |||
| Delivery format | 59 | 4.69 (1, 15.5) | .046 | ||||
| In-person | 22 | 62 | 0.41 [0.17, 0.64] | 0.45 | |||
| Other2 | 7 | 14 | 0.14 [−0.02, 0.30] | 0.00 | |||
| Intervention focus | 62 | 3.87 (2, 3.3) | .136 | ||||
| Individual | 16 | 45 | 0.25 [0.01, 0.49] | 0.32 | |||
| Dyad | 2 | 4 | 0.06 [−0.37, 0.49] | 0.00 | |||
| Group | 10 | 27 | 0.52 [0.16, 0.88] | 0.45 | |||
| Intervention type | 75 | 0.86 (3, 10.3) | .491 | ||||
| Cognitive behavioral therapy, cardio exercise, interpersonal, or supportive | 8 | 18 | 0.34 [−0.12, 0.80] | 0.49 | |||
| Mindfulness, music therapy, yoga, or relaxation | 9 | 29 | 0.36 [−0.05, 0.77] | 0.42 | |||
| Multicomponent | 8 | 20 | 0.38 [−0.01, 0.77] | 0.40 | |||
| Other3 | 4 | 9 | 0.13 [−0.20, 0.47] | 0.00 | |||
| Control group type | 67 | 8.54 (1, 18.0) | .009 | ||||
| Attention, education, or component control | 9 | 27 | 0.09 [−0.08, 0.26] | 0.00 | |||
| Wait-list or standard care | 20 | 49 | 0.46 [0.22, 0.71] | 0.45 | |||
| Intervention targets positive affect | 76 | 0.02 (1, 12.9) | .900 | ||||
| Yes | 10 | 21 | 0.32 [−0.13, 0.76] | 0.54 | |||
| No | 18 | 55 | 0.34 [0.15, 0.54] | 0.29 | |||
| Outcome measure | 71 | 1.92 (1, 14.8) | .186 | ||||
| PANAS | 16 | 53 | 0.23 [0.07, 0.40] | 0.17 | |||
| Other4 | 12 | 23 | 0.49 [0.11, 0.87] | 0.52 | |||
Notes:
Other cancer types included: cervical, head/neck, prostate, leukemia, melanoma, and mixed types.
Other delivery formats included: online, telephone, print, self-delivered, and combinations thereof.
Other intervention types included: writing interventions.
Other outcome measures included: ABS – Positive Affect, Affectivity Scale (positive affectivity sub-scale), Differential Emotions Scale (Enjoyment, Interest, and Positive Affect sub-scales), FACIT-Sp (Peace subscale), Fordyce Happiness Measure, Gratitude Questionnaire, Mood Report Form (positive affect subscale), and Positive States of Mind Scale.
There were no statistically significant differences in treatment efficacy as a function of demographic or clinical variables with the exception of cancer stage (p = .046). Studies with survivors at advanced cancer stages had lower average effect sizes (g = −0.03) than studies with survivors at more mixed stages (g = 0.38) or survivors in early cancer stages (g = 0.42). In terms of intervention characteristics, there were statistically detectable differences in intervention delivery formats (p=0.046) and type of control condition (p=.009). Interventions that were inperson (g=0.41) yielded greater improvements in positive affect than those that were delivered remotely (g=0.14). Studies comparing psychosocial interventions to standard care or wait-list control conditions (g=0.46) showed larger effects, on average, than studies comparing interventions to attention, education, or component control conditions (g=0.09). However, there were not detectable differences by intervention focus or for intervention type. Interestingly, average effects of interventions targeting a positive affect outcome were not statistically distinguishable from effects of interventions that did not. Lastly, there were no detectable differences between positive affect outcomes assessed by the PANAS compared to those positive affect outcomes assessed by other measures of positive affect.
Discussion
This project is the first systematic review that synthesizes the effects of psychosocial interventions on positive affect outcomes among cancer survivors. A number of important findings emerged. Overall, psychosocial interventions increased positive affect among cancer survivors, but studies using more rigorous methods to address potential biases had more modest effects. Notably, interventions with survivors at an early or mixed stage of cancer performed better than those with survivors at advanced stages and in-person interventions performed better than interventions delivered remotely.
The modest average effect of psychosocial interventions on positive affect (g=0.35) is consistent with the effects of interventions to manage common negative psychosocial sequelae in cancer such as pain (g=0.34 for pain severity, 0.40 for pain interference) [2], fatigue (g=0.26 to 0.30 across various interventions) [3], and depression (g=0.43) [1] and effects of positive psychology interventions more broadly (e.g., g=0.34) [42, 43, 90]. However, our results show that two-thirds of the true effects of psychosocial interventions on positive affect will fall between −0.05 and 0.75. This suggests substantial variation in the effects of psychosocial interventions on positive affect in survivors with cancer. In addition, the weighted effect size for the overall impact of interventions on positive affect includes a study that had a strong influence on the estimates [84]. If this study is excluded, the average effect size estimate is reduced to 0.29 and the between-study SD to 0.29. Interestingly, the outlying study is one of only a handful of studies in our review which included multicomponent interventions specifically designed to promote positive affect, an approach that would generally be expected to yield more beneficial effects [50, 67, 78, 83]. A final important caveat is related to our analyses of the potential for bias in our findings. These analyses revealed a mixed picture. On one hand, studies which used more stringent designs to reduce the risk of potential bias (e.g., randomization sequence generation, allocation concealment) yielded smaller effects. On the other hand, when considering risk of bias across the collection of studies as a whole, there was not clear evidence of small study effects, which can be a symptom of selective outcome reporting.
In an effort to better understand which interventions are effective with which groups of survivors, we examined several potential moderating variables. There was no clear evidence of differential benefit for psychosocial interventions across samples varying in sex, age, on-treatment versus off-treatment status, or cancer type. However, interventions conducted with survivors diagnosed with early stage cancer yielded greater improvements in positive affect than interventions conducted with survivors diagnosed with late stage cancer. There are several potential reasons for this. Survivors with early stage cancer have a favorable survival prognosis compared to survivors diagnosed with late stage cancer. Further, survivors with late stage cancer can experience rapid functional decline [91]. Psychologically, expectations for oncologic treatment, the meaning of physical symptoms, and the impact on family may vary based on cancer stage [92]. The experience of positive affect may also vary based on cancer stage. Positive affect in survivors with late stage cancer is often described in terms of peace, tranquility, and contentment as survivors acknowledge a likely terminal disease and shift their efforts to symptom relief and preparing for death [93–95]. In contrast, positive affect in survivors with earlier stage cancer may reflect more high activated aspects such as excitement as survivors focus on finishing treatment and restoring their health. Psychosocial interventions for survivors with late stage cancer may not be expected to improve high activated positive affect, but may be expected to improve low activated positive affect. Unfortunately, only four studies (accounting for five effects) included low activated measures of positive affect as an outcome – three of which included survivors with mixed stages of cancer [70, 73, 78] and one which included survivors with advanced stage cancer [85]. Thus, we were unable to explore these important distinctions.
We also observed differences in positive affect by intervention delivery. In-person interventions resulted in more positive affect benefits than did remotely delivered interventions (via internet, phone, etc.). Cancer can be an emotionally isolating disease, with loneliness increasing post-diagnosis, especially in the absence of social support [96]. As such, perhaps there is a more activating component that is inherent to in-person delivery of interventions that may mitigate loneliness and result in greater gains in positive affect. It might also be that remotely delivered interventions have yet to properly capture the effective elements from in-person interventions. In addition, the type of comparison condition resulted in differential impact. Intervention efficacy was more pronounced among RCTs using standard care or wait list controls than attention or educational controls, suggesting that these psychosocial interventions are clearly better than receiving no interventions to promote positive affect and modestly better than information or attention-focused tasks. Importantly, attention is an indispensable ingredient of all psychosocial interventions and so, in behavioral RCTs, comparison and not control is the primary function of attention control groups.[97]
Contrary to our expectations, we did not see differences in positive affect outcomes as a function of intervention type. Other meta-analytic findings suggest that interventions in which people practice multiple activities may be more effective than those in which people practice single activities [42] and certain components of positive affect interventions may resonate better with some people than others [98]. However, our data did not identify differences in therapeutic approach or combinations of approaches for impacting positive affect outcomes. Moreover, the relative infrequency with which study investigators used positive affect measures besides the PANAS meant we could not conduct comparisons between high activity and low activity positive affect as an outcome. Although we are unable to settle the question of “What works?”, “for whom?”, and “under what conditions?”, we can advance some tentative conclusions based on these moderator analyses. In short, in-person interventions that leverage group-based formats may prove particularly effective in fostering positive affect among survivors with early stage cancers.
This study is not without limitations. First, although we identified a small to medium average effect of interventions, there was substantial heterogeneity in our findings. This compromised our ability to detect significant moderating variables and identify those conditions for which these interventions would be optimally beneficial for participants. Second, not all studies clearly indicated which outcomes were primary or secondary. Indeed, 39% of our studies (k=11) included positive affect as an “unspecified” outcome. Due to increasing standardization in registering and reporting applicable trials to ClinicalTrials.gov [99], future studies will be required to report these details, allowing for more informative summaries of data for systematic reviews and meta-analyses. Third, many studies had a very limited follow-up. The average follow-up from baseline to post-intervention was 14.2 weeks and only 2 studies had post-intervention follow-ups of a year or longer. This is relevant because data from multicomponent interventions in HIV [10] suggest that longer term follow-ups may yield larger effects than immediate post-intervention assessments. Finally, we were unable to stratify positive affect outcomes based on low activated vs. high activated, as most studies only assessed high activated positive affect.
Despite those limitations, there are a number of strengths about this meta-analysis. Importantly, this is the first systematic review and meta-analysis of the impact of psychosocial interventions on positive affect among cancer survivors. Consistent with recommendations for reporting our meta-analysis, we followed standard guidelines for conducting and reporting our findings as suggested by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [58]. Our review drew from six databases; leveraged the scientific expertise of four Ph.D. reviewers (JMS, RH, SMS, & JTM), a medical librarian (MB), and a statistician (JEP); included multiple approaches to evaluate potential bias; and explored the potential impact of relevant demographic, clinical, and intervention-level moderators.
This meta-analysis revealed some important directions for future research. First, as already discussed, only a small number of studies included multiple strategies for reducing risk of bias. Although there was not clear evidence of small-study bias, differences in effect sizes were observed for those studies which included multiple strategies for reducing risk of bias. Enhancing the methodological rigor of evaluations of psychosocial interventions to increase positive affect would help to increase confidence in their efficacy and enhance their acceptability. Research teams interested in positive affect but without experience in clinical trial methodology should pair with those who can help design rigorous studies. Second, the most common post-intervention follow-up assessment was a 3 to 4-month follow-up. Little is known about whether benefits to positive affect are sustained over time. Studies with longer term follow-ups are warranted. Third, although remotely delivered interventions were not as beneficial as in-person interventions, that does not mean that all remote interventions are less helpful. Recent years have seen an increase in the sophistication of technology for assessment [100] and treatment delivery [101]. As the use of technology in psychosocial interventions improves, remote interventions could become equally effective. Fourth, given the differences observed in cancer survivors diagnosed at early stages compared to survivors diagnosed at advanced stages within our meta-analysis, researchers need to evaluate low versus high activated components of positive affect (c.f., [102]). Peace and contentment may be desirable emotional states for survivors with late stage cancer diagnoses and thus a more important intervention target than joy or excitement. Fifth, the relatively small number of studies meeting our inclusion criteria limited our ability to detect moderator effects, particularly among demographic and clinical variables and the significant moderator effects we did observe were only marginally so. This will likely change over time as more and more interventions to promote positive affect are being developed and tested to support cancer survivors.
In conclusion, our findings revealed that psychosocial interventions are effective in increasing positive affect among cancer survivors, though effect sizes vary. Notably, in-person interventions performed better than interventions delivered remotely and interventions were more effective for survivors with early stages of cancer than with advanced cancers. More work in this area is certainly needed but this meta-analysis suggests that psychosocial interventions may be an effective means to help more survivors thrive in the midst of cancer. As rates of cancer survivorship increase, these findings highlight that positive affect strategies can be an explicit target in evidence-based medicine and that positive affect has a role in patient-centered care. Psychosocial interventions to promote positive affect may provide tools to uniquely mobilize human strengths and equip survivors to manage the late and long-term psychosocial sequaellae of cancer and its treatment.
Supplementary Material
Acknowledgments
This research was supported by National Cancer Institute Grant No. R03 CA184560 (PI: Salsman). Dr. McLouth was supported by National Cancer Institute R25 CA122061 (PI: Avis). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Portions of this manuscript were presented as part of a symposium at the 2017 Annual Meeting of the Society of Behavioral Medicine: Moskowitz, J. T., Pustejovsky, J. E., Hernandez, R., Schueller, S. M., Berendsen, M., Salsman, J. M. (2017). Don’t worry, be happy? Metaanalysis of intervention trials on positive affect in cancer patients and survivors. In J.M. Salsman (Chair), Promoting psychological well-being in cancer patients and survivors: Meta-analyses of randomized clinical trials. Annals of Behavioral Medicine, 51(Suppl.): S1624.
Study authors received exempt approval from the Wake Forest University School of Medicine Institutional Review Board under IRB00034301. Study procedures described by the authors did not meet the federal definition of research involving human subjects, and research described in this manuscript constituted secondary analysis of published, de-identified data. Thus, informed consent was not applicable.
Footnotes
Compliance with Ethical Standards
The authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Contributor Information
John M. Salsman, Department of Social Sciences and Health Policy, Wake Forest School of Medicine, Wake Forest Baptist Comprehensive Cancer Center
James E. Pustejovsky, Department of Educational Psychology, University of Texas at Austin
Stephen M. Schueller, Department of Psychological Science, University of California, Irvine
Rosalba Hernandez, School of Social Work, University of Illinois at Urbana-Champaign.
Mark Berendsen, Galter Health Sciences Library, Northwestern University Feinberg School of Medicine.
Laurie E. Steffen McLouth, Department of Social Sciences and Health Policy, Wake Forest School of Medicine.
Judith T. Moskowitz, Department of Medical Social Sciences, Northwestern University Feinberg School of Medicine
References
- 1.Hart SL, et al. , Meta-analysis of efficacy of interventions for elevated depressive symptoms in adults diagnosed with cancer. J Natl Cancer Inst, 2012. 104(13): p. 990–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheinfeld Gorin S, et al. , Meta-analysis of psychosocial interventions to reduce pain in patients with cancer. J Clin Oncol, 2012. 30(5): p. 539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mustian KM, et al. , Comparison of Pharmaceutical, Psychological, and Exercise Treatments for Cancer-Related Fatigue: A Meta-analysis. JAMA Oncol, 2017. 3(7): p. 961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanton AL, Psychosocial Concerns and Interventions for Cancer Survivors. Journal of Clinical Oncology, 2006. 24(32): p. 5132–5137. [DOI] [PubMed] [Google Scholar]
- 5.Pressman SD and Cohen S, Does positive affect influence health? Psychological Bulletin, 2005. 131(6): p. 925–971. [DOI] [PubMed] [Google Scholar]
- 6.Fredrickson BL, The Role of Positive Emotions in Positive Psychology: The Broaden-and-Build Theory of Positive Emotions. American Psychologist, 2001. 56(3): p. 218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moskowitz JT, Acree M, and Epel ES, Positive Affect Uniquely Predicts Lower Risk of Mortality in People With Diabetes. Health Psychology, 2008. 27(1 Suppl.): p. S73–S82. [DOI] [PubMed] [Google Scholar]
- 8.Moskowitz JT, Positive Affect Predicts Lower Risk of AIDS Mortality. Psychosomatic medicine, 2003. 65(4): p. 620. [DOI] [PubMed] [Google Scholar]
- 9.Chida Y and Steptoe A, Positive Psychological Well-Being and Mortality: A Quantitative Review of Prospective Observational Studies. Psychosomatic Medicine, 2008. 70(7): p. 741–756. [DOI] [PubMed] [Google Scholar]
- 10.Moskowitz JT, et al. , Randomized controlled trial of a positive affect intervention for people newly diagnosed with HIV. J Consult Clin Psychol, 2017. 85(5): p. 409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charlson ME, et al. , Randomized controlled trials of positive affect and self-affirmation to facilitate healthy behaviors in patients with cardiopulmonary diseases: rationale, trial design, and methods. Contemp Clin Trials, 2007. 28(6): p. 748–62. [DOI] [PubMed] [Google Scholar]
- 12.Ogedegbe GO, et al. , A randomized controlled trial of positive-affect intervention and medication adherence in hypertensive African Americans. Arch Intern Med, 2012. 172(4): p. 322–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterson JC, et al. , Translating basic behavioral and social science research to clinical application: the EVOLVE mixed methods approach. J Consult Clin Psychol, 2013. 81(2): p. 217–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boutin-Foster C, et al. , Results from the Trial Using Motivational Interviewing, Positive Affect, and Self-Affirmation in African Americans with Hypertension (TRIUMPH). Ethn Dis, 2016. 26(1): p. 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diener E and Emmons RA, The independence of positive and negative affect. Journal of Personality and Social Psychology, 1984. 47(5): p. 1105–1117. [DOI] [PubMed] [Google Scholar]
- 16.Craske MG, et al. , Positive affect treatment for depression and anxiety: A randomized clinical trial for a core feature of anhedonia. J Consult Clin Psychol, 2019. 87(5): p. 457–471. [DOI] [PubMed] [Google Scholar]
- 17.Danner DD, Snowdon DA, and Friesen WV, Positive emotions in early life and longevity: findings from the nun study. Journal of Personality and Social Psychology, 2001. 80(5): p. 804–813. [PubMed] [Google Scholar]
- 18.Fredrickson BL and Levenson RW, Positive emotions speed recovery from the cardiovascular sequelae of negative emotions. Cognition & Emotion, 1998. 12(2): p. 191–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richman LS, et al. , Positive emotion and health: going beyond the negative. Health Psychology, 2005. 24(4): p. 422–429. [DOI] [PubMed] [Google Scholar]
- 20.Diener E, Subjective well-being. The science of happiness and a proposal for a national index. American Psychologist, 2000. 55(1): p. 34–43. [PubMed] [Google Scholar]
- 21.Koizumi M, et al. , Effect of having a sense of purpose in life on the risk of death from cardiovascular diseases. Journal of Epidemiology, 2008. 18(5): p. 191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verduin PJ, et al. , Purpose in life in patients with rheumatoid arthritis. Clinical Rheumatology, 2008. 27(7): p. 899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mascaro N and Rosen DH, Existential meaning’s role in the enhancement of hope and prevention of depressive symptoms. Journal of Personality, 2005. 73(4): p. 985–1013. [DOI] [PubMed] [Google Scholar]
- 24.Stauber S, et al. , Health-related quality of life is associated with positive affect in patients with coronary heart disease entering cardiac rehabilitation. J Clin Psychol Med Settings, 2013. 20(1): p. 79–87. [DOI] [PubMed] [Google Scholar]
- 25.Eaton RJ, Bradley G, and Morrissey S, Positive predispositions, quality of life and chronic illness. Psychology, Health & Medicine, 2014. 19(4): p. 473–489. [DOI] [PubMed] [Google Scholar]
- 26.Sharma A, et al. , Patient personality predicts postoperative stay after colorectal cancer resection. Colorectal Disease, 2008. 10(2): p. 151–156. [DOI] [PubMed] [Google Scholar]
- 27.Bruce J, et al. , Psychological, surgical, and sociodemographic predictors of pain outcomes after breast cancer surgery: a population-based cohort study. Pain, 2014. 155(2): p. 232–43. [DOI] [PubMed] [Google Scholar]
- 28.Voogt E, et al. , Positive and negative affect after diagnosis of advanced cancer. Psycho-Oncology, 2005. 14(4): p. 262–273. [DOI] [PubMed] [Google Scholar]
- 29.Ritterband LM and Spielberger CD, Depression in a cancer patient population. Journal of Clinical Psychology in Medical Settings, 2001. 8(2): p. 85–93. [Google Scholar]
- 30.Hou WK, Law CC, and Fu YT, Does change in positive affect mediate and/or moderate the impact of symptom distress on psychological adjustment after cancer diagnosis? A prospective analysis. Psychology & Health, 2010. 25(4): p. 417–431. [DOI] [PubMed] [Google Scholar]
- 31.Salsman J, et al. , Development and validation of the positive affect and well-being scale for the neurology quality of life (Neuro-QOL) measurement system. Quality of Life Research, 2013. 22(9): p. 2569–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calhoun LG and Tedeschi RG, Handbook of posttraumatic growth: Research and practice. 2014: Routledge. [Google Scholar]
- 33.Rasmussen H, Scheier M, and Greenhouse J, Optimism and Physical Health: A Metaanalytic Review. Annals of Behavioral Medicine, 2009. 37(3): p. 239–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howell RT, Kern ML, and Lyubomirsky S, Health benefits: Meta-analytically determining the impact of well-being on objective health outcomes. Health Psychology Review, 2007. 1(1): p. 83–136. [Google Scholar]
- 35.Watson D, Clark LA, and Tellegen A, Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 1988. 54(6): p. 1063–1070. [DOI] [PubMed] [Google Scholar]
- 36.Bradburn NM, The Structure of Psychological Well-being. 1969, Chicago, IL: Aldine Publishing. [Google Scholar]
- 37.Fredrickson BL, et al. , What good are positive emotions in crises? A prospective study of resilience and emotions following the terrorist attacks on the United States on September 11th, 2001. J Pers Soc Psychol, 2003. 84(2): p. 365–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruini C, Positive Psychology in the Clinical Domains: Research and Practice. 2017, Springer. [Google Scholar]
- 39.Adler NE, A. Page, and S. Institute of Medicine Committee on Psychosocial Services to Cancer Patients/Families in a Community, Cancer care for the whole patient : meeting psychosocial health needs. 2008, Washington, D.C.: National Academies Press. [PubMed] [Google Scholar]
- 40.Mancuso CA, et al. , Increasing physical activity in patients with asthma through positive affect and self-affirmation: a randomized trial. Archives of internal medicine, 2012. 172(4): p. 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohn MA, et al. , An online positive affect skills intervention reduces depression in adults with type 2 diabetes. The journal of positive psychology, 2014. 9(6): p. 523–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sin NL and Lyubomirsky S, Enhancing well-being and alleviating depressive symptoms with positive psychology interventions: a practice-friendly meta-analysis. J Clin Psychol, 2009. 65(5): p. 467–87. [DOI] [PubMed] [Google Scholar]
- 43.Bolier L, et al. , Positive psychology interventions: a meta-analysis of randomized controlled studies. BMC Public Health, 2013. 13: p. 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moyer A, et al. , Characteristics and methodological quality of 25 years of research investigating psychosocial interventions for cancer patients. Cancer Treat Rev, 2009. 35(5): p. 475–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haynes RB, et al. , Optimal search strategies for retrieving scientifically strong studies of treatment from Medline: analytical survey. Bmj, 2005. 330(7501): p. 1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montori VM, et al. , Optimal search strategies for retrieving systematic reviews from Medline: analytical survey. Bmj, 2005. 330(7482): p. 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Higgins JP, Green S Cochrane handbook for systematic reviews of interventions version 5.1. 0. The cochrane collaboration, 2011. 5(0). [Google Scholar]
- 48.Higgins JPT, et al. , The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ, 2011. 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hedges LV, Distribution theory for Glass’s estimator of effect size and related estimators. Journal of Educational Statistics, 1981. 6(2): p. 107–128. [Google Scholar]
- 50.Schnur JB, et al. , A randomized trial of a cognitive-behavioral therapy and hypnosis intervention on positive and negative affect during breast cancer radiotherapy. Journal of Clinical Psychology, 2009. 65(4): p. 443–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tukey JW, Exploratory data analysis. 1977. [Google Scholar]
- 52.Hedges LV, Tipton E, and Johnson MC, Robust variance estimation in metaregression with dependent effect size estimates. Research Synthesis Methods, 2010. 1(1): p. 39–65. [DOI] [PubMed] [Google Scholar]
- 53.Borenstein M, et al. , Basics of meta analysis: I2 is not an absolute measure of heterogeneity. Research synthesis methods, 2017. 8(1): p. 5–18. [DOI] [PubMed] [Google Scholar]
- 54.Pigott T, Advances in meta-analysis. 2012: Springer Science & Business Media. [Google Scholar]
- 55.Pustejovsky JE and Rodgers MA, Testing for funnel plot asymmetry of standardized mean differences. Res Synth Methods, 2019. 10(1): p. 57–71. [DOI] [PubMed] [Google Scholar]
- 56.Viechtbauer W, Conducting meta-analyses in R with the metafor package. Journal of Statistical Software, 2010. 36(3): p. 1–48. [Google Scholar]
- 57.Pustejovsky J, clubSandwich: Cluster-robust (sandwich) variance estimators with small-sample corrections. R package version 0.0. 0.9000. URL: https://github.com/jepusto/clubSandwich, 2015. [Google Scholar]
- 58.Moher D, et al. , Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine, 2009. 6(7): p. e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Antoni MH, et al. , How stress management improves quality of life after treatment for breast cancer. Journal of Consulting and Clinical Psychology, 2006. 74(6): p. 1143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Badger T, et al. , Telephone interpersonal counseling with women with breast cancer: symptom management and quality of life. Oncol Nurs Forum, 2005. 32(2): p. 273–9. [DOI] [PubMed] [Google Scholar]
- 61.Badger TA, et al. , Psychosocial interventions to improve quality of life in prostate cancer survivors and their intimate or family partners. Qual Life Res, 2011. 20(6): p. 833–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bower JE, et al. , Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial.[Erratum appears in Cancer. 2015 Jun 1;121(11):1910]. Cancer, 2015. 121(8): p. 1231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Branstrom R, et al. , Self-report Mindfulness as a Mediator of Psychological Well-being in a Stress Reduction Intervention for Cancer Patients-A Randomized Study. Annals of Behavioral Medicine, 2010. 39(2): p. 151–161. [DOI] [PubMed] [Google Scholar]
- 64.Burns DS, et al. , Music imagery for adults with acute leukemia in protective environments: a feasibility study. Support Care Cancer, 2008. 16(5): p. 507–13. [DOI] [PubMed] [Google Scholar]
- 65.Cadmus LA, et al. , Exercise and quality of life during and after treatment for breast cancer: Results of two randomized controlled trials. Psycho-Oncology, 2009. 18(4): p. 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carpenter KM, et al. , An online stress management workbook for breast cancer. Journal of Behavioral Medicine, 2014. 37(3): p. 458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheung EO, et al. , A randomized pilot trial of a positive affect skill intervention (lessons in linking affect and coping) for women with metastatic breast cancer. Psychooncology, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cole B, et al. , A randomized controlled trial of spiritually-focused meditation in patients newly diagnosed with acute leukemia. Blood, 2010. 116(21). [Google Scholar]
- 69.Cole BS, et al. , A randomised clinical trial of the effects of spiritually focused meditation for people with metastatic melanoma. Mental Health, Religion & Culture, 2012. 15(2): p. 161–174. [Google Scholar]
- 70.Cook EL and Silverman MJ, Effects of music therapy on spirituality with patients on a medical oncology/hematology unit: A mixed-methods approach. Arts in Psychotherapy, 2013. 40(2): p. 239–244. [Google Scholar]
- 71.Danhauer SC, et al. , Restorative yoga for women with breast cancer: findings from a randomized pilot study. Psycho-Oncology, 2009. 18(4): p. 360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Donnelly C, et al. , A randomised controlled trial testing the feasibility of a physical activity intervention in managing fatigue with gynaecological cancer survivors. Physiotherapy (United Kingdom), 2011. 97: p. eS294–eS295. [DOI] [PubMed] [Google Scholar]
- 73.Fauver R, The healing wisdom within: A preliminary experimental trial of psychospiritual integrative therapy for people with cancer. 2012. 73: p. 3948–3948. [Google Scholar]
- 74.Fredenburg HA and Silverman MJ, Effects of music therapy on positive and negative affect and pain with hospitalized patients recovering from a blood and marrow transplant: A randomized effectiveness study. The Arts in Psychotherapy, 2014. 41(2): p. 174–180. [Google Scholar]
- 75.Gadler D, The effects of written emotional expression on health-related quality of life and cognitive processing in early-stage breast cancer patients: An exploratory study. 2006. 66: p. 6269–6269. [Google Scholar]
- 76.Helgeson V, et al. , Education and peer discussion group interventions and adjustment to breast cancer. Archives of General Psychiatry, 1999. 56(4): p. 340–347. [DOI] [PubMed] [Google Scholar]
- 77.Hoffman DL, The effects of a practice of gratitude on quality of life and depression in head and neck cancer survivors. 2016, ProQuest Information & Learning: US. [Google Scholar]
- 78.Jafari N, et al. , Spiritual therapy to improve the spiritual well-being of Iranian women with breast cancer: a randomized controlled trial. Evidence-based complementary and alternative medicine : eCAM, 2013. 2013: p. 353262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kovacs AH, The design and evaluation of a brief intervention to enhance well-being among women with breast cancer completing chemotherapy. 2003. 64: p. 2924–2924. [Google Scholar]
- 80.Larson MR, et al. , A presurgical psychosocial intervention for breast cancer patients: psychological distress and the immune response. Journal of Psychosomatic Research, 2000. 48(2): p. 187–194. [DOI] [PubMed] [Google Scholar]
- 81.Mutrie N, et al. , Benefits of supervised group exercise programme for women being treated for early stage breast cancer: Pragmatic randomised controlled trial. BMJ: British Medical Journal, 2007. 334(7592): p. 517–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nakamura Y, et al. , Investigating efficacy of two brief mind–body intervention programs for managing sleep disturbance in cancer survivors: A pilot randomized controlled trial. Journal of Cancer Survivorship, 2013. 7(2): p. 165–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shao D, Gao W, and Cao FL, Brief psychological intervention in patients with cervical cancer: A randomized controlled trial. Health Psychol, 2016. 35(12): p. 1383–1391. [DOI] [PubMed] [Google Scholar]
- 84.Victoria Cerezo M, et al. , Positive psychology group intervention for breast cancer patients: a randomised trial. Psychol Rep, 2014. 115(1): p. 44–64. [DOI] [PubMed] [Google Scholar]
- 85.Wise M, et al. , Suffering in Advanced Cancer: A Randomized Control Trial of a Narrative Intervention. Journal of palliative medicine, 2018. 21(2): p. 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mroczek DK and Kolarz CM, The effect of age on positive and negative affect: a developmental perspective on happiness. J Pers Soc Psychol, 1998. 75(5): p. 1333–49. [DOI] [PubMed] [Google Scholar]
- 87.Adler NE, et al. , Additional validation of a scale to assess positive states of mind. Psychosom Med, 1998. 60(1): p. 26–32. [DOI] [PubMed] [Google Scholar]
- 88.Horowitz M, Adler N, and Kegeles S, A scale for measuring the occurrence of positive states of mind: a preliminary report. Psychosom Med, 1988. 50(5): p. 477–83. [DOI] [PubMed] [Google Scholar]
- 89.Peterman AH, et al. , Measuring spiritual well-being in people with cancer: The Functional Assessment of Chronic Illness Therapy--Spiritual Well-being Scale (FACIT-Sp). Annals of Behavioral Medicine, 2002. 24(1): p. 49–58. [DOI] [PubMed] [Google Scholar]
- 90.Weiss LA, Westerhof GJ, and Bohlmeijer ET, Can we increase psychological wellbeing? The effects of interventions on psychological well-being: A meta-analysis of randomized controlled trials. PloS one, 2016. 11(6): p. e0158092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Glare P, et al. , Predicting survival in patients with advanced disease. Eur J Cancer, 2008. 44(8): p. 1146–56. [DOI] [PubMed] [Google Scholar]
- 92.Kim Y and Given BA, Quality of life of family caregivers of cancer survivors: across the trajectory of the illness. Cancer, 2008. 112(11): p. 2556–68. [DOI] [PubMed] [Google Scholar]
- 93.Kuhl D, Exploring the Lived Experience of Having a Terminal Illness*. Journal of Palliative Care, 2011. 27(1): p. 43–52. [PubMed] [Google Scholar]
- 94.Cohen SR and Leis A, What determines the quality of life of terminally ill cancer patients from their own perspective? Journal of Palliative Care, 2002. 18(1): p. 48–58. [PubMed] [Google Scholar]
- 95.Austin P and MacLeod R, Finding peace in clinical settings: A narrative review of concept and practice. Palliative and Supportive Care, 2016. 15(4): p. 490–498. [DOI] [PubMed] [Google Scholar]
- 96.Deckx L, van den Akker M, and Buntinx F, Risk factors for loneliness in patients with cancer: a systematic literature review and meta-analysis. Eur J Oncol Nurs, 2014. 18(5): p. 466–77. [DOI] [PubMed] [Google Scholar]
- 97.Freedland KE, et al. , Usual and unusual care: existing practice control groups in randomized controlled trials of behavioral interventions. Psychosom Med, 2011. 73(4): p. 323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schueller SM, Person–Activity Fit in Positive Psychological Interventions. The Wiley Blackwell handbook of positive psychological interventions, 2014: p. 385–402. [Google Scholar]
- 99.ClinicalTrials.gov. Changes from Current Practice Described in the Final Rule. 2016. [cited 2018 14 Feb]; Available from: https://prsinfo.clinicaltrials.gov/FinalRuleChanges16Sept2016.pdf.
- 100.Low CA, et al. , Estimation of Symptom Severity During Chemotherapy From Passively Sensed Data: Exploratory Study. J Med Internet Res, 2017. 19(12): p. e420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nasi G, Cucciniello M, and Guerrazzi C, The performance of mHealth in cancer supportive care: a research agenda. J Med Internet Res, 2015. 17(1): p. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Scheibe S, et al. , Striving to feel good: ideal affect, actual affect, and their correspondence across adulthood. Psychol Aging, 2013. 28(1): p. 160–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.