Abstract
Subcritical water extraction (SWE) of pectin from fresh sunflower heads was optimized using the response surface methodology (RSM). The optimal conditions for the maximum yield of pectin (6.57 ± 0.6%) were found to be a pressure of 8 bar, temperature of 120 °C, time of 20 min, and liquid–solid ratio (LSR) of 7 mL/g. The degree of esterification (DE) of pectin was analyzed by titrimetry and Fourier transform infrared (FTIR) methods, which was low methoxyl pectin. The molecular weight (Mw), galacturonic acid (GalA) content, and surface tension of pectin were 11.50 kDa, 82%, and 45.38 mN/m (1.5% w/v), respectively. Moreover, thermogravimetric (TG) and differential scanning calorimetry (DSC) analysis confirmed that pectin had excellent thermal stability. FTIR and 1H NMR spectra confirmed its structure. This study demonstrated that SWE could be used as a productive and environmentally friendly method for extracting pectin from fresh sunflower heads.
1. Introduction
Pectin can be composed of as many as 17 different monosaccharides that contain more than 20 different linkages.1 The primary structural components of pectin include homogalacturonan (HG), rhamnogalacturonan I (RG-I), and rhamnogalacturonan II (RG-II). HG is a linear polymer composed of a 1,4-linked α-d-galacturonic acid (α-d-GalA) backbone. RG-I comprises the repeating disaccharide [-4)-α-d-GalA-(1–2)-α-l-Rha-(1-], whereas RG-II has a backbone of HG with complex side chains attached to the GalA residues.2 Low methoxyl pectin (LMP) with a degree of esterification (DE) lower than 50% has recently attracted considerable attention because of its applications in functional foods and pharmaceuticals.3 LMP is not only highly suitable for wound-healing applications but also has good compatibility with yogurt; moreover, it could be widely used as a carrier for delivering probiotics.4,5 Furthermore, LMP can be used as a thickening agent in acidic dairy products and can reinforce the firmness of Japonica rice noodles when Ca2+ is added to the mixture.6,7 Moreover, LMP gels can be used in low-calorie jams and jellies for glazing, retorting, microwaving, baking, and sterilizing or pasteurizing.8 Typically, LMP is obtained from natural sources or prepared from high methoxyl pectin; however, synthetically preparing LMP from high methoxyl pectin is expensive.3,9 Nevertheless, the yield and quality of pectin are affected by raw materials, stage of maturity, and extraction conditions.3 Note that sunflower heads, which are discarded during sunflower seed harvest, contain pectin that is naturally low-methoxylated. Because the heads are rich in pectin and contain 18–24% pectin after seed removal, they have been suggested as an excellent natural source of LMP.10 Traditionally, pectin can be extracted in multiple ways using hot water, dilute alkali solutions, salt solutions, enzymes, and acidic solutions.11−15
Previous studies on pectin extraction from sunflower heads employed dried mature sunflower heads as the raw material. The extracted pectin was tanned or black in color because of the presence of brown water-soluble pigments; however, commercial pectin products are expected to be a light colored or a colorless powder. The amount of pectin, composition, and physicochemical properties can be considerably affected by the decolorization step. Therefore, pigment removal is critical to the quality of pectin and has an impact on the overall cost. Furthermore, the current extraction methods have certain disadvantages such as being unsafe, expensive to operate, and can generate considerable environmental pollution.16−18 To address these limitations of LMP extraction methods, we utilized fresh sunflower heads as the raw material and looked for innovative extraction techniques. Subcritical water extraction (SWE) is a well-known environmentally friendly and green method for extracting natural materials. Subcritical water is water maintained in the liquid state at temperatures between 100 and 374 °C under pressure. In this state, the dielectric constant and polarity of water can be changed.19,20 Because water is nontoxic, inexpensive, readily available, and can be easily disposed, using subcritical water for extraction can be both cost-effective and environmentally friendly. To date, most of the SWE studies on pectin have used extraction pressures and extraction liquid–solid ratios (LSRs) in the range of 20–180 bar and 10–70 mL/g, respectively.21−23 In this study, we studied low extraction pressures to improve the production safety and smaller extraction LSRs to investigate the possibility of decreasing the amount of water required without affecting pectin loss. Experimental design (Box–Behnken design (BBD) in this work) is used to reduce the number of assays needed for optimizing conditions while the response surface methodology (RSM) is used to analyze the results of the experimental design.24
To our knowledge, the extraction of pectin from fresh sunflower heads by subcritical water technology has not been reported to date. In this study, we aimed to develop an optimized, ecofriendly, SWE protocol for obtaining pectin from fresh sunflower heads. Further, the fresh sunflower heads pectin (SFHP) was also characterized using Fourier transform infrared (FTIR) and proton nuclear magnetic resonance (1H NMR), the molecular weight (Mw) was determined using high-performance size exclusion chromatography (HPSEC), and thermal properties were evaluated using thermogravimetric (TG) and differential scanning calorimetry (DSC) analysis. The DE of pectin was also determined by both titrimetry and FTIR methodology, and we expect that SFHP will be well received by the food industry as a stabilizing agent.
2. Results and Discussion
2.1. Single-Factor Analysis for the Extraction of SFHP
2.1.1. Effect of Extraction Time on SFHP Yield
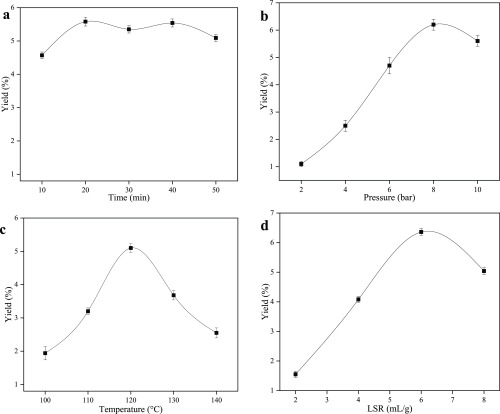
The influence of extraction time on SFHP yield was reported in this study. The effect of extraction time (10–50 min) on pectin yield was examined by fixing an LSR of 2 mL/g, pressure of 10 bar, and extraction temperature of 140 °C. As shown in Figure 1a, the extraction yield of SFHP increased with increase in extraction time to the peak value (5.58%) when the extraction time was 20 min. No increase in the yield above the peak value occurred from 20 to 50 min. The appropriate extraction time facilitated the mass transfer of pectin from plant tissues into solution. The cell wall was broken, and pectin was released. However, prolonged extraction time decreased the yield because of pectin decomposition.25 Therefore, in this study, we used 20 min as the time interval for all BBD experiments (Section 2.2).
Figure 1.
Effects of different reaction conditions on pectin yield: (a) time, (b) pressure, (c) temperature, and (d) LSR.
2.1.2. Effect of Pressure on SFHP Yield
The effect of extraction pressure (2–10 bar) on the yield of SFHP was examined by fixing the LSR to 2 mL/g, temperature to 140 °C, and extraction time to 30 min. The SFHP yields increased with the increase of pressure from 2 to 8 bar (Figure 1b). The plant material was heated in pressure, the cell structure tended to break down, and insoluble pectin was hydrolyzed into soluble pectin. Therefore, pectin yields increased. The pressure increased, the surface tension of water decreased, and the intensity of water increased, which was beneficial for pectin extraction. At pressures above 8 bar, no difference was found in the yield. This can be explained by possible interactions and aggregation of molecules or even by their re-adsorption under high pressures.26 Moreover, while optimizing the SWE of pectin, it was desirable that the extraction pressure was kept low, provided that the liquid state of the solvent was maintained.23 A lower extraction pressure will reduce equipment stress, thus reducing equipment operating costs and safety concerns.
2.1.3. Effect of Temperature on SFHP Yield
The effect of extraction temperature (100–140 °C) on pectin yield was examined by fixing the LSR to 2 mL/g, pressure to 10 bar, and extraction time to 30 min. The extraction temperature was a significant parameter that affected the pectin yield of the SWE process.27,28 As the temperature increased from 100 to 120 °C, the yield increased (Figure 1c). This may be attributed to plant tissue degradation at high temperatures; thus, the diffusivity of solvent into the cell walls would be accelerated and more pectin would be released and dissolved in the solvent, leading to a notable increase in the yield.29 However, the yield decreased as the temperature increased from 120 to 140 °C. This might be because of the degradation of pectin at high temperatures, which can damage the structure of pectin and lead to degradation. Our results of increased temperatures causing pectin degradation were corroborated by several other studies.30,31 Moreover, at high temperatures, the polarity of subcritical water could be affected, which subsequently reduces the efficacy of the solvent for pectin extraction.25
2.1.4. Effect of LSR on SFHP Yield
Figure 1d shows the result of the single-factor analysis of the extraction of SFHP. To understand the effect of LSR on the extraction yield, different LSRs (2, 4, 6, and 8 mL/g) were investigated by fixing the temperature to 140 °C, pressure to 10 bar, and extraction time to 30 min. The yield significantly increased from 1.55 to 6.36% with the increase in LSR from 2 to 8 mL/g. At an LSR of less than 1 mL/g, the mixture was difficult to separate. Several studies have confirmed that both very low and very high LSR are unsuitable for extracting the compounds of interest from their sample matrices.32 The solvent can increase the contact surface area between the plant matrix and the solvent, leading to increases in pectin extraction yields up to an LSR of 6 mL/g. However, excessive LSR overly dilutes the solution, resulting in pectin hydrolysis,33 and larger LSR can cause difficulties during the ethanolic precipitation. Optimal LSR can improve the diffusivity rate of the solvent into cells and can enhance desorption of the pectin from the plant cells of fresh sunflower heads. Thus, an appropriate LSR should be used to speed up the pigment removal without affecting pectin yield.
2.2. Statistical Analysis and Optimization of Pectin Extraction
The Box–Behnken experimental design (BBD) is a collection of mathematical and statistical techniques for building empirical models. Compared with other experimental designs, the advantage of BBD was the simultaneous investigation of individual and interactive effects of process variables on the response from a lower number of experiments.34 The experimented values for the yield of SFHP at different extraction conditions are shown in Table 1. The second-order equations of the extraction yield are expressed as follows
| 1 |
where Y is the yield of pectin and Xi represents the coded variable (X1 is the pressure, X2 is the temperature, and X3 is the LSR).
Table 1. Experimental Values for the Yield of SFHP from the BBD Experimental Design.
| pressure | temperature | LSR | yield (%) | |
|---|---|---|---|---|
| run | X1 (bar) | X2 (°C) | X3 (mL/g) | experimental values |
| 1 | 8 | 100 | 4 | 2.54 |
| 2 | 8 | 140 | 4 | 1.61 |
| 3 | 10 | 120 | 4 | 2.15 |
| 4 | 10 | 100 | 6 | 3.66 |
| 5 | 10 | 120 | 8 | 4.64 |
| 6 | 6 | 120 | 8 | 2.24 |
| 7 | 8 | 120 | 6 | 7.06 |
| 8 | 8 | 140 | 8 | 2.73 |
| 9 | 8 | 120 | 6 | 6.44 |
| 10 | 6 | 100 | 6 | 3.58 |
| 11 | 8 | 120 | 6 | 6.84 |
| 12 | 10 | 140 | 6 | 3.33 |
| 13 | 8 | 100 | 8 | 3.46 |
| 14 | 6 | 140 | 6 | 2.66 |
| 15 | 6 | 120 | 4 | 2.76 |
The analysis of variance (ANOVA) results are presented in Table 2. The corresponding variables would be more significant if the F-value became greater and the p-value became smaller. As shown in Table 2, the p-value of the model was 0.0001, indicating that the model was significant. The F-value was 66.69, indicating that there was only a 0.01% chance that this could occur because of the noise in the model. The “lack of fit” F-value of 0.16 indicated that the lack of fit was not significant relative to pure error. The quadratic regression model showed that the value of the determination coefficient (R2) was 0.99, which indicated that 99% of the variation could be explained by the model. For a good statistical model, adj R2 should be close to R2. As shown in Table 2, adj R2 was 0.98, which indicated that only 2% of the total variation was not explained by the model. The “pred R2” of 0.93 was in reasonable agreement with the “adj R2” of 0.98; moreover, a relatively low coefficient of variation value (3.64%) indicated good reliability of the experimental values. From the above analysis, the models could perfectly forecast the nature of the extraction process.
Table 2. Analysis of Variance (ANOVA) for the BBD Model.
| source | sum of squares | DF | mean square | F-value | p-value |
|---|---|---|---|---|---|
| model | 42.55 | 9 | 4.73 | 66.69 | 0.0001 |
| X1—pressure | 0.81 | 1 | 0.81 | 11.38 | 0.0198 |
| X2—temp | 1.06 | 1 | 1.06 | 14.93 | 0.0118 |
| X3—LSR | 2.01 | 1 | 2.01 | 28.36 | 0.0031 |
| X1X2 | 0.09 | 1 | 0.087 | 1.23 | 0.3183 |
| X1X3 | 2.27 | 1 | 2.27 | 31.95 | 0.0024 |
| X2X3 | 0.01 | 1 | 0.01 | 0.14 | 0.7226 |
| X12 | 8.93 | 1 | 8.93 | 125.95 | <0.0001 |
| X22 | 13.58 | 1 | 13.58 | 191.52 | <0.0001 |
| X32 | 19.15 | 1 | 19.15 | 270.18 | <0.0001 |
| residual | 0.35 | 5 | 0.02 | ||
| lack of fit | 0.16 | 3 | 0.05 | 0.53 | 0.7057 |
| pure | 0.20 | 2 | 0.10 | ||
| cor total | 42.9 | 14 | |||
| X | 0.99 | ||||
| adj R2 | 0.98 | ||||
| pred R2 | 0.93 |
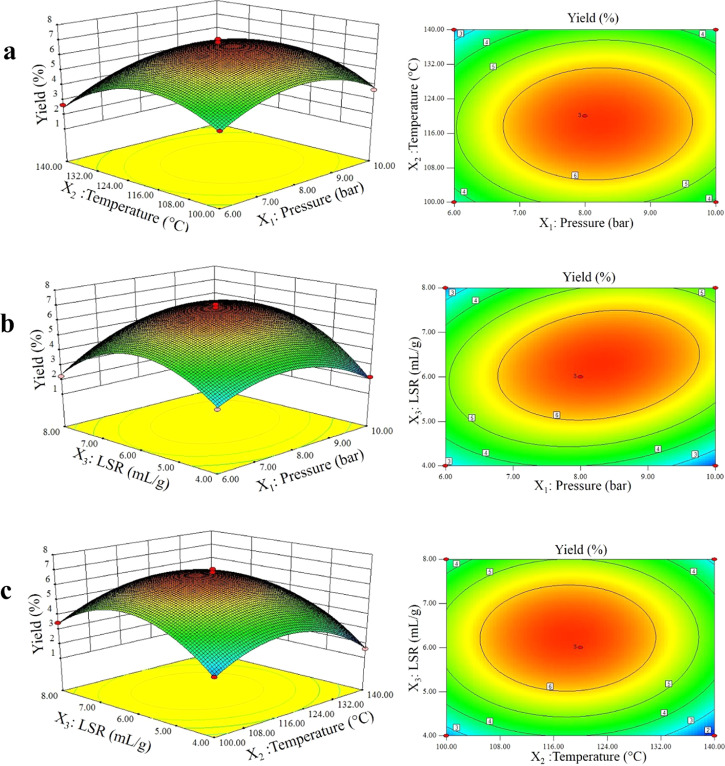
The response surfaces provided a method to visualize the relationship between the responses and the independent variables. In this work, response surfaces were obtained by maintaining one of the variables constant at a zero level while varying the other two variables. The response surface plot showed the magnitude of the response values. As shown in Figure 2, when other variables were fixed at a zero level, the slope of the response surface was relatively flat, which suggested that the extraction conditions had little effect on the yield. However, if the slope was relatively steep, it indicated that the extraction conditions considerably affected the yield.21Figure 2 showed that the extraction LSR played an important role in SFHP yield. Furthermore, the shape of contour plots reflected the extent of the interaction effect: an ellipse showed a significant interaction effect between factors, whereas a circle showed no significant impact. The maximum response value was obtained at the center of the ellipse-shaped region and gradually decreased from the center to the edge.
Figure 2.
Response surface and contour plots showing the effects of independent variables on the yield of SFHP. (a) Temperature vs pressure, (b) LSR vs pressure, and (c) LSR vs temperature.
Based on the response surface and contour plots, it was confirmed that moderate pressure, temperature, and LSR conditions would increase the pectin yield. Thus, we determined that the effect on the SFHP was reduced in the following order X3 > X2 > X1 according to the p-value. Based on the BBD-derived model, the recommended extraction conditions for the maximum SFHP yield were as follows: pressure of 8.3 bar, temperature of 120 °C, and LSR of 6.72 mL/g. Finally, an extraction run with slightly sub-optimal reaction conditions for SFHP yield (pressure of 8 bar, temperature of 120 °C, and LSR of 7 mL/g) was completed. At these conditions, the predicted yield was 6.85%, with a desirability value of 0.99. To confirm and ensure that the predicted values did not considerably deviate from the true experimental values, extractions were performed in triplicate using the slightly modified predicted optimal extraction conditions, and then an average pectin yield of 6.57 ± 0.6% was obtained. This value was in very good agreement with the model-predicted value, indicating that the model could be used safely for optimizing the extraction of SFHP.
2.3. Determination of the Molecular Weight
The functional properties of pectin significantly depend on the Mw.35 The Mw values of SFHP extracted by sodium citrate and ammonium oxalate from dried sunflower heads were 256.40 and 605.60 kDa, respectively.3,18 For extraction of pectin by sodium hexametaphosphate from dried sunflower heads, the Mw ranged from 39 to 52 kDa.36 Pectin was extracted from pistachio green hull with the Mw of 1.65 kDa.37 In this study, the Mw was 11.5 kDa. Therefore, the SFHP had a reasonable Mw. Moreover, in our previous study, the Mw value of pectin was affected by the drying process of the raw material.38 On the one hand, the pectin in fresh sunflower heads may have a low Mwrelated to the stage of maturity and the moisture content of the raw material. On the other hand, the low observed Mw under SWE may be because of pectin hydrolysis and decomposition under subcritical water temperature and pressure.33 When comparing SWE and other extraction methods,23 the lower Mw values were obtained from SWE methods. Certainly, these values agreed with the Mw range of 8–1000 kDa for the pectin extracted from miscellaneous fruit sources.39 Moreover, the Mw/Mn of 2.49 was much higher than 1, indicating that SFHP had a wide Mw distribution and was a heterogeneous natural polysaccharide.29 Pectin from the same source could have different structural differences because of different extraction methods and storage times, which further affected its functionality. For a fixed raw material, the extraction condition was the most important factor.37 Therefore, it was possible to produce pectin with specific characteristics using different methods and widen its application potential.40
2.4. Physicochemical Properties of SFHP
The DE and chemical parameters are listed in Tables 3 and 4, respectively. The SFHP had a pH value of 4.93, whereas high methoxyl pectin extracted from tobacco had a pH of 2.63.41 Similarly, the obtained SFHP had a DE between 17.7 and 19.4%, which was comparatively lower than other sunflower head pectins extracted by ammonium oxalate and sodium citrate with DE values of 31.7 and 49%, respectively.13,15 Note that the SWE process may have decreased the DE.23 Moreover, the DE from two different methods had a difference of 1.7%. Interestingly, the GalA content was found to be 82%, indicating that the SFHP was of good quality and satisfied the commercial pectin requirement (GalA > 65%).42 The SFHP was of light yellow color with the required lightness. Lighter colored pectin is a typical commercial product, which is generally preferable for food industry use. The possible reason for the lighter color may be the short heating time compared to the conventional method and raw material without pigmentation.23
Table 3. Comparison of Titrimetry and FTIR Methods on the DE of SFHP Extracted at Optimized Conditions.
| method | DE (%) |
|---|---|
| titrimetry | 19.4 ± 0.95 |
| FTIR | 17.7 ± 0.2 |
Table 4. Chemical Parameters of SFHP Extracted at Optimized Conditions.
| chemical parameter | SFHP |
|---|---|
| pH | 4.93 ± 0.03 |
| GalA (%) | 82 |
2.5. TG/DTG Analysis
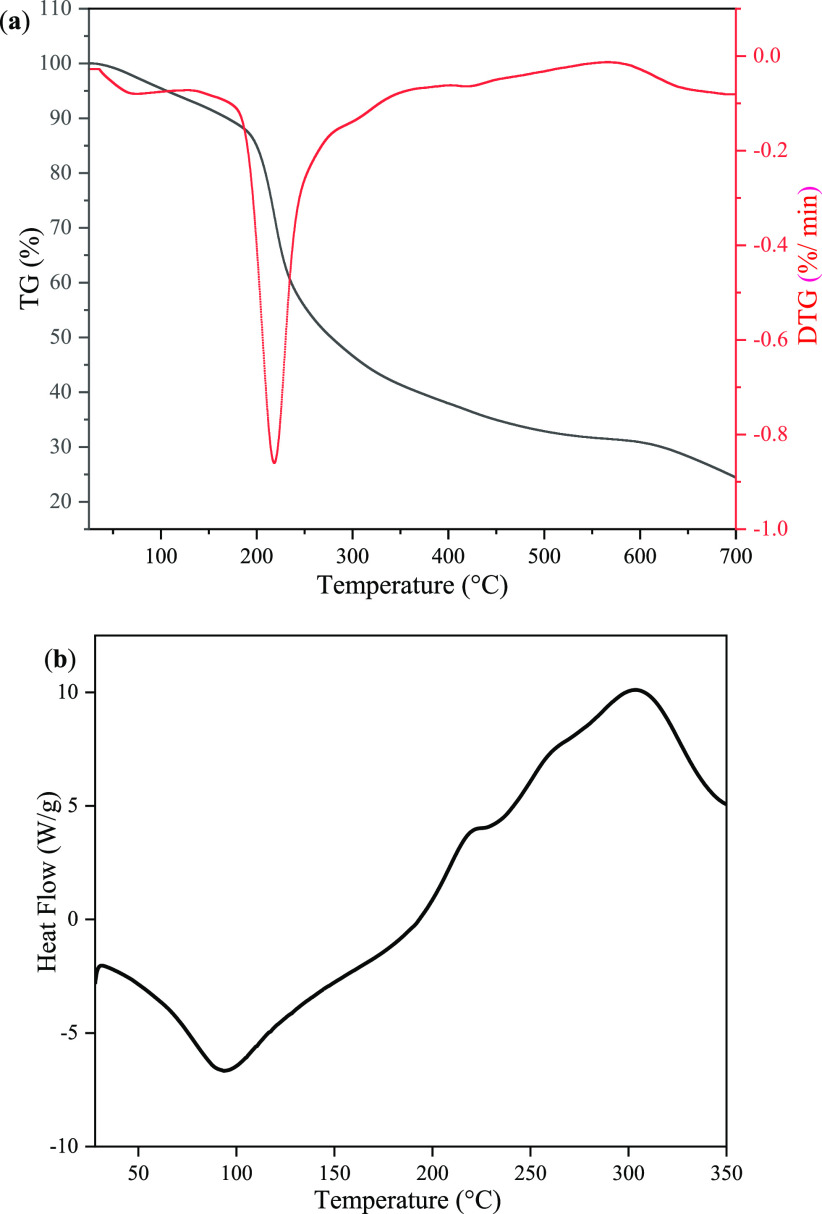
Figure 3a shows the TG and DTG curves of SFHP, revealing three regions: 50–193, 193–426, and 426–700 °C. The small weight loss in the first region (25%) could be explained by the vaporization of absorbed water in SFHP.43 In this temperature range, the maximum degradation rate was obtained at 210 °C. First, the depolymerization of the polysaccharide chain was observed, and then the galacturonic rings started to undergo extensive thermal degradation with the evolution of various gaseous products and the formation of a solid char. In fact, the solid char formed at 492 °C possesses relatively lower aromatization degree. The polyaromatic structures in the solid char may be grafted from many groups such as aliphatic and ketonic groups, which can be used to create links and loops between various aromatic clusters. When the pyrolysis temperature increases, these groups are partially destroyed. Consequently, the polyaromatic structures could further stack compactly,44 and the residual mass was 24.4% at 700 °C.
Figure 3.
(a) TG/DTG and (b) DSC thermogram analysis of SFHP extracted at optimized conditions.
2.6. Differential Scanning Calorimetry
Figure 3b shows the DSC curve, revealing two peaks: one endothermic peak at 95.6 °C as the melting temperature (Tm) and one exothermic peak as the degradation temperature (Td) at 304.5 °C. The endothermic peak was related to DE, Mw, and the GalA content of pectin.45 The Tm value was comparatively lower, which indicated that a lower DE and Mw made pectin absorb water. Therefore, more energy was required to completely remove water because the endothermic phenomenon was attributed to water evaporation. The presence of water might have resulted from hydrogen bonds among entities of GalA and structural transformations from stationary 4C1 chair conformation of the galacturonan ring to the inverse 1C4 chair conformation.46 Based on this study, it can be said that the SFHP had a strong water retention capacity. The smaller exothermic peak at 225 °C may be caused by impurities in pectin. Both the degradation temperature (Td) and degradation enthalpy (ΔHd) can reflect the relevant characteristics of thermal cracking. Pectin was pyrolyzed by a random breakdown of glycoside bonds prior to further decomposition.33 According to the abovementioned results, SFHP had a relatively good thermal stability. Therefore, SFHP could be used as an additive to food products, such as cakes, breads, and pastries, which can be subjected to high temperatures. Thus, high-temperature-resistant pectin could be more favored in the food industry.40
2.7. Surface Tension of SFHP
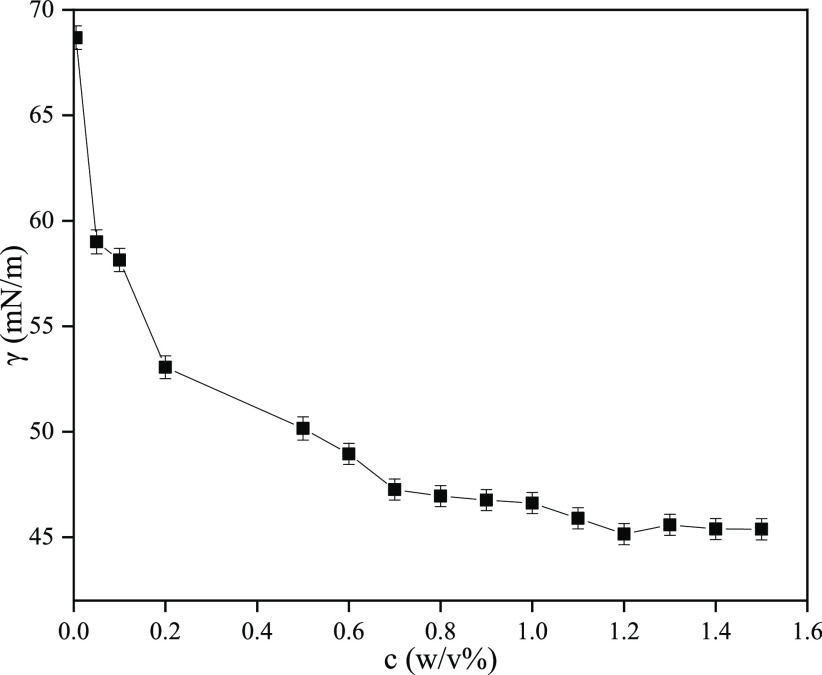
We determined the surface tension of pectin obtained under modified optimal extraction conditions. As shown in Figure 4, the surface tension decreased with the increase in pectin concentration from 0.005 to 1.1% (w/v). After 1.1%, there was little difference in the surface tension values. The lowest surface tension (45.38 mN/m) was obtained from the 1.5% (w/v) aqueous solution of SFHP. In previous studies, the surface tension values of commercial apple pectin solutions, pistachio green hull pectin, and sour orange peel pectin were 63, 49.75, and 42.14 mN/m at a concentration of 0.5% (w/v),37,46,47 respectively. In fact, the enzymatically modified apple pectin had a surface tension value of 55 mN/m because of changes in the blockwise distribution of carboxylic acid groups.46 Similarly, the surface tension values of sugar beet pulp pectin samples ranged from 48.3 to 58.7 mN/m at a concentration of 0.1% (w/v).48 Therefore, SFHP reduced the surface tension more efficiently than other reported pectins. The surface activity of pectins may be due to the presence of hydrophobic groups such as methoxy and acetyl groups. Surface tension is one of the most important properties in aerated food products. Note that the surface activity of pectin has a direct relation with its Mw.37 Generally, pectins with lower surface tension values are more appropriate for these products,47 which may prove to be a promising application of SFHP.
Figure 4.
Variations in Surface tension with SFHP concentrations in distilled water at 25 °C.
2.8. FTIR Spectrum of SFHP
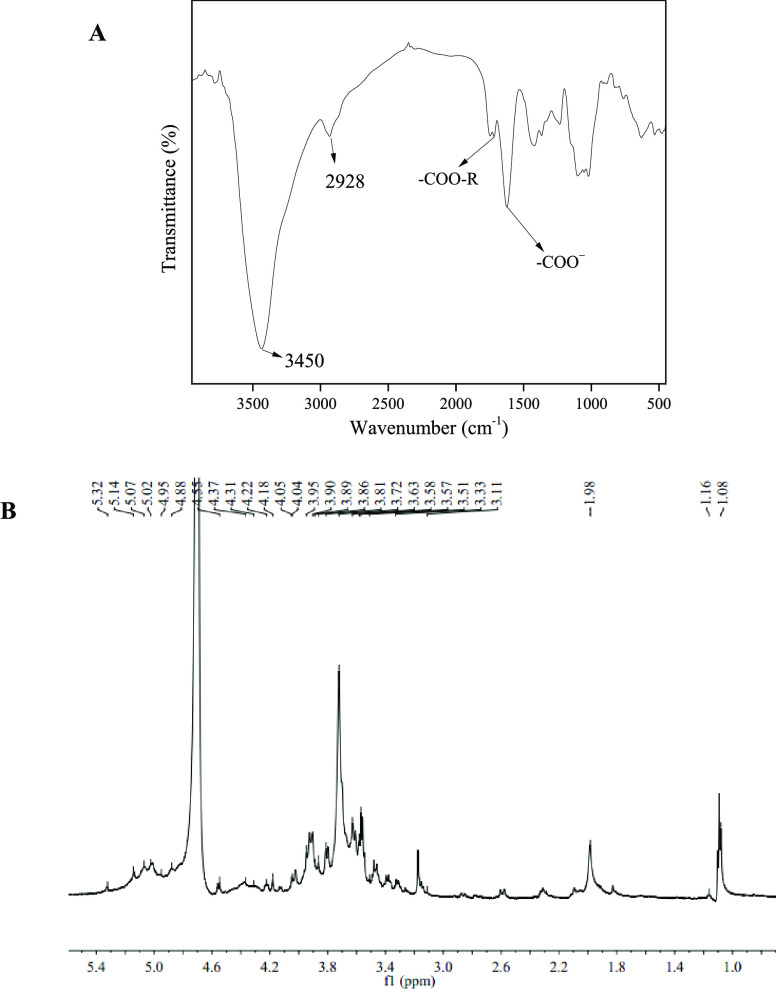
The FTIR spectrum was used to determine the primary functional groups present in SFHP. As shown in Figure 5A, the strong absorption peak at about 3418 cm–1 was attributed to the stretching vibration of the O–H groups present in the inter- and intramolecular hydrogen bonding of the GalA backbone. The peak at around 2936 cm–1 corresponded to the absorption of the stretching and bending vibrations of the C–H groups (CH, CH2, and CH3) in pectin.29 The peaks at 1631 and 1741 cm–1 were specific to the free carboxylic groups (−COO–) and esterified carboxyl (−COOR), respectively. Similarly, the peak at 1415 cm–1 was attributed to C–H stretching vibrations.37 Moreover, the absorption patterns between 1300 and 800 cm–1 were collectively referred to as the “fingerprint” region and reflected specific variations in pectin monosaccharide composition.32 Note that absorptions between 1096 and 1012 cm–1 were assigned to the R–O–R and C–C rings related to the glycosidic linkage between sugar units in the pectin structure.43 The peak at 1010–1100 cm–1 showed that there were α-glycoside bonds and β-glycoside bonds. In conclusion, there was an obvious polysaccharide structure in SFHP.37
Figure 5.
(A) FTIR and (B) 1H NMR spectra of SFHP extracted at optimized conditions.
2.9. 1H NMR Spectrum Analysis of SFHP
Figure 5B and Table 5 show the 1H NMR spectrum and the fraction of SFHP, respectively. A very large and sharp singlet at 3.72 ppm was attributed to the methoxy ester (−OCH3) of GalA. The signal near 1.98 ppm was attributed to the acetyl groups (−COCH3) of esterified GalA units,49 whereas those at 5.14, 3.81, 3.86, 4.37, and 3.64 ppm were assigned to H-1, H-2, H-3, H-4, and H-5 of GalA units,50 respectively. Similarly, the signal at 4.55 ppm was probably derived from branched groups of β-1,3, β-1,6, and β-1,3,6-linked galactose (Gal) residues.51 The chemical shift of H-1 (5.07, 5.02, and 4.88 ppm) indicated that the α-1,5, α-1,3,5, and α-1,3-linked arabinose (Ara) residues remained.52,53 The signals at 1.08 and 1.16 ppm were thought to be indicative of the presence of methyl groups of l-Rha. Note that the anomeric H-1 signals near 4.95 ppm belonged to α-1,2 and α-1,3,4 Rha residues.40 Thus, the 1H NMR spectrum further confirmed the presence of a pectin polysaccharide structure in the obtained SFHP.
Table 5. Chemical Shifts of the Glycosyl Residues of the SFHP Fraction from 1H NMR Spectra.
| chemical shifts, δ (ppm) |
|||||||
|---|---|---|---|---|---|---|---|
| glycosyl residues | H-1 | H-2 | H-3 | H-4 | H-5 | H-6 | –OCH3 |
| →4-α-GalAp-(1→ | 5.14 | 3.81 | 3.86 | 4.37 | 3.64 | 3.68 | |
| →3-α-d-Galp-(1→ | 5.32 | 3.72 | 3.72 | 4.29 | 3.86 | 3.93 | |
| →2-α-Rhap-(1→ | 4.95 | 4.05 | 3.90 | 3.33 | 3.51 | 1.15 | |
| →2,4-α-Rhap-(1→ | 4.95 | 4.05 | 3.90 | 3.63 | 3.51 | 1.24 | |
| →4-β-Galp-(1→ | 4.57 | 3.57 | 3.65 | 3.11 | 3.72 | 3.61 | |
| →3-β-Galp-(1→ | 4.55 | 3.57 | 4.04 | 3.95 | 3.63 | 3.61 | |
| →6-β-Galp-(1→ | 4.55 | 3.57 | 3.72 | 3.95 | 3.63 | 4.05 | |
| →3,6-β-Galp-(1→ | 4.55 | 3.57 | 4.04 | 3.95 | 3.63 | 4.05 | |
| →3,5-α-Araf-(1→ | 5.02 | 4.31 | 4.01 | 4.05 | 3.89 | ||
| →5-α-Araf-(1→ | 5.07 | 4.22 | 3.86 | 4.18 | 3.90 | ||
| →3)-α-Araf-1(→ | 4.88 | 3.57 | 3.61 | 3.72 | 3.93 | ||
3. Conclusions
In this study, a green extraction process using subcritical water was applied to fresh sunflower heads for the extraction of pectin. The extraction process was optimized using BBD, resulting in the near-optimal, but practical extraction conditions: pressure of 8 bar, extraction temperature of 120 °C, time of 20 min, and LSR of 7 mL/g with 6.57 ± 0.6% yield of SFHP. Also, the SFHP exhibited low Mw with a low DE (<50%), good surface tension lowering properties, and high GalA content. The SFHP was fully characterized and revealed that fresh sunflower heads were a good source of LMP. This extraction method was free of organic/toxic reagents; thus, this environmentally friendly and efficient use of fresh sunflower heads should contribute toward developing the use of LMP in the food industry.
4. Materials and Methods
4.1. Materials and Chemicals
Fresh sunflower heads (moisture content = 550 g/kg) were obtained from a local market in Shanxi, China. Sulfuric acid, sodium sulfate, and ethanol were obtained from Beijing Chemical Works (Beijing, China). Chromatographic grade acetonitrile, potassium bromide, sodium hydroxide, carbazole, and deuterium oxide (D2O, 99.9%) were purchased from Aladdin Reagents Co., Ltd. (Shanghai, China).
4.2. Pectin Extraction
We extracted pectin from fresh sunflower heads using a modified method.19 Before extraction, leaves, roots, soil, and all other impurities were removed from the fresh sunflower heads. After the heads were manually collected, they were cut into small pieces (1 × 1 cm–1), which were washed with water at 100 °C and slowly stirred for 20 min at a solid/water ratio of 1:10. The slurries were filtered through a cheesecloth to remove the soluble pigments and dust.54 The extraction conditions were optimized by single-factor experiments. To identify and set the range of extraction variables for optimizing the extraction process using the BBD, several extractions were performed by changing one parameter at a time while setting the others constant. The experimental variables were extraction time (10–50 min), LSR (2–8 mL/g), temperature (100–140 °C), and pressure (2–10 bar). Immediately after the SWE reaction, the extract was obtained by filtration using a 400-mesh gauze. After rotary evaporation, twice the volume of 95% (v/v) ethanol was added. Precipitation of jellified pectin from ethanol was allowed to occur for 24 h at 25 °C. Finally, the jellified pectin was filtered using a 300-mesh gauze, and the solid was dried to obtain the pectin yield percentage Y (%) using the following formula
| 2 |
where m0 (g) is the mass of dried pectin and m (g) is the mass of fresh sunflower heads.
4.3. Galacturonic Acid (GalA) Content
Because GalA was the dominant monosaccharide in the pectin structure, its content was determined as the indicator of pectin yield by the colorimetric carbazole method.18 We added 6 mL of H2SO4 (98% w/w) to 1 mL of the sample (50 mg/L), and then the mixture was agitated with a vortex mixer. The tubes were cooled in an ice–water bath incubated in boiling water for 15 min and then immediately cooled in an ice bath. After cooling to room temperature, 0.5 mL (1.5% w/v) of carbazole–ethanol was added. The tubes were shaken and the reaction was allowed to stand at room temperature for 30 min. The absorbance reading at 530 nm was obtained using a UV–vis spectrophotometer (PerkinElmer). The absorbance values were compared using the GalA calibration curve. The GalA yield can be obtained as follows
| 3 |
where c (mg/L) is the GalA content calculated by the calibration curve.
4.4. Determination of Pectin pH
SFHP (1.25 g) was dissolved in distilled water (50 mL) and the pH was measured at 25 °C using a pH meter.41
4.5. Determination of Pectin Esterification
4.5.1. FTIR Method
SFHP was analyzed using a Bruker Tensor 27 Fourier transform infrared spectrometer (Bruker, Germany). The dried sample (1 mg) and potassium bromide (100 mg) were ground together and pressed into pellets. The pellet was then scanned 32 times over 4000–400 cm–1 with a resolution of 2 cm–1, and the resultant spectra were smoothened to remove noise. Following the method of Pappas et al.,55 the pectin samples were prepared and a calibration curve was established. The DE was calculated by the ratio of the area of the peak at1741 cm–1 (COO–R) to the sum of the areas of the peaks at 1642 cm–1 (COO–) and 1741 cm–1 (COO–R).
4.5.2. Titrimetry Method
The DE of SFHP was determined by titration using a slightly modified method.43 Briefly, a dried sample (200 mg) was moistened with absolute ethanol (1 mL) and then dissolved in distilled water free of carbon dioxide (20 mL) at 40 °C. After dissolving the sample completely, five drops of phenolphthalein reagent were added, and then the sample was titrated with 0.1 mol/L NaOH (V1, mL). Then, 0.5 mol/L NaOH (20 mL) was added and the sample was shaken for 4 h to encourage a hydrolysis reaction. Next, 0.5 mol/L HCl (20 mL) was added and the sample was shaken until the pink color disappeared. Finally, five drops of phenolphthalein reagent were added and the mixture was titrated with 0.5 mol/L NaOH until a pale pink color persisted even after vigorous shaking (V2, mL). DE was computed using the following equation
| 4 |
4.6. Molecular Weight Determination
We determined the Mw of the extracted pectin using high-performance size exclusion chromatography (HPSEC) coupled with a Shodex GF-7M HQ (50 μm, 7.8 × 300 mm) and a refractive index detector (RID) 201H at 35 °C. The extract was dissolved in a NaSO4 solution (0.25 mg/mL) and passed through a 0.22 μm membrane filter. This was followed by manually injecting the extract through a 50 μL loop. The mobile phase was a 0.05 mol/L NaSO4 solution with a flow rate of 0.5 mL/min. The Mw was determined according to the calibration curve of dextran standards (2.8, 20.4, 62.9, 111.9, 212.5, 310.2, and 390 kDa).
4.7. Thermogravimetric Analysis
The thermal stability test was performed to evaluate the thermal degradation of SFHP. A Mettler Toledo TGA-1 thermogravimetric analyzer (Mettler Toledo, Switzerland) with flowing nitrogen (50 mL/min) was used to determine the thermal stability of SFHP (5 mg) in the temperature range of 25–700 °C at a rate of 10 °C/min. Within the heating range, the weight loss rates because of thermal decomposition were calculated by comparing the original weight with data of weight loss.
4.8. Differential Scanning Calorimetry
A Mettler Toledo DSC1 instrument was used to determine the thermal analysis of SFHP. The sample (5 mg) was added into a sealed aluminum pan with a pinhole alongside an empty pan used as a reference.
The temperature was heated from 25 to 350 °C at a rate of 10 °C/min. Nitrogen was the conveying gas employed at a flow rate of 20 mL/min.
4.9. Surface Tension
Surface tension values were measured using a Kruss-K100 tensiometer (Kruss, Germany) with a platinum ring (precision range ±0.5 mN/m). Samples (0.005–1.5% w/v) were prepared in distilled water, and the surface tension measurements were performed at 25 °C. A surface tension value of 72.5 mN/m was obtained for distilled water and was used as a reference. The platinum ring was washed with distilled water, flame-dried over an alcohol lamp, and cooled to room temperature prior to use.
4.10. Nuclear Magnetic Resonance Spectra
SFHP obtained under optimal conditions was dehydrated and dissolved in D2O (0.55 mL, 99.9%). Then, 1H NMR analysis was performed at 20 °C using a Varian Unity Bruker 600 MHz spectrometer (Bruker, Germany). The data were collected and analyzed using the MestReNova software.
4.11. Experimental Design
The type of the experimental design chosen is BBD, while RSM is the mathematical method used to analyze the results of the experimental design (i.e., finding the optimal conditions). The parameters ranges were fixed based on our preliminary experiments as follows: extraction pressure (6–10 bar), temperature (100–140 °C) and LSR (4–8 mL/g). However, a BBD with three variables in three levels was applied for evaluating the effect of the different factors on the extraction yield. To validate the theoretical accounts for the optimization process, an ANOVA was performed.
The optimal conditions were estimated using a second-order polynomial equation, and then three-dimensional response surface plots were drawn between each independent and dependent variables. The generalized form of the second-order polynomial equation is as follows
| 5 |
where Y is the response; Xi and Xj are variables; β0 is the model intercept coefficient; and βi, βii, and βij are the interaction coefficients of the linear, quadratic, and the second-order terms, respectively. All computations and graphics in this study were conducted using the statistical software Design Expert 7.0 and Origin 8.0.
Acknowledgments
The authors thank the participants for their involvement in this research. The authors also gratefully acknowledge the reviewers for providing valuable suggestions.
The authors declare no competing financial interest.
References
- Voragen A. G. J.; Coenen G. J.; Verhoef R. P.; Schols H. A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. 10.1007/s11224-009-9442-z. [DOI] [Google Scholar]
- Mohnen D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. 10.1016/j.pbi.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Hua X.; Wang K.; Yang R. J.; Kang J. Q.; Zhang J. N. Rheological properties of natural low-methoxyl pectin extracted from sunflower head. Food Hydrocolloids 2015, 44, 122–128. 10.1016/j.foodhyd.2014.09.026. [DOI] [Google Scholar]
- Kocaaga B.; Kurkcuoglu O.; Tatlier M.; Batirel S.; Guner F. S. Low-methoxyl pectin–zeolite hydrogels controlling drug release promote in vitro wound healing. J. Appl. Polym. Sci. 2019, 136, 47640 10.1002/app.47640. [DOI] [Google Scholar]
- Li M. Y.; Jin Y. X.; Wang Y. W.; Meng L.; Zhang N.; Sun Y.; Hao J. F.; Fu Q.; Sun Q. S. Preparation of bifidobacterium breve encapsulated in low methoxyl pectin beads and its effects on yogurt quality. J. Dairy Sci. 2019, 102, 4832–4843. 10.3168/jds.2018-15597. [DOI] [PubMed] [Google Scholar]
- Zidi D.; Gharsallaoui A.; Dupas-Farrugia C.; Attia H.; Ayadi M. A. Physicochemical and rheological changes of acidified camel milk added with commercial low methoxyl-pectin. Int. J. Biol. Macromol. 2019, 128, 347–353. 10.1016/j.ijbiomac.2018.12.244. [DOI] [PubMed] [Google Scholar]
- Nitta Y.; Yoshimura Y.; Ganeko N.; Ito H.; Okushima N.; Kitagawa M.; Nishinari K. Utilization of Ca2+-induced setting of alginate or low methoxyl pectin for noodle production from Japonica rice. LWT 2018, 97, 362–369. 10.1016/j.lwt.2018.07.027. [DOI] [Google Scholar]
- Li G. J.; Chang K. C. Viscosity and gelling characteristics of sunflower pectin as affected by chemical and physical factors. J. Agric. Food Chem. 1997, 45, 4785–4789. 10.1021/jf9708150. [DOI] [Google Scholar]
- Peng X.; Yang G.; Shi Y.; Zhou Y.; Zhang M.; Li S. Box-Behnken design based statistical modeling for the extraction and physicochemical properties of pectin from sunflower heads and the comparison with commercial low-methoxyl pectin. Sci. Rep. 2020, 10, 3595 10.1038/s41598-020-60339-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenborn D. P.; Xu M.; Chang K. C.; Schwarz J. G. Pigment removal and pectin loss during the continuous, countercurrent washing of sunflower heads. Trans. ASAE 1996, 39, 1781–1787. 10.13031/2013.27654. [DOI] [Google Scholar]
- Bianchi S.; Koch G.; et al. Hot water extraction of Norway spruce (Picea abies [Karst.]) bark: analyses of the influence of bark aging and process parameters on the extract composition. Holzforschung 2016, 70, 619–631. 10.1515/hf-2015-0160. [DOI] [Google Scholar]
- Wandee Y.; Uttapap D.; Mischnick P. Yield and structural composition of pomelo peel pectins extracted under acidic and alkaline conditions. Food Hydrocolloids 2019, 87, 237–244. 10.1016/j.foodhyd.2018.08.017. [DOI] [Google Scholar]
- Wang K.; Hua X.; Yang R. J.; Kang J. Q.; Zhang W. B. Hydrodynamic behavior and gelling properties of sunflower head pectin in the presence of sodium salts. Food Hydrocolloids 2014, 36, 238–244. 10.1016/j.foodhyd.2013.09.011. [DOI] [Google Scholar]
- Encalada A. M. I.; Perez C. D.; Flores S. K.; Rossetti L.; Fissore E. N.; Rojas A. M. Antioxidant pectin enriched fractions obtained from discarded carrots (Daucus carota L.) by ultrasound-enzyme assisted extraction. Food Chem. 2019, 289, 453–460. 10.1016/j.foodchem.2019.03.078. [DOI] [PubMed] [Google Scholar]
- Muñoz-Almagro N.; Rico-Rodriguez F.; Wilde P. J.; Montilla A.; Villamiel M. Structural and technological characterization of pectin extracted with sodium citrate and nitric acid from sunflower heads. Electrophoresis 2018, 39, 1984–1992. 10.1002/elps.201800130. [DOI] [PubMed] [Google Scholar]
- Wang D.; Zhang L.; Xu Y.; Qi X.; Wang X.; Wang X.; Zhang Q.; Li P. Optimization of an ultrasound-assisted extraction for simultaneous determination of antioxidants in sesame with response surface methodology. Antioxidants 2019, 8, 321 10.3390/antiox8080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharibzahedi S. M. T.; Smith B.; Guo Y. Pectin extraction from common fig skin by different methods: The physicochemical, rheological, functional, and structural evaluations. Int. J. Biol. Macromol. 2019, 136, 275–283. 10.1016/j.ijbiomac.2019.06.040. [DOI] [PubMed] [Google Scholar]
- Kang J.; Hua X.; Yang R. J.; Chen Y.; Yang H. Characterization of natural low-methoxyl pectin from sunflower head extracted by sodium citrate and purified by ultrafiltration. Food Chem. 2015, 180, 98–105. 10.1016/j.foodchem.2015.02.037. [DOI] [PubMed] [Google Scholar]
- Kruse A.; Dinjus E. Hot compressed water as reaction medium and reactant. J. Supercrit. Fluids 2007, 39, 362–380. 10.1016/j.supflu.2006.03.016. [DOI] [Google Scholar]
- Teoh W. H.; Mammucari R.; Vieira de Melo S. A. B.; Foster N. R. Solubility and solubility modeling of polycyclic aromatic hydrocarbons in subcritical water. Ind. Eng. Chem. Res. 2013, 52, 5806–5814. 10.1021/ie302124e. [DOI] [Google Scholar]
- Chen H. M.; Fu X.; Luo Z. G. Properties and extraction of pectin-enriched materials from sugar beet pulp by ultrasonic-assisted treatment combined with subcritical water. Food Chem. 2015, 168, 302–310. 10.1016/j.foodchem.2014.07.078. [DOI] [PubMed] [Google Scholar]
- Zhang J. X.; Wen C. T.; Chen M.; Gu J. Y.; Zhou J.; Duan Y. Q.; Zhang H. H.; Ma H. L. Antioxidant activities of Sagittaria sagittifolia L. polysaccharides with subcritical water extraction. Int. J. Biol. Macromol. 2019, 134, 172–179. 10.1016/j.ijbiomac.2019.05.047. [DOI] [PubMed] [Google Scholar]
- Liew S. Q.; Teoh W. H.; Tan C. K.; Yusoff R.; Ngoh G. C. Subcritical water extraction of low methoxyl pectin from pomelo (Citrus grandis (L.) Osbeck) peels. Int. J. Biol. Macromol. 2018, 116, 128–135. 10.1016/j.ijbiomac.2018.05.013. [DOI] [PubMed] [Google Scholar]
- Pasandide B.; Khodaiyan F.; Mousavi Z. E.; Hosseini S. S. Optimization of aqueous pectin extraction from Citrus medica peel. Carbohydr. Polym. 2017, 178, 27–33. 10.1016/j.carbpol.2017.08.098. [DOI] [PubMed] [Google Scholar]
- Getachew A. T.; Lee H. J.; Cho Y. J.; Chae S. J.; Chun B. S. Optimization of polysaccharides extraction from Pacific oyster (Crassostrea gigas) using subcritical water: structural characterization and biological activities. Int. J. Biol. Macromol. 2019, 121, 852–861. 10.1016/j.ijbiomac.2018.10.091. [DOI] [PubMed] [Google Scholar]
- Cvetanović A.; Švarc-Gajić J.; Zeković Z.; Gašić U.; Tešić Z.; Zengin G.; Mašković P.; Mahomoodally M. F.; Đurović S. Subcritical water extraction as a cutting edge technology for the extraction of bioactive compounds from chamomile: influence of pressure on chemical composition and bioactivity of extracts. Food Chem. 2018, 266, 389–396. 10.1016/j.foodchem.2018.06.037. [DOI] [PubMed] [Google Scholar]
- Liu J.; Chen P.; Yao W. J.; Wang J.; Wang L. Y.; Deng L. H.; He J.; Zhang G. F.; Lei J. D. Subcritical water extraction of betulinic acid from birch bark. Ind. Crops Prod. 2015, 74, 557–565. 10.1016/j.indcrop.2015.05.064. [DOI] [Google Scholar]
- Kim D. S.; Lim S. B. Subcritical water extraction of rutin from the aerial parts of common buckwheat. J. Supercrit. Fluids 2019, 152, 104561 10.1016/j.supflu.2019.104561. [DOI] [Google Scholar]
- Yang J. S.; Mu T. H.; Ma M. M. Optimization of ultrasound-microwave assisted acid extraction of pectin from potato pulp by response surface methodology and its characterization. Food Chem. 2019, 289, 351–359. 10.1016/j.foodchem.2019.03.027. [DOI] [PubMed] [Google Scholar]
- Liu J.; Li Y. C.; Liu W. Q.; Qi Q.; Hu X.; Li S. Y.; Lei J. D.; Rong L. Extraction of polysaccharide from Dendrobium nobile Lindl. by subcritical water extraction. ACS Omega 2019, 4, 20586–20594. 10.1021/acsomega.9b02550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchev I. N.; Kirtchev N. A.; Kratchanov C. Kinetic model of pectin extraction. Carbohydr. Polym. 1989, 11, 193–204. 10.1016/0144-8617(89)90050-7. [DOI] [Google Scholar]
- Hosseini S. S.; Khodaiyan F.; Yarmand M. S. Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties. Carbohydr. Polym. 2016, 140, 59–65. 10.1016/j.carbpol.2015.12.051. [DOI] [PubMed] [Google Scholar]
- Li W. J.; Fan Z. G.; Wu Y. Y.; Jiang Z. G.; Shi R. C. Eco-friendly extraction and physicochemical properties of pectin from jackfruit peel waste with subcritical water. J. Sci. Food Agric. 2019, 99, 5283–5292. 10.1002/jsfa.9729. [DOI] [PubMed] [Google Scholar]
- Prakash Maran J.; Sivakumar V.; Thirugnanasambandham K.; Sridhar R. Microwave assisted extraction of pectin from waste Citrullus lanatus fruit rinds. Carbohydr. Polym. 2014, 101, 786–791. 10.1016/j.carbpol.2013.09.062. [DOI] [PubMed] [Google Scholar]
- Asgari K.; Labbafi M.; Khodaiyan F.; Kazemi M.; Hosseini S. S. High-methylated pectin from walnut processing wastes as a potential resource: Ultrasound assisted extraction and physicochemical, structural and functional analysis. Int. J. Biol. Macromol. 2019, 1274–1282. 10.1016/j.ijbiomac.2019.10.224. [DOI] [PubMed] [Google Scholar]
- Iglesias M. T.; Lozano J. E. Extraction and characterization of sunflower pectin. J. Food. Eng. 2004, 62, 215–223. 10.1016/S0260-8774(03)00234-6. [DOI] [Google Scholar]
- Kazemi M.; Khodaiyan F.; Labbafi M.; Saeid Hosseini S.; Hojjati M. Pistachio green hull pectin: optimization of microwave-assisted extraction and evaluation of its physicochemical, structural and functional properties. Food Chem. 2019, 271, 663–672. 10.1016/j.foodchem.2018.07.212. [DOI] [PubMed] [Google Scholar]
- Xu X.Sunflower Head Extract as Corrosion Inhibitor for the Acid Pickling of Steel; North University of China, 2017. [Google Scholar]
- Corredig M.; Kerr W.; Wicker L. Molecular characterization of commercial pectins by separation with linear mix gel permeation columns in-line with multi-angle light scattering detection. Food Hydrocolloids 2000, 14, 41–47. 10.1016/S0268-005X(99)00044-2. [DOI] [Google Scholar]
- Wang W. J.; Ma X. B.; Jiang P.; Hu L. L.; Zhi Z. J.; Chen J. L.; Ding T.; Ye X. Q.; Liu D. H. Characterization of pectin from grapefruit peel: a comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocolloids 2016, 61, 730–739. 10.1016/j.foodhyd.2016.06.019. [DOI] [Google Scholar]
- Zhang M. L.; Zeng G. M.; Pan Y. Z.; Qi N. Difference research of pectins extracted from tobacco waste by heat reflux extraction and microwave-assisted extraction. Biocatal. Agric. Biotechnol. 2018, 15, 359–363. 10.1016/j.bcab.2018.06.022. [DOI] [Google Scholar]
- Zhang W.; Xie F.; Liu X. H.; Luo J.; Wu J. H.; Wang Z. W. Pectin from black tomato pomace characterization, interaction with gallotannin and emulsifying stability properties. Starch/Stärke 2019, 71, 1800172 10.1002/star.201800172. [DOI] [Google Scholar]
- Al-Amoudi R. H.; Taylan O.; Kutlu G.; Can A. M.; Sagdic O.; Dertli E.; Yilmaz M. T. Characterization of chemical, molecular, thermal and rheological properties of medlar pectin extracted at optimum conditions as determined by Box-behnken and Anfis models. Food Chem. 2019, 271, 650–662. 10.1016/j.foodchem.2018.07.211. [DOI] [PubMed] [Google Scholar]
- Zhou S.; Xu Y. B.; Wang C. H.; Tian Z. F. Pyrolysis behavior of pectin under the conditions that simulate cigarette smoking. J. Anal. Appl. Pyrolysis 2011, 91, 232–240. 10.1016/j.jaap.2011.02.015. [DOI] [Google Scholar]
- Wang X.; Chen Q. R.; Lü X. Pectin extracted from apple pomace and citrus peel by subcritical water. Food Hydrocolloids 2014, 38, 129–137. 10.1016/j.foodhyd.2013.12.003. [DOI] [Google Scholar]
- Lutz R.; Aserin A.; Wicker L.; Garti N. Structure and physical properties of pectins with block-wise distribution of carboxylic acid groups. Food Hydrocolloids 2009, 23, 786–794. 10.1016/j.foodhyd.2008.04.009. [DOI] [Google Scholar]
- Hosseini S. S.; Khodaiyan F.; Kazemi M.; Najari Z. Optimization and characterization of pectin extracted from sour orange peel by ultrasound assisted method. Int. J. Biol. Macromol. 2019, 125, 621–629. 10.1016/j.ijbiomac.2018.12.096. [DOI] [PubMed] [Google Scholar]
- Yapo B. M.; Robert C.; Etienne I.; Wathelet B.; Paquot M. Effect of extraction conditions on the yield, purity and surface properties of sugar beet pulp pectin extracts. Food Chem. 2007, 100, 1356–1364. 10.1016/j.foodchem.2005.12.012. [DOI] [Google Scholar]
- Kazemi M.; Khodaiyan F.; Hosseini S. S. Utilization of food processing wastes of eggplant as a high potential pectin source and characterization of extracted pectin. Food Chem. 2019, 294, 339–346. 10.1016/j.foodchem.2019.05.063. [DOI] [PubMed] [Google Scholar]
- Petkowicz C. L. O.; Vriesmann L. C.; Williams P. A. Pectins from food waste: extraction, characterization and properties of watermelon rind pectin. Food Hydrocolloids 2017, 65, 57–67. 10.1016/j.foodhyd.2016.10.040. [DOI] [Google Scholar]
- Yang B.; Prasad K. N.; Jiang Y. M. Structure identification of a polysaccharide purified from litchi (Litchi chinensis Sonn.) pulp. Carbohydr. Polym. 2016, 137, 570–575. 10.1016/j.carbpol.2015.10.088. [DOI] [PubMed] [Google Scholar]
- Barbieri S. F.; da Costa Amaral S.; Ruthes A. C.; de Oliveira Petkowicz C. L.; Kerkhoven N. C.; da Silva E. R. A.; Silveira J. L. M. Pectins from the pulp of gabiroba (Campomanesia xanthocarpa Berg): structural characterization and rheological behavior. Carbohydr. Polym. 2019, 214, 250–258. 10.1016/j.carbpol.2019.03.045. [DOI] [PubMed] [Google Scholar]
- Wang X.; Zhao X.; Lv Y.; Hu M.; Fan L.; Li Q.; Cai C.; Li G.; Yu G. Extraction, isolation and structural characterization of a novel polysaccharide from Cyclocarya paliurus. Int. J. Biol. Macromol. 2019, 132, 864–870. 10.1016/j.ijbiomac.2019.03.148. [DOI] [PubMed] [Google Scholar]
- Sarıçoban C.; Yilmaz M. T.; Karakaya M.; Tiske S. S. The effect of different levels of sunflower head pith addition on the properties of model system emulsions prepared from fresh and frozen beef. Meat Sci. 2010, 84, 186–195. 10.1016/j.meatsci.2009.08.046. [DOI] [PubMed] [Google Scholar]
- Pappas C. S.; Malovikova A.; Hromadkova Z.; Tarantilis P. A.; Ebringerova A.; Polissiou M. Determination of the degree of esterification of pectinates with decyl and benzyl ester groups by diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) and curve-fitting deconvolution method. Carbohydr. Polym. 2004, 56, 465–469. 10.1016/j.carbpol.2004.03.014. [DOI] [Google Scholar]