Abstract
Social interactions are mediated by recognition systems, meaning that the cognitive abilities or phenotypic diversity that facilitate recognition may be common targets of social selection. Recognition occurs when a receiver compares the phenotypes produced by a sender with a template. Coevolution between sender and receiver traits has been empirically reported in multiple species and sensory modalities, though the dynamics and relative exaggeration of traits from senders versus receivers have received little attention. Here, we present a coevolutionary dynamic model that examines the conditions under which senders and receivers should invest effort in facilitating individual recognition. The model predicts coevolution of sender and receiver traits, with the equilibrium investment dependent on the relative costs of signal production versus cognition. In order for recognition to evolve, initial sender and receiver trait values must be above a threshold, suggesting that recognition requires some degree of pre-existing diversity and cognitive abilities. The analysis of selection gradients demonstrates that the strength of selection on sender signals and receiver cognition is strongest when the trait values are furthest from the optima. The model provides new insights into the expected strength and dynamics of selection during the origin and elaboration of individual recognition, an important feature of social cognition in many taxa.
This article is part of the theme issue ‘Signal detection theory in recognition systems: from evolving models to experimental tests’.
Keywords: social cognition, individual recognition, communication, individual identity signals, social brain hypothesis
1. Introduction
Individual recognition allows animals to adaptively alter their behaviour and differentially allocate effort depending on with whom they interact [1–4]. In order for recognition to occur, senders must produce distinctive cues or signals that are then perceived and acted upon by receivers [5–8]. A major question, then, is how selection should jointly shape the interacting phenotypes of senders and receivers to facilitate individual recognition.
The still rapidly expanding literature on the evolution of organismal recognition systems has included not only empirical descriptions of how such systems operate [9–13] but also investigations of why they operate as they do, the latter drawing from the theoretical insights of engineering optimization (e.g. signal detection/acceptance threshold theory) and evolutionary game theory [2,14–17]. The tested theories typically focus on the evolutionary optima of thresholds that optimally balance rejection and acceptance errors [8,18–20]. However, relatively few theoretical studies have sought to predict the dynamics of the evolution of recognition systems and relate these predictions to the evolutionary dynamics (e.g. strength and timing of selection) underlying both recognition ability and the cues or signals mediating recognition. Given that recognition systems are the basis of social cognition [21], understanding the selective dynamics of recognition systems is likely to provide novel insights into the processes by which social cognition evolves.
With respect to individual recognition, the evolutionary dynamics of sender and receiver traits may provide insights into the processes shaping signal diversity and cognition, respectively. Previous models examining the evolution of sender phenotypes used for recognition have examined the extent of diversity expected within a population in relation to various scenarios dealing with competition or choice [2,14,15,22,23]. These models have not considered, however, the dynamic interplay of sender phenotypic diversity with receiver cognition. Similarly, there are many authors who have considered the importance of social cognition as a potential driver of cognitive evolution, typically ignoring the role of sender phenotypes in potentially facilitating recognition [24–26]. Verbal arguments have been put forth that the elaboration of sender phenotypes to facilitate recognition may influence the extent of investment in recognition-related cognition by receivers [27], though mathematical models examining the dynamic interplay between investment in sender diversity and receiver cognition have been lacking.
There is a wide diversity of contexts in which animals use individual recognition to navigate their social environment, which include competition, cooperation, care and mate choice, underscoring the importance of the evolution of individual recognition for a wide range of social phenomena. When animals compete, the recognition of individuals can prevent or reduce costs of suboptimal aggression levels towards individuals of varying fighting ability (‘dominance’ recognition) or of varying threat (e.g. ‘dear enemy’ recognition) [28,29]. In cooperative settings, recognition can assess the likelihood of returning the benefits of reciprocal cooperation (e.g. ‘cooperator’ recognition or ‘partner choice’) [16,30]. Care of offspring often involves costly investments and the recognition of parents and/or offspring can reduce costs of misappropriating efforts to an incorrect individual(s) [31,32]. Mate choice can also be facilitated by individual recognition where displays and mating choices are temporally separated [33].
We have developed a simple dynamical model of the coevolution of investment in discrimination ability, which is neurologically and temporally costly [34], and investment in individual distinctiveness in the context of individual recognition. We note that the cost of investing in identity information per se is different from investments in the production of the general signal. For example, pigments used in generating individually distinctive egg shell patterns in murres (Uria aalge) carry some costs, but differential patterning of pigments is not expected to be costly [35]. Similarly, the urinary proteins that signal scent mark identity in mice are metabolically costly to produce [36], though the genetic mechanisms generating individuality are likely not costly in themselves [37]. Even where identity information exists, costs of generating more apparent signals would still apply, such as the risk of detection by predators due to calling. We use this model to address a number of outstanding questions about the evolution of individual recognition: (i) What will be the relative investments in recognition by senders and receivers that are favoured by selection, and under what conditions will these investments be asymmetric? (ii) Does some degree of pre-existing diversity and/or discrimination ability facilitate the evolution of individual recognition? (iii) What are the expected dynamics of trait evolution during the course of elaboration? And, (iv) how does genetic relatedness influence the dynamics or equilibrium of investments in sender and receiver traits?
2. The basic model of coevolution of individual recognition
We develop a simple model of the coevolution of the costly investment d in discrimination ability of a receiver and costly investment v in exaggerating or amplifying pre-existing, individually distinctive traits of a sender (which corresponds to trait variation at the population level). The investments d and v could have any metric depending on the organism, but are multiplied by constants e and f, respectively, to obtain the fitness costs of those investments. The model we develop is applicable across a wide diversity of recognition systems. To highlight the generality of the model, table 1 details possible ways for organisms to increase their investment in discrimination and distinctiveness and possible costs associated with these investments. Increases in a receiver's d or a sender's v are assumed to increase the probability that the receiver will correctly classify a sender that is being assessed. As is true in individual recognition among conspecifics, individuals in the model act as both a sender and a receiver and possess a value for both d and v. The model assumes pairwise recognition, so we ask whether pairs of individuals each with their own values of d and v would recognize each other or not. When pairwise recognition occurs, a benefit is gained for both parties. For example, the recognition context could include the identification of the kinship, cooperative tendency, sex or dominance rank of a recipient. We further assume that a benefit b is gained if and only if both parties correctly identify each other. For example, where there is competition or conflict, recognition is thought to be beneficial because it reduces the likelihood of excessively costly aggression [52], the costs might be avoided only if the both parties correctly recognize each other's relative fighting ability. In the latter case, the benefit b could be thought of as the reduction of the cost inflicted by mutual aggression enabled by successful mutual recognition. In a cooperative scenario, the benefit b can be seen as the statistically expected enhancement of cooperative benefits received from a partner upon correct mutual recognition (e.g. if either partner does not recognize the other, they part without the possibility of the exchange of benefits).
Table 1.
Relating model variables to biological examples.
| description | example | references | |
|---|---|---|---|
| investment in discrimination (d) | |||
| perceptual abilities | ability to perceive and discriminate among differences in signal | detecting cuckoo eggs | [38] |
| learning/memory | acquiring and retrieving knowledge of signals | offspring recognition | [39,40] |
| pattern recognition | comparison of a signal against an internal template | species-specific song preference in crickets | [41] |
| cost of discrimination (e) | |||
| energetic costs | energy allocated to developing and maintaining tissue to detect signal | high energetic cost of brain tissue | [34,42] |
| tissue allocation costs | tradeoff costs of using tissue for recognition and not another purpose | paper wasps with individual recognition abilities have decreased the size of olfactory bulbs | [43] |
| sensory processing costs | neurological cost to processing and interpreting signal | extracting relevant information in noisy environments | [44,45] |
| detection costs | energy allocated to detecting signal | active sensing modifying behaviour to reduce stimulus ambiguity | [46,47] |
| investment in distinctiveness (v) | |||
| information content | the amount of information conveyed by the signal | facial colour variation in guenon monkeys | [48] |
| efficacy | the strength and/or reliability of the signal | environmental changes in chemical signals in lizards | [49] |
| cost of distinctiveness (f) | |||
| manufacturing costs | energy expended to manufacture signal | mouse major urinary proteins (MUPs) | [36] |
| byproduct costs | costs associated with other receivers detecting the signal | predatory bats eavesdropping on frog calls | [50] |
| opportunity costs | tradeoff costs of using signal for recognition and not for another purpose | tradeoff between individual recognition cues and quality cues | [27] |
| recognition costs | costs associated with being recognized by others | punishment of cheaters | [51] |
In particular, we assume that the probability p of successful mutual recognition for a focal individual exhibiting receiver investment d and sender investment v in a population in which everyone else exhibits d′ and v′ is given by the following equation:
| 2.1 |
where a is a constant that measures the intrinsic difficulty of discrimination. As required by assumption, p (which ranges from 0 to 1.0) increases as d or d′ increases or v or v′ increases, supporting the intuition that recognition should be more likely with greater investments in sender and receiver traits. Similarly, as a decreases (i.e. the recognition task is easier), then p increases. We further assume that investment in discrimination d entails a cost ed, where e is a constant that measures the efficiency of discrimination—the resource costs required to discriminate among individuals (lower e means intrinsically more efficient discrimination). We also assume that investment in exaggerating cue diversity v entails a cost fv, where f is a constant that measures the efficiency of exaggeration—the resource costs for producing a diverse cue (lower f means intrinsically more efficient exaggeration of underlying cue diversity). Example costs related to sender and receiver traits are described in table 1. Greater efficiency as reflected by lower values of e or f reflects lower costs to the organism in elaborating either the sender or receiver traits in question. We might expect, for example, animals with larger brains and well-developed sensory systems to pay less additional cost for an improvement in discrimination ability compared with the same abilities in an organism starting with relatively less investment in sensory or processing abilities.
We assume that the focal individual's overall fitness payoff W is equal to a baseline y, plus the successful discrimination benefit b times the probability p that both parties correctly recognize each other, minus the costs of investment in discrimination ability and individual distinctiveness (cue diversity exaggeration), i.e.
| 2.2 |
Assuming that d and v can evolve independently of each other to their respective optima, the evolutionarily stable strategy (ESS) values of d = d* and v = v* are obtained by solving the following equations:
| 2.3 |
The solutions d* and v* are seen to satisfy (see Maynard Smith [53]).
| 2.4 |
Equations (2.4) together imply that
| 2.5 |
from which we can derive the prediction that the relative values of the equilibrium investments will vary inversely with their costs. For example, as is intuitive, the lower the cost rate for investing in discrimination ability versus individual distinctiveness, the higher the optimal investment should be in discrimination ability relative to the investment in individual distinctiveness.
Equation (2.5) implies that d* = (f/e)v*, which can be back-substituted into equations (2.4) to yield solution with separated optimal investments satisfying two equations:
| 2.6 |
Joint solution of the equations in (2.6) yields six complicated polynomial roots, with up to two that are real. The lower real one corresponds to an unstable equilibrium and the larger real one corresponds to a stable equilibrium. An immediate consequence of both equations is that as b, the benefit of discrimination, increases both stable equilibrium investments v and d increase, as makes sense since increasing both investments should increase the chance of reaping the benefit of successful mutual recognition. (The latter is seen mathematically from the implicit function theorem by differentiating the left side equation in (2.6) with respect to e and f, respectively, in both cases yielding positive expressions.)
We are also interested in the coevolutionary dynamics for general d′ and v′, which are given by the selection gradients on d and v, Sd and Sv, respectively:
| 2.7 |
These selection gradients are given by
| 2.8 |
These are the selection gradients describing selection on a rare mutant d or v in a population in which everyone else adopts investments d′ and v′. Positive values of the selection gradient will result in future increases in the value of the investment by the population, negative values in decreases in the value of the investment, and zero values indicate selective equilibria, either stable or unstable. The analysis of the phase portraits of the coevolutionary dynamics generated by these selection gradients at different population values of d′ and v′ is illustrated in figures 1 and 2. The general analysis of these dynamics generates the following conclusions, where d* and v* now represent the non-trivial stable equilibrium.
Figure 1.
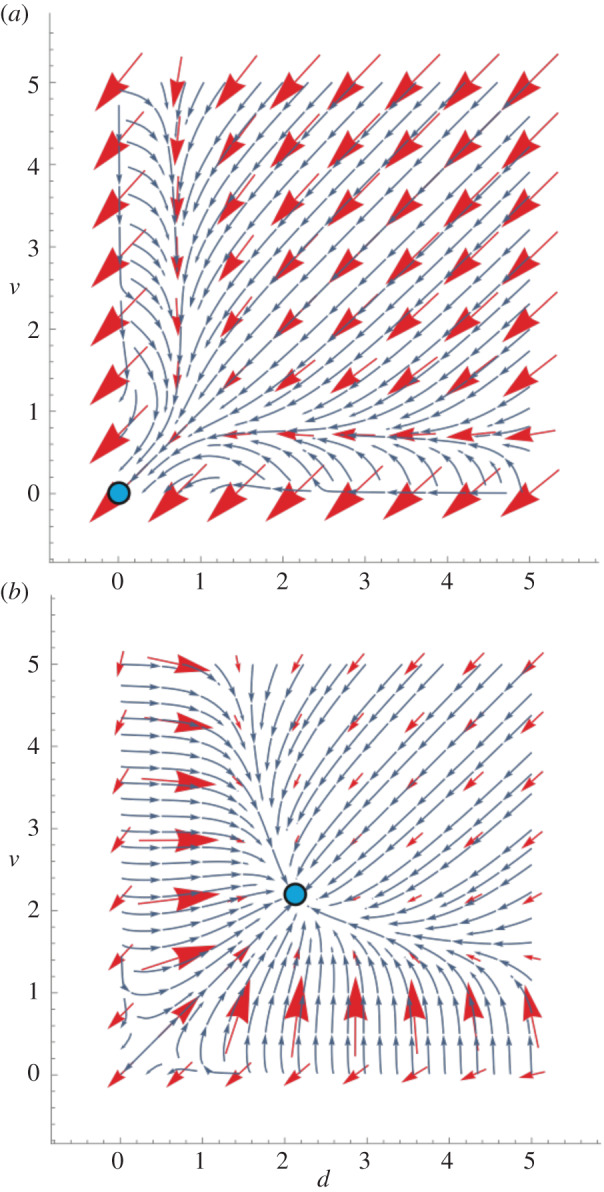
Coevolutionary dynamics for investment in discrimination ability d and investment in amplifying cues used in discrimination, v. Red arrows are eight particular directional selection gradients scattered evenly throughout the phase portrait; the stream flow-lines are the evolutionary trajectories starting at any given population values of d and v. The lengths of selection gradients measure vector-sum strengths of selection and consequently are proportional to the speed of joint changes in d and v. Blue dots are stable equilibria. (a) Coevolutionary phase portrait for discrimination parameter a = 0.80, benefit of discrimination b = 2, efficiency of discrimination e = 0.40 and efficiency of cue diversity amplification f = 0.40. The benefit of discrimination is too weak to give rise to and maintain a discrimination system and only the trivial equilibrium (d* = 0, v* = 0) is stable. (b) Increased benefit of discrimination: discrimination parameter a = 0.80, benefit of discrimination b = 8, efficiency of discrimination e = 0.40, efficiency of cue diversity amplification f = 0.40. A higher benefit of discrimination is sufficient to create a stable non-trivial equilibrium which is reached only if the beginning discrimination ability and/or cue diversity are sufficiently high. The strength of selection and the speed of progress to the equilibrium are higher farther from the equilibrium (in regions where selection moves the population towards the equilibrium) and for lower starting values of d and v. (Online version in colour.)
Figure 2.
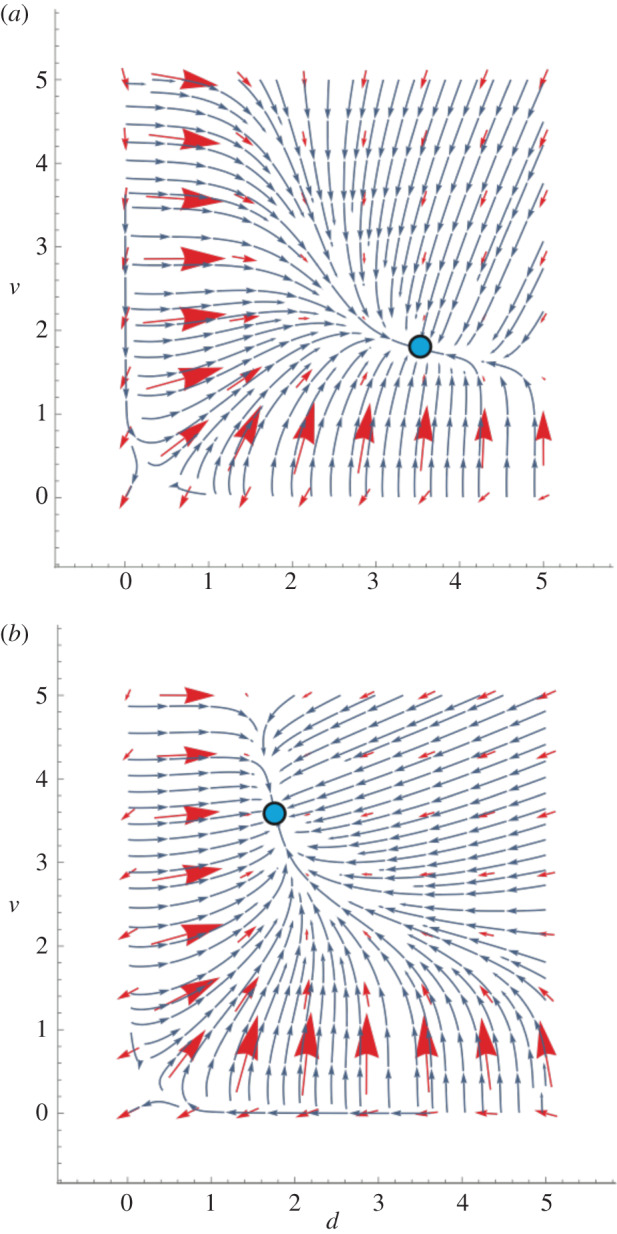
(a) Coevolutionary dynamics for investment in discrimination ability d and investment in amplifying cues used in discrimination, v. Red arrows are eight particular directional selection gradients scattered evenly throughout the phase portrait; the stream flow-lines are the evolutionary trajectories starting at any given population values of d and v. The lengths of selection gradients measure vector-sum strengths of selection and consequently are proportional to the speed of joint changes in d and v. Values of discrimination parameters: difficulty of discrimination a = 0.80, benefit of discrimination b = 8. Blue dots are stable equilibria. (a) The efficiency of discrimination, e = 0.20, is less than the efficiency of cue diversity amplification, f = 0.40. (b) The efficiency of discrimination, e = 0.40, is greater than the efficiency of cue diversity amplification, f = 0.20. A higher efficiency of discrimination (lower e) moves the discrimination system to a stable equilibrium of higher discrimination ability and lower cue diversity exaggeration, and vice versa. The strength of selection and the speed of progress to the equilibrium are still higher farther from the equilibrium and for lower starting values of d* and v* in regions where selection moves the population towards the non-trivial equilibrium. (Online version in colour.)
3. Results of the simple coevolutionary dynamics model
(a). Relative investment in sender and receiver traits depends on efficiencies
As one might expect, the equilibrium discrimination system becomes more sophisticated or exaggerated (i.e. d* and v* become higher) as the benefit of discrimination b increases, as the efficiency of discrimination increases (lower e), and as the efficiency of exaggerating cue diversity v increases (lower f). The relative investment in discrimination versus cue diversity exaggeration should depend directly on their relative efficiencies (figure 2).
(b). Pre-existing abilities are needed for recognition to evolve
If the benefit of discrimination b is sufficiently low, then d* = 0 and v* = 0, i.e. there is only a trivial equilibrium and no non-trivial discrimination system is stably maintained (figure 1). Thus, there is a threshold value of b above which a discrimination system can evolve to a stable equilibrium.
If the benefit of discrimination b is sufficiently high (such that it overcomes costs associated with recognition), then a non-trivial stable equilibrium of d* and v* exists, but this equilibrium can be reached only if the starting values of d′ and/or v′ are sufficiently high; otherwise, d′ and v′ decay to the trivial equilibrium (figure 1). If the starting values of d′ and/or v′ are sufficiently high to avoid attraction to the trivial equilibrium, the non-trivial equilibrium will be rapidly approached (figure 1). How large the benefit or initial starting values of d and v must be in order to reach the non-trivial equilibrium depends on the values of the other parameters in the model. It is not the case, however, that any value greater than zero will lead to the non-trivial equilibrium, as an unstable equilibrium in the lower left hand corner of the phase portrait can push sufficiently low non-zero values back down to zero (see figure 2a as an example). Thus, at least a moderate degree of pre-existing diversity in sender phenotypes or discrimination abilities in receivers is needed for individual recognition to evolve.
(c). Selection is stronger when populations are farther from the equilibrium
The rate of approach to the non-trivial equilibrium is generally higher the farther the starting state is from that equilibrium, as indicated by the fact that the selection gradients are of larger magnitude the farther d′ and v′ are away from the equilibrium (figures 1 and 2). The strongest selection and fastest change occurs when the investments are increasing from low values during the population's progress towards the equilibrium (figures 1 and 2).
4. Extending the simple model to include genetic relatedness among individuals
We can extend the basic model to the case of recognition between relatives of genetic relatedness r. A convenient way to do this is to assume that the fitness of a rare focal mutant is given by the following equation:
| 4.1 |
That is, when the mutant is rare, it has a chance r of interacting with a partner that shares its same genetically specified investment in discrimination ability d and v and a chance (1 − r) of interacting with a partner that exhibits the population values of d′ and v′. We can then solve for possible ESSs using the method in (2.3). The new (complicated) expressions can each be differentiated with respect to relatedness r to determine how the ESS (non-trivial stable equilibrium) will change as relatedness changes. These derivatives are, respectively,
| 4.2 |
Since both expressions are >0 at a non-trivial equilibrium, it follows from the implicit function theorem that increasing genetic relatedness r increases the stable investments in both discrimination ability and individual distinctiveness. The latter result makes sense because successful mutual recognition benefits both parties, so a focal individual is willing to increase its investments with a genetic relative because it now receives both a personal benefit and an indirect genetic benefit from successful mutual recognition.
5. Discussion
The predicted characteristics of the stable non-trivial equilibrium in our individual recognition model yield a number of biological predictions for existing individual recognition systems with mutual benefits for successful recognition: the most elaborate such systems should be those where (i) the benefits of mutual recognition are the greatest and (ii) the costs of investments in discrimination ability and individual distinctiveness are least. (iii) Increased relatedness among individuals that benefit from mutual recognition would lead to further exaggeration of traits. Additional dynamical models should be investigated for recognition contexts in which there are conflicts of interest (e.g. when discriminating individuals encounter not only neighbours or cooperators but also deceptive interlopers or defectors) and in which senders or receivers receive unequal benefits.
The coevolutionary model presented here makes a number of predictions regarding the expected patterns of investment in sender and receiver traits that can be tested with detailed studies of single species or using phylogenetic comparative analyses. Here, we highlight some of these predictions and point to current literature relevant to the model as well as systems that may be particularly fruitful for testing the coevolution of receiver cognition and sender identity signals.
-
(i)
Individual recognition systems should be most elaborated when the marginal benefits of accurate recognition are greatest. For example, in competitive contexts, we might expect greater investments in recognition when costs of misdirected aggression are higher, as might be the case for species with weaponry or otherwise costlier fights [54]. Similarly, the likelihood and costs of misdirecting parental care to the wrong individual can vary across species. For example, parent–offspring recognition systems are especially well developed in a variety of species nesting in high density colonies, where the risk of misdirected parental care is especially high, such as in colonial penguins [55], swallows [56], gulls [57], pinnipeds [39] and bats [58]. While there have been some studies highlighting variation in individual distinctiveness of eggs, plumage or vocalizations [59–61] in these groups, attempting to tie the patterns of evolution to the relative frequency and costs for misdirected parental care is a clear avenue for future research.
-
(ii)
Recognition systems should be most elaborated when the marginal costs of investing in discrimination ability or individual distinctiveness are smaller. This prediction has two major consequences. First, costs of sender and receiver traits will differ across clades. Most obviously, this relates to body size, which limits perceptual capabilities and the ability to produce detectable signals [27]. All else being equal, slight increases in sensory or neural tissue are likely to be less costly for larger species [34], as the same increase in complexity would represent a relatively small portion of total energy/mass budget. Second, we expect more sophisticated recognition systems in species that already possess substantial discrimination abilities or individually distinctive phenotypes. Discrimination abilities may be selected for in contexts other than individual recognition, such as mating, foraging or predator recognition [62,63]. The costs of modifying and elaborating the pre-existing sensory systems or neural circuits underlying these other forms of recognition should be lower than for species in which such sensory systems or circuitry are absent and must be built from scratch. Likewise, many species that lack individual recognition nevertheless have traits with individually distinctive features [64–67] that would facilitate the evolutionary origin of recognition in comparison with a species that lacks individual identity cues. As a corollary to prediction (ii), recognition systems with high costs for investing in discrimination ability and/or distinctiveness suggest a substantial benefit of recognition. For example, major urinary proteins (MUPs) are used for scent marking and as a signal of individual identity in house mice (Mus musculus domesticus) [37,68] and constitute upwards of 20% of transcripts produced in the liver in highly competitive individuals [36]. The extreme investment in individually distinctive scent marks in house mice suggests a large benefit of territory marking for territorial males in that species, likely because mate choice is partly determined by females monitoring individual scent marking abilities [69,70].
-
(iii)
A further prediction of our model is that there should be a positive correlation between discrimination ability and individual distinctiveness across species, as the investments in each trait will generally exhibit positive coevolution since the benefits of both traits are increased by higher benefits of recognition. The model does not predict equal investments in sender and receiver traits, but rather that both traits are likely to increase given a greater benefit of recognition. Furthermore, the relative investment in recognition ability versus individual distinctiveness should be inversely related to the ratio of their marginal costs. For example, relative investment in recognition ability should be highest in species having elaborate, previously established recognition systems and low levels of interindividual variability. Nevertheless, the model predicts that increased benefits of recognition will be associated with some degree of increase in sender and receiver traits. For example, humans are highly adept at recognizing other human faces and our faces show signatures of selection to advertize individual identity [71,72]. Comparative data from halictid bees provide evidence for increased investment in antennal sensilla density in social halictid bees relative to solitary species [73].
Our model also provides insight into the dynamics of the evolution of new recognition systems. We find that recognition systems should be hard to evolve unless the benefits of discrimination reach some threshold value, and then should evolve rapidly to a stable equilibrium, provided that the beginning values of d′ and v′ are sufficiently large. Thus, the evolution of novel recognition systems requires some degree of pre-existing discrimination and distinctiveness in a population. New recognition systems may be favoured to evolve in response to a temporary or permanent increase in the benefit of recognition or the relatedness of individuals, such as a change in environmental conditions or a population bottleneck. Similarly, changes in the initial values of d′ or v′ could also favour the evolution of a new recognition system. One possible scenario by which this could occur is through gene duplication. For example, cuticular hydrocarbon genes that convey chemical cues for nest-mate recognition in social insects, and opsin genes which are necessary for colour discrimination, evolved through repeated gene duplication events [74,75]. An interesting extension of these observations is that the type of signal (e.g. visual, chemical or acoustic) used by a new recognition system should favour the modality with the lowest cost of discrimination and distinctiveness (table 1). This would not be determined by solely the signal-to-noise ratio for a given modality but also its external costs such as how vulnerable that modality may be to eavesdropping predators.
Based on our model, we predict a difference in the evolutionary dynamics of recognition traits between recently evolved and established recognition systems. Our model furthers the understanding of the adaptive dynamics of sender–receiver coevolution in signalling systems by applying adaptive dynamics analyses to a new context–individual recognition [76,77]. Once at the equilibrium, selection should be weak and therefore relatively difficult to detect. By contrast, selection favouring recognition systems far from equilibrium, as when investment in discrimination ability and individual distinctiveness are initially low in the early stages of evolution, should be relatively strong and thus easier to detect. Indeed, greater rates of fitness gain when populations are further from the equilibrium may be a common feature of adaptation across a range of taxa and traits [78,79]. Perhaps counterintuitively, this would suggest that species from clades where recognition traits are otherwise minimal will show stronger evidence of selection on receiver cognition or sender identity signals compared with clades with ancestrally well-developed recognition abilities. For example, we would expect weak signatures of recent selection on recognition traits in species such primates, where ancestral sender and receiver states were likely relatively close to current optima [80,81]. By contrast, strong selection may be detectable in species starting from minimal individual recognition abilities. Intriguingly, these predictions appear supported by recent evidence on the evolutionary dynamics of loci likely involved in the individual recognition ability of co-nesting foundresses (nest-founding females) of the paper wasp Polistes fuscatus. Female P. fuscatus have diverse facial markings that they use to recognize other individuals [28]. Polistes fuscatus are cognitively specialized for learning and memory of conspecific facial images [82] and possess robust long-term memories for familiar individuals [83]. Mutual recognition in P. fuscatus is thought to be beneficial because it reduces aggression by mediating dominance interactions among co-foundresses [52] and facilitates tracking of individual contributions to nest building and egg laying [84]. A recent study investigating the evolutionary dynamics of individual recognition in P. fuscatus found evidence for recent strong selective sweeps on loci associated with cognition and vision [85]. By contrast, studies examining recent selection in species with older established recognition systems such as the great tit [86], parrot [87] or the great apes [88] have found relatively weak selection across loci associated with cognition. Similarly, attempts to link cognitive phenotypes to genetic loci in humans have identified hundreds of loci with a small phenotypic effect [89] consistent with an evolutionary process of weak selection across a large number of loci. These findings support our prediction that selection should be strong early in the evolution of a new recognition system but weak in established recognition systems that are presumably nearer to the equilibrium, similar to the expectation from Fisher's geometric model of adaptation [90,91]. Thus, signatures of selection on social cognition may be most prominent in species with relatively modest cognitive abilities that have resulted from recent selection for increased investments in recognition.
Data accessibility
This article has no additional data.
Authors' contributions
S.E.M., M.J.S. and H.K.R. designed and conceived of the study; H.K.R. analysed the model; S.E.M., M.J.S. and H.K.R. wrote and edited the paper.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by NIH grant no. DP2-GM128202 (M.J.S.), NSF CAREER grant no. DEB-1750394 (M.J.S.) and NSF Postdoctoral Fellowship grant no. DBI-1711703 (S.E.M.).
References
- 1.Wiley RH. 2013. Specificity and multiplicity in the recognition of individuals: implications for the evolution of social behaviour. Biol. Rev. 88, 179–195. ( 10.1111/j.1469-185X.2012.00246.x) [DOI] [PubMed] [Google Scholar]
- 2.Johnstone RA. 1997. Recognition and the evolution of distinctive signatures: when does it pay to reveal identity? Proc. R. Soc. Lond. B 264, 1547–1553. ( 10.1098/rspb.1997.0215) [DOI] [Google Scholar]
- 3.Tibbetts EA, Dale J. 2007. Individual recognition: it is good to be different. Trends Ecol. Evol. 22, 529–537. ( 10.1016/j.tree.2007.09.001) [DOI] [PubMed] [Google Scholar]
- 4.Gherardi F, Aquiloni L, Tricarico E. 2012. Revisiting social recognition systems in invertebrates. Anim. Cogn. 15, 745–762. ( 10.1007/s10071-012-0513-y) [DOI] [PubMed] [Google Scholar]
- 5.Sherman PW, Reeve HK, Pfennig DW, Krebs JR, Davies NB. 1997. Recognition systems. In Behavioural ecology: an evolutionary approach (eds J Krebs, N Davies), pp. 69–96. Oxford: Blackell Science. [Google Scholar]
- 6.Mateo JM. 2004. Recognition systems and biological organization: the perception component of social recognition. Ann. Zool. Fenn. 41, 729–745. [Google Scholar]
- 7.Tsutsui ND. 2004. Scents of self: the expression component of self/nonself recognition systems. Ann. Zool. Fenn. 41, 713–727. [Google Scholar]
- 8.Liebert AE, Starks PT. 2004. The action component of recognition systems: a focus on the response. Ann. Zool. Fenn. 41, 747–764. [Google Scholar]
- 9.Hurst JL, Payne CE, Nevison CM, Marie AD, Humphries RE, Robertson DHL, Cavaggioni A, Beynon RJ. 2001. Individual recognition in mice mediated by major urinary proteins. Nature 414, 631–634. ( 10.1038/414631a) [DOI] [PubMed] [Google Scholar]
- 10.Lightfoot JW, Wilecki M, Rödelsperger C, Moreno E, Susoy V, Witte H, Sommer RJ. 2019. Small peptide-mediated self-recognition prevents cannibalism in predatory nematodes. Science 364, 86–89. ( 10.1126/science.aav9856) [DOI] [PubMed] [Google Scholar]
- 11.Pask GM, et al. 2017. Specialized odorant receptors in social insects that detect cuticular hydrocarbon cues and candidate pheromones. Nat. Commun. 8, 297 ( 10.1038/s41467-017-00099-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tibbetts EA. 2004. Complex social behaviour can select for variability in visual features: a case study in Polistes wasps. Proc. R. Soc. Lond. B 271, 1955–1960. ( 10.1098/rspb.2004.2784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Ettorre P, Heinze J. 2005. Individual recognition in ant queens. Curr. Biol. 15, 2170–2174. ( 10.1016/j.cub.2005.10.067) [DOI] [PubMed] [Google Scholar]
- 14.Dale J, Lank DB, Reeve HK. 2001. Signaling individual identity versus quality: a model and case studies with ruffs, queleas, and house finches. Am. Nat. 158, 75–86. ( 10.1086/320861) [DOI] [PubMed] [Google Scholar]
- 15.Sheehan MJ, Miller C, Reeve HK. 2017. Identity signaling and patterns of cooperative behavior. Integr. Comp. Biol. 57, 580–588. ( 10.1093/icb/icx054) [DOI] [PubMed] [Google Scholar]
- 16.Crowley PH, Provencher L, Sloane S, Dugatkin LA, Spohn B, Rogers L, Alfieri M. 1996. Evolving cooperation: the role of individual recognition. BioSystems 37, 49–66. ( 10.1016/0303-2647(95)01546-9) [DOI] [PubMed] [Google Scholar]
- 17.Dugatkin LA, Earley RL. 2004. Individual recognition, dominance hierarchies and winner and loser effects. Proc. R. Soc. Lond. B 271, 1537–1540. ( 10.1098/rspb.2004.2777) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reeve HK. 1989. The evolution of conspecific acceptance thresholds. Am. Nat. 133, 407–435. ( 10.1086/284926) [DOI] [Google Scholar]
- 19.Lacy RC, Sherman PW. 1983. Kin recognition by phenotype matching. Am. Nat. 121, 489–512. ( 10.1086/284078) [DOI] [Google Scholar]
- 20.Rodriguez-Girones MA, Lotem A. 1999. How to detect a cuckoo egg: a signal-detection theory model for recognition and learning. Am. Nat. 153, 633–648. ( 10.1086/303198) [DOI] [PubMed] [Google Scholar]
- 21.Seyfarth RM, Cheney DL. 2015. Social cognition. Anim. Behav. 103, 191–202. ( 10.1016/j.anbehav.2015.01.030) [DOI] [Google Scholar]
- 22.Thom MDF, Dytham C. 2012. Female choosiness leads to the evolution of individually distinctive males. Evolution 66, 3736–3742. ( 10.1111/j.1558-5646.2012.01732.x) [DOI] [PubMed] [Google Scholar]
- 23.Dytham C, Thom MD.. 2019. Population fragmentation drives up genetic diversity in signals of individual identity. Oikos 29, 526–532. ( 10.1111/oik.06743) [DOI] [Google Scholar]
- 24.Byrne RW, Whiten A. 1988. Machiavellian intelligence: social expertise and the evolution of intellect in monkeys, apes, and humans. New York, NY: Oxford Univeristy Press. [Google Scholar]
- 25.Dunbar RIM. 1998. The social brain hypothesis. Evol. Anthropol. 6, 178–190. () [DOI] [Google Scholar]
- 26.González-Forero M, Gardner A. 2018. Inference of ecological and social drivers of human brain-size evolution. Nature 557, 554–557. ( 10.1038/s41586-018-0127-x) [DOI] [PubMed] [Google Scholar]
- 27.Sheehan MJ, Bergman TJ. 2016. Is there an evolutionary trade-off between quality signaling and social recognition? Behav. Ecol. 27, 2–13. ( 10.1093/beheco/arv109) [DOI] [Google Scholar]
- 28.Tibbetts EA. 2002. Visual signals of individual identity in the wasp Polistes fuscatus. Proc. R. Soc. Lond. B 269, 1423–1428. ( 10.1098/rspb.2002.2031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tumulty JP. 2018. Dear enemy effect. In Encyclopedia of animal cognition and behavior (eds Vonk J, Shackelford T). Berlin, Germany: Springer. [Google Scholar]
- 30.Cheney DL, Moscovice LR, Heesen M, Mundry R, Seyfarth RM. 2010. Contingent cooperation between wild female baboons. Proc. Natl Acad. Sci. USA 107, 9562–9566. ( 10.1073/pnas.1001862107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beecher MD, Beecher IM, Lumpkin S. 1981. Parent–offspring recognition in bank swallows (Riparia riparia): I. Natural history. Anim. Behav. 29, 87–94. ( 10.1016/S0003-3472(81)80155-8) [DOI] [Google Scholar]
- 32.Insley SJ. 2001. Mother–offspring vocal recognition in northern fur seals is mutual but asymmetrical. Anim. Behav. 61, 129–137. ( 10.1006/anbe.2000.1569) [DOI] [PubMed] [Google Scholar]
- 33.Hurst JL. 2009. Female recognition and assessment of males through scent. Behav. Brain Res. 200, 295–303. ( 10.1016/j.bbr.2008.12.020) [DOI] [PubMed] [Google Scholar]
- 34.Niven JE, Laughlin SB. 2008. Energy limitation as a selective pressure on the evolution of sensory systems. J. Exp. Biol. 211, 1792–1804. ( 10.1242/jeb.017574) [DOI] [PubMed] [Google Scholar]
- 35.Hauber ME, Bond AL, Kouwenberg A-L, Robertson GJ, Hansen ES, Holford M, Dainson M, Luro A, Dale J. 2019. The chemical basis of a signal of individual identity: shell pigment concentrations track the unique appearance of common murre eggs. J. R. Soc. Interface 16, 20190115 ( 10.1098/rsif.2019.0115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheehan MJ, Campbell P, Miller CH. 2019. Evolutionary patterns of major urinary proteins scent signals in house mice and relatives. Mol. Ecol. 28, 3587–3601. ( 10.1111/mec.15155) [DOI] [PubMed] [Google Scholar]
- 37.Sheehan MJ, Lee V, Corbett-Detig R, Bi K, Beynon RJ, Hurst JL, Nachman MW. 2016. Selection on coding and regulatory variation maintains individuality in major urinary protein scent marks in wild mice. PLoS Genet. 12, e1005891 ( 10.1371/journal.pgen.1005891) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lotem A, Nakamura H, Zahavi A. 1995. Constraints on egg discrimination and cuckoo-host co-evolution. Anim. Behav. 49, 1185–1209. ( 10.1006/anbe.1995.0152) [DOI] [Google Scholar]
- 39.Insley SJ. 2000. Long-term vocal recognition in the northern fur seal. Nature 406, 404–405. ( 10.1038/35019064) [DOI] [PubMed] [Google Scholar]
- 40.Kendrick KM, Da Costa AP, Broad KD, Ohkura S, Guevara R, Lévy F, Keverne EB.. 1997. Neural control of maternal behaviour and olfactory recognition of offspring. Brain Res. Bull. 44, 383–395. ( 10.1016/S0361-9230(97)00218-9) [DOI] [PubMed] [Google Scholar]
- 41.Schöneich S, Kostarakos K, Hedwig B. 2015. An auditory feature detection circuit for sound pattern recognition. Sci. Adv. 1, e1500325 ( 10.1126/sciadv.1500325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chittka L, Niven J. 2009. Are bigger brains better? Curr. Biol. 19, R995–R1008. ( 10.1016/j.cub.2009.08.023) [DOI] [PubMed] [Google Scholar]
- 43.Gronenberg W, Ash LE, Tibbetts EA. 2008. Correlation between facial pattern recognition and brain composition in paper wasps. Brain Behav. Evol. 71, 1–14. ( 10.1159/000108607) [DOI] [PubMed] [Google Scholar]
- 44.Corcoran AJ, Moss CF. 2017. Sensing in a noisy world: lessons from auditory specialists, echolocating bats. J. Exp. Biol. 220, 4554–4566. ( 10.1242/jeb.163063) [DOI] [PubMed] [Google Scholar]
- 45.Fries P, Reynolds JH, Rorie AE, Desimone R. 2001. Modulation of oscillatory neuronal synchronization by selective visual attention. Science 291, 1560–1563. ( 10.1126/science.1055465) [DOI] [PubMed] [Google Scholar]
- 46.Nelson ME, MacIver MA. 2006. Sensory acquisition in active sensing systems. J. Comp. Physiol. A 192, 573–586. ( 10.1007/s00359-006-0099-4) [DOI] [PubMed] [Google Scholar]
- 47.Mhatre N. 2015. Active amplification in insect ears: mechanics, models and molecules. J. Comp. Physiol. A 201, 19–37. ( 10.1007/s00359-014-0969-0) [DOI] [PubMed] [Google Scholar]
- 48.Allen WL, Higham JP. 2015. Assessing the potential information content of multicomponent visual signals: a machine learning approach. Proc. R. Soc. B 282, 20142284 ( 10.1098/rspb.2014.2284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baeckens S, Martín J, García-Roa R, Pafilis P, Huyghe K, Van Damme R.. 2018. Environmental conditions shape the chemical signal design of lizards. Funct. Ecol. 32, 566–580. ( 10.1111/1365-2435.12984) [DOI] [Google Scholar]
- 50.Bernal XE, Page RA, Rand AS, Ryan MJ. 2007. Cues for eavesdroppers: do frog calls indicate prey density and quality? Am. Nat. 169, 409–415. ( 10.1086/510729) [DOI] [PubMed] [Google Scholar]
- 51.Molles LE, Vehrencamp SL. 2001. Songbird cheaters pay a retaliation cost: evidence for auditory conventional signals. Proc. R. Soc. Lond. B 268, 2013–2019. ( 10.1098/rspb.2001.1757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sheehan MJ, Tibbetts EA. 2009. Evolution of identity signals: frequency-dependent benefits of distinctive phenotypes used for individual recognition. Evolution 63, 3106–3113. ( 10.1111/j.1558-5646.2009.00833.x) [DOI] [PubMed] [Google Scholar]
- 53.Smith JM. 1982. Evolution and the theory of games. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 54.Lane SM, Briffa M. 2017. The price of attack: rethinking damage costs in animal contests. Anim. Behav. 126, 23–29. ( 10.1016/j.anbehav.2017.01.015) [DOI] [Google Scholar]
- 55.Aubin T, Jouventin P. 1998. Cocktail-party effect in king penguin colonies. Proc. R. Soc. Lond. B 265, 1665–1673. ( 10.1098/rspb.1998.0486) [DOI] [Google Scholar]
- 56.Medvin MB, Stoddard PK, Beecher MD. 1993. Signals for parent-offspring recognition: a comparative-analysis of the begging calls of cliff swallows and barn swallows. Anim. Behav. 45, 841–850. ( 10.1006/anbe.1993.1105) [DOI] [Google Scholar]
- 57.Charrier I, Mathevon N, Jouventin P, Aubin T. 2001. Acoustic communication in a black-headed gull colony: how do chicks identify their parents? Ethology 107, 961–974. ( 10.1046/j.1439-0310.2001.00748.x) [DOI] [Google Scholar]
- 58.Balcombe JP, McCracken GF. 1992. Vocal recognition in Mexican free-tailed bats—do pups recognize mothers? Anim. Behav. 43, 79–87. ( 10.1016/s0003-3472(05)80073-9) [DOI] [Google Scholar]
- 59.Jouventin P, Aubin T. 2002. Acoustic systems are adapted to breeding ecologies: individual recognition in nesting penguins. Anim. Behav. 64, 747–757. ( 10.1006/anbe.2002.4002) [DOI] [Google Scholar]
- 60.Dale J. 2006. Intraspecific variation in coloration. In Function and evolution. Bird coloration (eds GE Hill, KJ McGraw), vol. 2, pp. 36–86. Cambridge, MA: Harvard University Press. [Google Scholar]
- 61.Buckley PA, Buckley FG. 1972. Individual egg and chick recognition by adult royal terns (Sterna maxima maxima). Anim. Behav. 20, 457–462.. ( 10.1016/S0003-3472(72)80009-5) [DOI] [Google Scholar]
- 62.Shaw KL, Lesnick SC. 2009. Genomic linkage of male song and female acoustic preference QTL underlying a rapid species radiation. Proc. Natl Acad. Sci. USA 106, 9737–9742. ( 10.1073/pnas.0900229106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bond AB, Kamil AC. 2002. Visual predators select for crypticity and polymorphism in virtual prey. Nature 415, 609–613. ( 10.1038/415609a) [DOI] [PubMed] [Google Scholar]
- 64.Job DA, Boness DJ, Francis JM. 1995. Individual variation in nursing vocalizations of Hawaiian monk seal pups, Monachus schauinlansi (Phocidae, Pinnepedia), and lack of maternal recognition. Can. J. Zool. 73, 975–983. ( 10.1139/z95-114) [DOI] [Google Scholar]
- 65.Knornschild M, Von Helversen O.. 2008. Nonmutual vocal mother–pup recognition in the greater sac-winged bat. Anim. Behav. 76, 1001–1009. ( 10.1016/j.anbehav.2008.05.018). [DOI] [Google Scholar]
- 66.Dreier S, D'Ettorre P. 2009. Social context predicts recognition systems in ant queens. J. Evol. Biol. 22, 644–649. ( 10.1111/j.1420-9101.2008.01668.x) [DOI] [PubMed] [Google Scholar]
- 67.Bergman TJ. 2010. Experimental evidence for limited vocal recognition in a wild primate: implications for the social complexity hypothesis. Proc. R. Soc. B 277, 3045–3053. ( 10.1098/rspb.2010.0580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheetham SA, Thom MD, Jury F, Ollier WER, Beynon RJ, Hurst JL. 2007. The genetic basis of individual-recognition signals in the mouse. Curr. Biol. 17, 1771–1777. ( 10.1016/j.cub.2007.10.007) [DOI] [PubMed] [Google Scholar]
- 69.Hurst JL, Beynon RJ. 2004. Scent wars: the chemobiology of competitive signalling in mice. BioEssays 26, 1288–1298. ( 10.1002/bles.20147) [DOI] [PubMed] [Google Scholar]
- 70.Ramm SA, Cheetham SA, Hurst JL. 2008. Encoding choosiness: female attraction requires prior physical contact with individual male scents in mice. Proc. R. Soc. B 275, 1727–1735. ( 10.1098/rspb.2008.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu Q, Song YY, Hu SY, Li XB, Tian MQ, Zhen ZL, Dong Q, Kanwisher N, Liu J. 2010. Heritability of the specific cognitive ability of face perception. Curr. Biol. 20, 137–142. ( 10.1016/j.cub.2009.11.067) [DOI] [PubMed] [Google Scholar]
- 72.Sheehan MJ, Nachman MW. 2014. Morphological and population genomic evidence that human faces have evolved to signal individual identity. Nat. Commun. 5, 4800 ( 10.1038/ncomms5800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wittwer B, Hefetz A, Simon T, Murphy LE, Elgar MA, Pierce NE, Kocher SD. 2017. Solitary bees reduce investment in communication compared with their social relatives. Proc. Natl Acad. Sci. USA 114, 6569–6574. ( 10.1073/pnas.1620780114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoffmann M, Tripathi N, Henz SR, Lindholm AK, Weigel D, Breden F, Dreyer C. 2006. Opsin gene duplication and diversification in the guppy, a model for sexual selection. Proc. R. Soc. B 274, 33–42. ( 10.1098/rspb.2006.3707) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Wilgenburg E, Symonds MRE, Elgar MA.. 2011. Evolution of cuticular hydrocarbon diversity in ants. J. Evol. Biol. 24, 1188–1198. ( 10.1111/j.1420-9101.2011.02248.x) [DOI] [PubMed] [Google Scholar]
- 76.Botero CA, Pen I, Komdeur J, Weissing FJ. 2010. The evolution of individual variation in communication strategies. Evolution 64, 3123–3133. ( 10.1111/j.1558-5646.2010.01065.x) [DOI] [PubMed] [Google Scholar]
- 77.Szalai F, Szamado S. 2009. Honest and cheating strategies in a simple model of aggressive communication. Anim. Behav. 78, 949–959. ( 10.1016/j.anbehav.2009.06.025) [DOI] [PubMed] [Google Scholar]
- 78.Good BH, McDonald MJ, Barrick JE, Lenski RE, Desai MM. 2017. The dynamics of molecular evolution over 60 000 generations. Nature 551, 45–50. ( 10.1038/nature24287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Westley PA. 2011. What invasive species reveal about the rate and form of contemporary phenotypic change in nature. Am. Nat. 177, 496–509. ( 10.1086/658902) [DOI] [PubMed] [Google Scholar]
- 80.Pascalis O, Bachevalier J. 1998. Face recognition in primates: a cross-species study. Behav. Processes 43, 87–96. ( 10.1016/S0376-6357(97)00090-9) [DOI] [PubMed] [Google Scholar]
- 81.Parr LA. 2011. The evolution of face processing in primates. Phil. Trans. R. Soc. B 366, 1764–1777. ( 10.1098/rstb.2010.0358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sheehan MJ, Tibbetts EA. 2011. Specialized face learning is associated with individual recognition in paper wasps. Science 334, 1272–1275. ( 10.1126/science.1211334) [DOI] [PubMed] [Google Scholar]
- 83.Sheehan MJ, Tibbetts EA. 2008. Robust long-term social memories in a paper wasp. Curr. Biol. 18, R851–R852. ( 10.1016/j.cub.2008.07.032) [DOI] [PubMed] [Google Scholar]
- 84.Reeve HK, Nonacs P. 1992. Social contracts in wasp societies. Nature 359, 823–825. ( 10.1038/359823a0) [DOI] [Google Scholar]
- 85.Miller SE, Legan AW, Henshaw M, Ostevik KL, Samuk K, Uy FM, Reeve HK, Sheehan MJ. 2018. Evolutionary dynamics of recent selection for enhanced social cognition. bioRxiv, 425215 ( 10.1101/425215) [DOI]
- 86.Laine VN, et al. 2016. Evolutionary signals of selection on cognition from the great tit genome and methylome. Nat. Commun. 7, ncomms10474 ( 10.1038/ncomms10474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wirthlin M, et al. 2018. Parrot genomes and the evolution of heightened longevity and cognition. Curr. Biol. 28, 4001–4008. ( 10.1016/j.cub.2018.10.050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cagan A, et al. 2016. Natural selection in the great apes. Mol. Biol. Evol. 33, 3268–3283. ( 10.1093/molbev/msw215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee JJ, et al. 2018. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat. Genet. 50, 1112 ( 10.1038/s41588-018-0147-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fisher RA. 1958. The genetical theory of natural selection. New York, NY: Dover Publications. [Google Scholar]
- 91.Tenaillon O. 2014. The utility of Fisher's geometric model in evolutionary genetics. Annu. Rev. Ecol. Evol. Syst. 45, 179–201. ( 10.1146/annurev-ecolsys-120213-091846) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.


