Abstract
To improve thermal stability and hardness of UV-cured materials, a series of UV-cured solvent-free coatings were prepared from allyl-terminated hyperbranched polycarbosilanes and thiol silicone resins. The silicone coatings prepared have pencil hardness of 4–9 H, water absorption no more than 0.04 wt %, and transmittance higher than 94%. The temperature for the coatings’ starting thermal decomposition is higher than 236 °C; especially, that of the coating prepared with G1 is as high as 371.1 °C. The UV-cured coatings in this work exhibit much higher pencil hardness than and superior thermal stability to those reported previously.
Introduction
Ultraviolet (UV)-cured coatings possess numerous merits including low VOC, energy saving, and fast curing speed.1−3 However, many UV-cured materials suffer from relatively poor thermal stability4 and low hardness.5−7 Materials with poor thermal stability are liable to decompose at relatively low temperature.8,9 Additionally, the coatings with low pencil hardness are very easy to be scratched.5−7 Nowadays, many efforts have been made to overcome these shortcomings.
Many experiments have confirmed that hyperbranched polymers can reduce the VOC emission of UV-cured coatings for reduction in the content of the reactive diluent and solvent.2,12−15 In addition, the functional groups on the surface of hyperbranched polymers will increase the cross-linking density, which will benefit preparation of coatings with moderately high hardness.12−14 As hyperbranched polymers, hyperbranched polycarbosilanes have Si–C bonds contributing to the relative stability of the carbosilane because of less sensitivity to nucleophilic attack.15
UV-cured silicone coatings have been proven to possess spectacular performances such as good UV resistance and thermal stability, wide operating temperature, high transparency, and low humidity absorption.1,4,10,11 Thiol-ene reactions initiated by UV have been widely applied to develop silicone materials under mild reaction conditions due to their advantages including high effectivity, no photoinitiator required, relative tolerance to lots of functional moieties, no oxygen inhibition, and less by-products.16,17 Recently, a kind of transparent flexible silicone material with thermal decomposition temperatures about 340 °C has been produced by UV-initiated thiol-ene reaction from hyperbranched polycarbosilanes and thiol silicone resins.18 Inspired by this interesting work, a class of UV-cured transparent solvent-free coatings with pencil hardness of 4–9 H, initial thermal decomposition temperature higher than 236 °C, transmittance higher than 94%, and water absorption no more than 0.04 wt % were prepared.
Results and Discussion
Effect of UV Curing Time
UV curing time has a significant impact as shown in Table 1. Even if the curing time is only 5 s, the curing degree is as high as 98.2%. The pencil hardness increases continuously from B to 8 H when the curing time increases from 5 to 30 s. If the curing time is longer than 30 s, it will have a less effect on the curing degree and pencil hardness, which is quite similar to the epoxy-modified silicone coating reported previously.1 The surface water contact angles are almost constant, 101.3–105.4°, and water absorption is no more than 0.04 wt %.
Table 1. Effect of UV Curing Timea.
| entry | curing time/s | curing degree/% | pencil hardness | surface water contact angle/deg | water absorption/wt % |
|---|---|---|---|---|---|
| 1 | 5 | 98.2 | B | 101.3 | 0.04 |
| 2 | 10 | 98.8 | 2 H | 103.4 | 0.02 |
| 3 | 15 | 98.5 | 3 H | 104.2 | 0.01 |
| 4 | 20 | 98.8 | 5 H | 104.3 | 0.02 |
| 5 | 30 | 99.6 | 8 H | 105.3 | 0.01 |
| 6 | 40 | 99.6 | 8 H | 104.6 | 0.03 |
| 7 | 50 | 99.7 | 8 H | 104.8 | 0.02 |
| 8 | 60 | 99.7 | 8 H | 105.4 | 0.04 |
Conditions: R/Si and thiol content of thiol silicone resin are 1.4 and 0.004 mol g–1, respectively. The terminated hyperbranched polycarbosilane is G1. n(thiol):n(allyl) = 1.4:1.
The Effect of the Molar Ratio of Thiol to Allyl
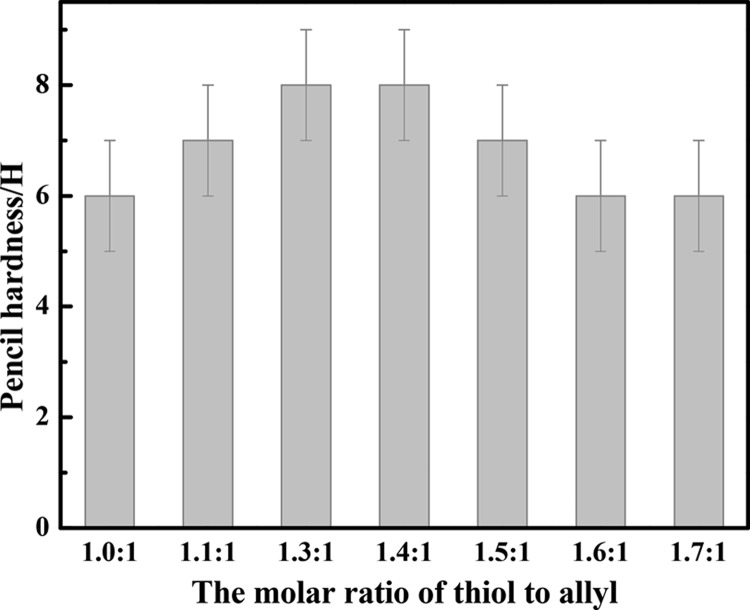
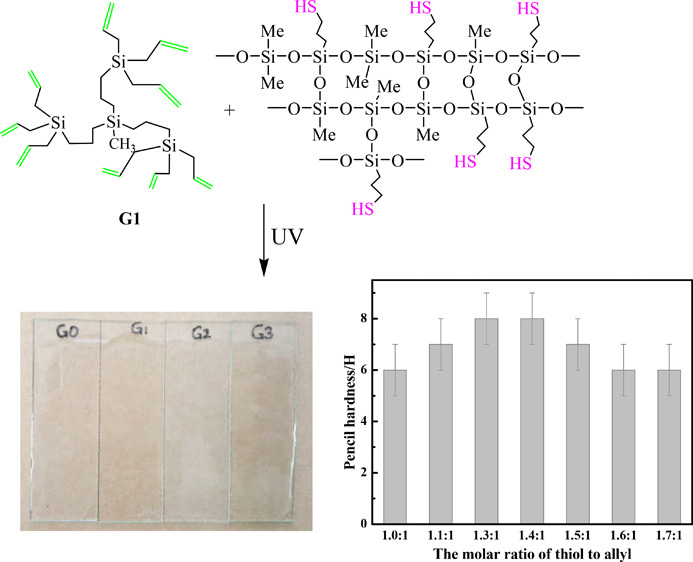
The effect of the molar ratio of thiol to allyl (n(thiol):n(allyl)) is shown in Figure 1. It is obvious that the silicone coatings have a fairly high pencil hardness (6–8 H). When n(thiol):n(allyl) increases from 1.3:1 to 1.4:1, the pencil hardness reaches the highest for the highest cross-linking density of cured materials. The pencil hardness of the UV-cured epoxy-modified silicone resin coating reported by our group can reach 5 H,1 while those of the UV-cured coatings reported by other groups are generally not higher than 4 H.2−4,12 As argued by previous works,12−14 the abundant functional groups on the surface of hyperbranched polymers will increase the cross-linking density, which will benefit preparation of coatings with moderately high hardness. Similarly, the much higher pencil hardness of the UV-cured coatings obtained might be contributed by plenty of allyls in the hyperbranched polycarbosilanes, which will react with the thiol group of the thiol silicone resin by UV-initiated thiol-ene reaction without an additional photoinitiator and increase the cross-linking density of the cured coatings. On the basis of these results, a conclusion might be drawn that the coatings have very high pencil hardness, which can be good candidates to overcome the problems such as scratches on the surface and marks produced during transportation or work.
Figure 1.
Effect of n(thiol):n(allyl) on the pencil hardness of the coatings. Conditions: R/Si of thiol silicone resin is 1.4. The hyperbranched polycarbosilane is G1. The coatings were all cured for 30 s.
The Effect of the R/Si Molar Ratio of Silicone Resin
The effect of R/Si molar ratios of thiol silicone resins is shown in Table 2. After being cured by UV for 30 s, curing degrees are higher than 99.0%, which implies that the coatings are almost cured entirely. The pencil hardness of the coatings is fairly high (4–9 H). The pencil hardness decreases with the increment of the R/Si molar ratio, which may be because a relatively high R/Si molar ratio will result in a low cross-linking density. Though there is a small growth of the water absorption when the R/Si molar ratio is increased, the water absorption is still no more than 0.03 wt %.
Table 2. Effect of R/Si Molar Ratios of Thiol Silicone Resinsa.
| entry | R/Si molar ratio | curing degree/% | pencil hardness | surface water contact angle/deg | water absorption/wt % |
|---|---|---|---|---|---|
| 1 | 1.3 | 99.8 | 9 H | 104.4 | 0.01 |
| 2 | 1.4 | 99.6 | 8 H | 105.3 | 0.01 |
| 3 | 1.5 | 99.3 | 5 H | 105.4 | 0.03 |
| 4 | 1.6 | 99.8 | 4 H | 106.4 | 0.03 |
Conditions: the thiol content of thiol silicone resin is 0.004 mol g–1. The terminated hyperbranched polycarbosilane is G1. n(thiol):n(allyl) = 1.4:1. The coatings were cured for 30 s.
The Effect of the Thiol Content of Silicone Resin
It is exhibited in Table 3 that thiol content of thiol silicone resins takes an important role. If the thiol content increases from 0.002 to 0.004 mol g–1, the pencil hardness increases from 5 H to 8 H. A further increment of the thiol content has a less effect on the pencil hardness. The water absorption is still quite low, which is 0.01–0.02 wt %. The curing degree is 97.5–99.6%, and the surface water contact angle is about 105° (Table 3 and Figure S1), which denotes that the curing degree and surface water contact angle are less influenced by the thiol content.
Table 3. Influence of the Thiol Content of Thiol Silicone Resina.
| entry | thiol content/mol g–1 | curing degree/% | pencil hardness | surface water contact angle/deg | water absorption/wt % |
|---|---|---|---|---|---|
| 1 | 0.002 | 97.5 | 5 H | 105.5 | 0.01 |
| 2 | 0.003 | 99.3 | 6 H | 105.4 | 0.01 |
| 3 | 0.004 | 99.6 | 8 H | 105.3 | 0.01 |
| 4 | 0.005 | 98.1 | 8 H | 104.6 | 0.02 |
| 5 | 0.006 | 98.7 | 8 H | 104.9 | 0.02 |
Conditions: R/Si of thiol silicone resin is 1.4. The terminated hyperbranched polycarbosilane is G1. n(thiol):n(allyl) = 1.4:1. The coatings were cured for 30 s.
The Effect of Generations of Hyperbranched Polycarbosilanes
The generations of allyl-terminated hyperbranched polycarbosilanes have a crucial impact on the pencil hardness of the coatings as shown in Table 4. As it can be seen, the pencil hardness of the coatings is in the order of G1 > G2 > G0 > G3. A relatively lower pencil hardness of coatings prepared from higher-generation hyperbranched polycarbosilanes with a bigger cross-linking network and the more defects of the molecular structure might be explained by the decrease of the cross-linking density and curing degree.19 To verify the cross-linking density of these coatings, DSC of the coatings was carried out as shown in Figure S2. Obviously, the glass transition temperature (Tg) of the coatings is in the order of G1 > G2 > G0 > G3, which can actually prove that the coatings’ cross-linking density is in the order of G1 > G2 > G0 > G3.1,20,21 Generally speaking, the coating prepared with G1 exhibits optimum comprehensive performance. Highly transparent materials can be applied to prepare or protect optical devices,22−25 and new silicone materials with high transmittance have drawn much attention. It can be obviously seen from Figures 2 and 3 that the coatings have transmittance higher than 80% (400–800 nm). Especially, the transmittances of the coatings prepared with G1, G2, and G3 are higher than 94.0% at 800 nm.
Table 4. Influence of Generations of Hyperbranched Polycarbosilanesa.
| entry | generation of hyperbranched polycarbosilanes | curing degree/% | pencil hardness | surface water contact angle/deg | water absorption/% |
|---|---|---|---|---|---|
| 1 | G0 | 99.6 | 4 H | 104.8 | 0.02 |
| 2 | G1 | 99.6 | 8 H | 105.3 | 0.01 |
| 3 | G2 | 89.7 | 5 H | 105.5 | 0.01 |
| 4 | G3 | 75.6 | <6 B | 95.1 | 0.01 |
Conditions: R/Si and thiol content of thiol silicone resin are 1.4 and 0.004 mol g–1, respectively. n(thiol):n(allyl) = 1.4:1. The coatings were cured for 30 s.
Figure 2.
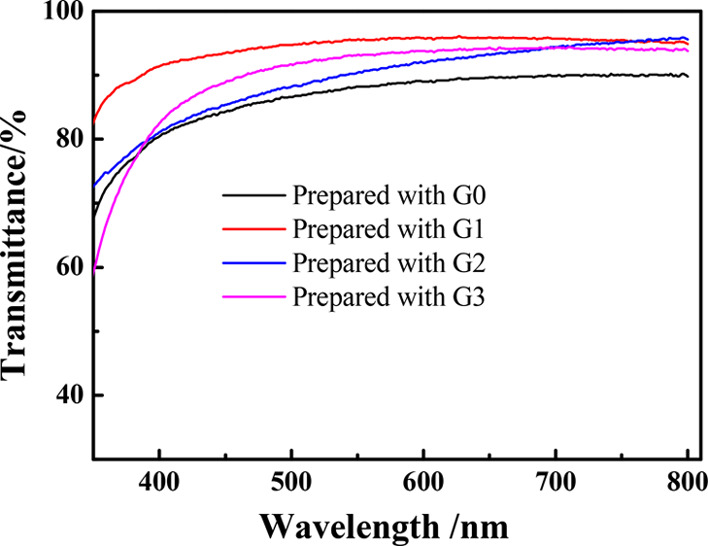
Transmittance of the coatings prepared with G0, G1, G2, and G3. Conditions: R/Si and thiol content of thiol silicone resin are 1.4 and 0.004 mol g–1, respectively. n(thiol):n(allyl) = 1.4:1. The coatings were cured for 30 s.
Figure 3.
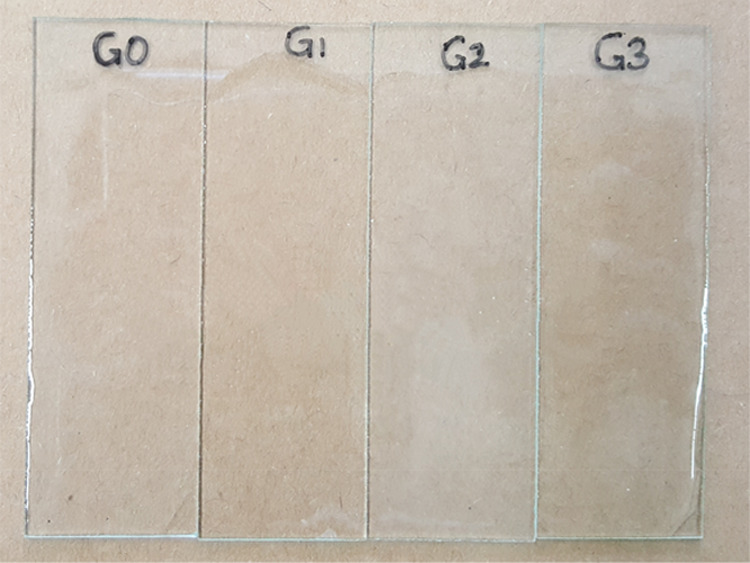
Photos of coatings prepared with G0, G1, G2, and G3. G0, G1, G2, and G3 stand for the coatings prepared with G0, G1, G2, and G3, respectively.
The FT-IR spectra of the coatings prepared with G0, G1, G2, and G3, G1, and thiol silicone resin are given in Figure 4. As can be seen from the FT-IR spectra of the coatings cured for 30 s, the characteristic stretching vibration absorption peak of =C–H at 3074 cm–1 and that of C=C in the allyl group at 1627 cm–1 of allyl-terminated hyperbranched polycarbosilanes disappeared. The tiny characteristic absorption peak of thiol groups at about 2550 cm–1 in the thiol silicone resin also vanished. The characteristic absorption peak of Si–O–Si at 1040 cm–1 and stretching vibration absorption peak of Si–CH3 at 2966 cm–1 were obviously in existence. These results imply that the coatings can be cured perfectly for only 30 s.
Figure 4.
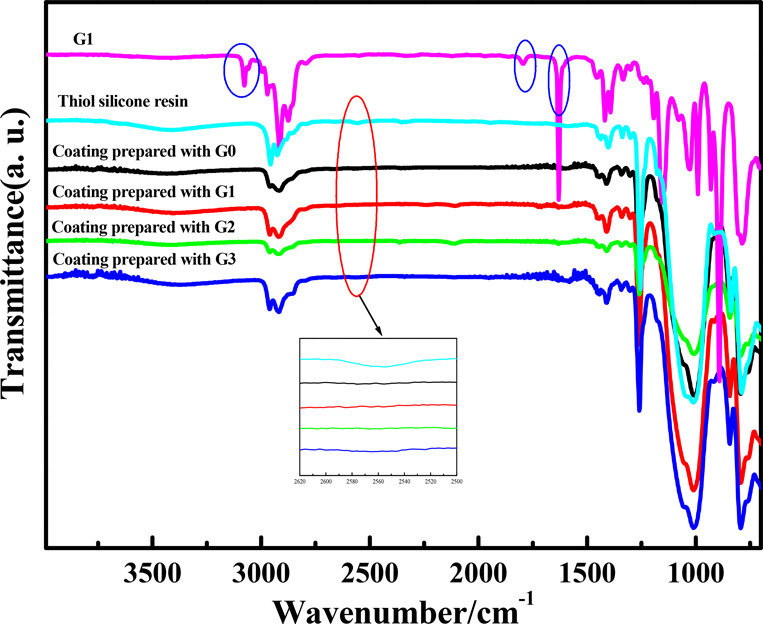
FT-IR spectra of the silicone coatings prepared, G1, and the thiol silicone resin with R/Si and thiol content of 1.4 and 0.004 mol g–1, respectively.
As can be seen from the TGA curves of the coatings prepared with G0, G1, G2, and G3 shown in Figure 5a, the mass residues of these coatings at 800 °C under an atmosphere of N2 are higher than 50 wt %. The starting thermal decomposition temperature (Td5) of these coatings is higher than 236 °C; especially, Td5 of the coating prepared with G1 is as high as 371.1 °C. The relatively lower Td5 of the coatings prepared with G3 may be attributed to the lowest curing degree. The coatings prepared in this work exhibit superior thermal stability to those UV-cured materials with Td5 in the range of 189–273.5 °C reported previously.25,26
Figure 5.
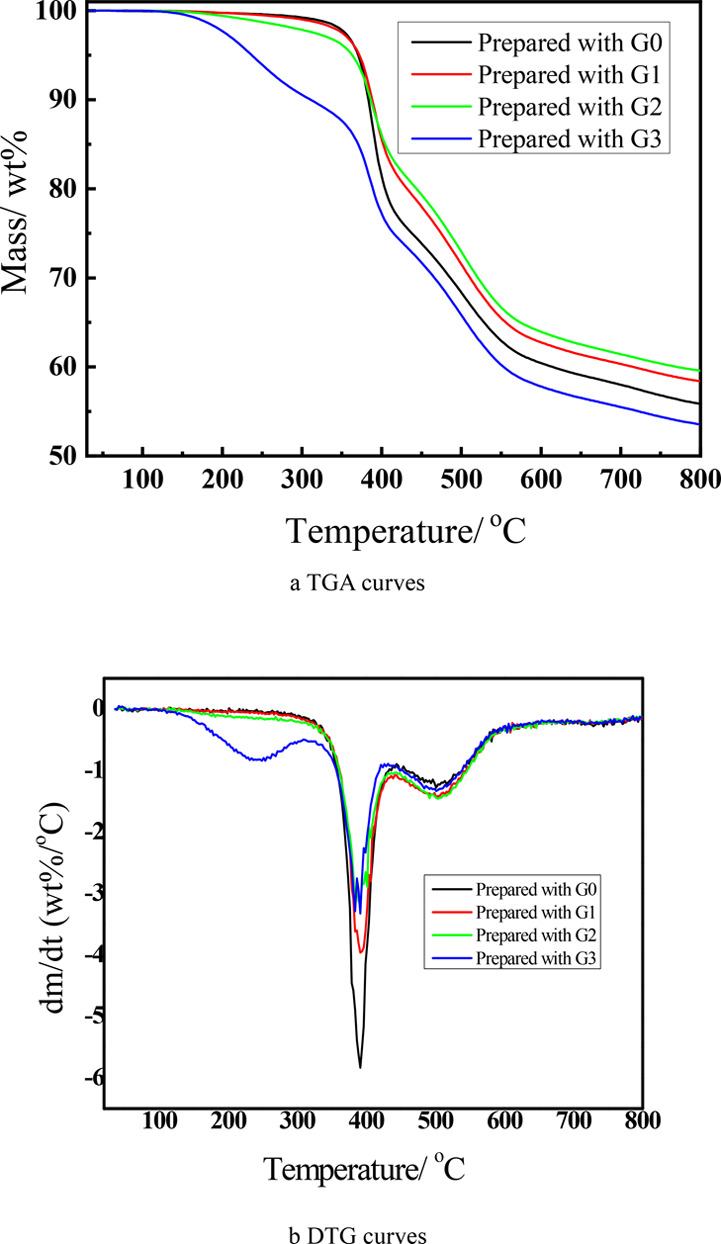
TGA (a) and DTG (b) curves of the coatings prepared with G0, G1, G2, and G3.
According to the DTG curves of these coatings shown in Figure 5b, the temperatures at which the maximum degradation speed took place (Tmax) for these coatings are about 391 °C. The excellent thermal stability for coatings prepared with G0, G1, and G2 can be proven that the thermal degradation process can be divided into two stages: fast degradation stage (320–425 °C) and carbonization stage (438–585 °C). By contrast, thermal degradation of the coating prepared with G3 can be divided into three stages: in addition to fast degradation stage and carbonization stage mentioned, there is an initiated degradation stage (122–308 °C), which might be due to the remaining allyl groups after the thiol-ene-initiated curing reaction for steric hindrance.
Conclusions
A class of UV-cured transparent solvent-free silicone coatings with high hardness, low water absorption, and fairly high transmittance were prepared from allyl-terminated hyperbranched polycarbosilanes and thiol silicone resins. The features for fabrication of the UV-cured silicone coatings were discussed. When n(thiol):n(allyl) = 1.4:1 and G1 is cured with the thiol silicone resin with R/Si and thiol content of 1.4 and 0.004 mol g–1 for 30 s, the coatings obtained have a pencil hardness of 9 H, water absorption no more than 0.04 wt %, transmittance higher than 94%, and Td5 as high as 371.1 °C. By comparison with those UV-cured materials reported previously, the coatings prepared in this work exhibit higher pencil hardness and superior thermal stability.
Experiments
Materials
3-Trimethoxysilylpropanethiol, ether, and tetrahydrofuran (THF) were from Beijing HWRK Chem. Co., Ltd. Ether and THF were distilled over potassium for 24 h before use. Dimethyl diethoxysilane, methyl trimethoxysilane, methyltrichlorosilane, and trichlorosilane (TCS) were the products of Shanghai Jiancheng Industry and Trade Co., Ltd. Ammonium chloride, magnesium sulfate anhydrous, and toluene were purchased from Sinopharm Chemical Reagent Co., Ltd., China. 3-Bromopropene, magnesium powder, and iodine were purchased from Adamas Reagent Co., Ltd. (Shanghai), Shanghai Lingfeng Chemical Reagent Co., Ltd., and TCI (Shanghai) Chemical Industrial Development Co., Ltd., respectively. Spiere’s platinum catalyst with a platinum concentration of 8000 ppm was prepared by our group. Thiol silicone resins with various thiol contents and R/Si were synthesized according to ref (18), and the thiol contents were calculated according to the 1H-NMR spectrum shown in Figure S3.
Preparation of Allyl-Terminated Hyperbranched Polycarbosilanes
Allyl-terminated hyperbranched polycarbosilanes were synthesized according to ref (27) (Figure S4), the 1H-NMR and 13C-NMR spectra of which are shown in Figures S5 and S6, respectively. The MALDI-TOF-MS analysis of products is summarized in Table S1.
Preparation of the UV-Cured Transparent Solvent-Free Silicone Coatings
As shown in Scheme S1, the solvent-free silicone coatings with thickness about 0.5 mm were prepared by dropping the mixtures in the middle of glass slides followed by spin-coating under a rotate speed of 3000 r/s for 30 s and then curing by UV with a laser wavelength of 365 nm and radiation intensity of 10.6 mW cm–2 (ZB1000, Changzhou Zibo Electron Technology Co., Ltd., the distance of the glass slides to the light is 20 cm). The thickness of the coatings was controlled by taking a mixture of equal mass onto the glass slides.
Characterization
NMR analysis was carried out using a 400 MHz Bruker AVANCE AV400 spectrometer in CDCl3 without tetramethylsilane. Fourier transform infrared (FT-IR) analysis was performed using a Nicolet 700 spectrometer (Nicolet Co., Ltd., America). MALDI-TOF-MS analysis was carried out using a Voyager DE RESIN MALDI-TOF-MS (Applied Biosystems, USA) using a mixture of 2,5-dihydroxybenzoic acid in tetrahydrofuran (0.078 mg mL–1) and sodium trifluoroacetate in tetrahydrofuran (0.068 mg mL–1) with a 1:1 mass ratio as the matrix. The samples were dissolved in THF (10 mg mL–1), and the solution of samples and matrix were mixed according to a 1:7 mass ratio. The transmittance spectra of samples were measured using a Unico UV 4802 UV/vis spectrophotometer (Unico Instrument Co., Ltd., Shanghai). The pencil hardness was measured with a BGD 562 pencil hardness meter (Zhenwei Testing Machinery Co. Ltd., Jiangdu, China) according to GBT6739-2006. Thermogravimetric analysis (TGA) was carried out using a TG 209C apparatus (Germany) at a heating rate of 10 °C min–1 under a N2 atmosphere. Differential scanning calorimetry (DSC) analysis was conducted using a DSC Q100 apparatus with a carrier gas flow rate of 20 mL min–1 under a nitrogen atmosphere. The curing degrees, water absorptions, and surface water contact angles were measured according to refs (18, 27), and (28).
Acknowledgments
This work was funded by the Open Fund of the Collaborative Innovation Centre for Fluorosilicon Fine Chemicals and Materials Manufacturing of Zhejiang Province (FSi2019A007) and General Scientific Research Project of Zhejiang Education Department (Y201943078).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01338.
1H-NMR characterization of thiol silicone resin. The synthesis procedure, 1H-NMR, and 13C-NMR spectra, and MALDI-TOF-MS of allyl-terminated hyperbranched polycarbosilanes G0, G1, G2, and G3. Scheme for preparation of the UV-cured transparent solvent-free silicone coatings, contact angles for the coatings with various contents of thiol, and DSC analysis of the coatings prepared with G0, G1, G2, and G3 (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Yang X.; Liu J.; Wu Y.; Liu J.; Cheng F.; Jiao X.; Lai G. Fabrication of UV–curable solvent–free epoxy modified silicone resin coating with high transparency and low volume shrinkage. Prog. Org. Coat. 2019, 129, 96–100. 10.1016/j.porgcoat.2019.01.005. [DOI] [Google Scholar]
- Mirshahi F.; Bastani S.; Sari M. G. Studying the effect of hyperbranched polymer modification on the kinetics of curing reactions and physical/mechanical properties of UV–curable coatings. Prog. Org. Coat. 2016, 90, 187–199. 10.1016/j.porgcoat.2015.10.015. [DOI] [Google Scholar]
- Ren Y.; Dong Y.; Zhu Y.; Xu J.; Yao Y. Preparation, characterization, and properties of novel ultraviolet–curable and flame–retardant polyurethane acrylate. Prog. Org. Coat. 2019, 129, 309–317. 10.1016/j.porgcoat.2018.11.009. [DOI] [Google Scholar]
- Zhang J.; Li L.; Guo R.; Zhou H.; Li Z.; Chen G.; Zhou Z.; Li Q. Preparation of novel UV–cured methacrylate hybrid materials with high thermal stability via thiol–ene photopolymerization. J. Mater. Sci. 2019, 54, 5877–5897. 10.1007/s10853-018-3157-8. [DOI] [Google Scholar]
- Favache A.; Daniel A.; Teillet A.; Pardoen T. Performance indices and selection of thin hard coatings on soft substrates for indentation and scratch resistance. Mater. Des. 2019, 176, 107827. 10.1016/j.matdes.2019.107827. [DOI] [Google Scholar]
- Domene-López D.; Sarabia-Riquelme R.; García-Quesada J. C.; Martin-Gullon I. Custom-made chemically modified graphene oxide to improve the anti–scratch resistance of urethane–acrylate transparent coatings. Coatings 2019, 9, 408–423. 10.3390/coatings9060408. [DOI] [Google Scholar]
- Chang C.-C.; Lin Z.-M.; Cheng L.-P. Preparation of superhydrophilic nanosilica/polyacrylate hard coatings on plastic substrate for antifogging and frost–resistant applications. J. Appl. Polym. Sci. 2019, 136, 48144–48154. 10.1002/app.48144. [DOI] [Google Scholar]
- Hui B.; Ye L. Highly heat–resistant silicon–containing polyurethane–imide copolymers: Synthesis and thermal mechanical stability. Eur. Polym. J. 2017, 91, 337–353. 10.1016/j.eurpolymj.2017.04.025. [DOI] [Google Scholar]
- Pagac J.; Hebda E.; Janowski B.; Sternik D.; Jancia M.; Pielichowski K. Thermal decomposition studies on polyurethane elastomers reinforced with polyhedral silsesquioxanes by evolved gas analysis. Polym. Degrad. Stabil. 2018, 149, 129–142. 10.1016/j.polymdegradstab.2018.01.028. [DOI] [Google Scholar]
- Karami Z.; Jazani O. M.; Navarchian A. H.; Karrabi M.; Vahabi H.; Saeb M. R. Well–cured silicone/halloysite nanotubes nanocomposite coatings. Prog. Org. Coat. 2019, 129, 357–365. 10.1016/j.porgcoat.2019.01.029. [DOI] [Google Scholar]
- Bakhshandeh E.; Bastani S.; Saeb M. R.; Croutxé-Barghorn C.; Allonas X. High–performance water–based UV–curable soft systems with variable chain architecture for advanced coating applications. Prog. Org. Coat. 2019, 130, 99–113. 10.1016/j.porgcoat.2019.01.033. [DOI] [Google Scholar]
- Zhang D.; Liu C.; Chen S.; Zhang J.; Cheng J.; Miao M. Highly efficient preparation of hyperbranched epoxy resins by UV–initiated thiol–ene click reaction. Prog. Org. Coat. 2016, 101, 178–185. 10.1016/j.porgcoat.2016.08.010. [DOI] [Google Scholar]
- Duan Q.; Wang S.; Wang Q.; Li T.; Chen S.; Miao M.; Zhang D. Simultaneous Improvement on Strength, Modulus, and Elongation of Carbon Nanotube Films Functionalized by Hyperbranched Polymers. ACS Appl. Mater. Int. 2019, 11, 36278–36285. 10.1021/acsami.9b12368. [DOI] [PubMed] [Google Scholar]
- Zheng Y.; Li S.; Weng Z.; Gao C. Hyperbranched polymers:advances from synthesis to applications. Chem. Soc. Rev. 2015, 44, 4091–4130. 10.1039/C4CS00528G. [DOI] [PubMed] [Google Scholar]
- Baldwin L. A.; Rueschhoff L. M.; Deneault J. R.; Cissel K. S.; Nikolaev P.; Cinibulk M. K.; Koerner H.; Dalton M. J.; Dickerson M. B. Synthesis of a two–component carbosilane system for the advanced manufacturing of polymer-derived ceramics. Chem. Mater. 2018, 30, 7527–7534. 10.1021/acs.chemmater.8b02541. [DOI] [Google Scholar]
- Zheng S.; Zlatin M.; Selvaganapathy P. R.; Brook M. A. Multiple modulus silicone elastomers using 3D extrusion printing of low viscosity inks. Addit. Manuf. 2018, 24, 86–92. 10.1016/j.addma.2018.09.011. [DOI] [Google Scholar]
- Zhang J.; Chen Y.; Brook M. A. Facile functionalization of PDMS elastomer surfaces using thiol–ene click chemistry. Langmuir 2013, 29, 12432–12442. 10.1021/la403425d. [DOI] [PubMed] [Google Scholar]
- Wu Y.; Liu J.; Jiao X.; Cheng F.; Lai G.; Yang X. UV-cured transparent flexible silicone materials with high tensile strength. ACS Omega 2020, 5, 6199–6206. 10.1021/acsomega.0c00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao J.-T.; Yuan L.; Guan Q.; Liang G.; Gu A. Water-phase synthesis of a biobased allyl compound for building UV-curable flexible thiol-ene polymer networks with high mechanical strength and transparency. ACS Sustain. Chem. Engin. 2018, 6, 7902–7909. 10.1021/acssuschemeng.8b01128. [DOI] [Google Scholar]
- Xie Q.; Liang S.; Liu B.; Fu K.; Zhan Z.; Lu L.; Yang X.; Lü F.; Huang Z. Structure, microparameters and properties of crosslinked DGEBA/MTHPA: A molecular dynamics simulation. Aip. Adv. 2018, 8, 075332 10.1063/1.5041283. [DOI] [Google Scholar]
- Liang B.; Zhao J.; Li G.; Huang Y.; Yang Z.; Yuan T. Facile synthesis and characterization of novel multi–functional bio–based acrylate prepolymers derived from tung oil and its application in UV–curable coatings. Ind. Crop. Prod. 2019, 138, 111585. 10.1016/j.indcrop.2019.111585. [DOI] [Google Scholar]
- Yang X.; Shao Q.; Yang L.; Zhu X.; Hua X.; Zheng Q.; Song G.; Lai G. Preparation and performance of high refractive index silicone resin–type materials for the packaging of light–emitting diodes. J. Appl. Polym. Sci. 2013, 127, 1717–1724. 10.1002/app.37897. [DOI] [Google Scholar]
- Oh Y.; Yoon I. S.; Lee C.; Kim S. H.; Ju B.-K.; Hong J.-M. Selective photonic sintering of Ag flakes embedded in silicone elastomers to fabricate stretchable conductors. J. Mater. Chem. C 2017, 5, 11733–11740. 10.1039/C7TC03828C. [DOI] [Google Scholar]
- Park S.; Lee J.; Ko H. Transparent and Flexible Surface-Enhanced Raman Scattering (SERS) Sensors Based on Gold Nanostar Arrays Embedded in Silicon Rubber Film. ACS Appl. Mater. Interfaces 2017, 9, 44088–44095. 10.1021/acsami.7b14022. [DOI] [PubMed] [Google Scholar]
- Deng X.-R.; Yang W.; Zhang Q.-H.; Hui H.-H.; Wei Y.-W.; Wang J.; Xu Q.; Lei X.-Y.; Chen J.-J.; Zhu J.-L. Fabrication of UV–curable silicone coating with high transmittance and laser–induced damage threshold for high–power laser system. J. Sol–Gel Sci. Technol. 2018, 88, 249–254. 10.1007/s10971-018-4807-7. [DOI] [Google Scholar]
- Liu C.; Li T.; Zhang J.; Chen S.; Xu Z.; Zhang A.; Zhang D. Preparation and properties of phosphorous–nitrogen containing UV–curable polymeric coatings based on thiol–ene click reaction. Prog. Org. Coat. 2016, 90, 21–27. 10.1016/j.porgcoat.2015.09.004. [DOI] [Google Scholar]
- Jiao X.; Zhang T.; Cheng F.; Fan Y.; Liu J.; Lai G.; Wu Y.; Yang X. UV-cured coatings prepared with sulfhydryl terminated branched polyurethane and allyl-terminated hyperbranched polycarbosilane. Coatings 2020, 10, 350. 10.3390/coatings10040350. [DOI] [Google Scholar]
- Liu J.; Jiao X.; Cheng F.; Fan Y.; Wu Y.; Yang X. Fabrication and performance of UV cured transparent silicone modified polyurethane–acrylate coatings with high hardness, good thermal stability and adhesion. Prog. Org. Coat. 2020, 144, 105673. 10.1016/j.porgcoat.2020.105673. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.