Abstract
Many animals are able to perform recognition feats that astound us—such as a rodent recognizing kin it has never met. Yet in other contexts, animals appear clueless as when reed warblers rear cuckoo chicks that bear no resemblance to their own species. Failures of recognition when it would seem adaptive have been especially puzzling. Here, we present a simple tug-of-war game theory model examining how individuals should optimally invest in affecting the accuracy of discrimination between desirable and undesirable recipients. In the game, discriminating individuals (operators) and desirable and undesirable recipients (targets and mimics, respectively) can all invest effort into their own preferred outcome. We demonstrate that stable inaccurate recognition will arise when undesirable recipients have large fitness gains from inaccurate recognition relative to the pay-offs that the other two parties receive from accurate recognition. The probability of accurate recognition is often determined by just the relative pay-offs to the desirable and undesirable recipients, rather than to the discriminator. Our results provide a new lens on long-standing puzzles including a lack of nepotism in social insect colonies, tolerance of brood parasites and male birds caring for extra-pair young in their nests, which our model suggests should often lack accurate discrimination.
This article is part of the theme issue ‘Signal detection theory in recognition systems: from evolving models to experimental tests'.
Keywords: brood parasite, nepotism, extra pair young, dear enemy effect, recognition systems, acceptance threshold
1. Introduction
A central tenet of behaviour ecology is that animals should be selected to invest their efforts or resources in strategies and decisions to maximize net fitness gains. In many social, sexual and species–interactions contexts, recognition systems allow animals to make optimal decisions of how to behave depending on who is interacting [1,2]. Animals need to be able to distinguish chicks from brood parasites [3], nest-mates from non-nest-mates [4], friends from foes [5], cooperators from defectors [6,7] and palatable from poisonous prey [8] among other challenges. Although animals benefit by making accurate choices, some individuals like brood parasitic chicks depend on their ability to generate inaccurate recognition decisions [9]. Indeed, some of the most enduring puzzles in evolutionary biology including parents raising heterospecific brood parasites [10–12], Batesian mimicry [13–15] and a lack of within nest nepotism in many polygynous or polyandrous social insects [16,17] have fascinated biologists because of the apparent failures of recognition systems. Whether such apparent recognition failures represent inherent constraints limiting recognition or adaptive investments in the face of recognition challenges has been a major question. The acceptance threshold theory provides a powerful framework for analysing how animals should invest in recognition systems [18].
Understanding of organismal recognition systems has moved forward significantly with the systematic empirical analysis within the framework of signal detection/acceptance threshold theory [19–24]. The tested theories generally focus on the setting of acceptance thresholds that optimally balance rejection and acceptance errors [18], with a given amount of overlap in the distributions of the recognition cues that are used by focal individuals (also known as operators or discriminators) to identify desirable and undesirable recipients of a particular action, such as provisioning or courtship. The degree of overlap and the shapes of the recognition cue distributions are often treated as fixed in the optimization, as it is reasonable at ecological timescales where acceptance thresholds are compared across recognition contexts, but the cues themselves have been established previously in development [25]. Over longer evolutionary timescales, the cue distributions and their overlap may coevolve with the discriminator's acceptance threshold to the extent that selection acts on the cue-associated phenotypes of the desirable and undesirable organismal recipients themselves. Johnstone [26] showed how the evolutionary game theory should be used to model the coevolution of a signaller's signal level with the signal detection thresholds of receivers and eavesdroppers in noisy environments, and the game theory is similarly required when a discriminator's acceptance threshold coevolves with the cues/signals of the desirable versus undesirable recipients being discriminated.
Of course, selection may act on much more than just the operator's acceptance threshold and the cues of the desirable and undesirable recipients. For example, selection altering the distinctiveness of cues/signals of desirable and undesirable recipients [27–31] might also generate selection on (i) the sensory or perceptual system of a discriminator to make these cues more easily discriminable [32], or (ii) the discriminator's degree of investment in learning so as to further sharpen discrimination [10,33–35]. Moreover, recipients might be favoured not only to alter the distinctiveness of their own cues/signals but also to behave in ways which would further modulate the discriminator's ability to perceive such distinctiveness, such as by manipulating (enhancing or diminishing) the recognition ability or motivation of operators [9]. In short, selection might act on many aspects of the phenotypes of both discriminators and recipients in ways that might affect the likelihood of the successful discrimination by discriminators.
In light of the many ways that selection can alter the outcome of an evolutionary recognition game among discriminators, desirable and undesirable recipients, we suggest that the next generation of recognition models should dispense with the assumptions of fixed sensory or perceptual constraints and fixed recipient cues/signals, and the assumptions that are implicit within the classic signal detection framework [36–39]. Instead, we seek to build a general evolutionary game among discriminators, desirable and undesirable recipients, with each deciding how much to invest (at a cost) in phenotypes that alter the overall probability of successful discrimination by discriminators. More specifically, we consider a recognition system involving an operator (discriminator), target (desirable recipient) and a mimic (undesirable recipient). Operators and targets are assumed to do best when the operator successfully discriminates between targets and mimics, but mimics are assumed to do best when this discrimination is unsuccessful (i.e. the operator chooses the mimic over the target). Because there are evolutionary conflicts of interests among some of the parties (between the operator or target and the mimic), we seek to solve for the investments by all parties that generate a stable resolution of the conflicts. A convenient framework for solving the stable resolution of conflicts is provided by a generalization of tug-of-war game theory [40–43], in which each player must decide how much to invest in attempting to win a conflict at the expense of some other component of fitness. The degree to which each player wins in tug-of-war theory depends not on its absolute investment, but on its investment relative to those of the other players [40]. The latter two features of the tug-of-war theory make it especially suitable as a model of conflict resolution in general. Thus, we propose a new framework for modelling recognition systems that is essentially an application of the tug-of-war theory to recognition systems that involve conflicts of interests among the interacting parties.
2. A model of simple tug-of-war among mimics, targets and operators
(a). The basic model with linear operator effort costs
Suppose there is a discrimination game involving an operator (discriminating individual), a target (the operator's desired recipient of an action) and a mimic (an individual who benefits more when it is confused with the target). We construe this game broadly, i.e. not applying to just games among predators, Batesian mimics and models. For example, the game structure could apply to nest-mate recognition in social species, in which colony guards must discriminate between nest-mates and potential non-nest-mate threats [19,23], and to parent–offspring recognition, as when male parents might benefit by discriminating between genetic offspring versus unrelated extra-pair young [44]. In both of the latter examples, there could be substantial fitness benefits to the targets (desirable recipients) for being properly identified by the operator and to the mimics for being able to induce acceptance errors by the operator.
Each individual in the game can invest effort in modifying the probability that the operator successfully discriminates in favour of the target (such investments may have been implemented before their interaction). Let p is the probability that the operator accepts or chooses the target instead of the mimic. We assume that both the operator and the target benefit more the higher the value of p, so p can be expressed as follows:
| 2.1 |
where etar is the effort (investment) of the target to promote discrimination (increase p), emim is the effort (investment) of the mimic to reduce discrimination (decrease p), eop is the effort (investment) of the operator to promote discrimination and a is a small positive constant. The structure of equation (2.1) encodes the assumption that, in the absence of a mimic or any mimic effort, the operator is guaranteed to accept the target and not a mimic, conveying benefits to both the operator and the target. For simplicity, the efforts are assumed to be set in advance of the actual recognition interactions (i.e. are not dynamically adjusted during or as a result of the interactions), but they need not be genetically fixed as they might be adjusted facultatively in different recognition contexts encountered by individuals. Our model also allows for the possibility that, for a given recognition context, a given individual may be in a model role in some interactions, but in a mimic or an operator role in others.
Next, we express the fitness pay-offs for each party. For the operator, we initially assume that its fitness pay-off is given by
| 2.2 |
where and are the fitness pay-offs to the operator for correct and incorrect discrimination, respectively. We define Δop as a pay-off differential for correct versus incorrect discrimination, i.e. . Thus, the operator's fitness pay-off can be re-written more simply as wop = pΔop − eop, focusing only on terms that are a function of its investment eop. This modification does not affect equilibria because only terms involving the selfish investments matter in the joint fitness maximization described in the following equation (i.e. maximizing a function and a constant yields the same solution as maximizing the function by itself).
Similarly, the target's fitness is given by
| 2.3 |
We assume that and can represent this difference as Thus, the target's fitness pay-off can be re-written as wtar = pΔtar − etar, including only terms that are a function of its investment etar (as discussed earlier, only these terms matter in the joint fitness maximization below).
Finally, the mimic's fitness is given by
| 2.4 |
We assume that and can represent this difference as . Note that we are assuming that both the operator and the target profit, although not necessarily to the same degree, by correct discrimination, but that the mimic profits from incorrect discrimination. Thus, the mimic's fitness pay-off can be re-written as wmim = (1 − p)Δmim − emim, including only terms that are a function of its investment emim (as discussed earlier, only these terms matter in the joint fitness maximization below).
We seek the set of efforts () that simultaneously maximize all three net fitness pay-offs, so we seek the critical points simultaneously satisfying
| 2.5 |
The stable non-trivial equilibria for the above model are as follows. If Δop < Δtar, then the operator invests zero effort, with the optimal effort of the target becoming and the optimal effort of the mimic becoming Assuming the latter two efforts are positive, plugging these efforts back into p yields, simply, p* = Δtar/Δtar + Δmim.
If, however, Δop > Δtar, we get the unrealistic result that the operator should invest an infinite effort in promoting discrimination, and the model and the mimic should invest zero effort. Based on both of these findings, we conclude that a linear cost for operators cannot explain recognition systems seen in nature. This raises the need to consider the more realistic situation in which the operator's effort cost at least eventually rises in a convex fashion with the increasing effort invested in promoting discrimination.
(b). The basic model with non-linear operator effort costs
We now consider what happens when the operator's cost for investing effort in promoting discrimination increases as the square of the effort invested. More steeply increasing costs to improve already highly accurate operator detection systems is suggested by research, demonstrating high costs of reducing sensory noise in already well-adapted bacterial systems [45]. Sensory systems and their refinement are also known to be costly in animals [46]. We shall keep the fitness expressions for the target and the mimic the same as earlier, but the operator's fitness now becomes
| 2.6 |
With this change, the optimal set of efforts becomes for the target, for the mimic and for the operator. These solutions correspond to local fitness maxima for each of the parties as the second derivatives of the fitness pay-offs with respect to investment is negative in all cases. The optimal efforts for each player depend on the pay-offs to other players to differing degrees. The target's optimal effort is influenced by both the mimic's and the operator's pay-offs, whereas the mimic's and the operator's optimal efforts are each influenced only by the target's pay-off.
The aforementioned solutions can be simplified further by introducing a parameter k that is just the ratio Δmim/Δtar. Then, the optimal investments become just
| 2.7 |
The properties of the solutions in equation (2.7) yield a number of predictions.
-
(i)
Plugging these optimal efforts back into p yields (assuming that all investments are positive), as in the model with linear operator investment costs, p* = (Δtar/Δtar + Δmim) = (1/1 + k). Thus, in both basic models, the optimal efforts yield a successful discrimination probability that depends only on the target's and the mimic's relative fitness benefits for their preferred discrimination outcomes. The reason for this is that the operator's optimal effort is given by Δop/2Δtar, an amount that is deducted from the target's optimal effort. That is, as the fitness benefit of successful discrimination to the operator increases relative to that for the target, the operator invests more in successful discrimination, but the target deducts exactly this amount from its own effort. The result is that the operator's effort has no net effect on the probability of successful discrimination at the equilibrium even though operators invest more the greater their pay-off for successful discrimination. Thus, stable accurate recognition by operators is expected when the pay-off to targets for successful operator discrimination is much higher than the pay-off to the mimic for failed operator discrimination. However, stable inaccurate recognition by operators is expected when the pay-off to mimics for failed operator discrimination is much higher than the pay-off to targets for successful operator discrimination.
-
(ii)
It is possible for the mathematical expression for the target's optimal effort in equation (2.7) to become negative, in particular if Δop > (2Δmim(Δtar)3/(Δmim + Δtar)2) − 2aΔtar. This is most likely to occur when the target's fitness benefit for successful discrimination is small relative to the operator's and mimic's benefits for their best outcomes (figure 1). In such a case, the target's optimal investment becomes zero, and only the operator and mimic invest in their best outcomes (the solutions for this case not given because of their complexity). It is unclear what biological situation would favour zero effort on the part of the target. In any event, we are particularly interested in recognition systems in which targets have a significant fitness interest in having the operator making a correct discrimination; therefore, we will restrict our attention to cases where all the solutions in (2.7) are non-zero, and thus, all parties make investments in the recognition tug-of-war.
-
(iii)
As is intuitive, each party's optimal investment increases its fitness pay-off for successful discrimination (the operator or target) or unsuccessful discrimination (the mimic), all other variables held constant [26]. This is reminiscent of the ‘life-dinner' principle, in which predator-avoidance investments by prey in a tug-of-war with predators should often exceed prey-capture investments by predators because the prey's life is at stake, whereas only a single dinner is at stake from the predator's perspective [47].
-
(iv)
The operator's optimal investment declines as the target's pay-off for correct operator discrimination increases but is not affected by the mimic's pay-off for incorrect operator discrimination. This result echoes findings in another work from this volume [35], which shows that optimal investments in individual recognition from senders (i.e. targets) and receivers (i.e. operators) will tend to be asymmetric. Interestingly, the target's investment increases as the mimic's pay-off for failed discrimination increases, up until the point at which the mimic's pay-off becomes equal or greater than the target's pay-off for successful discrimination. Past the latter point, the target decreases its investment as the mimic's pay-off increases. Conversely, the mimic's investment increases as the target's pay-off for correct discrimination increases, up until the point at which the target's pay-off becomes equal or greater than the mimic's pay-off for successful discrimination. Past the latter point, the mimic decreases its investment as the target's pay-off increases (figure 2). In other words, each of the latter two parties escalates its investment as the other's party's fitness pay-off for its best outcome increases, but only as long as its own fitness pay-off exceeds the fitness pay-off of the other party. It is as if each party starts ‘giving up' when the rival party's relative incentive to invest becomes stronger.
Figure 1.
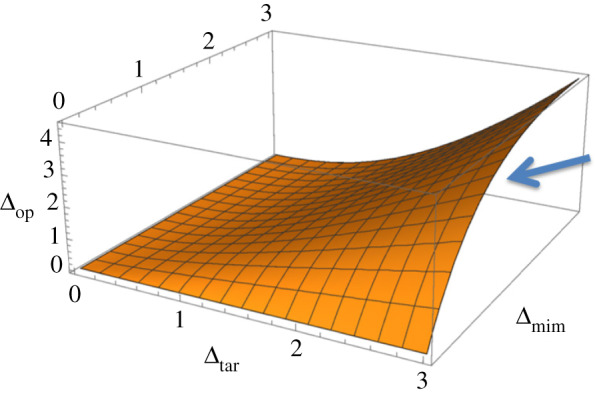
Conditions under which all three parties will invest effort in achieving their optimal recognition outcome. For values of Δop below the surface, the target (as indicated by the arrow), the mimic and the operator will have a positive optimal efforts. For values of Δop above the surface, the target will not exert any effort and only the mimic and operator exhibit positive optimal efforts. For this graph, a is taken to be positive but negligible. (Online version in colour.)
Figure 2.
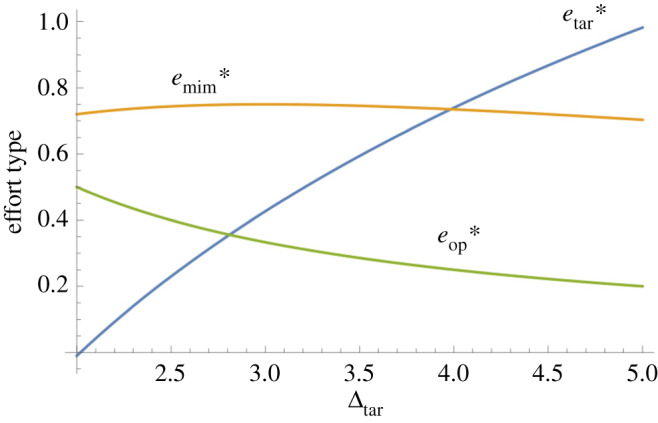
Optimal efforts of the operator (), mimic () and target () as a function of the fitness pay-off to the target (Δtar) for successful discrimination. For this graph, Δop = 2, Δmim = 3 and a = 0.01. The target's optimal effort steadily increases as its pay-off for successful discrimination increases, the operator's optimal effort steadily decreases as the target's pay-off for successful discrimination increases and the mimic's optimal effort increases and then decreases, as the target's pay-off for successful discrimination increases. (Online version in colour.)
(c). Variable interaction rates
In the aforementioned basic models, an operator, target and mimic reap pay-offs from affecting discrimination likelihood only when all are present together in a triad consisting of an operator, a target and a mimic. We refer to the latter kinds of triads as ‘recognition triads'. However, it is not generally realistic to assume that each player is equally likely to find itself in this triadic discrimination context. All three players may have different frequencies depending on the scenario. Consider the case of a brood parasitic cowbird (a mimic) interacting with host parents (operators) and host chicks (targets) [48]. The mimic in this case is always in a recognition triad; however, not all host nests are parasitized, so the rates are lower for operators and targets. Given that there are fewer parents than chicks in any given breeding cycle, the chances that a given target would be in a triad is lower than the probability for the operator in this case. Interspecific brood parasitism is a case where there is a single mapping between an individual and its potential role (host parent = operator, host chick = target, brood parasite = mimic). It need not be the case, however, that an individual plays one role in the model across different interactions. In the case of colonial nesting or breeding species, parents and offspring often have elaborate means to identify each other to aid the accurate delivery of parental care to the target offspring [33,49,50]. In these situations, depending on which parent's perspective we take as the operator, any particular offspring may be in a target or mimic role. Across the population as a whole, a given offspring is more often considered a mimic than a target from the perspective of adults, because there are many more non-parent adults than they have parents. However, from the perspective of the offspring, they are likely to find themselves in a target role more often than a mimic as parents do not choose between their offspring and all others, but perhaps a few other offspring at most in any given decision.
Thus, we can write the operator's fitness new pay-off as , the target's fitness new pay-off as wtar = utarpΔtar − etar and the mimic's fitness new pay-off as wmim = umim(1 − p)Δmim − emim, where uop, utar and umim are the operator's, target's and mimic's probabilities of finding themselves in a recognition triad, respectively. Each u value will be higher as the recognition triad scenario is more common for a given player. The u's can be thought of as reflecting the rates at which each party finds itself in a recognition triad, which is similar conceptually to the ‘interaction rate' of operators in the classical optimal acceptance threshold theory [18].
The optimal investments in this generalized model are given by
| 2.8 |
The optimal efforts in equation (2.8) are similar to that we saw in equation (2.7), but now taking into account the relative interaction rates of the three parties. We still find that the target's and operator's efforts are expected to be inversely related, all else being equal. Substituting the solutions in equation (2.8) back into the probability of correct operator discrimination yields
| 2.9 |
If k = Δmim/Δtar and j = umim/utar, then we obtain the following simple expression:
| 2.10 |
Thus, in the model with variable interaction rates, recognition is expected to be more accurate with increased probability that the target finds itself a recognition triad and/or the greater the fitness gain of the target relative to the mimic (figure 3).
Figure 3.
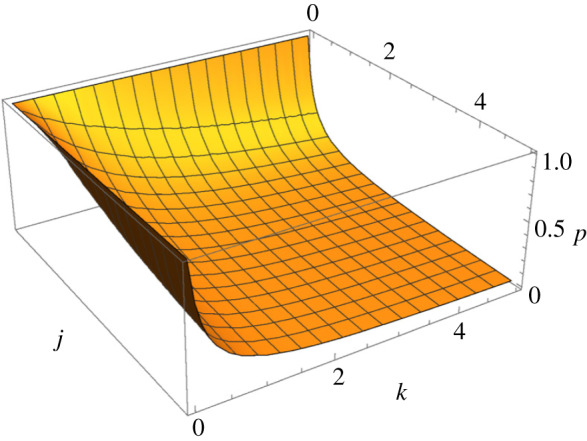
Overall probability p* of successful discrimination by the operator when all three parties are exhibiting non-zero optimal efforts. The variable j equals the ratio of the interaction rates, umim/utar and the variable k is the ratio of the fitness pay-offs Δmim/Δtar. High probability of correct operator recognition will occur when j is low (interaction rate for target is much higher than that for mimics), k is low (target's fitness pay-off for successful recognition is much higher than the mimic's pay-off for failed recognition), or both. Low probability of correct operator recognition will occur when j is high (interaction rate for the mimic is much higher than that for targets) and k is high (mimic's fitness pay-off for failed recognition is much higher than the target's pay-off for successful recognition). (Online version in colour.)
3. Discussion
Signal detection theory seeks to understand the probability of correct and incorrect recognition decisions in a system based on the relative benefits of accuracy, costs of errors and difficulty of discrimination. The game theory models presented earlier, unlike classic signal detection models [18], are unfettered by assumptions about the sensory, perceptual and cue/signal production mechanisms in recognition systems, instead allowing them to evolve freely for all three parties in a recognition triad. Thus, such models may yield a deeper understanding of many of the puzzling properties of recognition systems than would be obtained by applying the signal detection framework alone.
We illustrate a class of such puzzles that we think may be illuminated by our model, puzzles that involve otherwise unexpected failures of recognition.
(a). Lack of within-colony nepotism in social insects
A number of studies have shown that despite the simultaneous presence of multiple kin classes within social insect colonies, owing to queen multiple mating [51,52] or co-existence of multiple reproducing queens [53–55], there is little evidence that workers discriminate between more highly related kin (e.g. full-sisters) or less-highly related kin (e.g. half-sisters) [56–60]. First, this was puzzling, because one might think that selection would favour operators (e.g. discriminating workers) to favour their more highly related kin (targets), at least to the extent that the latter can reproduce. Of course, the less-highly related kin (mimics) could lose out from the latter discrimination and so would be expected to profit from trying to prevent it, perhaps by hiding their distinctive cues and/or exaggerating the cues shared by all colony members.
Suppose for example, we apply our aforementioned theory to the evolution of intracolonial nepotism among honeybee workers. Because of frequent multiple mating by the queen [51], a given worker is more likely to encounter brood that are half-sisters than full-sisters (e.g. if the queen mates with 20 different males and uses the sperm equally, a given worker will encounter a full-sister only 5% of the time on average). That means a focal larvae is much more likely to be in a context where it is in the mimic role than in the target role, meaning umim ≫ utar , because most of the workers who would care for it are related as half-sisters. According to the aforementioned theory, this means that the result is likely to be a weak or a failed recognition system because the mimic gains so much more cumulatively by hiding its kinship cues than the target would gain by exaggerating its kinship cues. This logic applies to every larvae, so it is not surprising that a lack of intracolony nepotism is what is observed. Similarly, the same logic also applies to worker–worker interactions where any given worker is most often in the role of mimic given that relative rarity of her full-sisters.
(b). Parent–offspring recognition—extra-pair young
In many bird species with bi-parental care, breeding males have genetically unrelated extra-pair young within their own nest [61–64], however several studies have shown that such males typically do not appear to discriminate against extra-pair young in either feeding or aggression [44,65–68]. In this case, the breeding male is the operator, the genetic young are potential targets and the extra-pair young are potential mimics. Our theory suggests that there are two possible explanations for this lack of nepotism. One is that not all nests usually have extra-pair young, so potential targets are often not in recognition triads, whereas extra-pair young usually co-occur with young that are genetic offspring of the breeding male, such that, again, umim > utar. Moreover, the consequence of successful discrimination might be the death of the extra-pair young, but only slightly more food for genetic offspring of the breeding male, so that Δmim > Δtar. Both of the aforementioned factors should act to reduce the accuracy of discrimination according to our model.
(c). Parent–offspring recognition—interspecific brood parasitism
The above logic also applies to interspecific brood parasitism in birds. Host parents (operators) would obviously benefit from discriminating between their own young (potential targets) and parasitic young (potential mimics) [3]. However, the percentage of hosts that are parasitized (population-wide) is often low [3], whereas parasitic brood are almost always at least initially surrounded by host brood within the same nest, so again we have umim ≫ utar. As in the case of recognition of extra-pair young, it again is also often true that Δmim > Δtar because not all brood parasites kill their host nest-mates [69,70] and not all potential targets occur in a nest with a mimic. Interestingly, in the above cases, the strategy of the brood parasitic young is not always to mimic the appearance of the host young throughout its development, but may involve other nefarious manipulations of the host's recognition system, leading to the bizarre cases in which the maturing parasitic young bear little resemblance to the host young [3,9,71,72]. Cuckoo chicks often bear little resemblance to the chicks of their hosts, although it is noteworthy that cuckoo chicks eject or kill the host eggs in their nests, meaning that at the chick stage, they are no longer in a recognition triad [73]. The absence of the appropriate target during the chick stage may explain some of the chick phenotypes found in cuckoos. Although we have not dynamically modelled the effects of mimic frequency on the evolutionary trajectories of investment in recognition systems, we can surmise that such an extension may explain some of the evolutionary dynamics inferred in brood parasite—host evolutionary dynamics. If mimics are successful and increase in frequency, then it would lead to increased rates of operators and targets being in recognition triads, leading to higher target investment and the mimic may give up. This pattern may explain the host-shifts that have been inferred for some host races of brood parasites [3].
In addition to explaining many puzzling features of failed recognition, our model predicts accurate recognition in many contexts where well-developed recognition systems are common.
(d). Parent–offspring recognition in colonial nesting/breeding species
In contrast with the puzzling failures of recognition in the contexts of extra-pair young and brood parasites, efficient and accurate parent–offspring recognition appears to the norm in many colonial breeding species including swallows [33], bats [74], pinnipeds [50,75], gulls [76,77] and penguins [49]. As with other parent–offspring scenarios discussed earlier, we can understand the evolution of recognition systems as a game among parents (operators), genetic young (targets) and other young (mimics). A major difference between colonial breeding species and other instances of parent–offspring recognition is the relative benefit of recognition errors to the mimic. Mimics in a colonial breeding scenario would benefit from inaccurate recognition by gaining food resources, just as in the extra-pair young or brood parasite scenarios. If potential mimic offspring are non-mobile, as when they are altricial and sufficiently young (e.g. not yet fledged), they would have a low chance of even being in a mimic role (umim low) because they would be unlikely to appear in the nest of unrelated parental adults. If the young in a colonial situation are mobile (as in precocial colonial birds), the cost of accurate recognition may just be one less feeding for a mimic, because the mimic can return to its natal nest after parasitizing an unrelated family group, in contrast with the likely death for a mimic extra-pair altricial young or young brood parasite trapped in a nest with an unrelated breeder that is much stronger than it. Even though the fitness benefits may be similar for parental feeding decisions, being identified as a non-genetic offspring of an operator in a colonial setting need not carry dire consequences. Therefore, Δmim is likely to be much lower in colonial species, increasing the probability of accurate recognition.
(e). Dear enemy effect
The ‘dear enemy' effect is a phenomenon where territory owners reduce their levels of aggression to neighbours, while maintaining high levels of aggression to unfamiliar challengers [5,78,79]. The recognition triad in the case of the ‘dear enemy' effect would include a focal territory owner (operator), a neighbour (target) and unfamiliar individuals (mimic). We might assume that receiving aggression from the operator has a similar cost for a target or mimic, so that Δtar and Δmim are similar in this scenario. The relative rate at which operators encounter targets is likely to be much higher than their encounter rate for mimics, which may drive this pattern. Times of instability owing to influxes of new animals or rapid territorial turnover would lead to increased mimic encounter rates and might be expected to reduce the likelihood of neighbour/stranger discrimination. Indeed, studies have described apparent failures of the ‘dear enemy' effect under such unstable conditions [80–82].
(f). Future theoretical extensions of optimal recognition
Here, we have presented a relatively simple and straightforward tug-of-war model to understand the optimal investments for accurate recognition depending on the strategy of individuals involved (i.e. operator, target or mimic). We extend the basic model to consider variable interaction rates, which suggests that the relative frequency of interactions and fitness differentials for targets and mimics are key factors explaining the likelihood of accurate recognition. Two obvious directions for future theoretical analysis would be to take genetics into account, both by considering relatedness and being more explicit about the genetic basis of the traits, e.g. using a quantitative genetics framework. Our models do not contain any relatedness terms, though many of the puzzling recognition failures in the literature our model appears to explain have involved variation in genetic relatedness within social insect colonies or in the case of extra-pair young [16]. Incorporating relatedness into future models will probably provide even crisper predictions regarding recognition system accuracy when some or all parties are genetically related. A third desirable extension to the model would be to relate the interaction rate terms more explicitly to the relative numbers of individuals in the different roles (model, mimic and operator), perhaps incorporating population dynamics and density dependence into the trait evolution using adaptive dynamics approaches.
Players in different roles may be the same individual in different contexts or at different times of their life. For example, in the context of recognizing young in a colonial breeding species, one's parent's target is another parent's mimic [83,84]. The young themselves will grow up later to be operators. Our model makes no assumptions about the types of traits that different players may use in the game, though in practice this may be unrealistic. It may not always be possible for individuals to shift rapidly between target and mimic roles in the most optimal way, especially if the traits used would be the same or similar (e.g. a given individual can only have one cuticular hydrocarbon profile at a time). Considering the genetic correlations among traits (but allowing those correlations to themselves evolve) may be a fruitful direction for additional theoretical studies. Nevertheless, we suggest that thinking about the fitness consequences and possible asymmetric interaction rates among (minimally), all three parties in recognition systems will be pivotal in understanding the evolutionary basis of variation in recognition accuracy across species and recognition contexts.
Data accessibility
This article has no additional data.
Authors' contributions
M.J.S. and H.K.R. conceived the research and designed the game. H.K.R. implemented the model. M.J.S. and H.K.R. wrote and edited the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by NIH grant no. DP2-GM128202 (M.J.S.) and NSF CAREER grant no. DEB-1750394 (M.J.S.)
References
- 1.Sheehan MJ, Bergman TJ. 2016. Is there an evolutionary trade-off between quality signaling and social recognition? Behav. Ecol. 27, 2–13. ( 10.1093/beheco/arv109) [DOI] [Google Scholar]
- 2.Sherman PW, Reeve HK, Pfennig DW. 1997. Recognition systems. In Behavioural ecology: an evolutionary approach (eds Krebs JR, Davies NB), pp. 69–96. Oxford: Blackwell Science Ltd. [Google Scholar]
- 3.Soler M. 2014. Long-term coevolution between avian brood parasites and their hosts. Biol. Rev. 89, 688–704. ( 10.1111/brv.12075) [DOI] [PubMed] [Google Scholar]
- 4.Breed MD. 2014. Kin and nestmate recognition: the influence of WD Hamilton on 50 years of research. Anim. Behav. 92, 271–279. ( 10.1016/j.anbehav.2014.02.030) [DOI] [Google Scholar]
- 5.Jaeger RG. 1981. Dear enemy recognition and the costs of aggression between salamanders. Am. Nat. 117, 962–974. ( 10.1086/283780) [DOI] [Google Scholar]
- 6.Axelrod R, Hamilton WD. 1981. The evolution of cooperation. Science 211, 1390–1396. ( 10.1126/science.7466396) [DOI] [PubMed] [Google Scholar]
- 7.Crowley PH, Provencher L, Sloane S, Dugatkin LA, Spohn B, Rogers L, Alfieri M. 1996. Evolving cooperation: the role of individual recognition. Biosystems 37, 49–66. ( 10.1016/0303-2647(95)01546-9) [DOI] [PubMed] [Google Scholar]
- 8.Mappes J, Alatalo RV. 1997. Batesian mimicy and signal accuracy. Evolution 51, 2050–2053. ( 10.1111/j.1558-5646.1997.tb05129.x) [DOI] [PubMed] [Google Scholar]
- 9.Davies NB, Kilner RM, Noble DG. 1998. Nestling cuckoos, Cuculus canorus, exploit hosts with begging calls that mimic a brood. Proc. R. Soc. Lond. B 265, 673–678. ( 10.1098/rspb.1998.0346) [DOI] [Google Scholar]
- 10.Medina I, Langmore NE. 2016. The evolution of acceptance and tolerance in hosts of avian brood parasites. Biol. Rev. 91, 569–577. ( 10.1111/brv.12181) [DOI] [PubMed] [Google Scholar]
- 11.Soler JJ, Soler M. 2017. Evolutionary change: facultative virulence by brood parasites and tolerance and plastic resistance by hosts. Anim. Behav. 125, 101–107. ( 10.1016/j.anbehav.2017.01.004) [DOI] [Google Scholar]
- 12.Avilés JM. 2017. Can hosts tolerate avian brood parasites? An appraisal of mechanisms. Behav. Ecol. 29, 509–519. ( 10.1093/beheco/arx150) [DOI] [Google Scholar]
- 13.Lindström L, Alatalo RV, Lyytinen A, Mappes J. 2004. The effect of alternative prey on the dynamics of imperfect Batesian and Müllerian mimicries. Evolution 58, 1294–1302. ( 10.1111/j.0014-3820.2004.tb01708.x) [DOI] [PubMed] [Google Scholar]
- 14.Kikuchi DW, Pfennig DW. 2010. Predator cognition permits imperfect coral snake mimicry. Am. Nat. 176, 830–834. ( 10.1086/657041) [DOI] [PubMed] [Google Scholar]
- 15.Sherratt TN. 2002. The evolution of imperfect mimicry. Behav. Ecol. 13, 821–826. ( 10.1093/beheco/13.6.821) [DOI] [Google Scholar]
- 16.Wenseleers T. 2007. Nepotism absent in insect societies—or is it? Mol. Ecol. 16, 3063–3065. ( 10.1111/j.1365-294X.2007.03313.x) [DOI] [PubMed] [Google Scholar]
- 17.Breed MD, Cook CN, McCreery HF, Rodriguez M. 2015. Nestmate recognition in eusocial insects: the honeybee as a model system. In Social recognition in invertebrates (eds Aquiloni L, Tricarico E), pp. 147–164. Berlin, Germany: Springer. [Google Scholar]
- 18.Reeve HK. 1989. The evolution of conspecific acceptance thresholds. Am. Nat. 133, 407–435. ( 10.1086/284926) [DOI] [Google Scholar]
- 19.Downs SG, Ratnieks FL. 2000. Adaptive shifts in honey bee (Apis mellifera L.) guarding behavior support predictions of the acceptance threshold model. Behav. Ecol. 11, 326–333. ( 10.1093/beheco/11.3.326) [DOI] [Google Scholar]
- 20.Hanley D, López AV, Fiorini VD, Reboreda JC, Grim T, Hauber ME. 2019. Variation in multicomponent recognition cues alters egg rejection decisions: a test of the optimal acceptance threshold hypothesis. Phil. Trans. R. Soc. B 374, 20180195 ( 10.1098/rstb.2018.0195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Starks PT, Fischer DJ, Watson RE, Melikian GL, Nath SD. 1998. Context-dependent nestmate discrimination in the paper wasp, Polistes dominulus: a critical test of the optimal acceptance threshold model. Anim. Behav. 56, 449–458. ( 10.1006/anbe.1998.0778) [DOI] [PubMed] [Google Scholar]
- 22.Noh H-J, Gloag R, Langmore NE. 2018. True recognition of nestlings by hosts selects for mimetic cuckoo chicks. Proc. R. Soc. B 285, 20180726 ( 10.1098/rspb.2018.0726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossi N, Baracchi D, Giurfa M, d'Ettorre P. 2019. Pheromone-induced accuracy of nestmate recognition in carpenter ants: simultaneous decrease in type I and type II errors. Am. Nat. 193, 267–278. ( 10.1086/701123) [DOI] [PubMed] [Google Scholar]
- 24.Steiger S, Müller JK. 2010. From class-specific to individual discrimination: acceptance threshold changes with risk in the partner recognition system of the burying beetle Nicrophorus vespilloides. Anim. Behav. 80, 607–613. ( 10.1016/j.anbehav.2010.06.018) [DOI] [Google Scholar]
- 25.Vernier CL, Krupp JJ, Marcus K, Hefetz A, Levine JD, Ben-Shahar Y. 2019. The cuticular hydrocarbon profiles of honey bee workers develop via a socially-modulated innate process. eLife 8, e41855 ( 10.7554/eLife.41855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnstone RA. 1998. Conspiratorial whispers and conspicuous displays: games of signal detection. Evolution 52, 1554–1563. ( 10.1111/j.1558-5646.1998.tb02236.x) [DOI] [PubMed] [Google Scholar]
- 27.Sheehan MJ, Nachman MW. 2014. Morphological and population genomic evidence that human faces have evolved to signal individual identity. Nat. Commun. 5, 1–10. ( 10.1038/ncomms5800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheehan MJ, Lee V, Corbett-Detig R, Bi K, Beynon RJ, Hurst JL, Nachman MW. 2016. Selection on coding and regulatory variation maintains individuality in major urinary protein scent marks in wild mice. PLoS Genet. 12, e1005891 ( 10.1371/journal.pgen.1005891) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pollard KA, Blumstein DT. 2011. Social group size predicts the evolution of individuality. Curr. Biol. 21, 413–417. ( 10.1016/j.cub.2011.01.051) [DOI] [PubMed] [Google Scholar]
- 30.Steiger S, Franz R, Eggert A-K, Müller JK. 2008. The Coolidge effect, individual recognition and selection for distinctive cuticular signatures in a burying beetle. Proc. R. Soc. B 275, 1831–1838. ( 10.1098/rspb.2008.0375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caves EM, Stevens M, Iversen ES, Spottiswoode CN. 2015. Hosts of avian brood parasites have evolved egg signatures with elevated information content. Proc. R. Soc. B 282, 20150598 ( 10.1098/rspb.2015.0598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheehan MJ, Jinn J, Tibbetts EA. 2014. Coevolution of visual signals and eye morphology in Polistes paper wasps. Biol. Lett. 10, 20140254 ( 10.1098/rsbl.2014.0254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loesche P, Stoddard PK, Higgins BJ, Beecher MD. 1991. Signature versus perceptual adaptations for individual vocal recognition in swallows. Behaviour 118, 15–25. ( 10.1163/156853991X00175) [DOI] [Google Scholar]
- 34.Sheehan MJ, Tibbetts EA. 2011. Specialized face learning is associated with individual recognition in paper wasps. Science 334, 1272–1275. ( 10.1126/science.1211334) [DOI] [PubMed] [Google Scholar]
- 35.Miller SE, Sheehan MJ, Reeve HK. 2020 Coevolution of cognitive abilities and identity signals in individual recognition systems. Phil. Trans. R. Soc. B 375, 20190467 ( 10.1098/rstb.2019.0467) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiley RH. 2006. Signal detection and animal communication. Adv. Study Behav. 36, 217–247. ( 10.1016/S0065-3454(06)36005-6) [DOI] [Google Scholar]
- 37.Rodriguez-Girones MA, Lotem A. 1999. How to detect a cuckoo egg: a signal-detection theory model for recognition and learning. Am. Nat. 153, 633–648. ( 10.1086/303198) [DOI] [PubMed] [Google Scholar]
- 38.Nesse RM. 2005. Natural selection and the regulation of defenses: a signal detection analysis of the smoke detector principle. Evol. Hum. Behav. 26, 88–105. ( 10.1016/j.evolhumbehav.2004.08.002) [DOI] [Google Scholar]
- 39.Getty T, Kamil AC, Real PG. 1987. Signal detection theory and foraging for cryptic or mimetic prey. In Foraging behavior (eds Kamil AC, Krebs JR, Pulliam HR), pp. 525–548. New York, NY: Plenum Press. [Google Scholar]
- 40.Reeve HK, Shen S-F. 2006. A missing model in reproductive skew theory: the bordered tug-of-war. Proc. Natl Acad. Sci. USA 103, 8430–8434. ( 10.1073/pnas.0603005103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen S-F, Reeve HK. 2010. Reproductive skew theory unified: the general bordered tug-of-war model. J. Theor. Biol. 263, 1–12. ( 10.1016/j.jtbi.2009.11.009) [DOI] [PubMed] [Google Scholar]
- 42.Johnstone RA. 2000. Models of reproductive skew: a review and synthesis (invited article). Ethology 106, 5–26. ( 10.1046/j.1439-0310.2000.00529.x) [DOI] [Google Scholar]
- 43.Buston PM, Zink AG. 2009. Reproductive skew and the evolution of conflict resolution: a synthesis of transactional and tug-of-war models. Behav. Ecol. 20, 672–684. ( 10.1093/beheco/arp050) [DOI] [Google Scholar]
- 44.Kempenaers B, Sheldon BC. 1996. Why do male birds not discriminate between their own and extra-pair offspring? Anim. Behav. 51, 1165–1173. ( 10.1006/anbe.1996.0118) [DOI] [Google Scholar]
- 45.Lan G, Sartori P, Neumann S, Sourjik V, Tu Y. 2012. The energy–speed–accuracy trade-off in sensory adaptation. Nat. Phys. 8, 422 ( 10.1038/nphys2276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niven JE, Laughlin SB. 2008. Energy limitation as a selective pressure on the evolution of sensory systems. J. Exp. Biol. 211, 1792–1804. ( 10.1242/jeb.017574) [DOI] [PubMed] [Google Scholar]
- 47.Dawkins R, Krebs JR. 1979. Arms races between and within species. Proc. R. Soc. Lond. B 205, 489–511. [DOI] [PubMed] [Google Scholar]
- 48.Kilner RM, Madden JR, Hauber ME. 2004. Brood parasitic cowbird nestlings use host young to procure resources. Science 305, 877–879. ( 10.1126/science.1098487) [DOI] [PubMed] [Google Scholar]
- 49.Jouventin P, Aubin T. 2002. Acoustic systems are adapted to breeding ecologies: individual recognition in nesting penguins. Anim. Behav. 64, 747–757. ( 10.1006/anbe.2002.4002) [DOI] [Google Scholar]
- 50.Insley SJ. 2001. Mother-offspring vocal recognition in northern fur seals is mutual but asymmetrical. Anim. Behav. 61, 129–137. ( 10.1006/anbe.2000.1569) [DOI] [PubMed] [Google Scholar]
- 51.Mattila HR, Seeley TD. 2007. Genetic diversity in honey bee colonies enhances productivity and fitness. Science 317, 362–364. ( 10.1126/science.1143046) [DOI] [PubMed] [Google Scholar]
- 52.Fjerdingstad EJ, Boomsma JJ. 1998. Multiple mating increases the sperm stores of Atta colombica leafcutter ant queens. Behav. Ecol. Sociobiol. 42, 257–261. ( 10.1007/s002650050437) [DOI] [Google Scholar]
- 53.Sheehan MJ, Botero CA, Hendry TA, Sedio BE, Jandt JM, Weiner S, Toth AL, Tibbetts EA. 2015. Different axes of environmental variation explain the presence vs. extent of cooperative nest founding associations in Polistes paper wasps. Ecol. Lett. 18, 1057–1067. ( 10.1111/ele.12488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kronauer DJ, Boomsma JJ. 2007. Multiple queens means fewer mates. Curr. Biol. 17, R753–R755. ( 10.1016/j.cub.2007.06.057) [DOI] [PubMed] [Google Scholar]
- 55.Hughes WOH, Ratnieks FLW, Oldroyd BP. 2008. Multiple paternity or multiple queens: two routes to greater intracolonial genetic diversity in the eusocial Hymenoptera. J. Evol. Biol. 21, 1090–1095. ( 10.1111/j.1420-9101.2008.01532.x) [DOI] [PubMed] [Google Scholar]
- 56.Atkinson L, Teschendorf G, Adams ES. 2008. Lack of evidence for nepotism by workers tending queens of the polygynous termite Nasutitermes corniger. Behav. Ecol. Sociobiol. 62, 805–812. ( 10.1007/s00265-007-0506-z) [DOI] [Google Scholar]
- 57.DeHeer CJ, Ross KG. 1997. Lack of detectable nepotism in multiple-queen colonies of the fire ant Solenopsis invicta (Hymenoptera: Formicidae). Behav. Ecol. Sociobiol. 40, 27–33. ( 10.1007/s002650050312) [DOI] [Google Scholar]
- 58.Zinck L, Châline N, Jaisson P. 2009. Absence of nepotism in worker–queen care in polygynous colonies of the ant Ectatomma tuberculatum. J. Insect Behav. 22, 196–204. ( 10.1007/s10905-008-9165-9) [DOI] [Google Scholar]
- 59.Strassmann JE, Seppä P, Queller DC. 2000. Absence of within-colony kin discrimination: foundresses of the social wasp, Polistes carolina, do not prefer their own larvae. Naturwissenschaften 87, 266–269. ( 10.1007/s001140050718) [DOI] [PubMed] [Google Scholar]
- 60.Strassmann JE, Klingler CJ, Arevalo E, Zacchi F, Husain A, Williams J, Seppä P, Queller DC. 1997. Absence of within–colony kin discrimination in behavioural interactions of swarm–founding wasps. Proc. R. Soc. Lond. B 264, 1565–1570. ( 10.1098/rspb.1997.0218) [DOI] [Google Scholar]
- 61.Brouwer L, et al. 2017. Multiple hypotheses explain variation in extra-pair paternity at different levels in a single bird family. Mol. Ecol. 26, 6717–6729. ( 10.1111/mec.14385) [DOI] [PubMed] [Google Scholar]
- 62.Griffith SC, Owens IP, Thuman KA. 2002. Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol. Ecol. 11, 2195–2212. ( 10.1046/j.1365-294X.2002.01613.x) [DOI] [PubMed] [Google Scholar]
- 63.Brouwer L, Griffith SC.. 2019. Extra-pair paternity in birds. Mol. Ecol. 28, 4864–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Westneat DF, Stewart IR. 2003. Extra-pair paternity in birds: causes, correlates, and conflict. Ann. Rev. Ecol. Evol. Syst. 34, 365–396. ( 10.1146/annurev.ecolsys.34.011802.132439) [DOI] [Google Scholar]
- 65.Lattore M, Nakagawa S, Burke T, Plaza M, Schroeder J. 2019. No evidence for kin recognition in a passerine bird. PLoS ONE 14, e0213486 ( 10.1371/journal.pone.0213486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Westneat DF, Clark AB, Rambo KC, Westneat DF. 1995. Within-brood patterns of paternity and paternal behavior in red-winged blackbirds. Behav. Ecol. Sociobiol. 37, 349–356. ( 10.1007/BF00174140) [DOI] [Google Scholar]
- 67.Riehl C, Strong MJ. 2015. Social living without kin discrimination: experimental evidence from a communally breeding bird. Behav. Ecol. Sociobiol. 69, 1293–1299. ( 10.1007/s00265-015-1942-9) [DOI] [Google Scholar]
- 68.Li J, Zhang Z, Lv L, Gao C, Wang Y. 2014. Do parents and helpers discriminate between related and unrelated nestlings in the cooperative breeding silver-throated tit? Ethology 120, 159–168. ( 10.1111/eth.12190) [DOI] [Google Scholar]
- 69.Kilner RM. 2005. The evolution of virulence in brood parasites. Ornithologic. Sci. 4, 55–64. ( 10.2326/osj.4.55) [DOI] [Google Scholar]
- 70.Servedio MR, Hauber ME. 2006. To eject or to abandon? Life history traits of hosts and parasites interact to influence the fitness pay-offs of alternative anti-parasite strategies. J. Evol. Biol. 19, 1585–1594. ( 10.1111/j.1420-9101.2006.01124.x) [DOI] [PubMed] [Google Scholar]
- 71.Noble DG, Davies NB, Hartley IR, McRae SB. 1999. The red gape of the nestling cuckoo (Cuculus canorus) is not a supernormal stimulus for three common hosts. Behaviour 136, 759–778. ( 10.1163/156853999501559) [DOI] [Google Scholar]
- 72.Tanaka KD, Morimoto G, Stevens M, Ueda K. 2011. Rethinking visual supernormal stimuli in cuckoos: visual modeling of host and parasite signals. Behav. Ecol. 22, 1012–1019. ( 10.1093/beheco/arr084) [DOI] [Google Scholar]
- 73.Davies NB. 2011. Cuckoo adaptations: trickery and tuning. J. Zool. 284, 1–14. ( 10.1111/j.1469-7998.2011.00810.x) [DOI] [Google Scholar]
- 74.Knornschild M, Von Helversen O.. 2008. Nonmutual vocal mother-pup recognition in the greater sac-winged bat. Anim. Behav. 76, 1001–1009. ( 10.1016/j.anbehav.2008.05.018) [DOI] [Google Scholar]
- 75.Charrier I, Mathevon N, Jouventin P. 2003. Vocal signature recognition of mothers by fur seal pups. Anim. Behav. 65, 543–550. ( 10.1006/anbe.2003.2073) [DOI] [Google Scholar]
- 76.Charrier I, Mathevon N, Jouventin P, Aubin T. 2001. Acoustic communication in a black-headed gull colony: how do chicks identify their parents? Ethology 107, 961–974. ( 10.1046/j.1439-0310.2001.00748.x) [DOI] [Google Scholar]
- 77.Beer CG. 1969. Laughing gull chicks: recognition of their parent's voices. Science 166, 1030–1032. ( 10.1126/science.166.3908.1030) [DOI] [PubMed] [Google Scholar]
- 78.Tumulty JP. 2018. Dear enemy effect. In Encyclopedia of animal cognition and behavior (eds Vonk J, Shackelford T). Berlin, Germany: Springer. [Google Scholar]
- 79.Temeles EJ. 1994. The role of neighbors in territorial systems—when are they dear enemies. Anim. Behav. 47, 339–350. ( 10.1006/anbe.1994.1047) [DOI] [Google Scholar]
- 80.Stoddard PK. 1996. Vocal recognition of neighbors by territorial passerines. In Ecology and evolution of acoustic communication in birds (eds Kroodsma DE, Miller EH), pp. 356–374. Ithaca, NY: Cornell University Press. [Google Scholar]
- 81.Hyman J. 2005. Seasonal variation in response to neighbors and strangers by a territorial songbird. Ethology 111, 951–961. ( 10.1111/j.1439-0310.2005.01104.x) [DOI] [Google Scholar]
- 82.Briefer E, Rybak F, Aubin T. 2008. When to be a dear enemy: flexible acoustic relationships of neighbouring skylarks, Alauda arvensis. Anim. Behav. 76, 1319–1325. ( 10.1016/j.anbehav.2008.06.017) [DOI] [Google Scholar]
- 83.Beecher MD. 1988. Kin recognition in birds. Behav. Genet. 18, 465–482. ( 10.1007/BF01065515) [DOI] [PubMed] [Google Scholar]
- 84.Beecher MD, Medvin MB, Stoddard PK, Loesche P. 1986. Acoustic adaptatios for parent-offspring recognition in swallows. Exp. Biol. 45, 179–193. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.


