Abstract
Children with reading difficulties (RD) share challenges in executive functions (EF). Neurobiological correlates provide evidence for EF challenges during reading among these readers, but an online cognitive load detection mechanism has yet to be developed. Nevertheless, eye-movement tracking can provide online data of reading patterns (pupil dilation, fixations) and, indeed, atypical eye-movement patterns of children with RD during reading have been documented. To identify eye-movement patterns related to increased cognitive load during reading in children with RD compared to typical readers, eye movements of 8–12-year-old English-speaking children were recorded during their reading of sentences with increasing difficulty (sentences that make sense, then sentences that do not make sense) and comparing incorrect and correct responses. Children with RD demonstrated greater pupil dilation when reading sentences that make sense than when reading sentences that do not make sense and also when reading incorrectly, compared to typical readers. Increased pupil dilation in children with RD when reading sentences correctly was positively correlated with phonological awareness capabilities. Higher phonological awareness and reading abilities were related to increased pupil dilation only in children with RD during correct reading, which is related to a heavier cognitive load. Results suggest that in addition to traditional findings of altered fixation patterns in children with RD, increased pupil dilation during reading may reflect EF challenges among this population. These findings can potentially be used to adapt online written materials for children with RD based on their fixation and pupil dilation patterns.
Keywords: Children, Eye movements, Executive functions, Reading, Reading difficulties
Introduction
Reading Difficulties and Challenges in Executive Functions
Reading is the skill of translating written graphemes into suitable sounds efficiently and fluently (Breznitz, 2006), and relies on intact phonological, semantic, and orthographic abilities (Horowitz-Kraus, 2016), as well as on cognitive control (also referred to as executive functions, or EF) (Horowitz-Kraus & Hutton, 2015a). Dyslexia, or reading difficulty (RD), is a continuous reading challenge with a neurobiological origin (Lyon, Shaywitz, & Shaywitz, 2003). RD is characterized by inaccurate and/or slower word recognition and reading comprehension, as well as poor spelling (Lyon et al., 2003). It has been proposed that individuals with RD also demonstrate challenges in other cognitive processes such as speed of processing, auditory sustained attention, fluency, visual-spatial abilities, and other skills in which EF are involved (Menghini et al., 2010) (Horowitz-Kraus, Holland, & Freund, 2016). Several EF capabilities are essential for reading including inhibition, working memory, processing speed, attention switching, and error monitoring (Booth, Boyle, & Kelly, 2014; Scharinger, Kammerer, & Gerjets, 2015). Correlation between EF and reading abilities has been shown in children and adults with RD, both behaviorally (Gooch, Thompson, Nash, Snowling, & Hulme, 2016; Smith-Spark, Henry, Messer, Edvardsdottir, & Ziecik, 2016) and in neuroimaging studies (Horowitz-Kraus, Vannest, Gozdas, & Holland, 2014).
Are There Physiological Measures for Challenges in Executive Functions?
Since individuals with RD struggle with reading, greater cognitive load imposes a major challenge to gathering the semantic information while reading words during sentence reading (LaBerge & Samuels, 1974). Therefore, it is not surprising that increased activation in frontal regions when reading sentences that make sense compared to sentences that do not make sense in typical readers was found (Rimrodt et al., 2009). The researchers suggested that children 9–14 years of age showed greater activation in regions related to cognitive control, an activation that was associated with the attempt to determine a semantic meaning for a sentence when it made sense. Children with RD, due to their actual struggle with reading, showed greater activation in frontal regions (inferior and middle frontal gyrus) than typical readers for sentence vs. word reading and also for sentences that make those that do not make sense, independent of the comprehension component (Rimrodt et al., 2009). The suggestion was that processing meaningful sentences is a more overloading task for children with RD than processing not-meaningful sentences and may be due to the additional semantic information included in sentences that makes sense and related to the activation of the inferior and middle frontal gyrus (Rimrodt et al., 2009). Support for these findings was also found in an electroencephalogram study (EEG), which demonstrated a decreased ability to monitor errors by individuals with RD when deciding if a sentence makes sense vs. judging if a word is real or not, as compared to typical readers (Horowitz-Kraus & Breznitz, 2011). These results suggest that sentence reading is more challenging than word reading, particularly when considering the load it puts on working memory and visual attention, and erroneous reading in individuals with RD may be a result of increased load, resulting in decreased brain activation in the anterior cingulate cortex (Horowitz-Kraus & Breznitz, 2011). The evidence generated by neuroimaging data (both EEG and fMRI), unfortunately, do not provide an online marker of the cognitive load shared by readers with RD, as the results can be generated only retrospectively, when processing the data. Online eye trackers, however, may be able to provide online information regarding the readers’ cognitive condition.
Atypical Eye-Movement Patterns in Children with Reading Difficulties
Several studies have demonstrated the correlation between atypical eye-movement patterns and RD, and indicated that some specific eye-movement measurements differentiate individuals with RD from typical readers (Kim, 2016; Nilsson Benfatto et al., 2016; Rayner, 1978; Zhan, Zhang, Mei, & Fong, 2016). Such measures include decreased fixation and saccade rates in both children with RD (Zhan et al., 2016) and children at-risk for RD (Nilsson Benfatto et al., 2016). Kim and colleagues have shown that eye movements, especially fixation frequency, number of fixations, and fixation duration, saccades and pupil dilation, can serve as an accurate biological marker to identify college students with RD using a simple text-reading task (Kim, 2016).
Recently, it has been suggested that pupil dilatation, which can be measured using an eye-tracker device, reflects challenges in EF (Sara, 2009; Wahn, Ferris, Hairston, & Konig, 2016). The biological explanation for the relationship between pupil dilation and EF has been related to the norepinephrine system (Sara, 2009; Wahn, Ferris, Hairston, & Konig, 2016). More specifically, the diameter of the pupil is affected by two muscles: the sphincter muscle, which is affected by the parasympathetic system, and the dilator muscle, affected by the sympathetic system, whereas the sympathetic activity triggers the dilation (Aston-Jones, 2005; Nieuwenhuis, 2011). It has been suggested that the locus-coeruleus norepinephrine (LC-NE) system (i.e., the norepinephrine system, part of the sympathetic system) receives signals related to greater task demands from brain regions associated with cognitive control such as the anterior cingulate cortex (ACC), the frontal, parietal cortices(Aston-Jones, 2005; Nieuwenhuis, 2011) as well as the superior colliculus (subcortical) (Foote, 1987). These regions are related to level of arousal, attentional control and cognitive load (for a review, see (Van der Wel, 2018)).
Hence, pupil dilation is impacted by task engagement, attentional load differences, and task experience, and increases with increased attentional load and decreases with increased task experience (Wahn et al., 2016). Pupil dilation was found to indicate increased attention load in adults (Scharinger et al., 2015), as well as in 10-year-old children (Karatekin, Marcus, & Couperus, 2007).
While these measures were related to general cognitive load in adults and children, studies have yet to determine, e.g., by examining pupil dilation in conditions with an increased difficulty level, whether the challenge in EF shared among individuals with RD during reading is reflected in eye-movement patterns. Such an online evaluation of the level of cognitive load imposed on the reader could potentially allow the written materials to be adapted to the readers’ reading ability and challenges, decrease their level of frustration and increase motivation to read in children with RD as well as in typical readers.
The aim of the current study was to determine eye-movement patterns related to an increased cognitive load during reading in children with RD compared to typical readers. First, we wanted to verify if children with RD demonstrate decreased EF abilities and whether these abilities are associated with lower reading. Rimrodt and colleagues showed an increased challenge in typical readers, as well as in children with RD, when comprehending sentences that make sense (Rimrodt et al., 2009). We, therefore, during a semantic judgement task, presented participants with a cognitive load by using sentences that make sense and sentences that do not make sense. We hypothesized that children with RD would demonstrate greater pupil dilation—indicating greater cognitive load for sentences that make sense (MS) compared to sentences that do not make sense (N-MS)—as well as more fixations for both the sentences that make sense vs. the sentences that do not make sense condition and incorrect vs. correct reading, especially for sentences that make sense. We also hypothesized that greater pupil dilation would be associated with longer and more fixations, as was previously suggested (Mathot, 2015), and that larger pupils are positively related to the number and duration of fixations—likely due to greater visual search in the case of cognitive load. We presumed that this would be even more evident in children with RD compared to typical readers.
Methods
Participants
The study participants (N=19) were 8–12-year-old children with RD (n=9; mean age=10.47 years, SD=1.67; 5 males) and age-matched typical readers (n=10; mean age=9.10 years, SD=1.45; 4 males). All participants were native English speakers, Caucasian, and from a middle-class background. Each participant displayed normal vision in both eyes and had normal hearing. Participants were right- or left-handed (children with RD: 6 right-handed; typical readers: 10 right-handed). None of the participants had symptoms of attention deficit hyperactive disorder (ADHD) or any other neurological/psychiatric comorbidity, which was verified using the Conners task (Conners, 1989). All participants were within the normal range of nonverbal IQ (children with RD: M=100, SD=6.442; typical readers: M=102.56, SD=7.248; t=−0.79, p=0.44), as measured by the Test of Nonverbal Intelligence, third edition [(TONI-3; Brown, Sherbenou, & Johnsen, 1997)]. Participants in the RD group had either received a previous RD diagnosis or parents had reported their children as having RD. Typical readers were healthy volunteers who responded to posted ads. To ensure the existence or absence of RD, all participants completed the same set of normative reading tests: a) phonologic awareness [Ellison subtest, from the CTOPP (Wagner, Torgesen, & Rashotte, 1999)]; b) timed word-efficient reading [TOWRE SWE, from the TOWRE (Torgesen, Wagner, & Rashotte, 1999)]; c) timed-decoding nonword reading (TOWRE PWE, from the TOWRE); d) non-timed word reading [Letter-Word, from the WJ III (Woodcock & Johnson, 1989)]; e) decoding [Word-Attack, from the WJ III]. To be included in the RD group, a z-score of −1 or below (more than one standard deviation below the mean) had to be reached in at least two of the administered reading-measures tests, following (Kovelman et al., 2012). All parents provided informed written consent and children over the age of 11 provided informed written assent prior to the study. Participants received a $15 gift card as compensation for their participation. The study was approved by the appropriate Institutional Review Board.
Study Procedure
After an initial phone screening to verify that the children did not have a neurological or psychiatric condition other than their reading challenges, their parents were invited to bring them to the hospital where several reading and EF measures were administered. Participants were assigned to one of two groups (RD or typical readers) following the administration of the reading measures. Behavioral data collection (reading and EF abilities) was done in a single session that lasted approximately three hours (allowing 1–2 breaks when needed). Following the behavioral-testing session, a 30-minute eye-movement session was conducted.
Behavioral Measures
Reading measures.
The scores for the reading measures listed in the Participants section, which were acquired during the screening session, were also used as the reading measures for the current study.
Executive function measures.
EF were measured using several subtests: a) visual attention abilities [Sky Search; TEA-Ch (Manly, Robertson, Anderson, & Nimmo-Smith, 1999)]; b) error monitoring [BRIEF(Gioia, 2000)]; c) inhibition [Wisconsin Card Sorting Task (Nyhus & Barcelo, 2009)]; d) organization skills [BRIEF (Gioia, 2000)]; e) speed of processing [Coding and Symbol Search; WISC-IV (Wechsler, 1999)]; f) switching and inhibition abilities [Stroop; D-KEFS(Dellis, 2001)]; g) working memory skills [BRIEF (Gioia, 2000)]. All t-test analyses were corrected for multiple comparisons using a Bonferroni correction.
Eye-Movement Task: Sentence Reading
To generate various cognitive-load measures during reading, a total of 80 sentences were presented to the participants: 40 sentences that ‘make sense’ (MS) and 40 sentences that ‘do not make sense’ (N-MS). Only the last word in the sentence generated the meaning of the sentence. This structure posed the semantic challenge: participants had to read the sentences to the end before making a decision. All sentences contained five words, with an equal word frequency, and were fully displayed on the screen (sentences used are available at: https://neuroimaging-center.technion.ac.il/wp-content/uploads/2017/11/Sentences.pdf). For each sentence, the participant was instructed to decide whether the sentence was MS or N-MS by clicking one of two buttons using their index finger. Responses (accuracy and reaction times) were recorded and segmented into correct and incorrect responses. The participants were instructed to respond as quickly and accurately as possible. After pushing the button, a fixation point appeared for 1 second, followed by the next sentence. The participants practiced the task by reading 10 sentences prior to the recorded task and using different stimuli than the test stimuli.
Eye-Movement Tools and Setup
A Tobii 60-Hz infrared-based eye tracker was used to record eye movements throughout the study. The eye tracker was calibrated to each participant’s pupil position before each task using a one-point calibration that moved across the screen, and that the participant had to visually track. The experiment was run in a quiet, dimly lit room. Participants sat in a chair in front of a 15-inch monitor while their eye-tracking data were recorded. The distance from their eyes to the screen was approximately 24 inches. The remote eye-tracking system was positioned below the monitor. Blinks were removed from the data. Fixation duration, number of fixations, and pupil dilation were recorded during the tasks.
Behavioral Data Analysis
To determine the differences in reading and EF measures between children with RD and typical readers, t-test analyses were conducted for each measure.
Eye-Movement Data Analysis
Participant responses were divided into four categories: 1) “make sense” sentences with correct responses (MS correct), 2) “make sense” sentences with incorrect responses (MS incorrect), 3) “not make sense” sentences with correct responses (NMS correct), and 4) “not make sense” sentences with incorrect responses (N-MS incorrect).
To determine whether heavier cognitive-loading conditions (i.e., MS incorrect) would differ from those with lighter cognitive loads (N-MS correct or MS correct), we parceled our results according to the response categories using the performance measures from the task, as well as reading time, response time, and number of responses per category. To compare the behavioral measures and the eye-movement measures between the groups in the cognitive-load conditions, t-test, paired t-test, and repeated measures (RM)-ANOVA analyses were performed for all eye-movement indicators that were acquired (pupil dilation in mm, number of fixations, and fixation duration in msec). A 2 × 2 RM-ANOVA for Group (children with RD; typical readers) and Response (Correct; Error) was conducted separately for reaction times, accuracy, sentence reading time, pupil dilation, and fixation time. To relate the reading and EF scores to the eye-movement measures, a Pearson correlation was performed. All data were corrected for multiple comparisons using a Bonferroni correction.
Correlation Analysis
To determine the association between reading skills and EF, a Pearson correlation analysis was performed. To define the relationships between eye-movement patterns and reading challenges and EF load, correlations between these measures were conducted. All data were corrected for multiple comparisons using a Bonferroni correction.
Results
Behavioral Data
Baseline measures.
No significant differences were found between children with RD and typical readers in nonverbal abilities (RD: M=100, SD=6.442; typical readers: M=102.56, SD=7.248, t=−0.79, p=0.44) or attention abilities (RD: M=10.13, SD= 5.59; typical readers: M=8.6, SD=2.5, t=0.72, p=0.49); see Table 1 for details.
Table 1.
Reading and EF behavioral measures for children with RD and typical readers
| Cognitive Ability | Measure | Children with reading difficulties A | Typical readers B | T (p value) | Contrast |
|---|---|---|---|---|---|
| Reading measures | |||||
| Phonological awareness | Ellison (CTOPP), standard deviation | 8.22(3.6) | 11.9(1.52) | −2.85** | B > A |
| Orthography (word reading timed) | Sight word efficiency (TOWRE, SWE), standard deviation | 82.56(11.8) | 97.7(8.46) | −3.18** | B > A |
| Phonological processing (decoding timed) | Pseudo word efficiency (TOWRE, PDE), standard deviation | 82.22(11.7) | 102.1(7.74) | −4.41*** | B > A |
| Phonological processing (decoding nontimed) | Word Attack (WJ III), standard deviation | 94(6.24) | 105(10.55) | −2.8* | B > A |
| Orthographical abilities (non-timed) | Letter-Word (WJ III), standard deviation | 91.56(7.65) | 102.10(19.20) | −1.60 p=0.13 | B > A |
| Executive Functions | |||||
| Visual attention | Sky Search (TEA-Ch, Time Per Target), standard deviation | 10.13(5.59) | 8.6(2.5) | 0.72 p=0.49 | A > B |
| Initiate | Initiate (Brief PRα), T score | 51.11(10.85) | 44.5(9.4) | 1.41 p=0.18 | A > B |
| Working memory | Working Memory (Brief PRα), T score | 52.67(0.07) | 44.1(7.77) | 2.20* | A > B |
| Error monitoring | Monitor (Brief PRα), T score | 50.67(9.16) | 41.9(9.74) | 2.02 p=0.06 | A > B |
| Perseverative Error (WCST), percentage | 67.75(3.33) | 61.56(4.13) | 3.42** | A > B | |
| Inhibition | Stroop, Color-Word Condition (Time, D- KEFS), standard deviation | 7.11(2.85) | 9.80(1.71) | −2.34* | B > A |
| Organization | Organize (Brief PRα), T score | 56.22(11.33) | 44.1(8.5) | 2.61* | A > B |
| Processing speed | Coding (WISC-IV), standard deviation | 7.33(3.12) | 9.00(2.98) | −1.19 p=0.25 | A < B |
| Symbol Search (WISC- IV), standard deviation | 8.89(2.37) | 10.60(1.78) | −1.77 p=0.10 | A < B | |
| General EF | General EF abilities (Brief PRα), T score | 51.67(7.45) | 42.3(9.06) | 2.47* | A > B |
p<0.05;
p<0.01;
p<0.001
Higher scores are related to a lower ability in the examined domain Group results are expressed as mean (standard deviation)
Executive functions and reading abilities.
Children with RD demonstrated a significantly lower reading ability in all the examined domains compared to typical readers. The RD group also demonstrated significantly lower inhibition, organization, working memory, and general EF skills, and had lower error-monitoring scores (i.e., sub-ability from the Wisconsin task) compared to typical readers; see Table 1 for details.
Behavioral Measures during the Sentence-Reading Task
Sentence-reading time.
No significant differences were demonstrated between children with RD and typical readers in reading times for the four response categories: MS correct, MS incorrect, N-MS correct, N-MS incorrect; see Table 2.
Table 2.
Behavioral and eye measurements during a sentence-reading task for children with reading difficulties and typical readers
| Children with RD | Typical readers | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MS IC | MS C | N-MS IC | N-MS C | MS IC | MS C | N-MS IC | N-MS C | |||
| A | B | C | D | E | F | G | H | |||
| Fixation duration (msec) |
102.4 (12.78) |
131.7 (109.3) |
56.63 (45.11) |
76.2 (40.69) |
65.1 (9.22) |
55.1 (17.43) |
48.59 (10.48) |
62.49 (37.84) |
A > E | 7.36*** |
| A > C | −2.89* | |||||||||
| E > G | −3.05* | |||||||||
| Number of fixations | 1.79 (0.385) |
1.47 (0.31) |
1.25 (0.31) |
1.58 (0.48) |
1.74 (0.436) |
2.08 (0.41) |
2.12 (0.44) |
1.93 (0.19) |
F > B | −3.66** |
| H > D | −2.1 p=0.06 |
|||||||||
| G > C | −4.92*** | |||||||||
| A > C | −3.70** | |||||||||
| G > E | −3.36** | |||||||||
| Pupil dilation (mm) | 4.19 (0.45) |
3.856 (0.58) |
3.78 (0.48) |
3.9 (0.46) |
3.85 (0.52) |
3.95 (0.25) |
3.92 (0.32) |
3.84 (0.44) |
A > C | 3.24* |
| A > D | 2.85* | |||||||||
| A > B | 2.62* | |||||||||
| D > B | −2.626* | |||||||||
| Reading time (msec) | 813.23 (18.58) |
808.47 (3.42) |
807.54 (7.04) |
811.01 (5.46) |
813.55 (8.16) |
809.81 (11.73) |
811.04 (9.12) |
815.96 (15.57) |
||
| Response time (msec) | 801.86 (328.7) |
586.43 (160.7) |
744.64 (172.5) |
655.98 (175.04) |
701.45 (166.49) |
570.21 (101.65) |
611.15 (227.8) |
740.14 (292.03) |
||
| Number of responses (average) | 3.44 (3.20) |
31 (7.3) |
3 (3.9) | 29.11 (7.88) |
8.6 (7.1) | 27.3 (8.2) |
4.7 (2.9) | 22.1 (11.89) |
B > C | 8.842*** |
| B > A | 8.952*** | |||||||||
| D > C | 8.184*** | |||||||||
| D > A | 7.652*** | |||||||||
| F > H | 2.493* | |||||||||
| F > G | 7.361*** | |||||||||
| F > E | 3.96** | |||||||||
| H > G | 4.365** | |||||||||
| E > A | −1.98 p=0.06 |
|||||||||
, p<0.05;
, p<0.01;
, p<0.001
Results are expressed as mean (standard deviation).
RD, reading difficulties; MS, sentences that make sense; IC, incorrect response; C, correct response; N-MS, sentences that do not make sense
Reading accuracy.
The RM-ANOVA revealed a significant main effect of Response, [F(1,19)=69.05, p<0.001, η2=0.802], demonstrating significantly greater accuracy rates compared to erroneous responses. No effect was found for Group; see Table 2.
Response time.
The RM-ANOVA revealed a significant Response × Type interaction ([F(1,19)=6.296, p<0.05, η2=0.276]), suggesting a significantly smaller difference in response time for incorrect and correct responses for the N-MS sentences compared to the MS sentences for both groups. For the four responses categories (MS correct, MS incorrect, N-MS correct, N-MS incorrect), response times were not significantly different in children with RD compared to typical readers (see Table 2).
Eye-Movement Measures During the Sentence-Reading Tasks
Pupil dilation.
RM-ANOVA revealed a significant main effect of Type [F(1,19)=4.917, p<0.05, η2=0.224], suggesting a greater pupil dilation for the MS vs N-MS sentences. A significant Group × Type × Response interaction ([F(1,19)=6.14, p<0.05, η2=0.265]) suggests that pupil dilation for the children with RD was significantly larger for correct vs. incorrect responses for the MS vs N-MS sentences, which overall were larger than for typical readers. Paired t-test results showed that children with RD had significantly larger pupil dilation during correct vs. incorrect responses for the MS sentences compared to pupil dilation during correct vs. incorrect responses for the N-MS sentences. Typical readers had no significant differences in pupil dilation when comparing the four responses categories (MS correct, MS incorrect, N-MS correct, N-MS incorrect) for this group; see Table 2 for details.
Number of fixations.
RM-ANOVA revealed Group × Type [F(1,19)=5.227, p<0.05, η2=0.235] and Group × Type × Response [F(1,19)=17.5, p<0.001, η2=0.50] interactions. Results suggest an overall lower number of fixations for children with RD and more fixations for MS sentences read incorrectly than when reading N-MS sentences incorrectly. Typical readers, however, exhibited the opposite pattern: a significantly higher number of fixations when reading N-MS sentences incorrectly than when reading MS sentences incorrectly (Table 2).
Fixation duration.
A significant main effect of Type was found [F(1,19)=0.835 p<0.05, η2=0.344], suggesting longer fixation durations for MS vs N-MS sentences. A significant Group × Type interaction was also found [F(1,17)=8.505, p<0.05, η2=0.333], suggesting that children with RD had significantly longer fixations for MS vs. N-MS sentences, while typical readers exhibited the opposite pattern. A t-test analysis revealed significantly longer fixation durations for children with RD compared to typical readers for incorrectly answered MS sentences; see Table 2 and Figure 1 for these results.
Figure 1. Duration of fixations in children with reading difficulties (RD) and in typical readers (TR) during a sentence-reading task.
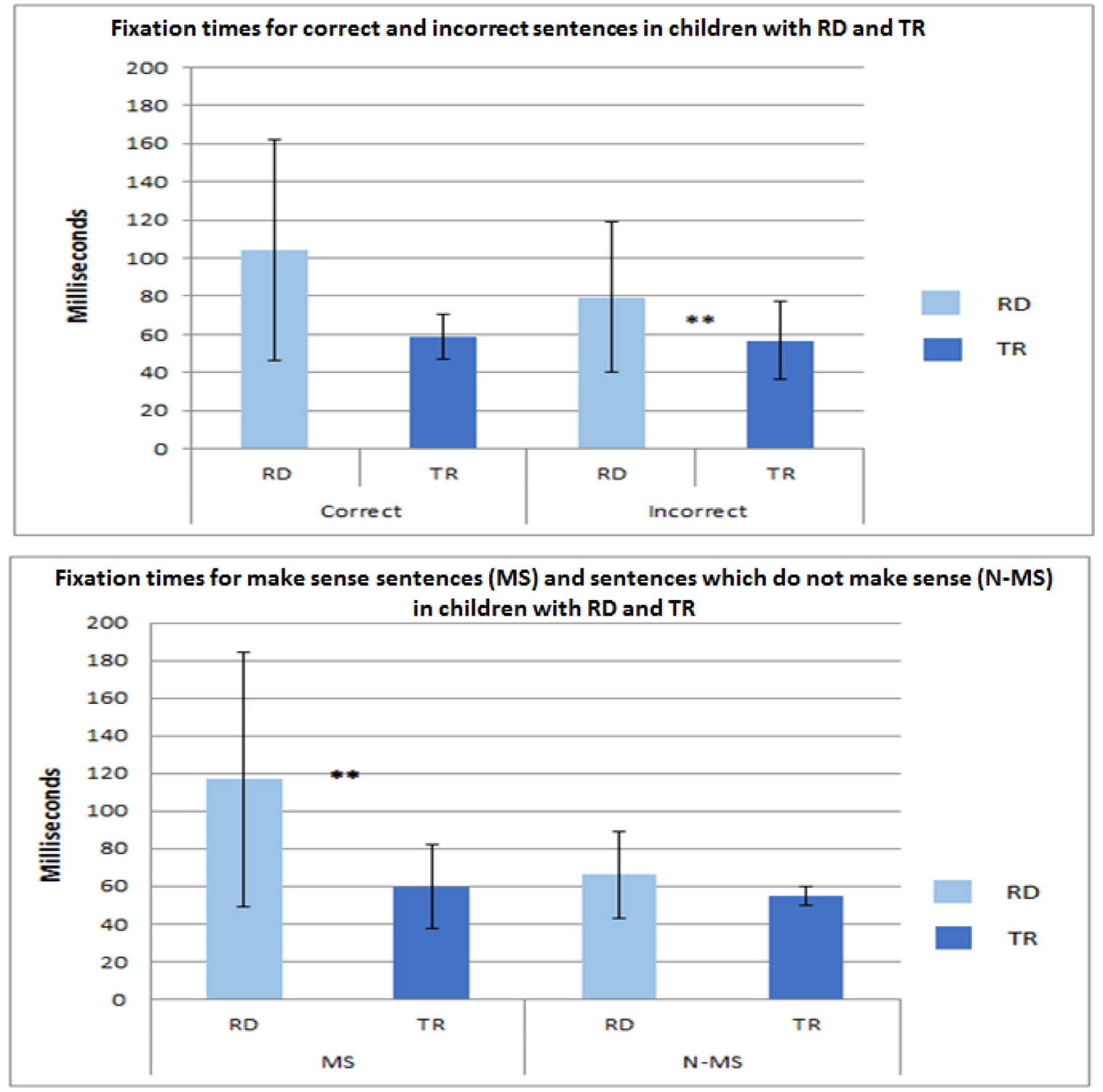
Fixation times for correct and Incorrect sentences (upper graph) and sentences that make sense (MS) and sentences that do not make sense (N-MS, lower graph). The Y axes represent duration of the fixations in milliseconds. *, p<0.01
Correlation Between Reading Skills and Executive Functions
Pearson correlation analysis revealed no significant correlation between EF and reading ability in children with RD. Typical readers exhibited positive correlation between reading (TOWRE, SWE, scaled score) and inhibition abilities (Stroop, scaled score), (r=0.704, p<0.05).
Correlation Between Eye-Movement Patterns and Reading, and Executive Functions Measures
Pupil dilation.
In children with RD, pupil dilation during correct responses for both MS and N-MS sentences was positively correlated with phonological processing (as measured by the CTOPP, Ellison scaled score: r=0.731, p<0.05 and r=0.709, p<0.05, respectively). Typical readers showed a positive correlation between pupil dilation and phonological awareness abilities only during incorrectly read N-MS sentences (r=0.665, p<0.05).
Number of fixations.
Negative correlation was found between the number of fixations during MS-sentence reading and accuracy rate for the error-monitoring subtest from the Wisconsin task [Perseverative Error Percent, (r=−0.724, p<0.05)]. Results suggest that the number of fixations decreased as the error-monitoring scores increased. In addition, children with RD showed negative correlation between the number of fixations when reading N-MS sentences incorrectly and organization skills [Brief PR, Organization of Materials (r=−0.718, p<0.05)]; more fixations were correlated with better organizational skills.
Discussion
The aim of the current study was to determine eye-movement patterns related to an increased cognitive load during reading in children with RD compared to typical readers, so as to exploit online cognitive load detection to assist in adapting reading materials for children. In support of our hypothesis, children with RD demonstrated greater pupil dilation when reading sentences with a greater semantic load, i.e., sentences that make sense and were answered correctly vs. sentences that were not answered correctly. Per our hypothesis, children with RD showed a positive correlation between phonological awareness abilities and pupil dilation. Contradictory to our hypothesis, however, no significant correlation was observed between pupil dilation and EF scores.
Eye Movement as a Reflection of Reading Challenges
Pupil dilation, number of fixations, and fixation duration have previously been shown to differ between individuals with RD, both in college (Kim, 2016; Zhan et al., 2016) and childhood (Nilsson Benfatto et al., 2016). These studies suggested that individuals with RD share specific eye-movement characteristics related to these measures, i.e., greater pupil dilation and more and longer fixations, compared to typical readers. In the current study, we have demonstrated that pupil dilation reflects the differences between children with RD and typical readers in reading tasks varying in cognitive-load demands, such as for sentences that do or do not make sense. Children with RD showed significant differences in pupil dilation patterns compared to typical readers. Inaccurate reading of sentences that make sense generated significantly larger pupil dilation in children with RD than did accurate reading of sentences that make sense, while typical readers did not demonstrate this pattern. In addition, inaccurate reading of sentences that make sense generated significantly larger pupil dilation in children with RD than inaccurate reading of sentences that do not make sense, while typical readers exhibited the opposite pattern. In line with previous findings, we found that the sentences that make sense were more challenging to process than the sentences that do not make sense, even among typical readers (Rimrodt et al., 2009).
Rimrodt and colleagues also demonstrated increased activation of frontal regions in children with RD when reading meaningful vs. non-meaningful sentences compared to typical readers, which was related to the effort to tease out the meaning of the sentence. Our results add an additional component to the story by providing evidence of pupil dilation specifically among children with RD when reading meaningful sentences. This finding also adds to the Laberge and Samuels model (LaBerge & Samuels, 1974), when reading is not automatic as in the case of those with RD, the attention resources are limited by the non-automatic reading and, therefore, a heavier load is allocated toward comprehension.
Number of fixations provided a sensitive indicator of children with RD using the four proposed response categories (MS correct, MS incorrect, N-MS correct, N-MS incorrect). The RM-ANOVA results revealed a significantly different pattern for children with RD compared to typical readers. Additionally, a significant difference for fixation duration in children with RD compared to typical readers was observed for inaccurate reading of sentences that make sense. These results suggest that inaccurate reading for this condition is related to fixation duration in children with RD. This is an extension of the proposed connection between number of fixations and pupil dilation (Mathot, 2015). Specifically, our results indicated that when children with RD read sentences that make sense, their fixation duration for inaccurate responses increased. Similar to the results regarding increased pupil dilation during the challenging reading condition in children with RD, there were more fixations when reading incorrectly the sentences that make sense than when reading incorrectly the sentences that do not make sense. Typical readers, however, exhibited the opposite pattern with a significantly higher number of fixations when reading incorrectly the sentences that do not make sense than when reading incorrectly the sentences that make sense. This suggests that these measures that were observed in the four reading conditions (MS correct, MS incorrect, N-MS correct, N-MS incorrect) may be part of the pathology of RD, i.e., specific for individuals with RD, and not generalizable to the healthy population. An additional study with a larger number of participants could further substantiate this point.
Assessing the Level of Cognitive Load in Reading Difficulties
In support of our original hypothesis, we found that better reading ability, reflected by better phonologic awareness test scores, correlated with pupil dilation in children with RD when reading accurately. Based on the findings that pupil dilation correlates with cognitive load in both adults (Scharinger et al., 2015; Wahn et al., 2016) and children (Karatekin et al., 2007), previous studies suggested that pupil dilation reflects the magnitude of cognitive load. The current study supports this point by showing that children with RD who were able to better recruit their EF abilities when reading managed to achieve better phonological awareness for better reading. Therefore, better phonological awareness abilities and thus better reading abilities are related to increased cognitive load for children with RD when experiencing accurate reading and can be measured by pupil dilation.
These results suggest that the set of proposed sentences that generates accurate reading could be used as an indicator of the level of cognitive load during reading within the population of children with RD. Future research with more participants is required to determine whether the eye-movement patterns can be used as a tool for assessing the general cognitive load in this group of readers. To accomplish this, more data is required for eye-movement patterns in non-linguistic tasks as well. Since impairment in phonological processing is a primary cause of RD (Vellutino, Fletcher, Snowling, & Scanlon, 2004), phonological awareness measured early in childhood is a predictor of future reading performance (Torgesen, Wagner, & Rashotte, 2012). It is, therefore, encouraging that phonological processing abilities were found to be related to specific eye-movement patterns in children with RD since, potentially, eye tracking could serve as an effective, low-cost, and fast early screener for RD that detects effort in utilizing phonological awareness. An additional larger-scale study should also use non-reading tasks for this purpose to assess this in younger children.
There have been a limited number of studies investigating cognitive load and its relationship with pupil dilation in children (Eckstein, Guerra-Carrillo, Miller Singley, & Bunge, 2017). A better understanding of this relationship requires additional research of the association between reading-task difficulties, reading-task experience, and reading-task engagement, which may lead to a way to change the cognitive load.
Study Limitations
Interpretation of the current study results should take into account the following limitations and recommendations. This study was conducted with a small sample, and results from a larger study population would likely give our results greater weight, especially for the correlation analysis. Our results did not show a correlation between EF skills and pupil dilation for children with RD; however, conducting the same study with a larger sample might yield more sensitive results. Inaccurate reading of meaningful text was shown to generate significantly greater pupil dilation for children with RD compared to typical readers. Only a relatively small number of sentences that make sense, however, generated incorrect answers. Therefore, to further corroborate our results, future research with more challenging sentences, more participants, and use of regression measurements is required.
Conclusions
The results of the current study provide physiological support for the cognitive load shared among children with RD when reading sentences. Current best practices for children with RD entail adaptation/leveling based on criterion-based mastery of content that is linked to reading development. Our results provide a basis for generation of a mechanism for online monitoring of cognitive load during reading, even within the current best practices programs. Such a tool has the potential to monitor and adapt the written intervention materials for children with RD. Additional larger-scale studies should address this possibility.
Acknowledgements
The study was carried out in the Pediatric Neuroimaging Research Consortium (PNRC) at the Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA. The study was supported by the Educational Neuroimaging Center, Faculty of Education in Science and Technology, Technion, Haifa, Israel. The authors thank J. Denise Wetzel for review and editing of the manuscript.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest: The authors declare that they have no conflict of interest.
References
- Aston-Jones G, & Cohen JD (2005). An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu Rev Neurosci, 28, 403–450. [DOI] [PubMed] [Google Scholar]
- Booth JN, Boyle JM, & Kelly SW (2014). The relationship between inhibition and working memory in predicting children’s reading difficulties. Journal of Research in Reading, 37(1), 84–101. [Google Scholar]
- Breznitz Z (2006). Fluency in reading: Synchronization of processes: Routledge. [Google Scholar]
- Brown L, Sherbenou R, & Johnsen S (1997). Test of nonverbal intelligence (3rd Ed.). Austin, TX: Pro-Ed. [Google Scholar]
- Conners CK (1989). Conners rating scales’ manual. North Towonada NY: Multihealth System. [Google Scholar]
- Dellis DC, Kaplan E, & Kramer JH (2001). Delis-Kaplan Executive Function System. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Eckstein MK, Guerra-Carrillo B, Miller Singley AT, & Bunge SA (2017). Beyond eye gaze: What else can eyetracking reveal about cognition and cognitive development? Dev Cogn Neurosci, 25, 69–91. doi: 10.1016/j.dcn.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote SL, & Morrison JH (1987). Extrathalamic modulation of cortical function. Annu Rev Neurosci, 10(67–95). [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L (2000). Behavior Rating Inventory of Executive Function Child Neuropsychology, 6(3). doi: 10.1076/chin.6.3.235.3152 [DOI] [PubMed] [Google Scholar]
- Gooch D, Thompson P, Nash HM, Snowling MJ, & Hulme C (2016). The development of executive function and language skills in the early school years. J Child Psychol Psychiatry, 57(2), 180–187. doi: 10.1111/jcpp.12458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz-Kraus T, Holland SK, & Freund LS (2016). Imaging executive functions in typically and atypically developed children. [Google Scholar]
- Horowitz-Kraus T, Vannest JJ, Gozdas E, & Holland SK (2014). Greater Utilization of Neural-Circuits Related to Executive Functions is Associated with Better Reading: A Longitudinal fMRI Study Using the Verb Generation Task. Frontiers in Human Neuroscience, 8, 447. doi: 10.3389/fnhum.2014.00447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz-Kraus T (2016). The Role of Executive Functions in the Reading Process In Khateb A & Bar-Kochva I (Eds.), Reading Fluency: Current Insights from Neurocognitive Research and Intervention Studies (pp. 51–63). Cham: Springer International Publishing. [Google Scholar]
- Horowitz-Kraus T, & Hutton JS (2015a). From emergent literacy to reading: how learning to read changes a child’s brain. Acta Paediatr, 104(7), 648–656. doi: 10.1111/apa.13018 [DOI] [PubMed] [Google Scholar]
- Horowitz-Kraus T, & Breznitz Z (2011). Error Detection Mechanism for Words and Sentences: A comparison between readers with dyslexia and skilled readers. International Journal of Disability, Development and Education, 58(1), 33–45. doi: 10.1080/1034912X.2011.548466 [DOI] [Google Scholar]
- Karatekin C, Marcus DJ, & Couperus JW (2007). Regulation of cognitive resources during sustained attention and working memory in 10-year-olds and adults. Psychophysiology, 44(1), 128–144. doi: 10.1111/j.1469-8986.2006.00477.x [DOI] [PubMed] [Google Scholar]
- Kim S (2016). Simple Sentence Reading and Specific Cognitive Functions in College Students with Dyslexia: An Eye-tracking Study (Vol. 1). [Google Scholar]
- Kovelman I, Norton ES, Christodoulou JA, Gaab N, Lieberman DA, Triantafyllou C, … Gabrieli JD. (2012). Brain basis of phonological awareness for spoken language in children and its disruption in dyslexia. Cereb Cortex, 22(4), 754–764. doi: 10.1093/cercor/bhr094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBerge D, & Samuels SJ (1974). Toward a theory of automatic information processing in reading. Cognitive Psychology, 6, 293–323. [Google Scholar]
- Lyon GR, Shaywitz SE, & Shaywitz BA (2003). A definition of dyslexia. Ann Dyslexia, 53(1), 1–14. doi: 10.1007/s11881-003-0001-9 [DOI] [Google Scholar]
- Manly T, Robertson IH, Anderson V, & Nimmo-Smith I (1999). TEA-Ch: The Test of Everyday Attention for Children Manual. Bury St. Edmunds, UK: Thames Valley Test Company Limited. [Google Scholar]
- Mathot S, Siebold A, Donk M, & Vitu F (2015). Large Pupils Predict Goal-Driven Eye Movements. J Exp Psychol Gen, 144(3), 513–521. [DOI] [PubMed] [Google Scholar]
- Menghini D, Finzi A, Benassi M, Bolzani R, Facoetti A, Giovagnoli S, Vicari S (2010). Different underlying neurocognitive deficits in developmental dyslexia: a comparative study. Neuropsychologia, 48(4), 863–872. doi: 10.1016/j.neuropsychologia.2009.11.003 [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, De Geus EJ, & Aston-Jones G (2011). The anatomical and functional relationship between the P3 and autonomic components of the orienting response. . Psychophysiology, 48, 162–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson Benfatto M, Öqvist Seimyr G, Ygge J, Pansell T, Rydberg A, & Jacobson C (2016). Screening for Dyslexia Using Eye Tracking during Reading. PLoS One, 11(12), e0165508. doi: 10.1371/journal.pone.0165508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyhus E, & Barcelo F (2009). The Wisconsin Card Sorting Test and the cognitive assessment of prefrontal executive functions: a critical update. Brain Cogn, 71(3), 437–451. doi: 10.1016/j.bandc.2009.03.005 [DOI] [PubMed] [Google Scholar]
- Rayner K (1978). Eye movements in reading and information processing. Psychol Bull, 85(3), 618–660. [PubMed] [Google Scholar]
- Rimrodt SL, Clements-Stephens AM, Pugh KR, Courtney SM, Gaur P, Pekar JJ, & Cutting LE (2009). Functional MRI of sentence comprehension in children with dyslexia: beyond word recognition. Cereb Cortex, 19(2), 402–413. doi: 10.1093/cercor/bhn092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ (2009). The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci, 10(3), 211–223. doi: 10.1038/nrn2573 [DOI] [PubMed] [Google Scholar]
- Scharinger C, Kammerer Y, & Gerjets P (2015). Pupil Dilation and EEG Alpha Frequency Band Power Reveal Load on Executive Functions for Link-Selection Processes during Text Reading. PLoS One, 10(6), e0130608. doi: 10.1371/journal.pone.0130608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Spark JH, Henry LA, Messer DJ, Edvardsdottir E, & Ziecik AP (2016). Executive functions in adults with developmental dyslexia. Res Dev Disabil, 53–54, 323–341. doi: 10.1016/j.ridd.2016.03.001 [DOI] [PubMed] [Google Scholar]
- Torgesen JK, Wagner RK, & Rashotte CA (1999). Test of word-reading efficiency (TOWRE). In. Austin, TX: Pro-Ed. [Google Scholar]
- Torgesen JK, Wagner RK, & Rashotte CA (2012). Test of Word Reading Efficiency–Second Edition (TOWRE-2) Austin, TX: Pro-Ed. [Google Scholar]
- Van der Wel P, & van Steenbergen H (2018). Pupil dilation as an index of effort in cognitive control tasks: A review. Psychonomic Bulletin & Review, 25, 2005–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellutino FR, Fletcher JM, Snowling MJ, & Scanlon DM (2004). Specific reading disability (dyslexia): what have we learned in the past four decades? J Child Psychol Psychiatry, 45(1), 2–40. [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK, & Rashotte CA (1999). Comprehensive test of phonological processing (CTOPP). In. Austin, TX: Pro-Ed. [Google Scholar]
- Wahn B, Ferris DP, Hairston WD, & Konig P (2016). Pupil Sizes Scale with Attentional Load and Task Experience in a Multiple Object Tracking Task. PLoS One, 11(12), e0168087. doi: 10.1371/journal.pone.0168087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1999). Wechsler Intelligence Scale for Children (WISC-III) (Third Edition ed.). New York: The Psychological Corporation. [Google Scholar]
- Woodcock RW, & Johnson MB (1989). Woodcock-Johnson Psycho-Educational Battery—Revised (WJ-R). In. Allen, TX: Developmental Learning Materials. [Google Scholar]
- Zhan Z, Zhang L, Mei H, & Fong PS (2016). Online Learners’ Reading Ability Detection Based on Eye-Tracking Sensors. Sensors (Basel), 16(9). doi: 10.3390/s16091457 [DOI] [PMC free article] [PubMed] [Google Scholar]


