Abstract
Most cooperative breeders live in discrete family groups, but in a minority, breeding populations comprise extended social networks of conspecifics that vary in relatedness. Selection for effective kin recognition may be expected for more related individuals in such kin neighbourhoods to maximize indirect fitness. Using a long-term social pedigree, molecular genetics, field observations and acoustic analyses, we examine how vocal similarity affects helping decisions in the long-tailed tit Aegithalos caudatus. Long-tailed tits are cooperative breeders in which help is typically redirected by males that have failed in their own breeding attempts towards the offspring of male relatives living within kin neighbourhoods. We identify a positive correlation between call similarity and kinship, suggesting that vocal cues offer a plausible mechanism for kin discrimination. Furthermore, we show that failed breeders choose to help males with calls more similar to their own. However, although helpers fine-tune their provisioning rates according to how closely related they are to recipients, their effort was not correlated with their vocal similarity to helped breeders. We conclude that although vocalizations are an important part of the recognition system of long-tailed tits, discrimination is likely to be based on prior association and may involve a combination of vocal and non-vocal cues.
This article is part of the theme issue ‘Signal detection theory in recognition systems: from evolving models to experimental tests’.
Keywords: cooperative breeding, kin recognition, kin discrimination, call similarity, acoustics
1. Introduction
Kin selection is often invoked to explain the evolution of cooperation in kin-structured communities and is expected to result in selection for some mechanism to discriminate kin from non-kin [1]. A recognition mechanism that permits the differential treatment of conspecifics according to their genetic similarity [2] enables individuals to avoid inbreeding [3,4] and maximize inclusive fitness [1,5] in populations where kin and non-kin associate beyond reproductive maturity. However, although the adaptive functions of kin recognition are well known, the proximate mechanisms, including sensory cues and cognitive thresholds, are often difficult to determine. Our current understanding of kin recognition in social animals is that discriminating individuals acquire cues to kinship from a referent (oneself, a subset of kin or the local environment), which are used to form internal templates [6] at a sensitive phase during development [7]. However, it may also be possible that cues and templates are genetically determined [8]. Templates are later compared with the phenotypes of encountered conspecifics, and discriminatory behaviour is performed based on the perceived similarity between templates and encountered phenotypes [9]. Thus, any cue that reliably covaries with relatedness may be used to discriminate kin from non-kin.
Most animal societies exhibit a substantial degree of kin structure, whereby individuals are organized in to more or less discrete family units of parents and their retained offspring [10–12]. If the probability of encountering a relative is high, individuals can maximize indirect fitness by indiscriminately cooperating within their group [13,14] and avoid inbreeding by selecting partners from outside the group [15]. In less viscous societies, such spatial cues to kinship may be unreliable. For example, in a small number of cooperatively breeding birds, cooperation occurs after natal dispersal, across extended networks of relatives known as kin neighbourhoods [16]. Here, the relatedness among spatially clustered individuals is less predictable, so kin recognition based on the phenotypic cues of potential social partners may be necessary [17]. In such situations, selection should favour effective discrimination, but any recognition system is prone to error because phenotypic cues overlap between non-kin and kin of varying relatedness owing to individual variation [6]. Thus, kin recognition is likely to involve a certain rate of acceptance errors, where non-kin are perceived as kin, and rejection errors, where kin are perceived as non-kin [2,6]. The accuracy of kin recognition, and hence the frequency of such errors, depends on their relative costs, which, in turn, is determined by the probability of encountering a relative and the fitness consequences of the associated behaviours [18]. This theoretical framework is supported empirically by intraspecific studies showing shifts in acceptance thresholds as the costs of error change [19], and by comparative analyses that demonstrate stronger kin discrimination in cooperatively breeding vertebrates where the benefits of helping are greater [20], and when the average relatedness within a group is lower and more variable [13].
Kin recognition often requires prior association; individuals learn the phenotypes of kin encountered during a sensitive phase and distinguish these familiar individuals from unfamiliar ones later in life [7]. Alternatively, recognition may involve phenotype matching, whereby individuals form a generalized template against which the phenotypes of unknown individuals are compared [21]. Phenotype matching does not require a period of previous association between matching individuals [22], but relies on a positive correlation between template–phenotype similarity and degree of genetic relatedness [23]. Whether kin are recognized through prior association or phenotype matching can be difficult to determine; both mechanisms involve matching phenotypes to learned cues, yet they differ in template specificity [6], such that mechanisms involving phenotype matching permit individuals to recognize unfamiliar kin and distinguish between kin of varying relatedness.
Kin recognition based on familiarity may often be sufficient for individuals to maximize inclusive fitness by directing help towards relatives, and prior association is indeed thought to be the most common mechanism of kin recognition in cooperatively breeding birds [24–26]. However, studies on long-tailed tits Aegithalos caudatus [27] and bell miners Manorina melanophrys [28], species in which helping occurs within kin neighbourhoods, found that helpers modify provisioning effort according to their degree of relatedness to recipient broods. In such situations, the risk of caring for non-kin is high, so kin recognition mechanisms with low error rates are likely to be selected for [13]. Moreover, finely tuned adjustment of provisioning behaviour in relation to kinship could indicate a relatively sophisticated mechanism of kin recognition that involves phenotype matching. Vocalizations are used as kin recognition cues in both species [29,30] and more widely in birds [31], although olfactory kin recognition has also been described in a few species [32–34]. In bell miners, a relationship between genetic relatedness and vocal similarity has been reported [30], but whether this relationship exists in other species remains to be tested.
This study aimed to identify the mechanism permitting kin-directed cooperation and flexible helper investment in long-tailed tits, a kin-neighbourhood cooperative breeder that exhibits effective kin recognition in the absence of spatial cues [35]. Helpers are failed breeders that redirect their care following unsuccessful attempts at independent breeding. Around 50% of successful nests receive help [36], typically from one or two helpers, but not all failed breeders choose to become helpers [37]. Although our study population is kin-structured during breeding, most neighbours are non-kin and help is directed towards close kin more often than expected by indiscriminate helping [38]. Furthermore, helpers provision more closely related broods at higher rates [27]. Helpers are overwhelmingly male, and gain indirect fitness benefits by increasing the productivity of related broods [39,40]. By contrast, no direct fitness benefits of helping have been identified [41,42]. Vocalizations play a major role in the coordination of cooperative behaviour [29]. Previous studies have demonstrated individuality in the churr call: a short-range contact call often used at the nest [43]. Playback and cross-fostering experiments have shown that individuals can recognize siblings using the churr call, and that these calls are learned during development [29]. However, whether churr call similarity is used to assess relatedness when making helping decisions remains untested.
Here, we quantified variation in churr call structure within and between adult long-tailed tits and determined which sound parameters explained this variation. We also tested for an association between call similarity and relatedness, and examined whether degree of vocal similarity influenced helping decisions by analysing the churr call similarity of helpers to the breeders they helped and to nearby breeders they did not help. Finally, we investigated whether long-tailed tit helpers adjust their provisioning effort according to how similar their churr calls are to the helped breeders.
2. Methods
(a). Study site and field methods
Fieldwork was carried out on a population of 31–46 breeding pairs of long-tailed tits in the Rivelin Valley, Sheffield, UK (53°38′ N 1°56 W) from 2015 to 2017. The site is approximately 2.5 km2 and comprises predominantly deciduous woodland, scrub and farmland. This population of ca 50 pairs (range: 18–72) has been studied extensively since 1994. The population is open: approximately 40% of breeders hatched in the study site, and are referred to as ‘native’ (A. E. Leedale and B. J. Hatchwell 2017, unpublished data), while the remaining ‘immigrant’ adults are assumed to have dispersed into the study area during their first winter, because individuals show high breeding site fidelity following their first breeding year [40]. Almost all individuals (ca 95%) were uniquely colour-ringed for field identification. Native birds were ringed as 11-day-old nestlings and immigrants were captured in mist nests and ringed under a British Trust for Ornithology licence during breeding. A sample of 5–30 µl of blood was taken by brachial venepuncture under Home Office licence. All nesting attempts were closely monitored to record breeding events and life-history traits such as timing of breeding, clutch size, incubation period and brood size, and the identity of parents and helpers. Nest locations were recorded using GPS receivers to an accuracy of 8 m. For most nests, provisioning behaviour was observed every 2 days from day 2 of the nestling period (day 0 = day of hatching; long-tailed tit broods hatch synchronously) to fledging (typically day 16 or 17) or until nest failure. Most observation periods lasted 1 h, during which the identities and visit rate of all carers were recorded. For further details of provisioning observations, see [27,39].
The churr call is disyllabic, consisting of an initial syllable of one or two unique elements, followed by a second syllable comprising a single element repeated several times [44]. The churr calls of adult carers were recorded at the nest using a Sennheiser ME67/K6 shotgun microphone onto a Roland R-05 v. 1.03 WAV/MP3 recorder, with a sample rate of 48 kHz, WAV-16bit accuracy, an input level of 60 dB and a low-cut frequency of 400 Hz. All recordings were made in dry conditions between 06.00 and 18.00 British summer time. Birds were recorded at a distance of approximately 3–15 m, to minimize the effects of sound degradation and reverberation. Birds were identified by their colour ring combinations. During recording, bird identity (ID) was dictated into the microphone after each call. In total, 213 recordings were made, containing 1116 churr calls from 98 individuals (mean = 11.39 ± 10.24 s.d. per bird; range 1–42).
(b). Bioacoustic analysis
Recordings were digitized with 16-bit accuracy at a sampling rate of 48 kHz. Spectrograms were produced in Avisoft SAS-Lab Pro v. 4.52 (Avisoft Bioacoustics, Raimund Specht, Berlin, Germany) using a 256-point fast Fourier transform length with a Hamming window, 100% frame size and 50% window overlap, generating a frequency resolution of 188 Hz and a time resolution of 2.7 ms. All recordings were visualized spectrographically to assess quality. Some background noise was removed using a high-pass filter of 1.5 kHz, though recordings with extreme background noise were excluded. The sampling frequency was converted to 22.05 kHz for further analysis. As long-tailed tit calls range from 2 to 9 kHz, this re-sampling does not affect the acoustic signal. All useable calls were stored and measured in Luscinia v. 2.16.10.29.01 (https://rflachlan.github.io/Luscinia/).
A subset of data was tested for individual repeatability. To determine the minimum number of calls required to capture individual variation, the cumulative repertoire size (number of distinct calls, based on number of syllables) was plotted against the number of calls considered to that point, for 100 churr calls, 10 from each of 10 birds recorded on at least 2 days in 2015. The resulting plots generally levelled off before the number of calls reached six (mean calls needed to reach asymptote = 5.5 ± 2.89 s.d., range 2–10). Therefore, repeatability tests were carried out on all calls from individuals with recordings of at least six calls from at least 2 days between 2015 and 2017: 907 churr calls from 54 individuals (mean = 17.46 ± 10.02 s.d. per bird; range 6–42). Within-individual repeatability was tested using two approaches. The first approach compared within and between-individual variation in overall call structure using dynamic time warping (DTW), implemented in Luscinia. The second tested the individual repeatability of specific vocal characteristics (defined in the electronic supplementary material, table S1).
DTW is a distance-based programming technique used to search for an optimal alignment of two signals, which has been implemented for use in bioacoustics. The algorithm calculates a distance score between signals based on certain acoustic features, with greater distance meaning lower similarity. The acoustic features used in the DTW analysis were weighted as: time, 1; fundamental frequency, 2; change in fundamental frequency, 2; compression factor, 0.1; minimum element length, 10; time s.d. weighting, 1; ArcTan transform weight for frequency slope, 0.02; maximum warp, 100%. These settings generated a DTW algorithm that correctly matched visually similar vocalizations, assessed using a dendrogram and multidimensional scaling plot. This is also in line with previous studies suggesting that frequency parameters show greater individuality than temporal parameters and are particularly important for kin recognition in this species [43]. Pairwise comparisons of individual calls generated a matrix of DTW scores for each pair of calls. To compare call similarity within and between individuals, pairwise comparisons were assigned a value according to whether the comparison was made between calls from the same individual (0) or from two individuals (1). The DTW scores were aggregated and the mean call similarity within and between individuals was compared. Because this analysis contained calls from across years, the measures of call similarity were also compared within and between years.
(c). Relatedness
Individuals were genotyped at 17 microsatellite loci. Genetic relatedness was estimated using coefficient of relatedness (rQG) [45] in SPAGeDi v. 1.1.5 [46]. This relatedness estimate is reliable when tested against our social pedigree [27]. For further details on genotyping, see [47,48]. The population allele frequencies used in analyses were generated using all genotyped individuals (1994–2017, n = 3304) in CERVUS v. 3.0.7 [49] to ensure non-zero frequencies for all alleles. To calculate social relatedness among dyads, an additive relationship matrix was generated from the social pedigree (1994–2017, n = 3068) in R v. 3.5.0 [50], using the nadiv package [51]. For further details on social and genetic relatedness estimates, see [38]. Hereafter, genetic relatedness refers to the rQG coefficients calculated from the microsatellite markers, whereas kinship refers to social relationships derived from the pedigree.
(d). Call similarity, relatedness and helping
Vocal similarity between individuals (1116 calls from 98 individuals) was quantified by: (i) DTW analysis and (ii) the difference in repeatable (R > 0.2) acoustic parameters (table 2), measured as Euclidean distances using the R package, spaa [52]. To investigate how vocal similarity varied with relatedness, we tested for a relationship between churr call similarity and both genetic relatedness and kinship. For the latter, three degrees of kinship were considered: first-order (r = 0.5), second-order (r = 0.25) or non-kin (r < 0.25); non-kin relationships included only those birds for which the parentage of both birds in the dyad was known.
Table 2.
The correlation between churr call dissimilarity and relatedness in long-tailed tits based on DTW analysis and the difference (Δ) in a range of acoustic parameters. The results and significance values from Mantel tests are reported for dyadic comparisons among breeders based on degree of kinship calculated from the social pedigree (n = 80), and genetic relatedness estimates (n = 88).
| measure of call dissimilarity | relatedness variable | Mantel R | 95% CI lower limit | 95% CI upper limit | p-value |
|---|---|---|---|---|---|
| DTW | kinship | −0.06 | −0.08 | −0.05 | 0.001 |
| Δ bandwidth (Hz) | kinship | −0.04 | −0.05 | −0.03 | 0.028 |
| Δ mean frequency (Hz) | kinship | −0.04 | −0.05 | −0.02 | 0.029 |
| Δ max. frequency (Hz) | kinship | −0.01 | −0.02 | 0.01 | 0.500 |
| Δ duration (ms) | kinship | −0.03 | −0.04 | −0.01 | 0.116 |
| DTW | genetic | −0.01 | −0.02 | 0.01 | 0.819 |
| Δ bandwidth (Hz) | genetic | −0.03 | −0.04 | −0.01 | 0.281 |
| Δ mean frequency (Hz) | genetic | 0.01 | −0.01 | 0.02 | 0.661 |
| Δ max. frequency (Hz) | genetic | 0.02 | 0.01 | 0.04 | 0.278 |
| Δ duration (ms) | genetic | −0.03 | −0.05 | −0.01 | 0.227 |
Helpers observed in 2015–2017 were related to male but not female parents of the broods they provisioned (see Results), so our analyses focused on helpers' vocal similarity to breeding males. If individuals use vocal similarity as a cue to relatedness, in order to direct helping effort towards close kin, helpers were expected to be more vocally similar to the breeders they helped than the breeders they did not help. For each helper, vocal similarity to male breeders at their first chosen nest in a given year (n = 19) was compared with their mean vocal similarity to a sample of potential males (excluding those helped) nesting within 750 m that year (n = 272), the range in which the majority of failed breeders travel to provide help (mean = 337.4 m ± 253.4 s.d., 95% confidence interval (CI) = 744.1 m, n = 220). Helping distance was calculated as the distance between a helper's last failed breeding attempt and the nest at which they first appeared as a helper in the same year.
To investigate whether helpers use vocal similarity to modify their provisioning effort, we tested for a relationship between the provisioning rates of helpers and their vocal similarity to the helped males. Because vocal similarity is a putative cue to relatedness [53], we also tested for a relationship between provisioning rate and relatedness, using genetic relatedness estimates and kinship from the social pedigree. Although the fitness consequences of helping depend on genetic relatedness, pedigree data are essential for understanding how accurately individuals are able to recognize kin, particularly when the mechanism depends on socially learned cues [29]. Provisioning rate was therefore expected to correlate most strongly with kinship.
(e). Statistical analysis
Statistical analyses were carried out using R v. 3.5.0 [50]. Overall similarity in call structure within and between individuals was compared using a generalized linear mixed-effects model (GLMM) fitted by restricted maximum likelihood (REML) in the lme4 package [54]. The square root of DTW distance score was fitted as the dependent variable, comparison type (within or between individuals) as a fixed effect and bird ID 1 and bird ID 2 as nested random effects. To test for differences across years, within or between years was also fitted as a fixed effect and year 1 and year 2 fitted as nested random effects. Individual repeatability based on call parameters was carried out using multiple GLMMs in the rptR package [55,56]. Year and ID were set as grouping variables, allowing for effects of year and ID to be tested. Gaussian models were used to test the repeatability of continuous variables and Poisson models were used to test the repeatability of count variables. To test for sex differences in call characteristics, GLMMs were built with each sound parameter as the dependent variable, sex as a fixed effect and bird ID and year as random effects. The significance of fixed effects was reported using Satterthwaite's degrees of freedom in the lmerTest package [57].
The relationships between vocal similarity and relatedness among adult breeders were analysed using Mantel tests based on Spearman correlations of ranked distances with 10 000 permutations using the R package, ecodist [58]. The relatedness of helpers to breeders that were helped and those that were not helped was compared using a Pearson's χ2 test for kinship data and a general linear model (GLM) fitted in lme4 for genetic relatedness estimates. Vocal similarity within observed helper–breeder dyads was compared to the mean vocal similarity within potential dyads for each focal helper using Wilcoxon signed-rank tests. Significance values were based on two-tailed tests and sample sizes are reported with the results. The vocal similarity of helpers to: (i) helped kin, (ii) helped non-kin and (iii) non-kin that were not helped was compared using a GLMM fitted with DTW score measured as a continuous variable with a Gamma distribution and log link, and helper ID as a random effect.
To investigate whether helper provisioning rates varied with respect to their vocal similarity to male breeders, we used linear mixed models fitted by REML. Genetic relatedness, kinship and vocal similarity were expected to be closely correlated, so their effect on provisioning rate was analysed in three separate models. In each model, the provisioning rate of helpers (number of visits h−1) was the response variable. In the first model, the explanatory variables were: nestling age, brood size, group size and genetic relatedness, all of which influence the provisioning rates of helpers [36]. In the second model, the explanatory variables were: nestling age, brood size, group size and kinship. In the third model, the explanatory variables were: nestling age, brood size, group size and vocal similarity. Genetic relatedness was the rQG estimate between helpers and male breeders, measured as a continuous variable. Kinship was the relationship between helpers and male breeders according to the social pedigree (three factor levels: r = 0, r = 0.25 and r = 0.5). Vocal similarity was the DTW score of churr calls between helpers and male breeders. Nestling age was measured in days from hatching (day 0). Brood size was the number of chicks in the nest on day 11, a good indicator of brood size from hatching because nestling starvation is rare [37]. Group size was the number of adults that provisioned a nest (parents and helpers). Bird identity and nest identity were included as random effects, to control for non-independence of repeated observations of feeding rates by the same birds, and repeated observations of feeding rates at the same nest. All explanatory covariates were initially included in full models and then dropped sequentially unless doing so significantly reduced the amount of variance explained, generating three minimum adequate models containing either genetic relatedness, kinship or vocal similarity as explanatory variables.
3. Results
(a). Individual repeatability
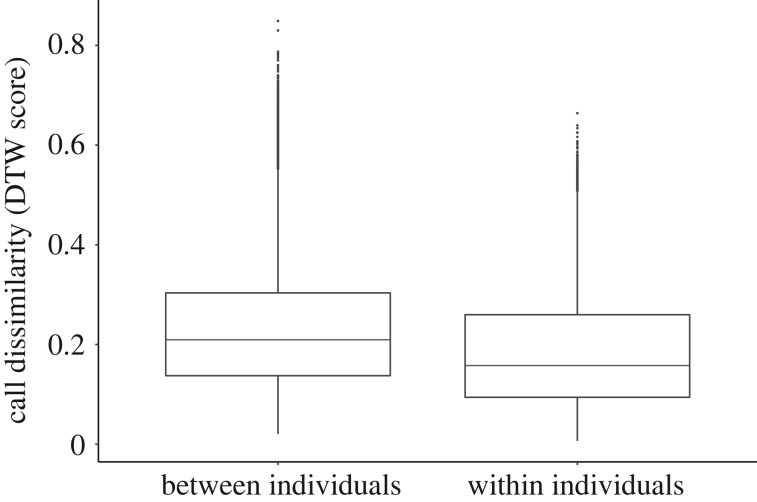
Visual inspection of spectrograms suggested that churr calls from the same individual were more similar in acoustic structure than those of different individuals. This was confirmed quantitatively, because the distance score from DTW for within-individual comparisons was significantly lower than that for between-individual comparisons (GLMM, estimate ± s.e. = −0.08 ± 0.008, t1561 = −9.9, p < 0.001; figure 1). Whether comparisons were made between calls recorded in the same or different years did not affect DTW distance score (GLMM, estimate = −0.003 ± 0.005, t4917 = −0.65, p = 0.55). Churr calls were repeatable within individuals based on all of the parameters tested, with maximum fundamental frequency across the churr call showing the greatest individual repeatability (table 1). There was no effect of recording year or sex on any of the parameters tested (electronic supplementary material, tables S2 and S3).
Figure 1.
Dissimilarity of the long-tailed tit churr call (n = 907 calls from 54 birds) within and between individuals, measured using distance scores generated by dynamic-time warping analysis in Luscinia.
Table 1.
Repeatability of long-tailed tit churr call parameters based on caller identity (n = 907 calls from 54 birds).
| call parameter | R ± s.e. | 95% CI | p-value |
|---|---|---|---|
| duration (ms) | 0.33 ± 0.05 | 0.22, 0.42 | <0.001 |
| number of repeats | 0.07 ± 0.02 | 0.02, 0.09 | <0.001 |
| fundamental frequency (Hz) | 0.29 ± 0.05 | 0.19, 0.38 | <0.001 |
| maximum fundamental frequency (Hz) | 0.5 ± 0.07 | 0.35, 0.61 | <0.001 |
| bandwidth (Hz) | 0.21 ± 0.05 | 0.13, 0.3 | <0.001 |
| Weiner entropy | 0.19 ± 0.04 | 0.11, 0.27 | <0.001 |
(b). Call similarity, relatedness and helping
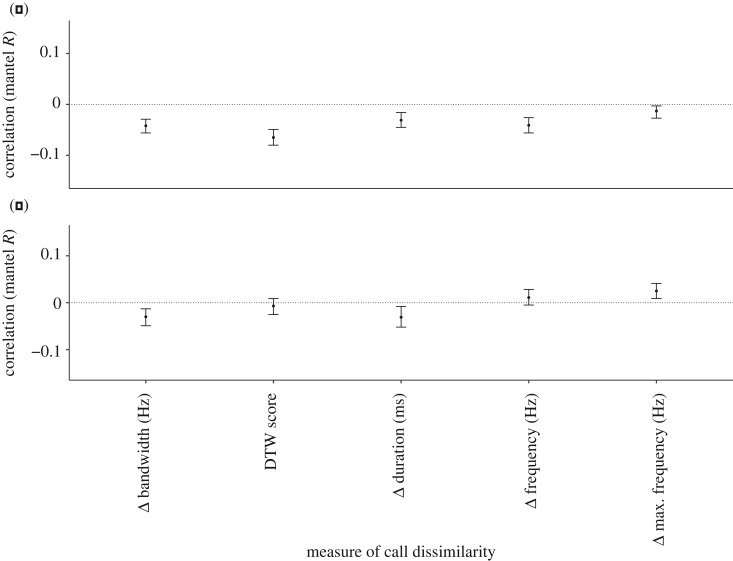
Although there was substantial variation in vocal similarity among breeders in all pedigree categories (electronic supplementary material, figure S1), churr call similarity correlated with kinship in several acoustic parameters: DTW score (Mantel test, R = −0.06, p < 0.01), difference in mean frequency (R = −0.04, p = 0.03) and difference in frequency bandwidth (R = −0.04, p = 0.03; figure 2a and table 2). By contrast, churr call similarity did not correlate with genetic relatedness (figure 2b and table 2).
Figure 2.
The relationship between churr call dissimilarity and relatedness in long-tailed tits based on DTW analysis (DTW score) and the difference (Δ) in a range of acoustic parameters. Mantel R correlations are shown for dyadic comparisons among breeders based on (a) degree of kinship calculated from the social pedigree (n = 80) and (b) genetic relatedness estimates (n = 88).
Based on the social pedigree 32% (6 out of 19) of helpers in 2015–2017 were known first-order relatives of the male, 16% (3 out of 19) were second-order relatives of the male and 55% (10 out of 19) were apparently unrelated to the male. Thus, the mean relatedness of helpers to male breeders from the social pedigree was r = 0.19 ± 0.2 s.d. (n = 19). The mean genetic relatedness of helpers to male breeders was r = 0.17 ± 0.2 s.d. (n = 15), showing that estimates of kinship from our pedigree closely match genetically estimated relatedness. By contrast, there were no cases of help given to known female kin, and the mean genetic relatedness of helpers to females was r = −0.04 ± 0.12 s.d. (n = 13). As expected, the kinship between helpers and males that they did not help within 750 m was significantly lower: 10.6% (n = 226) of relationships in which kinship was known were first-order kinships, 4.4% were second-order kinships and 84.9% dyads were unrelated (Pearson's χ2 test, χ2 = 17.3, p < 0.001). Likewise, the mean genetic relatedness of helpers to breeding males within 750 m that were not helped was r = 0.07 ± 0.18 s.d. (n = 272), significantly lower than that observed for males that were helped (GLM, t = 2.55, p = 0.01).
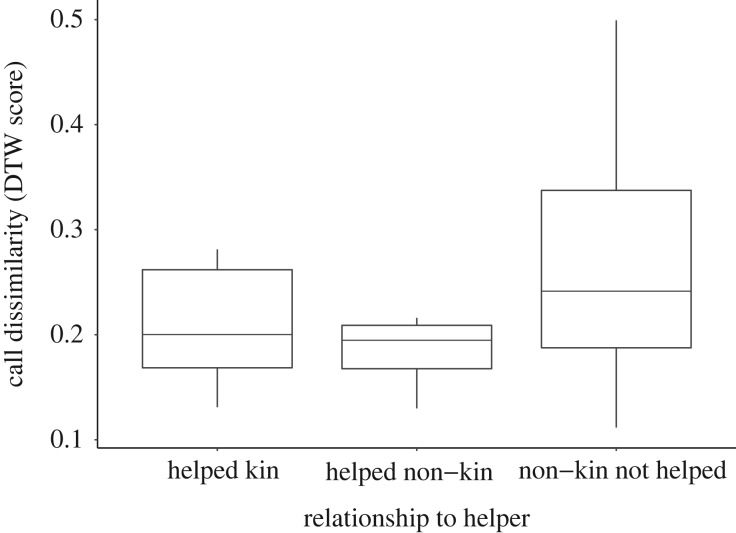
The decision of which male to help was positively associated with call similarity, as predicted. Failed breeders helped males that had more similar churr calls than those they did not help, based on DTW score (Wilcoxon signed-rank test: v = 20, n = 19, p < 0.01; table 3). Furthermore, there was no significant difference in the call similarity of helpers to helped kin and helped non-kin (GLMM: t = −0.29, n = 19, p = 0.77). By contrast, call similarity of helpers to non-kin that were helped was significantly greater than to non-kin that were not helped (GLMM: t = −2.52, n = 19, p = 0.01; figure 3). For full model outputs, see the electronic supplementary material, table S4.
Table 3.
Dissimilarity of churr calls between helpers and helped male breeders (n = 19) compared with the mean call dissimilarity of those helpers to the potential males they did not help (n = 272). Potential males were those breeding within 750 m of the helpers last failed nest in the same year. Call dissimilarity was measured using DTW analysis, and the difference (Δ) in a range of acoustic parameters.
| measure of call dissimilarity | helped males (n = 19 dyads) | potential males (n = 252 dyads) | Wilcoxon's signed rank |
|
|---|---|---|---|---|
| mean ± s.d. | mean ± s.d. | v | p-value | |
| DTW score | 0.21 ± 0.06 | 0.26 ± 0.06 | 20 | 0.002 |
| Δ bandwidth (Hz) | 149.54 ± 138.48 | 193.93 ± 103.52 | 61 | 0.18 |
| Δ mean frequency (Hz) | 274.07 ± 207.49 | 318.93 ± 117.59 | 63 | 0.21 |
| Δ max. frequency (Hz) | 243.61 ± 179.51 | 326.04 ± 126.57 | 47 | 0.05 |
| Δ duration (ms) | 25.68 ± 16.79 | 30.96 ± 11.21 | 60 | 0.17 |
Figure 3.
Dissimilarity of churr calls among helper–breeder dyads: helped kin, helpers and related (r ≥ 0.25) breeding males they helped (n = 9); helped non-kin, helpers and unrelated breeding males they helped (n = 8); and non-kin not helped, helpers and unrelated breeding males within helping range (less than or equal to 750 m) that they did not help (n = 218). Call dissimilarity was measured using DTW analysis. Boxplots represent median ± interquartile range. A full model output is reported in the electronic supplementary material, table S4.
Finally, we examined whether helper effort was modified according to relatedness and/or call similarity, analysing the provisioning data that were available for 14 of the 19 cases of helping. These included 41 observation periods of 14 helpers at 11 nests over 3 years (mean duration of observation = 180.1 min ± 145.3 s.d. nest−1, range = 1–8 h, mean feeding rate (visits h−1) = 5.05 ± 2.56 s.d. helper−1, range = 1–10.4). The provisioning rate of helpers increased with kinship between helper and male breeder (GLMM: χ2 = 5.68, p = 0.02), an effect that increased with group size (GLMM: χ2 = 11.61, p < 0.001). The provisioning rate of helpers also increased with genetic relatedness between helper and male breeder when group size was large (GLMM: χ2 = 5.94, p = 0.01). By contrast, the vocal similarity between helper and male breeder had no effect on helper provisioning rate (GLMM: χ2 = 0.01, p = 0.9). For full model comparisons, see the electronic supplementary material, table S5.
4. Discussion
Vocalizations are clearly a critical component of the kin-selected cooperative breeding system of long-tailed tits. Our results show, along with previous studies, that these calls are individual-specific [43] and that churr call similarity is positively associated with kinship [53], although this was the case for the social pedigree but not for genetic relatedness estimated from microsatellite data. This finding is consistent with previous experimental studies, indicating that churr calls are learned from provisioning adults in early development [29]. The sample of helpers included in this study showed a strong preference for kin relative to their availability, a finding that is again consistent with previous observational and experimental studies [35,38]. Importantly, we found that churr calls offer a potential mechanism to facilitate this kin preference because the calls of helpers were more similar to males they helped than to those they did not. Moreover, some helpers assisted unrelated recipients, and we found that call similarity between helpers and these non-kin recipients was greater than that between helpers and non-kin they could have helped. By contrast, there was no difference in the call similarity of helpers to kin and non-kin recipients. Finally, contrary to expectations, we found that although the provisioning effort of helpers was correlated with kinship, again supporting the findings of a previous study [27], helper effort was not predicted by call similarity to the helped male breeder.
Previous studies have revealed a strong preference for kin by helpers in long-tailed tits [35,38], as reported in many other cooperatively breeding vertebrates (e.g. [13,20,24,30,59]). Studies of other species have also shown that kin recognition is achieved using vocal cues (e.g. [60–62]), and this study provides further direct evidence that vocal similarity is the mechanism of kin recognition that permits kin-directed helping in long-tailed tits. However, there remains the persistent puzzle that a significant proportion of helpers in this species help broods to which they are unrelated [27,38,41], even though they appear to gain no benefit from doing so [42]. As vocalizations are learned in the nest, it is possible that helpers (whether related or unrelated) could gain future direct benefits through increasing the chances of being identified as kin by the grown offspring of the broods they helped. However, reciprocal helping is rare; in just 3.7% cases, did helpers choose to help breeders that had helped at their natal nest. Indeed, the high annual mortality rate provides little opportunity for reciprocity from helped broods [42], and most helping occurs among siblings [27,35]. It therefore seems unlikely that the opportunity to be identified as kin by helped broods could drive helper decisions. Instead, our results support an earlier suggestion that this counterintuitive behaviour arises from recognition errors [41].
The theoretical framework of the acceptance threshold model argues that an actor categorizes conspecifics depending on an acceptance threshold: a degree of template–phenotype dissimilarity below which it will accept and above which it will reject conspecifics as kin [6]. Our results suggest that long-tailed tits use degree of vocal similarity to recognize close kin, but also that their threshold for kin discrimination does lead to acceptance errors, with non-kin sometimes helped despite there being no known benefit of doing so [38,41,42]. There are two reasons why long-tailed tit helpers may be ‘generous’ with their help and inclined to make acceptance errors. First, although failed breeders may prefer to help close kin, given that relatives are clustered within the range that most helping occurs [38], there might still be a reasonable chance of gaining some indirect fitness by helping an unfamiliar individual because they could be more distantly related. This suggestion is supported by the finding of Leedale et al. [38] that the frequency of helping second-order relatives was as expected from random choice among nearby males. Secondly, Hatchwell et al. [41] argued that the costs of helping are low in long-tailed tits because there is no cost of lost breeding opportunity (all helpers are breeders that have failed to reproduce successfully) and help is provided for only a short period during the nestling and post-fledging stage. By contrast, the potential benefit of helping, via the increased recruitment of relatives is high. Therefore, a permissive threshold for acceptance of another individual as kin should be selected for [6]. The critical finding from that previous study [41] is that even with low mean relatedness between helpers and the brood they care for (r = 0.17), Hamilton's rule for the evolution of altruistic helping was satisfied.
Our finding that social pedigree was a better predictor of vocal similarity than genetic relatedness estimates was expected, given that churr calls are learned [29]. Several other species of cooperatively breeding birds have family- or group-specific vocalizations that are also consistent with this mechanism [60,63–66]. Learned kin recognition cues in any sensory modality are expected to be reliable if they are acquired at a time when associating individuals are close kin. In long-tailed tits, churr calls develop in the nest, learned from tutors that are likely to be first-order relatives, increasing vocal similarity among first-order kin relative to the general population [29]. Nevertheless, a continuous positive correlation between call similarity and relatedness, rather than a threshold effect, could arise if calls are learned from parents; for example, half-siblings that share one parent could be less vocally similar than full siblings that share two parents, or uncles, aunts and even cousins could conceivably retain some family-specific vocal traits. Although genetic relatedness estimates are reliable when tested against our social pedigree [27], the variation and overlap in genetic relatedness estimates for first-order, second-order and non-kin (electronic supplementary material, figure S1) may explain the weak correlation between vocal similarity and genetic relatedness estimates compared with pedigree kinship [53].
Our results do not exclude a genetic influence on vocal variation; indeed, high individual repeatability suggests some innate individual differences in long-tailed tits. Yet, any recognition system that relies entirely on genetically acquired cues may be susceptible to rejection errors because mutation and recombination would cause even close kin to be genetically dissimilar at some loci [67,68]. Genetic recognition cues have been reported in several non-avian taxa [69–70], but in social birds, kin recognition typically requires a critical period of learning during which recognition templates are formed [7]. However, very little is known about how socially learned recognition cues develop; for example, which adults act as tutors, or whether offspring can distinguish between helpers and parents during learning is unknown in any cooperative breeder. Further investigation into the learning and development of vocal cues in situations where there are multiple potential tutors is a worthwhile avenue for further study.
Familiarity is the most widely supported mechanism of kin recognition in cooperatively breeding birds [71,72], with kin association during extended brood care providing the sensitive period during which reliable recognition templates can form [7]. In long-tailed tits, it is very likely that first-order kin are associated during this crucial period, but there are instances in which this is not the case. First, extra-pair paternity occurs at low rates and results in half-siblings being raised together [73]. Second, offspring presumably acquire recognition templates from any second-order kin or non-kin that helped them as a nestling. Third, pair-bonds that last more than 1 year may produce full siblings that have not been raised together, although the high annual mortality rate [42], high divorce rate [74] and low probability of successful reproduction [39] together make this a rare event. But, despite these complicating factors, the pattern that long-tailed tits usually help at the nest of individuals with whom they have had close prior association [29,75] supports the idea that familiarity is the principal driver of helping decisions. Familiarity is also a stronger predictor of cooperative behaviour than genetic relatedness in Galápagos mockingbirds Nesomimus parvulus [24] and Seychelles warblers Acrocephalus sechellensis [26]. In the latter species, helpers provision the offspring of breeding females that provisioned them as a nestling, suggesting the context of prior association influences helper decisions [76].
Although kinship to male breeders explained a considerable amount of the variation in the provisioning rates of individual helpers, helpers did not adjust their provisioning rates according to vocal similarity to those breeders, suggesting that churr call similarity alone is not responsible for the fine-tuning of helping effort in relation to kinship. Therefore, although vocalizations may convey kinship cues, assessment of relatedness based solely on degree of call similarity is unlikely. This contrasts with studies of the bell miner, which identified ‘mew’ call similarity as the cue to relatedness that allows helpers to make fine-scale adjustments in their provisioning effort [28,30]. However, whether the reported relationship between provisioning effort and call similarity in bell miners is continuous or threshold-based is unclear. Bell miners live in complex societies in which membership of a coterie does not guarantee kinship and there is no evidence of a period of call learning, suggesting that ‘mew’ calls are innate [30]. Such genetically acquired cues would permit bell miners to recognize relatives in a population where familiarity does not signal kinship. By contrast, the social structure of long-tailed tits is relatively simple. The proximity of non-kin and kin of variable relatedness within breeding populations also necessitates active kin recognition, but the period of more or less exclusive association between close kin in early life provides an opportunity to learn the identity of kin that is missing in bell miners.
5. Conclusion
Our results indicate that vocal similarity is part of a combination of cues that allows individual long-tailed tits to recognize familiar individuals. The positive relationship between provisioning effort and relatedness to the brood may reflect a decision to help more familiar kin at a higher rate than less familiar individuals that are likely to be more distant kin. Discrimination based on prior association or familiarity would permit this adjustment. Regarding the precise cues used for discrimination, whether they are learned or genetic, a recognition mechanism that depends on variation in a single trait may be unstable; individuals bearing common cues are more likely to be accepted as kin than those with rare cues, leading to phenotypic convergence or fixation, and subsequent breakdown of the recognition system [77]. A recognition system based on multi-component kin ‘signatures’ would be less vulnerable to such processes.
Supplementary Material
Acknowledgements
We are grateful to all those who have contributed to the long-tailed tit project, and thank René van Dijk and Stuart Sharp for discussion. Molecular analyses were conducted at the NERC Biomolecular Analysis Facility at the University of Sheffield, with support from Terry Burke, Deborah Dawson, Natalie dos Remedios and Maria-Elena Mannarelli.
Ethics
An ethical review of licensed procedures was undertaken by the University of Sheffield's ASPA Ethical Review Process (Project Applications and Amendments Sub-Committee).
Data accessibility
The datasets and code supporting this article are available in the Dryad Digital Repository: https://doi.org/10.5061/dryad.sbcc2fr2p [78].
Authors' contributions
B.J.H. conceived and managed the long-tailed tit study and supervised the project with E.J.H.R. A.E.L. and B.J.H. designed the study and collected data. A.E.L. performed all analyses and wrote the manuscript. R.F.L. supported bioacoustic analysis.
Competing interests
We declare we have no competing interests.
Funding
This work was funded by the Natural Environment Research Council (NERC, UK, 1517208 and NE/I027118/1).
References
- 1.Hamilton WD. 1964. The genetical evolution of social behaviour (I and II). J. Theor. Biol. 7, 1–52. ( 10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 2.Sherman PW, Reeve HK, Pfennig DW. 1997. Recognition systems. In Behavioral ecology: an evolutionary approach (eds Krebs JR, Davies NB), pp. 69–96. Cambridge, UK: Blackwell Science Ltd. [Google Scholar]
- 3.Pusey AE, Wolf M. 1996. Inbreeding avoidance in animals. Trends Ecol. Evol. 11, 201–206. ( 10.1016/0169-5347(96)10028-8) [DOI] [PubMed] [Google Scholar]
- 4.Koenig WD, Haydock JL. 2004. Incest and incest avoidance. In Ecology and evolution of cooperative breeding in birds (eds Koenig WD, Dickinson JL), pp. 142–156. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 5.Bourke AFG. 2011. Principles of social evolution. Oxford series in ecology and evolution (eds Harvey PH., May RM., Godfray CH., Dunne JA). Oxford, UK: Oxford University Press. [Google Scholar]
- 6.Reeve HK. 1989. The evolution of conspecific acceptance thresholds. Am. Nat. 133, 407–435. ( 10.1086/284926) [DOI] [Google Scholar]
- 7.Komdeur J, Hatchwell BJ. 1999. Kin recognition: function and mechanism in avian societies. Trends Ecol. Evol. 14, 237–241. ( 10.1016/S0169-5347(98)01573-0) [DOI] [PubMed] [Google Scholar]
- 8.Queller DC, Ponte E, Bozzaro S, Strassmann JE. 2003. Single-gene Greenbeard effects in the social amoeba Dictyostelium discoideum. Science 299, 105–106. ( 10.1126/science.1077742) [DOI] [PubMed] [Google Scholar]
- 9.Lacy RC, Sherman PW. 1983. Kin recognition by phenotypic matching. Am. Nat. 121, 489–512. ( 10.1086/284078) [DOI] [Google Scholar]
- 10.Hatchwell BJ. 2009. The evolution of cooperative breeding in birds: kinship, dispersal and life history. Phil. Trans. R. Soc. B 364, 3217–3227. ( 10.1098/rstb.2009.0109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riehl C. 2013. Evolutionary routes to non-kin cooperative breeding in birds. Proc. R. Soc. B 280, 20132245 ( 10.1098/rspb.2013.2245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubenstein DR, Abbot P. 2017. Comparative social evolution. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 13.Cornwallis CK, West SA, Griffin AS. 2009. Routes to indirect fitness in cooperatively breeding vertebrates: kin discrimination and limited dispersal. J. Evol. Biol. 22, 2445–2457. ( 10.1111/j.1420-9101.2009.01853.x) [DOI] [PubMed] [Google Scholar]
- 14.Duncan C, Gaynor D, Clutton-Brock T, Dyble M. 2019. The evolution of indiscriminate altruism in a cooperatively breeding mammal. Am. Nat. 193, 841–851. ( 10.1086/703113) [DOI] [PubMed] [Google Scholar]
- 15.Varian-Ramos CW, Webster MS. 2012. Extrapair copulations reduce inbreeding for female red-backed fairy-wrens, Malurus melanocephalus. Anim. Behav. 83, 857–864. ( 10.1016/j.anbehav.2012.01.010) [DOI] [Google Scholar]
- 16.Dickinson JL, Hatchwell BJ. 2004. Fitness consequences of helping. In Ecology and evolution of cooperative breeding in birds (eds Koenig WD, Dickinson JL), pp. 48–66. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 17.Gamboa GJ, Reeve HK, Holmes WG. 1991. Conceptual issues and methodology in kin-recognition research—a critical discussion. Ethology 88, 109–127. ( 10.1111/j.1439-0310.1991.tb00267.x) [DOI] [Google Scholar]
- 18.Agrawal A. 2001. Kin recognition and the evolution of altruism. Proc. R. Soc. Lond. B 268, 1099–1104. ( 10.1098/rspb.2001.1611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Downs SG, Ratnieks FLW. 2000. Adaptive shifts in honey bee (Apis mellifera L.) guarding behavior support predictions of the acceptance threshold model. Behav. Ecol. 11, 326–333. ( 10.1093/beheco/11.3.326) [DOI] [Google Scholar]
- 20.Griffin AS, West SA. 2003. Kin discrimination and the benefit of helping in cooperatively breeding vertebrates. Science 302, 634–636. ( 10.1126/science.1089402) [DOI] [PubMed] [Google Scholar]
- 21.Holmes WG, Sherman PW. 1983. Kin recognition in animals. Am. Sci. 71, 46–55. [Google Scholar]
- 22.Tang-Martinez Z. 2001. The mechanisms of kin discrimination and the evolution of kin recognition in vertebrates: a critical re-evaluation. Behav. Process. 53, 21–40. ( 10.1016/S0376-6357(00)00148-0) [DOI] [PubMed] [Google Scholar]
- 23.Mateo JM. 2004. Recognition systems and biological organization: the perception component of social recognition. Ann. Zool. Fenn. 41, 729–745. [Google Scholar]
- 24.Curry RL. 1988. Influence of kinship on helping behaviour of Galápagos mockingbirds. Behav. Ecol. Sociobiol. 22, 141–152. ( 10.1007/BF00303549) [DOI] [Google Scholar]
- 25.Hatchwell BJ, Ross DJ, Fowlie MK, McGowan A. 2001. Kin discrimination in cooperatively breeding long-tailed tits. Proc. R. Soc. Lond. B 268, 885–890. ( 10.1098/rspb.2001.1598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komdeur J, Richardson DS, Burke T. 2004. Experimental evidence that kin discrimination in the Seychelles warbler is based on association and not on genetic relatedness. Proc. R. Soc. Lond. B 271, 963–969. ( 10.1098/rspb.2003.2665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nam K-B, Simeoni M, Sharp SP, Hatchwell BJ. 2010. Kinship affects investment by helpers in a cooperatively breeding bird. Proc. R. Soc. B 277, 3299–3306. ( 10.1098/rspb.2010.0737) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright J, McDonald PG, te Marvelde L, Kazem AJN, Bishop CM. 2010. Helping effort increases with relatedness in bell miners, but ‘unrelated’ helpers of both sexes still provide substantial care. Proc. R. Soc. B 227, 437–445. ( 10.1098/rspb.2009.1360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharp SP, McGowan A, Wood MJ, Hatchwell BJ. 2005. Learned kin recognition cues in a social bird. Nature 434, 1127–1130. ( 10.1038/nature03522) [DOI] [PubMed] [Google Scholar]
- 30.McDonald PG, Wright J. 2011. Bell miner provisioning calls are more similar among relatives and are used by helpers at the nest to bias their effort towards kin. Proc. R. Soc. B 278, 3403–3411. ( 10.1098/rspb.2011.0307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bradbury JW, Vehrencamp SL. 1998. Principles of animal communication. Sunderland, MA: Sinauer. [Google Scholar]
- 32.Coffin HR, Watters JV, Mateo JM. 2011. Odour-based recognition of familiar and related conspecifics: a first test conducted on captive Humboldt penguins (Spheniscus humboldti). PLoS ONE 6, 1–4. ( 10.1371/journal.pone.0025002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krause ET, Kruger O, Kohlmeier P, Caspers BA. 2012. Olfactory kin recognition in a songbird. Biol. Lett. 8, 327–329. ( 10.1098/rsbl.2011.1093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonadonna F, Sanz-Aguilar A. 2012. Kin recognition and inbreeding avoidance in wild birds: the first evidence for individual kin-related odour recognition. Anim. Behav. 84, 509–513. ( 10.1016/j.anbehav.2012.06.014) [DOI] [Google Scholar]
- 35.Russell AF, Hatchwell BJ. 2001. Experimental evidence for kin-biased helping in a cooperatively breeding vertebrate. Proc. R. Soc. Lond. B 268, 2169–2174. ( 10.1098/rspb.2001.1790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hatchwell BJ, Sharp SP, Beckerman AP, Meade J. 2013. Ecological and demographic correlates of helping behaviour in a cooperatively breeding bird. J. Anim. Ecol. 82, 486–494. ( 10.1111/1365-2656.12017) [DOI] [PubMed] [Google Scholar]
- 37.MacColl ADC, Hatchwell BJ. 2004. Determinants of lifetime fitness in a cooperative breeder, the long-tailed tit Aegithalos caudatus. J. Anim. Ecol. 73, 1137–1148. ( 10.1111/j.0021-8790.2004.00887.x) [DOI] [Google Scholar]
- 38.Leedale AE, Sharp SP, Simeoni M, Robinson EJH, Hatchwell BJ. 2018. Fine-scale genetic structure and helping decisions in a cooperatively breeding bird. Mol. Ecol. 27, 1714–1726. ( 10.1111/mec.14553) [DOI] [PubMed] [Google Scholar]
- 39.Hatchwell BJ, Russell AF, MacColl ADC, Ross DJ, Fowlie MK, McGowan A. 2004. Helpers increase long-term but not short-term productivity in cooperatively breeding long-tailed tits. Behav. Ecol. 15, 1–10. ( 10.1093/beheco/arg091) [DOI] [Google Scholar]
- 40.McGowan A, Hatchwell BJ, Woodburn RJW. 2003. The effect of helping behaviour on the survival of juvenile and adult long-tailed tits Aegithalos caudatus. J. Anim. Ecol. 72, 491–499. ( 10.1046/j.1365-2656.2003.00719.x) [DOI] [Google Scholar]
- 41.Hatchwell BJ, Gullett PR, Adams MJ. 2014. Helping in cooperatively breeding long-tailed tits: a test of Hamilton's rule. Phil. Trans. R. Soc. B 369, 20130565 ( 10.1098/rstb.2013.0565) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meade J, Hatchwell BJ. 2010. No direct fitness benefits of helping in a cooperative breeder despite higher survival of helpers. Behav. Ecol. 21, 1186–1194. ( 10.1093/beheco/arq137) [DOI] [Google Scholar]
- 43.Sharp SP, Hatchwell BJ. 2006. Development of family specific contact calls in the long-tailed tit Aegithalos caudatus. Ibis 148, 649–656. ( 10.1111/j.1474-919X.2006.00568.x) [DOI] [Google Scholar]
- 44.Sharp SP, Hatchwell BJ. 2005. Individuality in the contact calls of cooperatively breeding long-tailed tits (Aegithalos caudatus). Behaviour 142, 1559–1575. ( 10.1163/156853905774831918) [DOI] [Google Scholar]
- 45.Queller DC, Goodnight KF. 1989. Estimating relatedness using genetic markers. Evolution 43, 258–275. ( 10.1111/j.1558-5646.1989.tb04226.x) [DOI] [PubMed] [Google Scholar]
- 46.Hardy OJ, Vekemans X. 2002. SPAGeDi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol. Ecol. Notes 2, 618–620. ( 10.1046/j.1471-8286.2002.00305.x) [DOI] [Google Scholar]
- 47.Simeoni M, Dawson DA, Ross DJ, Châline N, Burke T, Hatchwell BJ. 2007. Characterization of 20 microsatellite loci in the long-tailed tit Aegithalos caudatus (Aegithalidae, AVES). Mol. Ecol. Notes 7, 1319–1322. ( 10.1111/j.1471-8286.2007.01868.x) [DOI] [Google Scholar]
- 48.Adams MJ, Robinson MR, Mannarelli M-E, Hatchwell BJ. 2015. Social genetic and social environment effects on parental and helper care in a cooperatively breeding bird. Proc. R. Soc. B 282, 20150689 ( 10.1098/rspb.2015.0689) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalinowski ST, Taper ML, Marshall TC. 2007. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 16, 1099–1106. ( 10.1111/j.1365-294X.2007.03089.x) [DOI] [PubMed] [Google Scholar]
- 50.R Core Team 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 51.Wolak ME. 2012. nadiv: an R package to create relatedness matrices for estimating non-additive genetic variances in animal models. Methods Ecol. Evol. 3, 792–796. ( 10.1111/j.2041-210X.2012.00213.x) [DOI] [Google Scholar]
- 52.Zhang J. 2016. spaa: SPecies Association Analysis. R package version 0.2.1 See https://CRAN.R-project.org/package=spaa.
- 53.Leedale AE. 2018. Functions and mechanisms of kin recognition in long-tailed tits. PhD thesis, University of Sheffield, Sheffield, UK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 55.Nakagawa S, Schielzeth H. 2010. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev. 85, 935–956. ( 10.1111/j.1469-185x.2010.00141.x) [DOI] [PubMed] [Google Scholar]
- 56.Schielzeth H, Nakagawa S, Stoffel M. 2017. rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Meth. Ecol. Evol. 8, 1639–1644. ( 10.1111/2041-210x.12797) [DOI] [Google Scholar]
- 57.Kuznetsova A, Brockhoff PB, Christensen RHB. 2017. lmerTest package: tests in linear mixed effects models. J Stat. Softw. 82, 1–26. ( 10.18637/jss.v082.i13) [DOI] [Google Scholar]
- 58.Goslee S, Urban D. 2007. The ECODIST package for dissimilarity-based analysis of ecological data. J Stat. Softw. 22, 1–19. ( 10.18637/jss.v022.i07) [DOI] [Google Scholar]
- 59.Emlen ST, Wrege PH. 1988. The role of kinship in helping decisions among white-fronted bee-eaters. Behav. Ecol. Sociobiol. 23, 305–315. ( 10.1007/BF00300577) [DOI] [Google Scholar]
- 60.Hopp SL, Jablonski P, Brown JL. 2001. Recognition of group membership by voice in Mexican jays, Aphelocoma ultramarina. Anim. Behav. 62, 297–303. ( 10.1006/anbe.2001.1745) [DOI] [Google Scholar]
- 61.Payne RB, Payne LL, Rowley I. 1988. Kin and social relationships in splendid fairy-wrens: recognition by song in a cooperative bird. Anim. Behav. 36, 1341–1351. ( 10.1016/S0003-3472(88)80203-3) [DOI] [Google Scholar]
- 62.Price JJ. 1998. Family- and sex-specific vocal traditions in a cooperatively breeding songbird. Proc. R. Soc. Lond. B 265, 497–502. ( 10.1098/rspb.1998.0322) [DOI] [Google Scholar]
- 63.Akçay Ç, Hambury KL, Arnold JA, Nevins AM, Dickinson JL. 2014. Song sharing with neighbours and relatives in a cooperatively breeding songbird. Anim. Behav. 92, 55–62. ( 10.1016/j.anbehav.2014.03.029) [DOI] [Google Scholar]
- 64.Akçay Ç, Swift RJ, Reed VA, Dickinson JL. 2013. Vocal kin recognition in kin neighborhoods of western bluebirds. Behav. Ecol. 24, 898–905. ( 10.1093/beheco/art018) [DOI] [Google Scholar]
- 65.Radford AN. 2005. Group-specific vocal signatures and neighbour-stranger discrimination in the cooperatively breeding green woodhoopoe. Anim. Behav. 70, 1227–1234. ( 10.1016/j.anbehav.2005.04.002) [DOI] [Google Scholar]
- 66.Crane JMS, Pick JL, Tribe AJ, Vincze E, Hatchwell BJ, Russell AF. 2015. Chestnut-crowned babblers show affinity for calls of removed group members: a dual playback without expectancy violation. Anim. Behav. 104, 51–57. ( 10.1016/j.anbehav.2015.02.022) [DOI] [Google Scholar]
- 67.Dawkins R. 1976. The selfish gene. Oxford, UK: Oxford University Press. [Google Scholar]
- 68.Keller L, Ross KG. 1998. Selfish genes: a green beard in the red fire ant. Nature 394, 573–575. ( 10.1038/29064) [DOI] [Google Scholar]
- 69.Gamboa GJ, Reeve HK, Ferguson ID, Wacker TL. 1986. Nestmate recognition in social wasps: the origin and acquisition of recognition odours. Anim. Behav. 34, 685–695. ( 10.1016/S0003-3472(86)80053-7) [DOI] [Google Scholar]
- 70.Green JP, Holmes AM, Davidson AJ, Paterson S, Stockley P, Beynon RJ, Hurst JL. 2015. The genetic basis of kin recognition in a cooperatively breeding mammal. Curr. Biol. 25, 2631–2641. ( 10.1016/j.cub.2015.08.045) [DOI] [PubMed] [Google Scholar]
- 71.Riehl C, Stern CA. 2015. How cooperatively breeding birds identify relatives and avoid incest: new insights into dispersal and kin recognition. Bioessays 37, 1303–1308. ( 10.1002/bies.201500120) [DOI] [PubMed] [Google Scholar]
- 72.Leedale AE, Li J, Hatchwell BJ. 2020. Kith or Kin? Familiarity as a cue to kinship in social birds. Front. Ecol. Evol. 8, 77 ( 10.3389/fevo.2020.00077) [DOI] [Google Scholar]
- 73.Hatchwell BJ, Ross DJ, Chaline N, Fowlie MK, Burke T. 2002. Parentage in the cooperative breeding system of long-tailed tits, Aegithalos caudatus. Anim. Behav. 64, 55–63. ( 10.1006/anbe.2002.3033) [DOI] [Google Scholar]
- 74.Hatchwell BJ, Russell AF, Ross DJ, Fowlie MK. 2000. Divorce in cooperatively breeding long-tailed tits: a consequence of inbreeding avoidance? Proc. R. Soc. Lond. B 267, 813–819. ( 10.1098/rspb.2000.1076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Napper CJ, Hatchwell BJ. 2016. Social dynamics in nonbreeding flocks of a cooperatively breeding bird: causes and consequences of kin associations. Anim. Behav. 122, 23–35. ( 10.1016/j.anbehav.2016.09.008) [DOI] [Google Scholar]
- 76.Richardson DS, Burke T, Komdeur J. 2003. Sex-specific associative learning cues and inclusive fitness benefits in the Seychelles warbler. J. Evol. Biol. 16, 854–861. ( 10.1046/j.1420-9101.2003.00592.x) [DOI] [PubMed] [Google Scholar]
- 77.Crozier RH. 1986. Genetic clonal recognition abilities in marine invertebrates must be maintained by selection for something else. Evolution 40, 1100–1101. ( 10.1111/j.1558-5646.1986.tb00578.x) [DOI] [PubMed] [Google Scholar]
- 78.Leedale AE, Lachlan RF, Robinson EJH, Hatchwell BJ. 2020. Data from: Helping decisions and kin recognition in long-tailed tits: is call similarity used to direct help towards kin? Dryad Digital Repository. ( 10.5061/dryad.sbcc2fr2p) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Leedale AE, Lachlan RF, Robinson EJH, Hatchwell BJ. 2020. Data from: Helping decisions and kin recognition in long-tailed tits: is call similarity used to direct help towards kin? Dryad Digital Repository. ( 10.5061/dryad.sbcc2fr2p) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The datasets and code supporting this article are available in the Dryad Digital Repository: https://doi.org/10.5061/dryad.sbcc2fr2p [78].