Abstract
The current investigation was aimed at in vivo MAOA inhibitory activity of coumarins angelicin, bergapten, and scopoletin isolated from the roots of Angelica archangelica. The isolated compounds were screened for MAOA (pdb ID 2Z5y) binding through molecular docking studies. The molecular docking results displayed that bergapten has a maximum affinity for MAOA, followed by angelicin and scopoletin. In silico prediction of physicochemical parameters indicated that maximum blood–brain barrier (BBB) permeability was observed with angelicin (2.3), followed by bergapten (2.0) and least with scopoletin (0.644). In consonance to the results of molecular docking studies, appreciable in vivo antidepressant activity of angelicin and bergaptan was observed over the mouse model of reserpine-induced depression. The modulation of MAOA in the antidepressant effect of extract and its isolated fractions was also determined. Biochemical examination of the brain tissue indicated that bergapten has maximum MAOA inhibitory activity while scopoletin fails to inhibit brain MAOA.
Introduction
Depression is an enfeebling mental disorder that has become a leading cause of disability and mortality worldwide with a previous year prevalence of 5%.1 It adversely affects the quality of life, reduces functional abilities, and is often associated with anxiety.2 It is a complex mental disorder associated with affective, cognitive, and physical symptoms characterized by reduced energy, loss of interest, and motivational symptoms such as anergia and fatigue.3 The pathophysiology of depression is very heterogenous and of pleiotropic nature. Anatomically, depression is characterized by disturbances in composite neuroplastic circuits and neuronal atrophy in brain.4 Several biochemical changes in the brain including biogenic amine imbalance, reduction in brain-derived neurotrophic factor (BDNF), increased oxidative stress, hypothalamic pituitary adrenal (HPA) axis perturbation, and generation of inflammatory cytokines mediate the disruption of synaptic transmission and neuronal plasticity. Drugs used for the therapeutic management of depression primarily target the monoaminergic pathways. Antidepressant agents are associated with adverse effects including metabolic, cardiovascular, and sleep disorders or aberrant behavior that are of serious concern.5 The increasing evidence of depression and its ill effects on society as well as the serious concerns with the use of conventional antidepressant drugs addresses a need to develop newer lead molecules with better tolerance and efficacy.
The plant Angelica archangelica (wild celery), belonging to family Apiaceae, is a perennial herb native of European and Asian countries. It is believed to have significant importance in the traditional herbal medicinal system.6 Preclinical evidence indicates the broad pharmacological potential of the A. archangelica plant including antianxiety activity, antitumor activity, hepatoprotective activity, antiepileptic activity, and antifungal activity.7,8 Phytochemical investigation revealed that it is a coumarin-rich plant mainly characterized by the presence of imperatorin, osthole, umbelliferone, and bergapten. The other nuclei found in this plant are monoterpenes such as R-pinen, δ-3-caren, limonene, β-phellandren, and R-phellandren.9,10 Chemically, coumarin is a 1,2-benzopyrone nucleus that forms pharmacologically active molecule and is documented to have neuroprotective, antihypertensive, anti-inflammatory, antibacterial, antifungal, antiviral, antioxidant, anticancer, antitubercular, anticonvulsant, antiadipogenic, anticoagulant, antihyperglycemic properties.11 The high pharmacological potential of coumarin may be attributed to its interaction with various cellular targets including matrix metalloproteinase, protein kinases, and oxidoreductase enzymes.12 Previous studies in our lab have also reported the analgesic and anti-inflammatory activity of coumarins in the acute as well as chronic pain models and fibromyalgia. The molecular insights into these activities suggested the interaction of coumarins with iNOS, COX, NMDA, and NFκB.13,14 Wszelaki et al. reported the in vitro cholinesterase inhibitory activity of various coumarins.15
In recent years, herbal molecules have gained intense research popularity. Coumarins have the possibility of becoming a lead molecule in the generation of efficacious novel agents for the therapeutic management of neuronal disorders. Therefore, the current investigation was aimed at in vivo MAOA inhibitory activity of isolated coumarins from the plant A. archangelica.
Results and Discussion
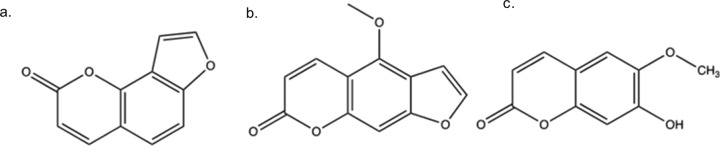
Extraction and Isolation of Coumarins
Dried roots of A. archangelica were subjected to Soxhlet extraction, and the purification over silica provided three compounds. The mass and nuclear magnetic resonance (NMR) spectral analyses of the isolated compounds assured the presence of coumarins (Figure 1a–c) in the extract. Compounds C (angelicin) and G (bergapten) were found to possess furanocoumarin nucleus, whereas compound H (scopoletin) was a simple coumarin. These findings were in line with the previous reports suggesting the presence of coumarins in the A. archangelica extract.6,15
Figure 1.
Chemical structure of compound isolated from (a) fraction C, (b) fraction G, and (c) fraction H.
In Silico Docking Studies
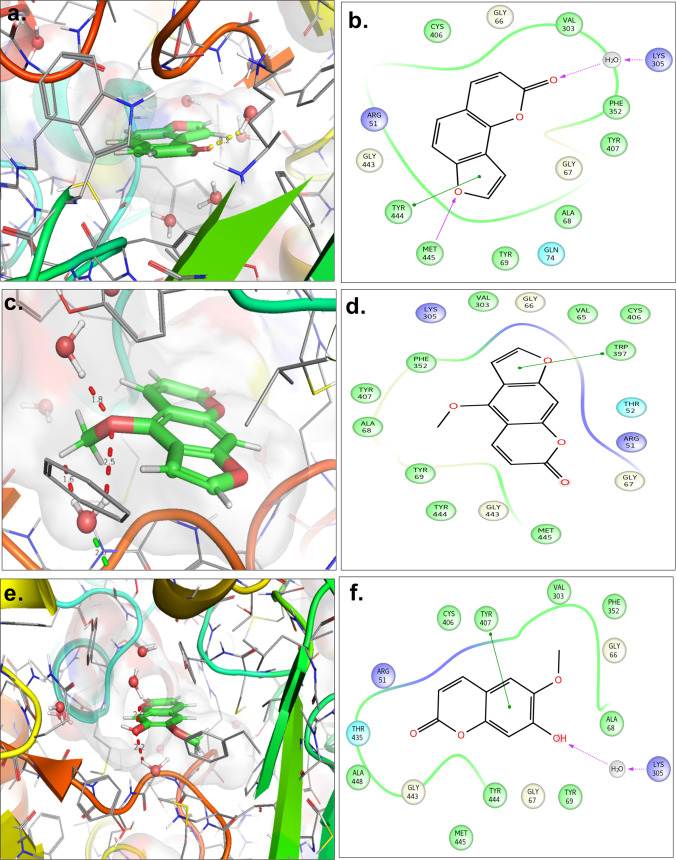
Prior to the in vivo experiments, molecular docking studies were performed to screen the binding affinities of angelicin, scopoletin, and bergapten into the active site of MAOA. The best ligand poses of the ligands including their corresponding ligand interaction diagrams are displayed in Figure 2. Angelicin forms a hydrogen bond with MET445 and H2O molecule present in the active site. It also exhibited π–π interaction with TYR444. This complex was having a G score of −6.25 kcal/mol and docking energy of −42.93 kcal/mol. Likewise, the docking results of scopoletin correspond to the G score of −7.93 kcal/mol and docking energy of −38.16 kcal/mol. It mainly showed H-bond with the water molecule and π–π interaction with TYR407. The best docking pose of bergapten was having two hydrogen bonds with the two water molecules and π interaction with TRP397 of MAO. This pose exhibited a G score of −6.50 kcal/mol and a docking energy of −38.25 kcal/mol.
Figure 2.
3D representation of compound C (a), compound G (c), and compound H (d) within the active site of MAOA; 2D molecular interactions of compound C (b), compound G (d), and compound H (e) within the active site of MAOA.
Prediction of Physicochemical Properties and ADME Parameters
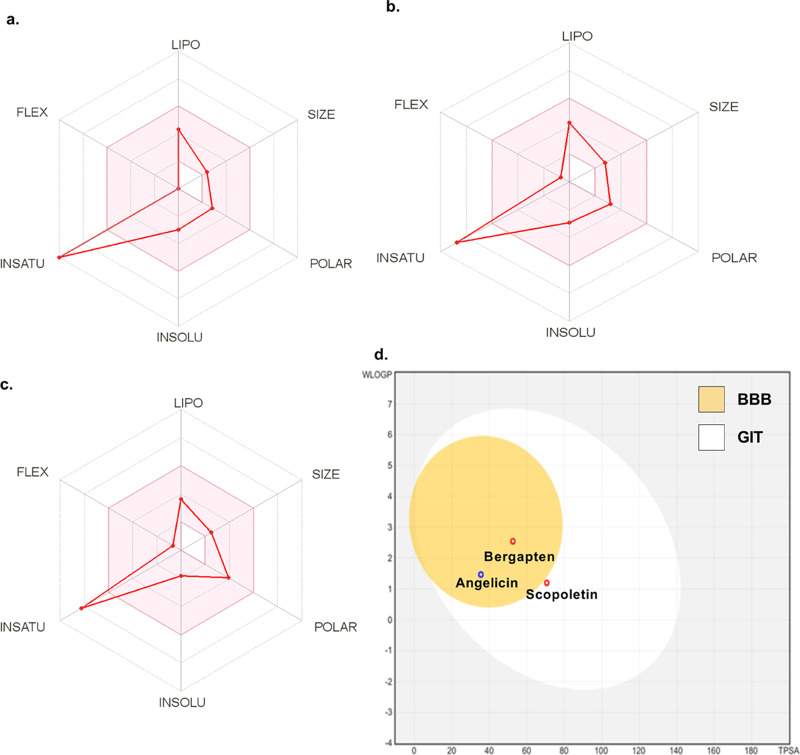
Swiss ADME tool was used to predict the physicochemical properties and ADME parameters of the three coumarins.16 All the three compounds pass Lipinski’s rule of five with minimum variation. The range of various properties (lipophilicity: XLOGP3 between −0.7 and +5.0; size: MW 150–500 g/mol; solubility: log S < 6; polarity: TPSA 20–130 Å2; flexibility: rotatable bonds >9; saturation: fraction of sp3-hybridized carbons in sp3 > 0.25) addressing bioavailability was represented by the pink area in bioavailability radar (Figure 3a–c). The radar indicates that all of the compounds fall under an optimum range of these physicochemical properties that points toward the drug-likeness behavior of these compounds. The drug-likeness behavior of these compounds can be graded as maximum for bergapten, followed by angelicin and least for scopoletin. The blood–brain barrier (BBB) permeability depicted as Conc.(brain)/Conc.(blood) was calculated using the preADMET tool (https://preadmet.bmdrc.kr/adme/). Among these three coumarins, the maximum BBB permeability was observed with angelicin (2.3), followed by bergapten (2.0) and least with scopoletin (0.644), as also depicted in the BOILED egg model (Figure 3d). The use of Swiss ADME tool for prediction of pharmacokinetic parameters has been well reported in the literature.17
Figure 3.
Bioavailability radar plots depicting predicted physicochemical parameters of angelicin (a), bergapten (b), and scopoletin (c). The optimum range for each parameter is shown by the pink area. The boiled egg plot (d) displaying the comparative prediction of BBB permeation ability and absorption of different compounds.
In Vivo Antidepressant Activity
The antidepressant activity of the extract and the coumarins isolated from the A. archangelica extract was explored in the mouse model of reserpine-induced depression. It is a well-established animal model used to evaluate the activity of the novel agents. Reserpine being a catecholamine depletory triggers neuronal monoaminergic dysfunction, leading to depression.18 In the current investigation, the mouse immobility time in forced swim test (FST) was measured as an indicator of depression. It was observed that the administration of reserpine at a dose of 0.5 mg/kg increased immobility time in the FST test compared to the control animal. Observations are in line with the literature reports.19 Further, we have observed that administration of the extract (400 mg/kg) and the test compounds bergapten and angelicin significantly reduced immobility time compared to the reserpine group, and the effect of these compounds was comparable to the standard drug clorgyline. However, no significant response was observed with scopoletin treatment, suggesting that scopoletin failed to alleviate reserpine-induced depression. A comparative analysis of the results indicated that the antidepressant potential of angelicin was higher than that of the extract, whereas the activity of bergapten was comparable to that of the extract and scopoletin was ineffective (Figure 4a). These observations of the in vivo studies were in agreement with the in silico prediction, suggesting that the least BBB permeability of scopoletin might be responsible for its ineffectiveness. The activity of the A. archangelica extract has been reported in other neuronal disorders such as anxiety and epilepsy.7,20 Coumarins are also well explored in the animal models of acute and chronic pain.13,14 We observed that the antidepressant activity of furanocoumarins (bergapten and angelicin) was higher than that of coumarin (scopoletin); furthermore, it has been observed that linear furanocoumarin (bergapten) is more efficacious than angular furanocoumarin (angelicin) in a mouse model of depression. These findings are in line with the previous reports suggesting the higher photosensitizing ability of linear furanocoumarin over coumarins.21 The low activity of scopoletin might be attributed to the presence of a polar anionic hydroxyl group, as suggested by previous reports.22,23
Figure 4.
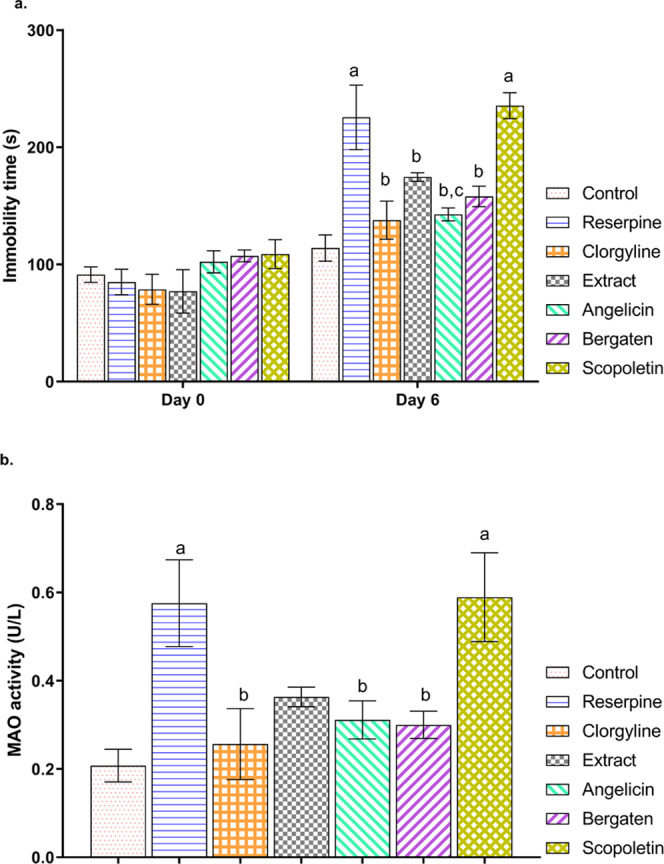
(a) Effect of extract and isolated fractions on the immobility activity (s) in FST and (b) on Brain MAOA time. Values are represented as mean ± SEM, analyzed by one-way/two-way ANOVA, followed by Tukey’s test. ap < 0.05 vs control, bp < 0.05 vs reserpine, cp < 0.05 vs extract.
Brain MAOA Activity
MAOA is a promising target of antidepressant drugs, and the MAO inhibitors are indicated in the therapeutic management of depression.24 We analyzed the activity of MAOA in the brain of mice. It was observed that there is a significant increase in the brain MAOA activity in the reserpine-exposed mice compared to the control mice. These results were evidenced by the previous reports suggesting increased MAOA levels in depressed subjects.25 Treatment with the A. archangelica extract and its isolated fractions angelicin and bergapten reduced the MAOA activity in comparison to the reserpine group. The results were comparable to the standard MAOA inhibitor clorgyline as well as with the reported coumarins and their derivatives.26,27 Scopoletin did not alter the reserpine-induced rise in the MAOA activity (Figure 4b). The results of biochemical analysis were in line with the behavioral examinations observed in FST.
Conclusions
In conclusion, we reported the isolation of compounds of the coumarin family from the A. archangelica extract. The root extract and the isolated compounds (angelicin and bergapten) displayed appreciable antidepressant activity in the mouse model of reserpine induced depression. Biochemical examinations of brain tissue suggested that these two compounds have a tendency to block the MAOA activity that could be responsible for their antidepressant effect in mice. The results of in vivo behavioral examinations and MAOA inhibitory activity of isolated coumarins were anticipated by the in silico docking and prediction analysis, indicating the maximum activity achieved with angelicin and bergapten, while the isolated scopoletin was found to be ineffective.
Materials and Methods
1H NMR spectra were recorded on 400 and 500 MHz NMR spectrometers (Bruker) with the corresponding recording of 13C NMR spectra at 100 and 125 MHz using tetramethylsilane as the internal standard and CDCl3 as the solvent. Chemical shifts are given in parts per million (ppm), and J values are given in hertz. Signals were represented as s—singlet, d—doublet, and dd—double–doublet. Mass spectra were recorded by HRMS (Bruker micrOTOF QII High-resolution mass spectrophotometer). Column chromatography was performed using silica gel (60–120 mesh) as the stationary phase. Bergapten was provided by Sugam Herbal Creation, Uttarakhand, India. Angelicin was purchased from Xenon Biosciences, India. Scopoletin, solvents, and other chemicals used were purchased from Sigma-Aldrich (India).
Plant Material
Roots of A. archangelica were obtained from Sri Venkateswara University, India. The plant material was identified and authenticated by Dr. A.S. Soodan, Professor, Department of Botanical and Environmental Sciences, Guru Nanak Dev University, Amritsar, Punjab, India (Ref no. 1012 Bot. & Env. Sc). Roots were cleaned thoroughly and dried under shade. These were ground to obtain a fine powder.
Extraction
The dried root powder was extracted over the soxhlet extractor using methanol as the solvent. The root material was extracted at 60 °C for 36 h. The extract was obtained by evaporating the solvent in a rota evaporator and freeze drying over a lyophilizer.
Isolation
The standardized extract was fractionated using column chromatography. The extract (3.7 g) was adsorbed over silica gel G, packed in a column, and eluted with a gradient solvent system of hexane and ethyl acetate. The column was eluted with hexane to remove hexane-soluble impurities followed by elution with a gradient solvent comprising 5% ethyl acetate in hexane. The fractions were pooled together on the basis of their thin-layer chromatography (TLC) profile. The TLC profiles of fractions were compared to the standard coumarins applied. The polarity of the eluting solvent was increased to 10% using ethyl acetate, and fraction pooling was done based on their TLC profile. The fractions obtained as a single spot in TLC were kept separate. The column elution was continued by increasing the polarity of the solvent to 15% with ethyl acetate, and the fractions were pooled together based on their TLC similarities. The chromatographic conditions are depicted in Figure 5. The pure fractions obtained were identified using mass spectroscopy, and structure elucidation was achieved through NMR spectroscopy.
Figure 5.
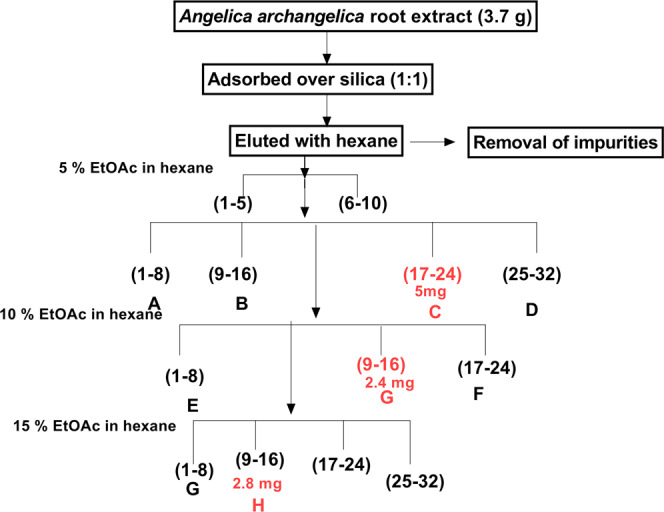
Schematic representation of column chromatography of the A. archangelica extract (EtOAc—ethyl acetate).
Characterization of Isolated Compounds
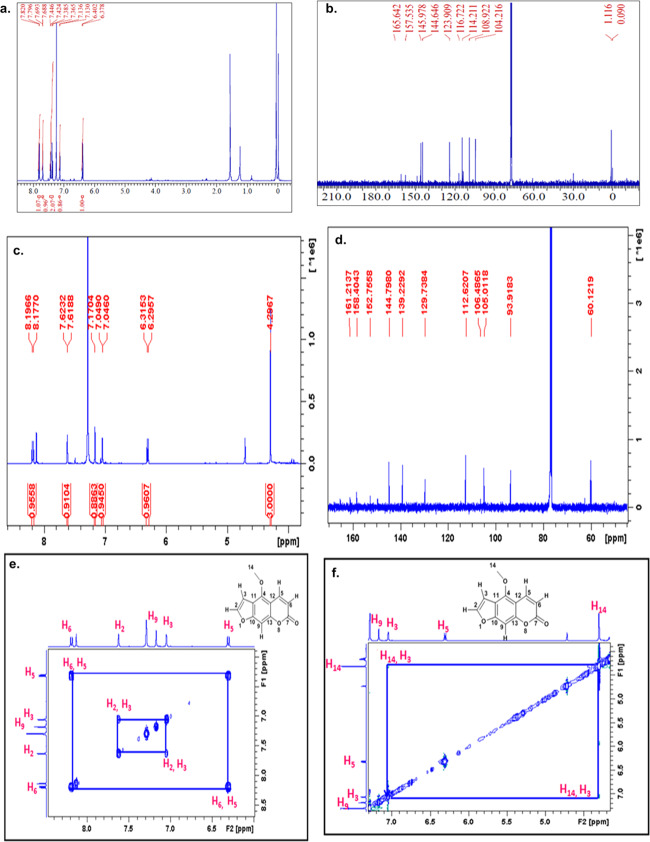
Compound C was isolated as a white solid; its molecular formula was established as C11H6O3 by an ion peak (m/z 225.6621), [M + K]+ and mp = 137–139.5 °C (lit. mp = 138–139 °C).281H NMR (400 MHz, CDCl3) δ 6.39 (d, J = 9.6 Hz, 1H), 7.13 (d, J = 2.3 Hz, 1H), 7.41 (dd, J = 8.3, 8.5 Hz, 2H), 7.69 (d, J = 2.3 Hz, 1H), 7.81 (d, J = 9.6 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 104.21 (CH), 108.92 (CH), 113.60 (ArC), 114.21 (CH), 116.77 (ArC), 123.90 (CH), 144.64 (CH), 145.97 (ArC), 157.53 (ArC), 161.31 (C=O) assured that compound C isolated is angelicin (Figure 6a,b). The percent content of isolated compound C, i.e., angelicin, was found to be 0.135% w/w. Compound G was isolated as needle-shaped crystals and showed a peak at m/z 239.0933, [M + Na]+, in HRMS that corresponds to bergapten. Further, NMR-based structure elucidation assured the identification of compound G as bergapten. 1H NMR (500 MHz, CDCl3) δ: 4.29 (s, 3H, CH3), 6.29–6.31 (d, J = 9.4 Hz, 1H, ArH), 7.04–7.05 (m, 1H, ArH), 7.17 (s, 1H, ArH), 7.61–7.62 (m, 1H, ArH), 8.17–8.19 (d, J = 9.4 Hz, ArH). 13C NMR (125 MHz, CDCl3) δ: 60.12, 93.91 (ArCH), 105.01 (ArCH), 106.48 (ArC), 112.62 (ArCH), 139.22 (ArCH), 144.79 (ArCH), 149.6 (ArC), 152.75 (ArC), 158.40 (ArC), 161.21 (C=O) (Figure 6c–f). These data correspond to bergapten. The percent content of isolated compound G, i.e., bergapten, was found to be 0.064% w/w. It is clear from the NOESY spectrum that the methoxy group (C14) shows correlation with one of the aromatic protons, which is (H3) δ: 7.04–7.05, hence eliminating the possibility of psoralen. The m/z peak, 231.1191 [M + K]+, of compound H corresponds to scopoletin. The structure of scopoletin was further assured by NMR data: 1H NMR (400 MHz, CDCl3, 25 °C, TMS) δ 3.96 (s, 3H), 6.13 (s, 1H), 6.24 (d, J = 9.1 Hz, 1H), 6.85 (s, 1H), 6.92 (s, 1H), 7.59 (d, J = 9.6 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 56.69 (OCH3), 103.12 (CH), 107.69 (CH), 113.37 (CH), 143.13 (CH) (Figure 7a,b). The percent content of isolated compound H, i.e., scopoletin, was found to be 0.075% w/w.
Figure 6.
(a) 1H NMR spectrum of isolated fraction C; (b) 13C NMR spectrum of isolated fraction C; (c) 1H NMR spectrum of isolated fraction G; (d) 13C NMR spectrum of isolated fraction G; (e) COSY spectrum of isolated fraction G; and (f) NOESY spectrum of isolated fraction G.
Figure 7.
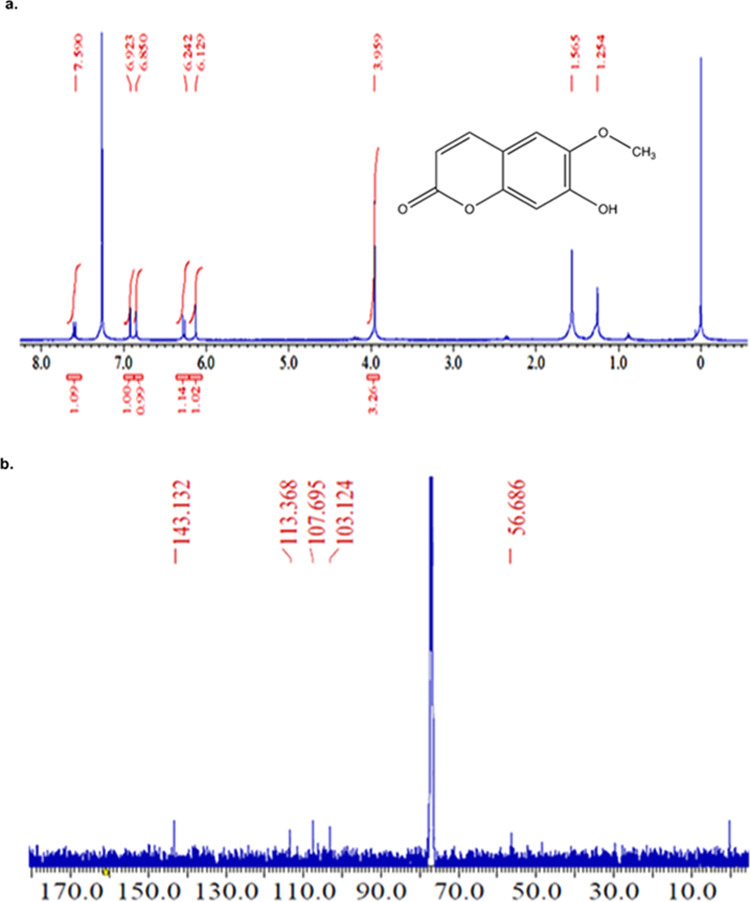
(a) 1H NMR spectrum of isolated fraction H and (b) 13C NMR spectrum of isolated fraction H.
In Silico Studies
The X-ray structure coordinates of human monoamine oxidase A (MAOA) (PDB entry: 2Z5Y; resolution, 2.17 Å) were downloaded from the Protein Data Bank. The X-ray structure consists of 513 amino acid chain sequencing complexed with 7-methoxy-1-methyl-9H-β-carboline (harmine) investigated previously.29 To explore the MAOA binding modes of compounds, we have docked at the binding site of harmine by defining the binding site with a radius of 6.0 Å. The preparation of the protein was performed employing Protein Preparation wizard as implemented in the Schrodinger suite. The structure of compounds were drawn on ChemDraw Ultra (2013), and its energy was minimized by MM2 force field in Chem 3D Ultra software and further optimized.30,31 All the compounds were used as protonated in aqueous solution and docked into the prepared binding site employing extra-precision mode, as implemented in glide software of Schrodinger. The best binding poses were selected based on their Glide scores, and thus ranked accordingly. These Glide scores are in kcal/mol. All of the above-said calculations were performed using the Schrodinger suite of programs.
Prediction of Physicochemical Properties and ADME Parameters
Swiss ADME tool is used to predict the physicochemical properties and ADME parameters of three compounds isolated from extract.16 The blood–brain barrier permeability depicted as (Conc.(brain)/Conc.(blood)) was calculated using the preADMET tool (https://preadmet.bmdrc.kr/adme/).
In Vivo Studies
Animals
Swiss albino mice weighing 25–30 g were procured from the Indian Institute of Integrative Medicine IIIM, Jammu, and were housed in the central animal house of the university at standard atmospheric conditions (25 ± 5 °C) with a 12 h light–dark cycle. The experimental protocols were approved by the Institutional Animal Ethics Committee, and the procedures were performed under ethical guidelines (226/CPSCEA2016/05).
Antidepressant Activity
The antidepressant activity was evaluated using a mouse model of reserpine-induced depression.14,32 The animals were subcutaneously administered with reserpine at a dose of 0.5 mg/kg for 3 consecutive days. The extract (400 mg/kg, po), isolated fractions [angelicin (10 mg/kg, ip); bergapten (10 mg/kg, ip); scopoletin (10 mg/kg, ip)], and standard drug (clorgyline, 3 mg/kg, ip) treatment was done continuously for a period of 5 days, 1 h before reserpine administration for the first 3 days. The animal behavior was evaluated before drug administration (day 0) and 24 h after the last dose of drug administration (day 6).
The Test
Forced swim test was used to evaluate the antidepressant activity of test compounds following the procedure already described in the literature.14,33 The depressive behavior in the mice was evaluated in terms of immobility time in the forced swim test. The results obtained were statistically analyzed using two-way ANOVA and were expressed as mean ± SEM.
In Vivo MAOA Activity
On the 6th day following behavioral analyses, the animals were sacrificed under light anesthesia (ketamine, 50 mg/kg, ip) and the brain tissues were isolated for the determination of MAOA activity using commercially available ELISA-based monoamine oxidase assay kit. The assay is based on the principle that one unit of MAO catalyzes the formation of 1 μM H2O2 per minute. The results were expressed as MAO activity (U/L), statistically analyzed using one-way ANOVA, and expressed as mean ± SEM using GraphPad Prism software (version 8.3.0).
Acknowledgments
The research was funded by the Department of Science and Technology, DST, India, under DST-SERB project awarded to R.B. (EMR/2016/005878). A.K. is thankful to the University Commission of India (UGC, India) for providing fellowship under UGC-MANF scheme (Award no. F1-17.1/2017-18/MANF-2017-18-PUN-84339). The authors gratefully acknowledge the infrastructure support provided under the university DST-PURSE scheme and UGC-RUSA scheme.
The authors declare no competing financial interest.
References
- Wang Y.-H.; Li J.-Q.; Shi J.-F.; Que J.-Y.; Liu J.-J.; Lappin J. M.; Leung J.; Ravindran A. V.; Chen W.-Q.; Qiao Y.-L.; et al. Depression and anxiety in relation to cancer incidence and mortality: a systematic review and meta-analysis of cohort studies. Mol. Psychiatry 2019, 10.1038/s41380-019-0595-x. [DOI] [PubMed] [Google Scholar]
- Wyman L.; Crum R. M.; Celentano D. Depressed mood and cause-specific mortality: a 40-year general community assessment. Ann. Epidemiol. 2012, 22, 638–643. 10.1016/j.annepidem.2012.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampogna G.; Del Vecchio V.; Giallonardo V.; Luciano M.; Fiorillo A. Diagnosis, Clinical Features, and Implications of Agitated Depression. Psychiatr. Clin. North Am. 2020, 43, 47–57. 10.1016/j.psc.2019.10.011. [DOI] [PubMed] [Google Scholar]
- Li Y.; Jia Y.; Wang D.; Zhuang X.; Li Y.; Guo C.; Chu H.; Zhu F.; Wang J.; Wang X.; et al. Programmed cell death 4 as an endogenous suppressor of BDNF translation is involved in stress-induced depression. Mol. Psychiatry 2020, 10.1038/s41380-020-0692-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanowska A. J.; Rao K. V.; Childress S.; El-Alfy A.; Matsumoto R. R.; Kelly M.; Stewart G. S.; Sufka K. J.; Hamann M. T. Secondary metabolites from three Florida sponges with antidepressant activity. J. Nat. Prod. 2008, 71, 186–189. 10.1021/np070371u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat Z.; Kumar D.; Shah M. Angelica archangelica Linn. is an angel on earth for the treatment of diseases. Int. J. Nutr., Pharmacol., Neurol. Dis. 2011, 1, 36. 10.4103/2231-0738.77531. [DOI] [Google Scholar]
- Kumar D.; Bhat Z. A.; Shah M. Anti-anxiety activity of successive extracts of Angelica archangelica Linn. on the elevated T-maze and forced swimming tests in rats. J. Tradit. Chin. Med. 2012, 32, 423–429. 10.1016/S0254-6272(13)60049-7. [DOI] [PubMed] [Google Scholar]
- Sigurdsson S.; Ögmundsdóttir H. M.; Hallgrimsson J.; Gudbjarnason S. Antitumour activity of Angelica archangelica leaf extract. In Vivo 2005, 19, 191–194. [PubMed] [Google Scholar]
- Härmälä P.; Kaltia S.; Vuorela H.; Hiltunen R. A furanocoumarin from Angelica archangelica. Planta Med. 1992, 58, 287–289. 10.1055/s-2006-961461. [DOI] [PubMed] [Google Scholar]
- Wedge D. E.; Klun J. A.; Tabanca N.; Demirci B.; Ozek T.; Baser K. H. C.; Liu Z.; Zhang S.; Cantrell C. L.; Zhang J. Bioactivity-guided fractionation and GC/MS fingerprinting of Angelica sinensis and Angelica archangelica root components for antifungal and mosquito deterrent activity. J. Agric. Food Chem. 2009, 57, 464–470. 10.1021/jf802820d. [DOI] [PubMed] [Google Scholar]
- Venugopala K. N.; Rashmi V.; Odhav B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed Res. Int. 2013, 2013, 963248 10.1155/2013/963248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.; Wang X.; Xu W.; Farzaneh F.; Xu R. The structure and pharmacological functions of coumarins and their derivatives. Curr. Med. Chem. 2009, 16, 4236–4260. 10.2174/092986709789578187. [DOI] [PubMed] [Google Scholar]
- Singh G.; Kaur A.; Kaur J.; Bhatti M. S.; Singh P.; Bhatti R. Bergapten inhibits chemically induced nociceptive behavior and inflammation in mice by decreasing the expression of spinal PARP, iNOS, COX-2 and inflammatory cytokines. Inflammopharmacology 2019, 27, 749–760. 10.1007/s10787-019-00585-6. [DOI] [PubMed] [Google Scholar]
- Kaur A.; Singh L.; Singh N.; Bhatti M. S.; Bhatti R. Ameliorative effect of imperatorin in chemically induced fibromyalgia: Role of NMDA/NFkB mediated downstream signaling. Biochem. Pharmacol. 2019, 166, 56–69. 10.1016/j.bcp.2019.05.012. [DOI] [PubMed] [Google Scholar]
- Wszelaki N.; Paradowska K.; Jamróz M. K.; Granica S.; Kiss A. K. Bioactivity-guided fractionation for the butyrylcholinesterase inhibitory activity of furanocoumarins from Angelica archangelica L. roots and fruits. J. Agric. Food Chem. 2011, 59, 9186–9193. 10.1021/jf201971s. [DOI] [PubMed] [Google Scholar]
- Daina A.; Michielin O.; Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznichenko O.; Quillévéré A.; Martins R. P.; Loaëc N.; Kang H.; Lista M. J.; Beauvineau C.; González-García J.; Guillot R.; Voisset C.; et al. Novel cationic bis (acylhydrazones) as modulators of Epstein–Barr virus immune evasion acting through disruption of interaction between nucleolin and G-quadruplexes of EBNA1 mRNA. Eur. J. Med. Chem. 2019, 178, 13–29. 10.1016/j.ejmech.2019.05.042. [DOI] [PubMed] [Google Scholar]
- Zhang S.; Liu X.; Sun M.; Zhang Q.; Li T.; Li X.; Xu J.; Zhao X.; Chen D.; Feng X. Reversal of reserpine-induced depression and cognitive disorder in zebrafish by sertraline and Traditional Chinese Medicine (TCM). Behav. Brain Funct. 2018, 14, 13 10.1186/s12993-018-0145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antkiewicz-Michaluk L.; Wąsik A.; Możdżeń E.; Romańska I.; Michaluk J. Antidepressant-like effect of tetrahydroisoquinoline amines in the animal model of depressive disorder induced by repeated administration of a low dose of reserpine: behavioral and neurochemical studies in the rat. Neurotoxic. Res. 2014, 26, 85–98. 10.1007/s12640-013-9454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak S.; Wanjari M.; Jain S.; Tripathi M. Evaluation of antiseizure activity of essential oil from roots of Angelica archangelica Linn. in mice. Indian J. Pharm. Sci. 2010, 72, 371. 10.4103/0250-474X.70487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri B.; Hall A.. Phytochemical Dictionary: A Handbook of Bioactive Compounds from Plants; CRC Press, 1998. [Google Scholar]
- Nigg H.; Nordby H.; Beier R.; Dillman A.; Macias C.; Hansen R. Phototoxic coumarins in limes. Food Chem. Toxicol. 1993, 31, 331–335. 10.1016/0278-6915(93)90187-4. [DOI] [PubMed] [Google Scholar]
- Penta S.Advances in Structure and Activity Relationship of Coumarin Derivatives; Academic Press, 2015. [Google Scholar]
- Finberg J. P.; Rabey J. M. Inhibitors of MAO-A and MAO-B in psychiatry and neurology. Front. Pharmacol. 2016, 7, 340 10.3389/fphar.2016.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J. H.; Ginovart N.; Boovariwala A.; Sagrati S.; Hussey D.; Garcia A.; Young T.; Praschak-Rieder N.; Wilson A. A.; Houle S. Elevated monoamine oxidase a levels in the brain: an explanation for the monoamine imbalance of major depression. Arch. Gen. Psychiatry 2006, 63, 1209–1216. 10.1001/archpsyc.63.11.1209. [DOI] [PubMed] [Google Scholar]
- Kong L. D.; Tan R. X.; Woo A. Y. H.; Cheng C. H. K. Inhibition of rat brain monoamine oxidase activities by psoralen and isopsoralen: implications for the treatment of affective disorders. Pharmacol. Toxicol. 2001, 88, 75–80. 10.1034/j.1600-0773.2001.d01-86.x. [DOI] [PubMed] [Google Scholar]
- Gnerre C.; Catto M.; Leonetti F.; Weber P.; Carrupt P.-A.; Altomare C.; Carotti A.; Testa B. Inhibition of monoamine oxidases by functionalized coumarin derivatives: biological activities, QSARs, and 3D-QSARs. J. Med. Chem. 2000, 43, 4747–4758. 10.1021/jm001028o. [DOI] [PubMed] [Google Scholar]
- Steck W.; Bailey B. Characterization of plant coumarins by combined gas chromatography, ultraviolet absorption spectroscopy, and nuclear magnetic resonance analysis. Can. J. Chem. 1969, 47, 3577–3583. 10.1139/v69-591. [DOI] [Google Scholar]
- Son S.-Y.; Ma J.; Kondou Y.; Yoshimura M.; Yamashita E.; Tsukihara T. Structure of human monoamine oxidase A at 2.2-Å resolution: the control of opening the entry for substrates/inhibitors. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 5739–5744. 10.1073/pnas.0710626105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins K. R.ChemDraw 6.0 Ultra CambridgeSoft Corporation, 100 Cambridge Park Drive, Cambridge, MA 02140. http://www.camsoft.com. Commercial Price: 1395. Academic Price: 699; ACS Publications, 2000. [Google Scholar]
- Lee C.; Yang W.; Parr R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785 10.1103/PhysRevB.37.785. [DOI] [PubMed] [Google Scholar]
- Park B.-K.; Kim Y. R.; Kim Y. H.; Yang C.; Seo C.-S.; Jung I. C.; Jang I.-S.; Kim S.-H.; Lee M. Y. Antidepressant-like effects of Gyejibokryeong-hwan in a mouse model of reserpine-induced depression. BioMed Res. Int. 2018, 2018, 5845491 10.1155/2018/5845491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt R. D.; Bertin A.; Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [PubMed] [Google Scholar]