Abstract

To recover potassium from feldspar, a biowaste, i.e., eggshell, was used. The chief composition of eggshells is calcite. As it is a rich source of Ca, hence it is used with HCl to produce calcium chloride. Feldspar is an aluminosilicate mineral that bears potassium in the interstitial sites. To unlock the potassium from the interstitial sites, it was roasted with calcium chloride prepared by mixing eggshell and hydrochloric acid. At the roasting temperature, CaCl2 melts and penetrates into the aluminosilicate matrix to replace K with Ca. Potassium ion released from the silicate matrix combines with chloride ions to form potassium chloride, which solubilized in water during the leaching process of the roasted feldspar. For elucidation of the mechanism of the roasting process, the shrinking core model was applied to the roast–leach data, and diffusion through the product layer was inferred as the rate-determining step. The order of the roasting process was found to be 2.158 and activation energy calculated to be 155.3 kJ/mol. Apart from potassium, sodium and excess calcium also got co-leached. To recover potassium from the leach liquor selectively, sodium perchlorate was added to precipitate potassium as KClO4. Further, potassium perchlorate was thermally decomposed to give fertilizer grade potassium chloride (purity: 99.81%).
1. Introduction
As the population is increasing rapidly so is the demand for food. The improvement in the agricultural yields depends on the progress of fertilizers. Potassium is the most essential micronutrient for agricultural sector in combination with nitrogen and phosphorus. It increases productivity and enhances resistive power in plants toward adverse effect of pests and environment.1 Human beings and animals acquire this element from plants, which in turn acquire it from soil. Soil should be fortified with potassium as a source to all members of the food chain.
In the present scenario, nearly 90% of the potassium products are used as fertilizers by the agricultural sector. Most of the potassium salts are commonly mined in the northern hemisphere due to the availability of soluble potash ores like sylvinite, which is a mixture of sylvite and halite.2,3 Canada tops the chart of both the world potash reserve with 26% of total reserve and world leading producer with 28% of total production followed by Russia, Belarus, Germany, Brazil, China, the USA, Israel, and Chile.4 India is an agriculture-based nation, and it fulfils all its potash requirement through imports. India’s entire requirement of potash fertilizer is met through imports because it is deprived of potassium-rich minerals. Potassium-rich minerals are not available in India; however, K-bearing silicates like feldspar, glauconitic sandstone, nepheline syenite, and so forth are available in abundance. The Indian mineral yearbook has reported that around 634 million tons of feldspar is distributed in various districts in Rajasthan, Andhra Pradesh, and Karur district of Tamil Nadu. It also reports that major parts of glauconitic sandstone are present in Rajasthan followed by Madhya Pradesh and Uttar Pradesh. Jena et al.5 have reported that nepheline syenite is available in the eastern part of India. In order to reduce potash imports and dependency on other nations, we need to explore these K-bearing silicates as a potential source of meeting potash requirements of India.
As stated above, feldspar is the most abundant mineral and comprises more than half of the earth’s crust. India has plenty resources of feldspar, so it could contribute to the attempt for indigenous production of potash fertilizer.
Feldspar possesses a silicon–oxygen–aluminum tetrahedron in which potassium is placed in the interstitial sites to balance the charge of the matrix.6,7 Other potassium-containing aluminosilicate minerals are nepheline syenite, glauconitic sandstone, mica, and so forth.4,8 Jena et al. used acid for leaching potassium from the aluminosilicate matrix, and the recovery was nearly 20%.5 Shekhar et al. lixiviated an aluminosilicate mineral with acid to extract potassium, and the recovery was very poor.9 Base leaching of feldspar resulted in production of slow releasing potassium compounds.2,10 In order to overcome low and slow release of potassium from acid and base leaching, respectively, researchers employed salt roasting of the aluminosilicate matrix followed by water leaching of the roasted product. Zhang et al. have extracted more than 80% potassium from roasting feldspar with calcium chloride and calcium carbonate followed by water leaching.11 Shekhar et al. have reported 98% recovery of potassium from glauconitic sandstone using sulfation roasting followed by water leaching.12 At 900 °C, the extraction of potassium is about 93% w/w. Jena et al. have recovered up to 90% potassium from nepheline syenite by utilizing microwave-assisted chloridizing roasting.13 Output of the literature discussed above clearly suggests that salt roasting accompanied by water leaching proves to be the best method for recovery of potassium from aluminosilicate minerals.
Most of the literature that has adopted the roasting–leaching process has suggested calcium chloride as the best roasting agent on the basis of potassium recovery.5,14−17 In all these studies, laboratory-grade calcium chloride was used.
Roasting agents play a crucial role in production of soluble potassium salts from the silicate ores. The cost of potassium fertilizers is directly affected by the cost of these reagents. We have tried to use a roasting agent cheaper than all the roasting agents discussed above. Using a cheaper agent will reduce the production cost of potassium fertilizers, which in turn benefits the farmers and people of an agriculture-based nation. For this purpose, we worked on synthesizing calcium chloride from a no-cost source of calcium. We used a biowaste, i.e., eggshell, as a no-cost precursor for synthesis of calcium chloride. India ranks third in the production of eggs after China and the USA. After the preparation of various products from eggs, eggshells are simply dumped. Some researchers have explored the adsorption properties of the eggshells.18,19 Wei et al. have calcined the eggshell to utilize it as a catalyst for biodiesel production.20 In order to remove heavy metals like Cd2+, Pb2+, and Cu2+ from aqueous solutions, eggshell is used as an efficient and cheaper adsorbent.21 Rivera et al. have used eggshells for the synthesis of hydroxyapatite in phosphate solution at an elevated temperature, which was utilized in biomedical application.22
In this present article, we have used eggshells as a constituent of the roasting agent for extracting potassium from feldspar. The novelty of our work is the application of a no-cost fluxing agent in production of potash. Apart from the cost, the process suggested in our work offers the following advantages:
-
(1)
The production of CaCl2 in our process is in situ, whereas the conventional process needs a separate production step.
-
(2)
The conventional process for CaCl2 production, i.e., the Solvay process, produces Na2CO3 along with CaCl2. Production of pure CaCl2 requires separation of CaCl2 from Na2CO3, whereas our process produces CaCl2 without employing any separation process as the byproduct of our process, i.e., CO2, evaporates during in situ production, and H2O helps in proper mixing of feldspar and calcium chloride.
Various roasting parameters were optimized, and kinetic studies have also been carried out to determine the activation energy, order, and the rate-determining stage of the process. To precipitate potassium from the leach liquor, which was generated by lixiviating the roasted feldspar with water, sodium perchlorate was used. The obtained precipitate on thermal decomposition gives pure potassium chloride.
2. Results and Discussions
2.1. Characterizations
The detailed chemical analysis of ore samples (as their respective oxide) is reported in Table 1, which was obtained through various conventional and instrumental methods like ICP-OES, atomic absorption spectrometry, and flame photometry. The feldspar (FS) sample constitutes 11.6% K2O as our target metal along with 1.43% Na2O, 14.79% Al2O3, and 67.19% SiO2 as the major components.
Table 1. Composition of Feldspar.
| compound | SiO2 | Al2O3 | Na2O | K2O | Fe2O3 | CaO | MgO | others |
|---|---|---|---|---|---|---|---|---|
| wt %a | 67.19 | 14.79 | 1.43 | 11.6 | 0.51 | 1.84 | 0.71 | 1.89 |
wt %: weight percentage of elements in terms of their oxides
The mineral phases of the sample are presented in Figure 1. The diffraction pattern shows the peaks, which match well with microcline (KAlSi3O8), orthoclase (KAlSi3O8), and quartz (SiO2). Microcline has the monoclinic crystal lattice, whereas the orthoclase phase has the triclinic crystal lattice, and these two are the potassium-bearing phases. From the XRD image, the major peaks are found to be microcline, which is the chief contributor to the potassium. Peaks for other elements like sodium, iron, calcium, and magnesium are not observed as their content is too little to be detected by the instrument.
Figure 1.
XRD image of feldspar.
The preliminary composition analysis through elemental mapping of FS is presented in Figure 2. The mapping of Si, Al, O, and K reveals the fact that K is locked inside the interstitial spaces of the aluminosilicate framework. This framework is very stable and hard to break through a simple leaching process using any acids or bases. Hence, release of potassium is only possible through breaking of the silicate matrix.
Figure 2.
Elemental mapping of feldspar.
To break this matrix various methods were employed from which chloridizing roasting was proved to be an efficient method. It helps in breaking the aluminosilicate network to release the maximum amount of potassium, which was recovered through water leaching.
The complete elemental analysis of eggshell powder (ESP) is provided in Table 2 from which it was confirmed that the calcium is present as the major element.
Table 2. Composition of Eggshell.
| compound | CaO | MgO | Al2O3 | SiO2 | K2O | P2O5 | ZnO | LOI |
|---|---|---|---|---|---|---|---|---|
| wt %a | 52.97 | 0.884 | 0.206 | 0.353 | 0.12 | 0.522 | 0.69 | 44.25 |
wt %: weight percentage of elements in terms of their oxides
From X-ray diffraction studies, it was found that almost all the peaks entirely match with the calcite phase as shown in Figure 3. Any other phases were not observed, which refers to the minor concentration of respective elements. A higher percentage of loss of ignition refers to decomposition of calcium carbonate to give off carbon dioxide. On the basis of data obtained from XRD and wet chemical analysis, it was found that eggshells, which were used in the present study, comprise nearly 95% calcium carbonate.
Figure 3.
XRD pattern of eggshell.
2.2. Extraction Studies
Various roasting parameters like particle size of feldspar, roasting agent doses, roasting temperature, roasting time, and so forth, were varied to study their effect on recovery of potassium from FS. As in our previously reported articles,23,24 calcium chloride was selected as the best roasting agent after taking various inorganic salts as roasting agents for nepheline syenite and feldspar. Therefore, eggshell powder was utilized together with hydrochloric acid to extract potassium from feldspar.
It was found that for complete conversion of calcium carbonate to calcium chloride, hydrochloric acid consumption was 1.5 times the stoichiometric requirement. Thereafter, in each experiment, HCl was added accordingly.
2.2.1. Mode of CaCl2 Preparation
Analysis of leach liquor generated by lixiviating roasted FS samples with water revealed that recovery of potassium with in situ-prepared CaCl2 is greater in comparison to ex situ-prepared calcium chloride (Figure 4). In situ preparation of CaCl2 resulted in higher potassium because the excess HCl leached K present in the non-interstitial sites of FS.
Figure 4.

Effect of ex situ and in situ preparation of CaCl2 on K recovery.
2.2.2. Particle Size Effect on Extraction
Increase in the recovery of potassium was observed on reduction of the particle size, which is presented in Figure 5. It clearly indicates that on reducing the particle size of the sample, the surface area increases, which enhances the interaction between feldspar and the additive, resulting in the hike in extraction of potassium. It was also inferred that on varying the size of particles between 45 and 25 μm, there is a minimal change in extraction efficiency; hence, for further studies, a particle size of 45 μ was taken.
Figure 5.

Particle size effect on K recovery.
2.2.3. Effect of ESP Dose
In order to study the influence of reagent dosage, the ESP dose was varied from 4 to 18 g for each 10 g of FS along with time from 10 to 40 min as depicted in Figure 6. A roasting temperature of 900 °C was maintained for these experiments. All the leaching experiments were carried out in a water medium. From the results, it was found that on increasing the amount of the reagent, potassium recovery increases, and when the ESP dose reaches 18 g for 10 g of FS, extraction of potassium from FS was completely done within 30 min. Hence, there is no need to increase the dosage and time further.
Figure 6.
Variation of K recovery with ESP dose (g per 10 g of FS) and time; temperature: 900 °C.
2.2.4. Temperature Effect
A positive effect was observed for both the temperature and time on variation of the roasting temperature from 600 to 900 °C for a time period of 10 to 40 min. The melting point of calcium chloride is 772 °C; hence, calcium chloride formed from the reaction of ESP and HCl melted at this temperature and started penetrating into the silicate matrix. Further increase in temperature accelerated the conversion of the stable microcline and orthoclase phases into the water-soluble sylvite phase and insoluble phases, such as anorthite, quartz, and wollastonite. Around 99% of potassium was recovered from the 18 g dose of ESP taken for each 10 g of FS roasted at 900 °C for 30 min as shown in Figure 7. Further increase in the temperature had a negligible effect on recovery of potassium. Therefore, the roasting temperature was fixed at 900 °C in all other roasting experiments.
Figure 7.
Effect of the temperature on recovery of potassium at an ESP dose of 18 g per 10 g of feldspar.
2.2.5. Extraction Mechanism
The mechanism of the roasting process could be explained on the basis of characteristic phases of FS obtained after roasting and leaching as compared to the phase of the raw FS ore. From eq 1, it is evident that CaCO3 that constitutes the ESP reacts with HCl to form CaCl2, which breaks the silicate matrix efficiently by the thermochemical conversion during the roasting process. XRD patterns (Figure 8) obtained for the roasted FS sample indicate that microcline (KAlSi3O8), orthoclase (KAlSi3O8), and quartz (SiO2) phases present in the raw FS have been converted into sylvite (KCl), quartz (SiO2), wollastonite (CaSiO3), and anorthite (CaAl2Si2O8) on roasting.15 The potassium present in orthoclase and microcline phases of FS reacts with the calcium chloride to produce potassium chloride as it has the minimum standard free energy change for the formation as compared to other metal chlorides.25 Molten salt additives are considered as a better fluxing agent for the materials comprising silicate ores.26 The probable reaction during roasting of feldspar with calcium chloride is as follows
| 1 |
Figure 8.
XRD pattern of roasted feldspar and leached residue.
The XRD analysis of the water-leached residue (Figure 8) of roasted NS proves that sylvite (KCl) has completely leached in the water medium, whereas the water insoluble phases of anorthite (CaAl2Si2O8), quartz (SiO2), and wollastonite (CaSiO3) remain in the residue.
2.3. Leaching Studies
All the leaching experiments were carried out in double-distilled water. Various leaching parameters, such as leaching time, temperature, and rpm (revolution per minute) of the digital stirrer, were studied for the roasted product obtained at the optimum roasting conditions. It was found that variation of different leaching parameters had a nominal effect on the extraction of potassium. It is inferred that formation of the sylvite phase in the roasting process is highly soluble in water, resulting in immediate leaching.
At the optimum roasting conditions of the 18 g dose of ESP taken for each 10 g of FS roasted at 900 °C for 30 min followed by water leaching, there is 99% of potassium recovery. With the similar conditions, the scale was increased to 100 g of feldspar and similar recovery was found, resulting in leach liquor comprising 18.3 g/L potassium chloride. This solution was further taken for precipitation studies.
2.4. Kinetic Study
The roasting process between feldspar and calcium chloride prepared from ESP and HCl is a fluid–solid reaction. At the eutectic point of the temperature, calcium chloride melts and acts as the fluid and feldspar as the solid. Particles of potash feldspar were assumed to be spherical in shape, and shrinking core model equations are utilized to analyze the rate-controlling step.27,28 In the roasting reaction system, steps occurring in succession are as follows: (1) molten calcium chloride diffuses through the film or diffusion layer to reach the particle surface of feldspar, (2) molten calcium chloride diffuses across the product layer as a form of internal diffusion, and (3) a chemical reaction takes place between molten calcium chloride and the unreacted core of solid potash feldspar. Each step mentioned above offers a resistance to the reaction. The step that provides maximum resistance becomes the slowest step, which determines the rate of the roasting process.
On the basis of the shrinking core model, the reaction is assumed to proceed on the external surface of the solid sample, and this surface starts shrinking further to the center of the solid as the reaction proceeds, resulting in an inert solid layer, called “ash layer” or “product layer”, around the core of the reacted solid.
When diffusion of calcium chloride through the film or diffusion layer becomes the rate-controlling step, the integrated form of the rate equation can be expressed as
| 2 |
When diffusion of calcium chloride across the product layer becomes the rate-controlling step, the following integrated rate equation can be used to express the roasting kinetics
| 3 |
When the chemical reaction occurring between the molten calcium chloride and unreacted core becomes the rate-controlling step, the subsequent expression describes the kinetics of the process
| 4 |
In all the above equations, the variables are as follows:
X = extraction percentage of potassium.
kf = rate constant of diffusion through film.
kd = rate constant of diffusion through product layer.
kr = rate constant of chemical reaction.
t = duration of reaction.
Various experimental conversion data based on shrinking core models were put into a graph against time, and regression analysis was done to ascertain the rate-determining stage of NS roasting. The R-squared value of various expressions for variation of the ESP dose has been produced. From Figure 9, it can be observed that diffusion through the product layer expression 1 – 3(1 – X)2/3 + 2(1 – X) is providing the best fit to the roasting data. From this regression analysis, we can ascertain that the rate-limiting stage of NS roasting follows diffusion through the product layer model.
Figure 9.
(a) Fitting of roast–leach data to the product layer diffusion model for various ESP doses. (b) Order of roasting of feldspar with various ESP doses; temperature: 900 °C.
The values of the reaction constant (k) for various ESP doses was obtained by drawing a slope for the linear trend lines for points plotted by taking the time as x coordinates and the respective value of 1 – 3(1 – X)2/3 + 2(1 – X) as y coordinates. To obtain the order of the roasting process for FS with ESP, the slope of the linear trend line was drawn for points plotted by taking log(ESP dose) as x coordinates and log(k) as y coordinates.29,30 The order of roasting FS with ESP was around 2.158 as shown in Figure 9. The order reported in our work is 2.158. The digit left of the decimal in the order value suggests that the reaction kinetics was influenced by the concentration of two entities viz. KAlSi3O8 and CaCl2. Digits right of the decimal suggest that the reaction was also being influenced fractionally by some impurity originating from the ore or the chemical reagents.
Coefficients of regression for different models under variation of temperatures have been procured. The model that has the closest fit to the data obtained from roasting was the 1 – 3(1 – X)2/3 + 2(1 – X) expression as shown in Figure 10.
Figure 10.
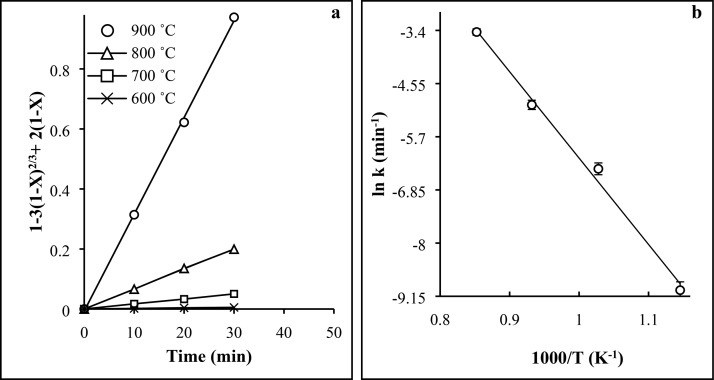
(a) Fitting of roast–leach data to the product layer diffusion model for various temperatures. (b) Arrhenius plot for roasting at the 600–900 °C temperature range using 18 g ESP per 10 g FS.
The slope drawn for linear trend lines between 1 – 3(1 – X)2/3 + 2(1 – X) versus time provided the values of the reaction constant (k) for corresponding temperatures. Figure 10 presented the Arrhenius plot, i.e., ln(k) versus (1/T) where the temperature is in Kelvin. The activation energy from the Arrhenius plot is estimated to be 155.3 kJ/mol.
Analysis of the shrinking core models and the amount of the activation energy calculated for nepheline syenite roasting suggests that the rate-controlling model is diffusion through the product layer.11,31
2.5. Production of KCl
Potassium chloride was prepared in two stages: at first, potassium was precipitated as potassium perchlorate by adding sodium perchlorate. Then, it was thermally decomposed to give potassium chloride.
2.5.1. Precipitation Process
The liquor obtained after leaching of the roasted sample at optimum conditions comprised considerable amounts of sodium and calcium together with potassium. As all forms of potassium salts are water soluble in nature, potassium was selectively recovered by a precipitation method. Sodium perchlorate was used to generate potassium perchlorate salt, which was insoluble in water at room temperatures. Sodium perchlorate is crystalline, hygroscopic, and highly soluble in water in comparison to other alkali perchlorates. Generally, the precipitation process is inversely proportional to temperatures. At room temperatures, the requirement of sodium perchlorate was more than the stoichiometric amount. Hence, the temperature was decreased to −10 °C starting from 10 °C with a stoichiometric amount of sodium perchlorate. During this process, around 30 g/L of sodium perchlorate was used for leach liquor with 18.3 g/L of potassium chloride. From Figure 11, it was inferred that on lowering the temperature, the precipitation of potassium perchlorate increases. At −7 °C, around 99.97% of potassium was recovered as its perchlorate salt, utilizing a stoichiometric amount of sodium perchlorate.32
Figure 11.

Temperature effect on precipitation of potassium.
The precipitate obtained in this process was filtered and thoroughly washed with cold water. After that, it was dried in the oven for the complete removal of moisture and characterized in XRD. All the peaks obtained in XRD perfectly matches with the KClO4 phases, which are shown in Figure 12. From 1 L of leach liquor containing 18.3 g/L of potassium chloride, around 34 g of potassium perchlorate was obtained.
Figure 12.

XRD of potassium perchlorate and potassium chloride.
2.5.2. Thermal Decomposition of Potassium Perchlorate
Extraction of potassium from the feldspar was proposed for agricultural application. Potassium perchlorate has very little solubility in water; it is necessary to alter the insoluble salt into water-soluble salt of potassium. Therefore, potassium perchlorate was subjected to thermal decomposition at temperature around 600 °C in the furnace for 1 h.
| 5 |
White powder of potassium chloride was obtained, having crystalline texture. To confirm the formation of potassium chloride, phase detection was carried out in XRD where the peaks completely match with the standard KCl (Figure 12), which bears the crystal structure of face-centered cubic. The XRD peak with 100% intensity [002] refers to 3.14 Å as the d-spacing value at 28.31 as a 2θ value. The purity of the potassium chloride was analyzed and calculated to be 99.81%. In this final process, around 18 grams of potassium chloride was produced from 34 grams of potassium perchlorate.
3. Conclusions
One of the indigenously available minerals, feldspar has been utilized in this study to prepare potassium salt for agricultural application. Eggshell is used in combination with hydrochloric acid as precursors of calcium chloride, which was used as the roasting agent for feldspar. Complete extraction of potassium was achieved by roasting feldspar with eggshell powder and 1.5 times the stoichiometric amount of hydrochloric acid. The optimized conditions were feldspar to ESP in a ratio of 1:1.8 at 900 °C for 30 min followed by leaching with water. To understand the mechanism of the roasting process, the potash recovery results were analyzed using a shrinking core model and diffusion through a product layer was concluded as the rate-determining step. The order of the roasting process was found to be 2.158, and activation energy was calculated to be 155.3 kJ/mol. In the purification stage, the temperature of the leach liquor was lowered to −7 °C by using a water bath filled with an azeotropic mixture of 60% ethylene glycol and 40% water. Thereafter, sodium perchlorate was added to the leach liquor in which more than 99.9% of potassium was precipitated as potassium perchlorate. The potassium perchlorate obtained after precipitation was thermally decomposed at 600 °C to get pure crystals of potassium chloride, which was 99.81% pure.
4. Experimental Section
4.1. Material Collection & Characterization
Feldspar mineral was obtained from local mines situated in Rajasthan. The collected sample was crushed and sieved for various particle sizes. Wet chemical analyzing techniques were used for quantitative estimation of the elements present in the sample. Potassium and sodium were determined by a flame photometer (model: CL378, maker: Elico). Calcium and magnesium contents were analyzed by ICP-OES (model: Optima 8300, maker: Perkin Elmer). Aluminum and iron were analyzed by atomic absorption spectroscopy (model: AA200, maker: Perkin Elmer). A fusion method was implemented to determine the silica content using a Pt crucible. Identification of mineral phases for feldspar at different stages of the experiment was carried out using an X-ray diffraction technique (model: JSM 6510, maker: PAN-analytical). The elemental mapping and morphology study were carried out through scanning electron microscopy (model: SEM, ZEISS EVO 18).
Eggshells were collected from various local fast-food stores and bakeries. They were properly washed with deionized water to remove dirt and impurity. Then, the sample was sundried followed by crushing and grinding to fine powder for further experimental purpose (Figure 13). One complete chicken eggshell gives around 5–6 g of eggshell powder. To determine the elemental composition of eggshells, wet chemical methods were employed and analyzed in respective instrumental methods as described above. Hydrochloric acid was obtained from Merck (purity: 99.9%, assay: 37%). All the leaching processes were carried out with double-distilled water.
Figure 13.
Image of eggshell and eggshell powder.
4.2. Roasting of Feldspar Followed by Water Leaching
To carry out the roasting processes, eggshell powder (ESP) was mixed with feldspar (FS) along with hydrochloric acid. A balanced chemical equation for the reaction between CaCO3, present in ESP and hydrochloric acid is given below
| 6 |
From eq.1, it can be inferred that for the formation of 1 mol of calcium chloride, the requirement for CaCO3 and HCl is in the molar ratio of 1:2. The above equation holds true for a case where both the reacting compounds are 100% pure. In our case, ESP is not 100% pure CaCO3. Therefore, the HCl requirement will be greater than 1 mol in our case. To optimize the amount of HCl consumption for complete conversion of CaCO3 to CaCl2, experiments were conducted with 1, 1.5, and 2 times the stoichiometric requirement.
Carbon dioxide emitted during the reaction of CaCO3 and HCl was collected by suitable arrangements and was sequestrated by passing it through lime solution.
| 7 |
Two modes of CaCl2 preparation viz. ex situ and in situ were compared to find the best manner for recovery of potassium. In the ex situ mode, 10 g of ESP and a requisite amount of HCl were mixed for around 10 min. The resulting mixture was added to 10 g of feldspar kept in an alumina crucible. This crucible was placed in a programmable muffle furnace for roasting. In the in situ mode, 10 g of FS was mixed thoroughly and uniformly with 10 g of finely ground eggshell powder in a particular weight ratio in alumina crucibles. A requisite amount of hydrochloric acid was then added to it and again mixed properly to make a frothy paste. The frothy paste samples were roasted inside a programmable muffle furnace. Effects of the roasting temperature and time were studied by varying the respective parameters with the help of a programmable furnace while other parameters were kept constant.
To choose an appropriate size for the feldspar sample for maximum recovery, the mineral was subjected to grinding followed by size analysis using various sieves of different mesh sizes. Feldspar minerals of various sizes, such as 25, 45, 75, 90, 106, and 300 μ, were obtained after sieving. All these size fractions of feldspar samples were taken for roasting with ESP in the ratio 1:1. The roasting temperature was maintained at 900 °C for a period of 60 min. The product obtained after roasting was lixiviated in water at room temperatures.
After completion of the process, roasted masses were further cooled inside the furnace to room temperature. The product was taken out of the crucibles with the help of a spatula and ground with the help of a mortar and pestle. Leaching was carried out in double-distilled water for 30 min with the stirring speed of 250 rpm at room temperature in glass beakers. Leach liquor was separated from the undissolved fraction by filtration and analyzed in a flame photometer. The recovery percentage of potassium was calculated using the following equation
| 8 |
4.3. Precipitation of Potassium Salt
The leach liquor rich in potassium was subjected to precipitation in a jacketed glass reactor, which was connected to a temperature-controlled water bath supplied by Julabo. The water bath was filled with an azeotropic mixture of 60% ethylene glycol and 40% distilled water, which was circulated in the external jacket. The temperature variation was done from 10 to −10 °C. Sodium perchlorate was added to the liquor when it attained the desired temperature. After completion of the precipitation process, the raffinate was checked for the residual potassium in the flame photometer. The precipitate obtained was filtered, washed thoroughly, and dried in the oven overnight at 80 °C. Phase detection was studied using an X-ray diffraction technique. Subsequently, the precipitate was calcined in a muffle furnace at 600 °C to obtain the final product. The purity of the product was analyzed by dissolving a specific amount in water and analyzing the concentration in flame photometer.
| 9 |
Acknowledgments
The authors wish to acknowledge the director of CSIR-IMMT Bhubaneswar for his kind consent to publish this research article. The authors also appreciatively recognize the technical assistance provided by the institute staff for the characterization studies.
The authors declare no competing financial interest.
References
- Kinekar B. K. Potassium Fertilizer Situation in India : Current Use and Perspectives. Karnataka J. Agric. Sci. 2011, 24, 1–6. [Google Scholar]
- Ciceri D.; Close T. C.; Barker A. V.; Allanore A. Fertilizing Properties of Potassium Feldspar Altered Hydrothermally. Commun. Soil Sci. Plant Anal. 2019, 50, 482–491. 10.1080/00103624.2019.1566922. [DOI] [Google Scholar]
- Skorina T.; Allanore A. Aqueous Alteration of Potassium-Bearing Aluminosilicate Minerals: From Mechanism to Processing. Green Chem. 2015, 17, 2123–2136. 10.1039/C4GC02084G. [DOI] [Google Scholar]
- Prakash S.; Verma J. P.. Global Perspective of Potash for Fertilizer Production. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; 2016, pp 327–331, 10.1007/978-81-322-2776-2_23. [DOI] [Google Scholar]
- Jena S. K.; Dhawan N.; Rao D. S.; Misra P. K.; Mishra B. K.; Das B. Studies on Extraction of Potassium Values from Nepheline Syenite. Int. J. Miner. Process. 2014, 133, 13–22. 10.1016/j.minpro.2014.09.006. [DOI] [Google Scholar]
- Ciceri D.; de Oliveira M.; Stokes R. M.; Skorina T.; Allanore A. Characterization of Potassium Agrominerals : Correlations between Petrographic Features , Comminution and Leaching of Ultrapotassic Syenites. Miner. Eng. 2017, 102, 42–57. 10.1016/j.mineng.2016.11.016. [DOI] [Google Scholar]
- Crundwell F. K. The Mechanism of Dissolution of Minerals in Acidic and Alkaline Solutions: Part II Application of a New Theory to Silicates, Aluminosilicates and Quartz. Hydrometallurgy 2014, 149, 265–275. 10.1016/j.hydromet.2014.07.003. [DOI] [Google Scholar]
- Burat F.; Kangal O.; Onal G. An Alternative Mineral in the Glass and Ceramic Industry: Nepheline Syenite. Miner. Eng. 2006, 19, 370–371. 10.1016/j.mineng.2005.10.015. [DOI] [Google Scholar]
- Shekhar S.; Mishra D.; Agrawal A.; Sahu K. K. Physico-Chemical Treatment of Glauconitic Sandstone to Recover Potash and Magnetite. J. Cleaner Prod. 2017, 147, 681–693. 10.1016/j.jclepro.2017.01.127. [DOI] [Google Scholar]
- Ciceri D.; de Oliveira M.; Allanore A. Potassium Fertilizer Via Hydrothermal Alteration Of K-Feldspar Ore. Green Chem. 2017, 19, 5187–5202. 10.1039/C7GC02633A. [DOI] [Google Scholar]
- Zhang Y.; Asselin E.; Li Z. Laboratory and Pilot Scale Studies of Potassium Extraction from K-Feldspar Decomposition with CaCl2 and CaCO3. J. Chem. Eng. Japan 2016, 49, 111–119. 10.1252/jcej.15we078. [DOI] [Google Scholar]
- Shekhar S.; Mishra D.; Agrawal A.; Sahu K. K. Physical and Chemical Characterization and Recovery of Potash Fertilizer from Glauconitic Clay for Agricultural Application. Appl. Clay Sci. 2017, 143, 50–56. 10.1016/j.clay.2017.03.016. [DOI] [Google Scholar]
- Jena S. K.; Dhawan N.; Rath S. S.; Rao D. S.; Das B. Investigation of Microwave Roasting for Potash Extraction from Nepheline Syenite. Sep. Purif. Technol. 2016, 161, 104–111. 10.1016/j.seppur.2016.01.039. [DOI] [Google Scholar]
- Sheng H.; Lv L.; Liang B.; Li C.; Yuan B.; Ye L.; Yue H.; Liu C.; Wang Y.; Zhu J.; Xie H. Aqueous Carbonation of the Potassium-Depleted Residue from Potassium Feldspar–CaCl2 Calcination for CO2 Fixation. Environ. Earth Sci. 2015, 6871–6879. 10.1007/s12665-015-4412-9. [DOI] [Google Scholar]
- Yuan B.; Li C.; Liang B.; Lü L.; Yue H.; Sheng H.; Ye L.; Xie H. Extraction of Potassium from K-Feldspar via the CaCl 2 Calcination Route. Chin. J. Chem. Eng. 2015, 23, 1557–1564. 10.1016/j.cjche.2015.06.012. [DOI] [Google Scholar]
- Ye L.; Yue H.; Wang Y.; Sheng H.; Yuan B.; Lv L.; Li C.; Liang B.; Zhu J.; Xie H. CO2 Mineralization of Activated K-Feldspar + CaCl2 Slag To Fix Carbon and Produce Soluble Potash Salt. Ind. Eng. Chem. Res. 2014, 53, 10557–10565. 10.1021/ie500992y. [DOI] [Google Scholar]
- Tanvar H.; Dhawan N. Recovery of Potash Values from Feldspar. Sep. Sci. Technol. 2019, 1398–1406. 10.1080/01496395.2019.1588317. [DOI] [Google Scholar]
- Tsai W. T.; Yang J. M.; Lai C. W.; Cheng Y. H.; Lin C. C.; Yeh C. W. Characterization and Adsorption Properties of Eggshells and Eggshell Membrane. Bioresour. Technol. 2006, 97, 488–493. 10.1016/j.biortech.2005.02.050. [DOI] [PubMed] [Google Scholar]
- Park H. J.; Jeong S. W.; Yang J. K.; Kim B. G.; Lee S. M. Removal of Heavy Metals Using Waste Eggshell. J. Environ. Sci. 2007, 19, 1436–1441. 10.1016/S1001-0742(07)60234-4. [DOI] [PubMed] [Google Scholar]
- Wei Z.; Xu C.; Li B. Application of Waste Eggshell as Low-Cost Solid Catalyst for Biodiesel Production. Bioresour. Technol. 2009, 100, 2883–2885. 10.1016/j.biortech.2008.12.039. [DOI] [PubMed] [Google Scholar]
- Ahmad M.; Usman A. R. A.; Lee S. S.; Kim S.-C.; Joo J.-H.; Yang J. E.; Ok Y. S. Eggshell and Coral Wastes as Low Cost Sorbents for the Removal of Pb 2 + , Cd 2 + and Cu 2 + from Aqueous Solutions. J. Ind. Eng. Chem. 2012, 18, 198–204. 10.1016/j.jiec.2011.11.013. [DOI] [Google Scholar]
- Rivera E. M.; Araiza M.; Brostow W.; Castaño V. M.; Díaz-Estrada J. R.; Hernández R.; Rodríguez J. R. Synthesis of Hydroxyapatite from Eggshells. Mater. Lett. 1999, 41, 128–134. 10.1016/S0167-577X(99)00118-4. [DOI] [Google Scholar]
- Samantray J.; Anand A.; Dash B.; Ghosh M. K.; Behera A. K. Production of Potassium Chloride from K-Feldspar Through Roast – Leach – Solvent Extraction Route. Trans. Indian Inst. Met. 2019, 72, 2613–2622. 10.1007/s12666-019-01730-z. [DOI] [Google Scholar]
- Samantray J.; Anand A.; Dash B.; Ghosh M. K.; Behera A. K.. Nepheline Syenite — An Alternative Source for Potassium and Aluminium. In Rare Metal Technology; Springer: 2019; pp 145–159. [Google Scholar]
- Darken L. S.; Gurry R. W.. Physical Chemistry of Metals; McGrwa-Hill Book Co. Inc.: 1987. [Google Scholar]
- Zhang H.; Sun D. S.; Bao H. The Extraction of Potassium from Feldspar by Molten Salt Leaching Method with Composite Additives. Adv. Mater. Res. 2012, 524-527, 1136–1139. 10.4028/www.scientific.net/AMR.524-527.1136. [DOI] [Google Scholar]
- Levenspiel O. Chemical Reaction Engineering. Ind. Eng. Chem. Res. 1999, 38, 4140–4143. 10.1021/ie990488g. [DOI] [Google Scholar]
- Habashi F.Principles of Extractive Metallurgy. 1. General Principles; Gordon and Breach: 1969. [Google Scholar]
- Miller J. D.; Wan R.-Y. Reaction Kinetics for the Leaching of MnO2 by Sulfur Dioxide. Hydrometallurgy 1983, 10, 219–242. 10.1016/0304-386X(83)90007-5. [DOI] [Google Scholar]
- Anand A.; Singh R.; Ghosh M. K.; Sanjay K. Factorial Design for Process Optimization and Generation of Kinetic Data for Yttrium and Europium Leaching. Miner. Process. Extr. Metall. 2018, 0, 1–9. 10.1080/25726641.2018.1505209. [DOI] [Google Scholar]
- Zhong Y.; Gao J.; Chen P.; Guo Z. Recovery of Potassium from K-Feldspar by Thermal Decomposition with Flue Gas Desulfurization Gypsum and CaCO3: Analysis of Mechanism and Kinetics. Energy Fuels 2017, 31, 699–707. 10.1021/acs.energyfuels.6b01915. [DOI] [Google Scholar]
- George D. A. R.; Riley J. M.; Ross J. R.. Method for Recovering and Producing Potassium Salts, US3,429,657A, 1969.