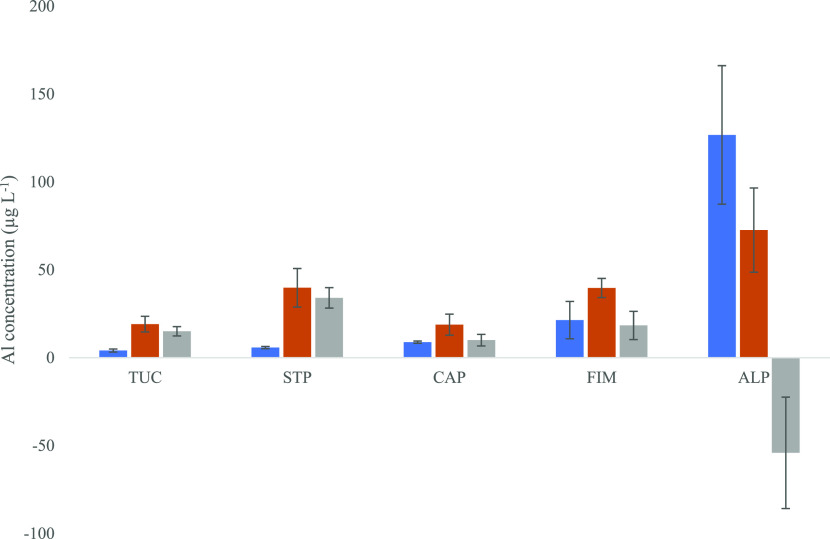Abstract
This study investigated the aluminum content in one of the most consumed daily beverages: coffee. The total Al concentration in 10 different samples of coffee beans and their water-extractable fraction were determined. We then tested the influence of different brewing methods on the concentration of the extracted Al in the final beverage. Metal analyses were performed using graphite furnace atomic absorption spectroscopy (GF-AAS) after microwave-assisted acid digestion. The results showed highly variable Al contents in coffee beans (1.5–15.5 mg kg–1), of which ∼2–10% were water-extractable. The brewing technique had a major influence on the Al content in the beverage: significantly higher Al concentrations (72.57 ± 23.96 μg L–1) occurred in coffee brewed in an aluminum moka pot. Interestingly, using ground coffee with this method even reduced the Al content in the final beverage compared to the brewing water used. Coffee brewed from Al capsules did not contain significantly higher Al concentrations compared to other methods.
Introduction
Aluminum has become an indispensable material used in industry, engineering, and modern everyday life. The metallic form of the element does not occur naturally in the environment, as it is usually present as ionic Al3+, bound to oxygen and enclosed in silicates and other minerals.1 Regarding its relevance to life, the status of aluminum is quite extraordinary: it is the third most common element in the Earth’s crust,2 yet no biological function for living organisms has been recognized.3 It actually displays a high toxicity toward many biota.4 The anthropogenic distribution of the metal Al and its compounds has drastically increased its presence in the environment in recent decades,5 and organisms, including humans, are put into contact with the metal on a daily basis. Al and its compounds are present in antiperspirants, vaccines, medicines and medical products, food packaging and preparation, and a long list of everyday items. This is especially worrying because many studies indicate a possible connection between the metal and the negative effects on human health. Al exposure and uptake have been linked to neurodegenerative diseases (e.g., Alzheimer’s disease (AD), Parkinson’s disease) and other severe illnesses such as breast cancer.6−9 Even though these findings are controversially discussed,10,11 the Al uptake by humans has been studied well: the consumed Al can be absorbed in the gastrointestinal tract. The chemical speciation and the respective chemical environment are major determinants for its uptake.12 Once absorbed, it can bind to the plasma proteins ferritin, albumin, and transferrin,13 thereby crossing the brain–blood barrier.14 Recent research showed that the aluminum content of plasma ferritin in AD patients was significantly higher when compared to that of control groups. This observation was especially evident in early-stage AD patients, suggesting that Al is released from ferritin due to a loss of the functional capacity of the protein in the second phase of the illness.15 The dysfunction of ferritin and transferrin activity through Al has also been linked to other medical conditions.9 As Al disrupts efficient iron homeostasis, several studies suggest that the metal potentially triggers the growth of certain breast cancer cell types, as well as ductal carcinoma in situ and primary invasive breast cancer.16−18 Furthermore, free iron resulting from an ineffective iron metabolism and the formation of an aluminum superoxide radical complex as a promotor have been described to react following the Fenton reaction, creating hydroxyl radicals.16,19 These radicals have a pro-oxidative effect and can induce lipid peroxidation and DNA and biomolecule damages. With the ongoing discussion concerning the potential health risks associated with aluminum, world health authorities, e.g., the European Food Safety Authority (EFSA), have set thresholds for intake levels that should avoid the negative effects associated with Al. Authorities often set a tolerable daily intake (TDI) for pollutants, but due to the accumulation pattern of the metal in the human body, it was more suitable to calculate a tolerable weekly intake (TWI). The TWI of 1 mg kg–1 bodyweight/week was defined by EFSA.12 There are various ways for humans to be exposed to metallic Al and Al compounds. One potential source is the air we breathe. Air polluted by Al particles has been described in industrial areas,20 with no further information on the chemical speciation of the metal given. Additionally, the individual inhalation of Al through cigarette smoke21 or aerosols of antiperspirants,22 for example, can significantly contribute to the daily uptake of Al compounds. Uptake via the skin needs to be considered as well, especially through the long-term application of antiperspirants containing Al salts.7 Another major factor is the oral uptake of Al compounds through food. Anthropogenic sources in food include contact materials (e.g., cookware),23 food additives,24 and pharmaceuticals such as antacids.25 Additionally, naturally occurring Al in foodstuff can contribute to dietary uptake. Data on the chemical speciation and concentration in foodstuff are not well established because analytical methods usually determine the total Al content.12 Some foods that are naturally high in the total Al content include oats, potatoes, and spinach.4 A beverage that has been recognized for high concentrations of Al is tea.26 It is brewed out of leaves from the tea plant (Camellia sinensis), which tolerates4 and accumulates Al from soil.27 The acidic soil conditions used for tea cultivation increase the uptake from the ground.28 Besides tea, coffee, which is brewed from the roasted seeds of Coffea spp., is one of the most consumed nonalcoholic beverages. Soil conditions similar to those used for tea have been proven to be ideal for cultivating coffee.29 Even though the Al uptake and accumulation behavior of coffee plants are still uncertain, a tolerance of the plants toward Al has been recognized.30 The two most commonly cultivated species for coffee consumption are Coffea arabica and Coffea canephora, making up 56 and 44% of worldwide production, respectively.31 Commercially available coffee beans are often sold as a blend of both species, with C. arabica usually being the dominant component due to its richer flavor profile. However, C. canephora production has recently increased because the plant can be grown at lower altitudes and is more resistant toward disease32 due to its higher caffeine content. The two species can be distinguished based on their caffeine content33 and metal composition.34,35 Regardless of the Al content in the raw beans, all of the subsequent production steps, postharvesting processes, storage, and packaging could enhance the metal concentration in the beans or ground coffee powder. Al has many different applications during postharvesting treatments, processing, and packing, including its use in airtight containers for transport or as easy-to-use capsules (CAPs). The Al content of the final beverage depends on several aspects: the Al content in the coffee beans, its water solubility, and the different brewing methods, during which the metal in brewing devices can partially dissolve.36 Depending on the pH and other factors, partial dissolution from cookware can take place, releasing Al3+ as a bioavailable cation in the beverage.36 A number of coffee-related publications have dealt with the effect of metals in brewing water on the sensory perception of coffee.37−39 However, fewer publications discuss the metal content and more specifically the Al content in brewed coffee (BC). Additionally, there is a lack of knowledge on the connection between the content in brewing water and the final content of aluminum in the beverage. This also includes a lack of information on the Al content in coffee brewed with ready-to-use Al capsules. In this study, we investigated the Al content in ground coffee beans from different regions of the world and tested the influence of different brewing methods under household conditions, including ready-to-use Al capsules. In addition, we tested whether aluminum is dissolved during the brewing process when brewing water and/or coffee comes into contact with the metal, potentially enhancing the Al content in the beverage. Finally, we estimate the contribution of coffee consumption to the weekly uptake of Al.
Results and Discussion
Aluminum in Ground Coffee Beans (GCBs)
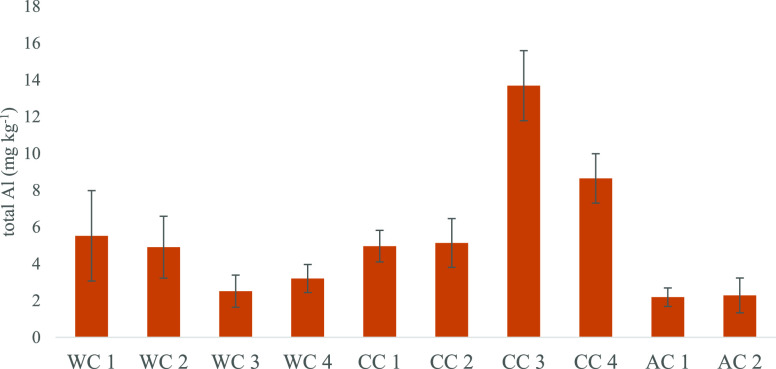
Aluminum concentrations in GCB varied between 1.54 mg kg–1 in WC3 and 15.51 mg kg–1 in CC3 (Table 1). The mean contents were between 2.18 and 13.68 mg kg–1 dw, with relatively high standard deviations (SDs) within the respective samples (Figure 1). Our findings are in good accordance with the values reported by Anderson et al.40 Those authors described significant differences in the Al content due to different geographical origins of the GCB, with very similar findings in our study. The two samples showing significantly (p < 0.01) higher concentrations of the total Al compared to all others were CC3 and CC4. The geographical origin of these two samples was unknown, but these were the only two consisting of a C. arabica and C. canephora blend, which potentially explains this difference. Especially manganese and zinc concentrations in GCB have been recognized as indicators for the differentiation of C. arabica and C. canephora.41 Distinctions between the two species based on Al have not been investigated yet, but a different uptake and storage behavior as described for many other metals is conceivable.
Table 1. Total Al Content (mg kg–1 dw) in Ground Coffee Beans from 10 Different Coffee Samples for WC1–4 (n = 6), 10 Single Beans WC1 (sb) “Single Beans” (n = 10), CC1–4 (n = 3), and for AC1 and AC2 (n = 5); Means Followed by the Same Letter Do Not Differ Significantly.
| sample ID | min | median | max | mean | SD |
|---|---|---|---|---|---|
| WC1 | 3.21 | 5.37 | 8.40 | 5.52a | 2.46 |
| WC1 (sb) | 2.59 | 3.77 | 7.18 | 4.02a | 1.35 |
| WC2 | 3.01 | 4.98 | 6.70 | 4.90a | 1.68 |
| WC3 | 1.54 | 2.46 | 3.63 | 2.51b | 0.87 |
| WC4 | 2.31 | 3.11 | 4.04 | 3.20a | 0.77 |
| CC1 | 3.74 | 5.32 | 5.68 | 4.96a | 0.86 |
| CC2 | 4.04 | 4.74 | 6.61 | 5.13a | 1.33 |
| CC3 | 11.71 | 13.82 | 15.51 | 13.68c | 1.90 |
| CC4 | 7.74 | 7.99 | 10.19 | 8.64d | 1.35 |
| AC1 | 1.56 | 2.21 | 2.74 | 2.18b | 0.50 |
| AC2 | 1.51 | 1.80 | 3.82 | 2.28b | 0.94 |
Figure 1.
Mean ± SD content of the total Al in ground coffee beans.
Geographical origin might not be the sole factor for the metal content in beans. Regional soil composition, which has a major influence on the uptake of metals by coffee plants, can differ drastically.42 Accordingly, variety, species, and geographical differences might be the cause of the significantly higher total Al content in samples CC3 and CC4. Even coffee beans originating from the same region can have experienced diverse soil compositions, resulting in different Al contents. This explains high variations within the same GCB sample of the respective coffee batches and also shows that Al is not homogeneously distributed; this finding aligns well with the data of Fraňková et al.,43 who reported similar variability within the subsamples from seven different GCB samples. Unequal distribution even on the level of single beans was further demonstrated in our analyses of 10 beans originating from the same batch of WC1. Although all beans from this batch stem from one coffee farm and differences due to varying soil composition can be ruled out, the results showed a high variation of the Al content between 2.59 and 7.18 mg kg–1 dw (mean = 4.02 ± 1.35 mg kg–1 dw) (Table 1). In addition to species-specific differences in the Al uptake and different exposure of coffee plants to Al in different soil types, postharvesting processes and packaging can influence the metal contents in coffee beans.34 The two samples CC3 and CC4, with the highest total Al content, were the only two pre-ground coffee bean samples with Al-coated packaging. Al is a commonly used packaging material for pre-ground coffee beans due to its aroma preservation properties; nonetheless, the possibility of Al leaching has also been recognized.44 Such leaching into foodstuff can be efficiently inhibited by coating the metal with plastic.45 The inside surface of the packaging for our samples was coated with such a plastic layer. This can be assumed to have impeded additional leaching of Al into GCB from CC3 and CC4. We draw a similar conclusion on the total Al content of GCB in capsules, where the beans were not directly in contact with the Al packaging and showed significantly lower values than most other GCB samples (Figure 1).
Water Extractability of Al from GCB
The determination of the water-extractable fraction of the total Al in GCB showed that the lowest mean content occurred in WC4 with 8.30 ± 1.66 μg L–1 and the highest mean content in CC3 with 114.4 ± 15.3 μg L–1. The content of the water-extractable fraction of the total Al for the other GCB is presented in Table 2. These results showed that approximately 2.7–12.1% of the total Al content in the GCB was water-extractable.
Table 2. Water-Extractable Al in Eight Different Coffee Brands (n = 5): Mean Concentrations ± SD of Al in Water Extract (μg L–1) and Water-Extractable Fraction of the Total Al Found in Ground Coffee Beans (%).
| sample ID | Al concentration (μg L–1) | water-soluble fraction (%) |
|---|---|---|
| WC1 | 17.05 ± 1.48 | 5.3 ± 2.3 |
| WC2 | 8.94 ± 1.63 | 2.9 ± 1.0 |
| WC3 | 16.09 ± 2.32 | 10.2 ± 3.6 |
| WC4 | 8.30 ± 1.66 | 3.9 ± 0.9 |
| CC1 | 8.94 ± 1.29 | 2.7 ± 0.5 |
| CC2 | 14.01 ± 1.62 | 4.1 ± 1.0 |
| CC3 | 114.4 ± 15.34 | 12.1 ± 1.7 |
| CC4 | 59.75 ± 3.43 | 10.0 ± 1.4 |
A previous study came to similar conclusions, with less than 10% of the total Al leaching into brewing water.46 WC3, CC3, and CC4 exceeded the 10% mark and were significantly different from all other samples (but not so from each other). WC3 contained the least amount of the total Al in GCB, whereas CC3 and CC4 showed the highest concentrations. Many factors can influence the water extractability of Al from GCB, including roasting, processing, and grind size.43 The degree of roasting determines the cell structure and chemical composition of the roasted coffee beans.47 The WC1–4 samples were all lightly roasted, leading to a reduction of organic degradation during the roasting process compared to that of medium and dark roasts.48 CC3 and CC4, in contrast, were dark roasts, potentially increasing the water-extractable fraction of Al due to the pyrolysis of organic matter and the subsequent release of Al ions bound to organic complexes. Finally, even the highest degree of metal leaching (12.1%) demonstrated that the major fraction of Al in the samples is not water-extractable.
Al in Brewing Water
We analyzed Al leaching from the respective brewing devices into brewing water for coffee preparation. To generate household conditions, we used commercially available mineral water with a mean content of 4.1 μg L–1 Al (Table 3) and analyzed the dissolved Al concentration in the water after the brewing procedures without inserting coffee powder.
Table 3. Characteristics, Major Dissolved Components, and the Total Al in the Used Mineral Water.
| pH | conductivity (μS cm–1) | Al3+ (μg L–1) | Na+ (mg L–1) | K+ (mg L–1) | Mg2+ (mg L–1) | Ca2+ (mg L–1) | Cl– (mg L–1) | SO42– (mg L–1) |
|---|---|---|---|---|---|---|---|---|
| 7.22 | 846 | 4.1 | 14.2 | 1.8 | 39.5 | 95 | 23 | 221 |
Unsurprisingly, the steel pot (STP) did not leach Al into the brewing water (mean = 4.8 ± 0.7 μg L–1 total Al), whereas the filter machine (FIM) clearly contained some component, possibly the tubing, where the water came in contact with aluminum, yielding a higher mean metal concentration of 21.4 ± 10.6 μg L–1 (Table 4).
Table 4. Total Al in Water Used for Coffee Brewing After Passing through the Respective Brewing Devices without Using Ground Coffee Beans.
| water type | Al (μg L–1) |
|---|---|
| mineral water, n = 19 | 4.07 ± 0.87 |
| mineral water, after “Turkish coffee”, n = 6 | 4.30 ± 0.55 |
| mineral water after steel pot, n = 6 | 4.80 ± 0.70 |
| mineral water after Al pot, n = 6 | 126.7 ± 39.4 |
| mineral water after filter machine, n = 6 | 21.4 ± 10.6 |
| mineral water after capsule machine, n = 6 | 8.82 ± 0.64 |
The highest values were found in the brewing water from the aluminum pot (ALP). The metallic Al used in alloys for such devices is relatively reactive.44 Nonetheless, Lamberti et al.44 demonstrated that corrosion of Al and subsequent leaching from the surface of such devices is significantly reduced due to the formation of an aluminum (oxy) hydroxide barrier layer, which acts as protection. This layer is apparently stable in a pH range from 4 to 8.5.36 Our experiments clearly demonstrated Al leaching from this pot during the brewing process, resulting in a mean concentration of 126.7 ± 39.4 μg L–1 Al in brewing water. Even though leaching might have been reduced, pitting corrosion and slow uniform dissolution of the Al oxide layer can still occur during the cooking process.36
Al in Brewed Coffee (BC)
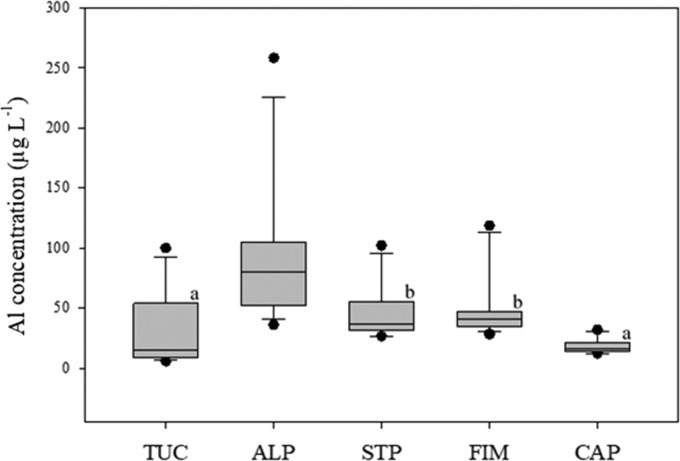
To further elucidate the origin of the extracted Al in BC, we then used different methods, including examining the role of different materials of coffee-brewing machines. Analyzing the results, we found that the main contributor to the extracted Al concentration in BC was the brewing devices and not GCB. Therefore, the results of all GCB for each brewing method were merged and subsequently treated as one group. The Al concentrations in BC from different brewing devices are depicted in Figure 2 and Table 5, clearly showing a significantly (p < 0.001) higher mean Al contamination using an ALP as the brewing device, with a mean Al concentration of 72.57 ± 23.96 μg L–1. The pH of BC was 4.4–4.8, thus not affecting the protective Al oxide layer of this brewing device’s inside surface. The importance of the brewing device material is underlined by examining the Al concentration in BC from the STP method. This method used the same extraction technique with a different alloy composition without Al (stainless steel), resulting in a significantly (p < 0.001) lower mean Al concentration of 39.68 ± 5.44 μg L–1 compared to the aluminum alloy ALP. At the same time, the STP method showed significantly (p < 0.001) higher mean values than the Turkish coffee (TUC) method, with a mean concentration of 19.08 ± 4.45 μg L–1 of the total Al. The differentiating factor between the two methods is the pressure used during the extraction. We concluded that the pressure induced by water vapor increased the solubility of Al in GCB and thus enhanced the concentration in the beverage significantly.
Figure 2.
Mean ± SD content of the total Al in BC using different brewing methods; methods followed by the same letter do not differ significantly from each other (p < 0.01).
Table 5. Mean ± SD Content of the Total Al in Brewed Coffee (μg L–1) from Different Brewing Methods (n = 40, Except for the CAP Method: n = 10), the Resulting Weekly Uptake (mg), and the Percentage of the TWI for a 70 kg Person Consuming 0.5 L Coffee per Day.
| brewing method | total Al (μg L–1) | estimated weekly uptake (mg) | % of TWI |
|---|---|---|---|
| TUC | 19.08 ± 4.45 | 0.067 | 0.10 |
| ALP | 72.57 ± 23.96 | 0.254 | 0.36 |
| STP | 39.77 ± 10.97 | 0.139 | 0.20 |
| FIM | 39.68 ± 5.44 | 0.139 | 0.20 |
| CAP | 18.26 ± 6.01 | 0.064 | 0.09 |
Interestingly, there was no statistical difference between the mean total Al concentration of the simple brewing method TUC (19.08 ± 4.45 μg L–1 total Al) and the CAP method (18.26 ± 6.01 μg L–1 total Al). One reason for the low mean concentration of the extracted Al in BC from aluminum capsules might be the previously discussed plastic layer on the inside capsule surface preventing contact between brewing water and Al for most of the brewing process. A direct contact of Al from the capsule and the brewing water is at the pierced surfaces, which let the water pass through. However, the contact time of the brewing water and the capsule Al might be too short for high leaching to occur. Moreover, the temperature of the brewing water was lower than for most other methods, which could have further decreased the leaching.
The comparison of the mean total Al concentration of brewing water (Table 4) to BC was especially interesting for the ALP. For this method, the total Al content in the BC with a mean value of 72.57 ± 23.96 μg L–1 was significantly (p = 0.011) lower than that in the brewing water using the same method (Figure 3), with the mean total Al content being 126.7 ± 39.4 μg L–1. Müller et al.45 described a similar observation. They stated that the Al concentration in BC was lower than in the tap water used in the preparation but provided no further explanation. The mean content of the total Al in other BC samples was still higher than in the brewing water of the respective brewing devices. One interpretation is that Al removal and leaching occurred simultaneously. Retention of Al in GCB during the brewing process apparently increased with increasing Al concentrations in the brewing water (Figure 3). Our observations showed that the brewing methods significantly influenced the uptake of Al. The methods FIM and STP showed no significant differences in the Al concentration of BC. However, the Al content in the brewing water was significantly (p = 0.022) higher for the FIM method. One potential conclusion is that slower extraction processes increase the absorption efficiency of GCB.
Figure 3.
Mean ± SD content of the total Al in brewing water (left columns) and brewed coffee (middle columns); the right columns show the difference between the two.
Although the binding mechanisms of roasted GCB and metals are still not fully understood, this phenomenon has already been described for other metals such as Cd, Pb, and Fe.49,50 Tokimoto et al.50 were able to remove lead from drinking water with GCB residue and also showed that proteins in the GCB residue were mainly responsible for removing the metal. No detailed binding mechanism was provided, but the assumption is that binding occurred due to the presence of functional groups of these proteins. Functional groups that can form bonds to metals are found not only in proteins but also occur abundantly in humic substances.51 Klöckling et al.52,53 investigated the presence of humic substances in roasted GCB and reported the formation of antisoluble lead humates in aqueous solutions in the presence of coffee powder and lead. Al shares a high affinity to humic substances with Pb and other metals.54 Tipping55 investigated cation binding by humic substances and reported that Al mainly binds to sites that are specific for humic substances. This includes single carboxyl and phenolic groups that are assumed to form multidentate binding sites by folding of the respective humic substances. Besides complexation, charged macromolecules in humic substances can also function as nonspecific binding sites because they accumulate counterions in their proximity. Other metals even experience a competitive effect, and their binding is diminished by the high affinity of Al3+ toward humic substances. The relatively low pH of 4.4–4.8 during the extraction, while the brewing water passes through the GCB, would additionally favor Al binding because dissolved Al3+ predominates at pH values under 5.56 Other Al species occurring at higher pH such as Al(OH)2+ and Al(OH)4– are presumed to have much lower binding affinities55 toward humic substances. Therefore, the uptake of Al from GCB might be related to the complexation of the metal due to the functional groups in humic substances and the reactivity of the Al3+ species toward them. Retention could also be linked to the protein content of GCB and the resulting formation of complexes. Clearly, additional research is required for a better understanding of this phenomenon.
Dietary Intake and Tolerable Weekly Intake
Many studies have shown that the intake of Al can vary considerably within the general population. The average daily uptake through diet ranges from 1.6 to 13 mg, equivalent to 0.16–1.3 mg kg–1 Al intake per week for an adult weighing 70 kg.12,57,58 Accordingly, the dietary ingestion of Al makes up 16–130% of the TWI (1 mg kg–1 bw/week). Comparing all of the different coffee-preparing techniques from our study, the worst-case scenario is using the ALP method. The estimated weekly intake of Al through BC from the ALP method would be 0.25 mg (Table 5). This accounts for only 0.36% of the TWI, assuming that an average person weighs 70 kg and drinks approximately 0.5 L59 of coffee a day. Even though only 0.1% of the bioavailable Al is ingested through the intestinal mucosa, it is important to note that the water-soluble character of an Al compound can influence the bioavailability by at least a 10-fold.12 The Al in BC measured in our study was present as a soluble. Thus, although the contribution from coffee seems to be relatively low, it could present a significant impact on the body load compared to other Al species in foodstuff, which might be less bioavailable. Additionally, due to today’s aging population, the risk of aluminum intoxication might also rise because the permeability of the intestinal mucosa is suggested to increase with age.60
Conclusions
The determination of the total Al in ground coffee beans showed a high variability of the aluminum content in beans, with only 2–10% of the metal being water-extractable. The major influence on the Al content in brewed coffee was the respective brewing method and the material of the cookware. The significantly highest content of the total Al was recorded in brewed coffee originating from a device completely made of Al alloy (aluminum moka pot). For this brewing method, the water used during brewing extracted significantly higher quantities of Al from the brewing device compared to that of all others. Interestingly, the Al content in the final beverage was lower compared to that of the brewing water, which points to ad/absorption processes in the ground coffee beans. We attribute this to the chelating and binding properties of proteins and humic substances in coffee powder. Furthermore, brewed coffee from Al capsules did not show significantly higher total Al concentrations than other brewing devices. Concerning the weekly uptake of Al from different brewing methods, even brewed coffee from the Al moka pot contributed only a small portion of the TWI. Nonetheless, caution is still advised, and brewing methods without Al components should be preferred.
Materials and Methods
Overview of Experiment
Four different brands of coffee beans, four different brands of pre-ground coffee beans, and two brands of coffee in aluminum capsules were purchased. The total Al in ground coffee beans was determined, and the water-extractable fraction was analyzed. All brands of coffee beans were used for brewing coffee in four different devices (Turkish coffee, aluminum moka pot, steel moka pot, and filter machine), and a capsule machine was utilized to brew the two brands of capsules. The total Al content was determined in all brewed coffee samples. Additionally, the total Al content in the respective water passing through the brewing device without the ground coffee beans was quantified to estimate the contribution of the brewing device to the final concentration of the total Al in the beverage. The single steps of the analysis are described in the following.
Coffee Bean Samples
Four brands of coffee were purchased as whole beans in 1 kg bags at a wholesale trade, labeled in the following text as “WC1”, “WC2”, “WC3”, and “WC4”. All beans in these samples were pure C. arabica, with WC1 originating from Ethiopia, WC2 from Guatemala, WC3 from Kenya, and WC4 from Nicaragua. The beans of these samples were ground using the coffee mill “Mahlkönig Mühle EK” (Hemro Manufacturing Germany GmbH, Hamburg, Germany). The grain size was kept at 0.3 mm, yielding a coffee powder referred to as “fine”, generally used for moka pots, espresso, and filter preparations.61 GCBs were homogenized by shaking after grinding and stored in polypropylene (PP) bags until further processing. Ten single beans from WC1 were kept without grinding and stored in PP bags as well. Four coffee samples were purchased pre-ground in 1 kg bags from a supermarket (“CC1”, “CC2”, “CC3”, “CC4”). GCB samples CC1 and CC2 originated from Mexico and were both 100% C. arabica. CC3 and CC4 samples were a blend of C. arabica and C. canephora. Information on the origin of the latter two samples was unavailable. Packaging of all pre-ground coffee beans suggested all types of preparation techniques, indicating a “fine” grinding process. Additionally, two different types of pre-ground coffee beans in aluminum capsules (“AC1” and “AC2”), ready to use for a capsule machine, were purchased.
Sample Preparation for Total Al Analysis in Ground Coffee Beans
All GCB samples were prepared in triplicates. GCBs (0.2 g) were weighed into PTFE tubes using an analytical balance (0.1 mg precision). After adding 4.5 mL of Millipore water, 4.5 mL of 69% HNO3 (Trace Metal, Fisher Chemical, Loughborough, United Kingdom), and 1 mL of hydrogen peroxide (≥30% for trace analysis, Sigma-Aldrich Chemie GmbH, Steinheim, Germany), digestion was carried out using the microwave digestion system “Mars Xpress” (CEM Corporation, Matthews). The 10 coffee beans from WC1 were weighed individually and digested as described above. After digestion, samples were transferred into 20 mL PP volumetric flasks and brought to volume with ultrapure water and stored there until further analysis.
Water-Extractable Fraction of Al in Ground Coffee Beans
To estimate the water-extractable fraction of Al in GCB, we used Millipore water (18.2 MΩ·cm at 25 °C). For the extraction with Millipore water, the brewing method commonly known as an ibrik or Turkish coffee was carried out in quintuplets for all GCB samples. The preparation for the Turkish coffee method is further described in the next section.
Preparation of Brewed Coffee
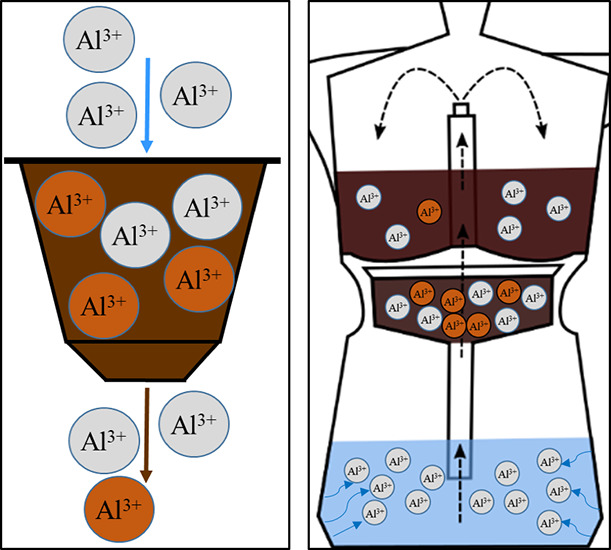
The brewing method Turkish coffee (TUC) was mimicked by using a 300 mL glass flask as a brewing device. The respective flasks were acid-cleaned prior to use. Moreover, we used a moka pot made of aluminum alloy (“aluminum pot” (ALP)) and a moka pot made of stainless steel (“steel pot” (STP)) (Figure 4). These devices were newly purchased and rinsed with a mild detergent prior to first use. Then, the brewing cycle was carried out 10 times with mineral water without the use of coffee powder to passivate the metal surface. The additional brewing devices used were a commercially available filter machine (FIM) (Figure 5) and a commercially available machine to brew coffee from Al capsules (CAPs). Both had been in regular use before the experiment.
Figure 4.
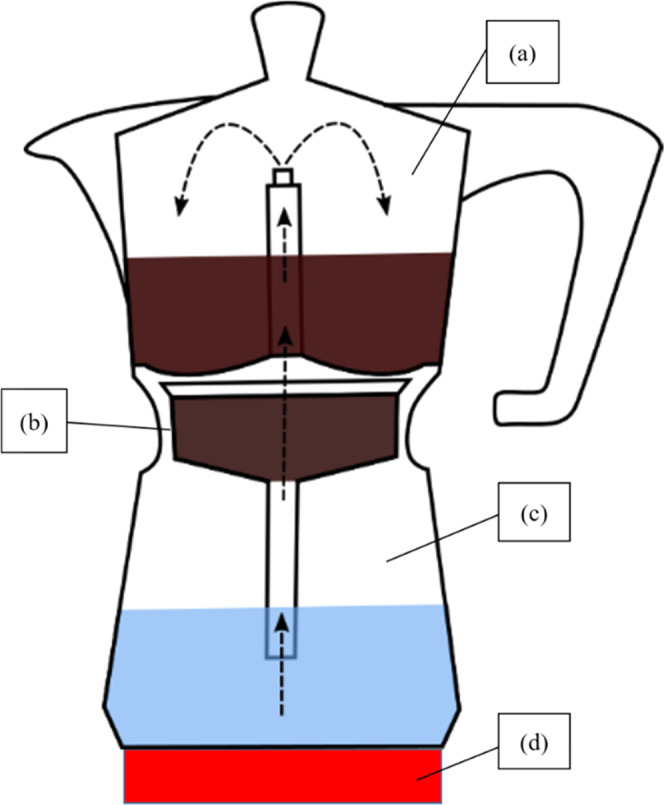
Sketch of a typical moka pot used for brewing coffee, commercially available in both aluminum and stainless steel. It consists of three parts that can be screwed together: (a) collection chamber, (b) basket chamber, (c) bottom chamber, and (d) heating plate. Arrows: pathway of water in the device.
Figure 5.
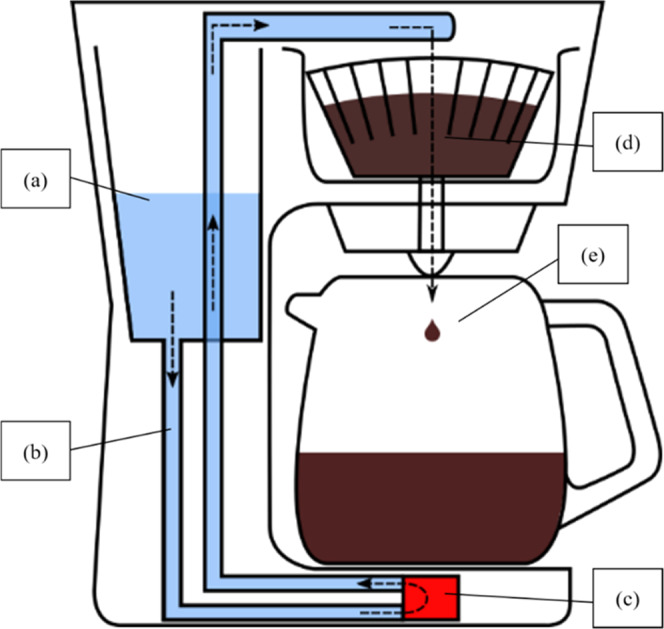
Sketch of the commercially available filter machine. (a) Water reservoir, (b) internal tubing system, (c) heating element, (d) paper coffee filter and GCB, and (e) glass pot. Arrows: pathway of water in the device.
All eight GCB samples (WC1–4 and CC1–4) were brewed in quintuplets for the brewing methods TUC, ALP, STP, and FIM. As brewing water, we used commercially available mineral water for which the major dissolved components were determined. This was achieved by using the flame atomic absorption spectrometer AAnalyst 200 (Perkin Elmer, Waltham), the photometer Spectroquant NOVA 60a (Merck Millipore, Burlington), and the conductivity meter FiveGo F3 (Mettler-Toledo, Columbus). This mineral water correlated to the composition of the Austrian tap water, thus mirroring household conditions.
Brewed coffee was prepared using 14 g of GCB and 200 mL of mineral water except for the CAP method, where 6 g of coffee (amount in each capsule) and 110 mL of mineral water were used based on the producers’ specifications. Five capsules from each brand were used for the preparation of brewed coffee from the capsule machine. The pH of BC was determined using a pH probe (pH100, VWR International, Radnor).
Turkish Coffee (TUC)
Ground coffee beans were put into a 300 mL glass flask; water was added and brought to boil on a heating plate. The flask was removed immediately, and, after 10 min of cooling at room temperature, 40 mL of liquid was taken and centrifuged for 10 min at 4000 rpm. Five milliliters of the supernatant was pipetted into a PP vial and kept for further analysis.
Aluminum Pot (ALP) and Steel Pot (STP)
The aluminum pot and steel pot followed the same device setup. The devices consisted of three individual parts: the bottom chamber, the basket chamber, and the collection chamber (Figure 4). The bottom chamber of the moka pot was filled with water, and the GCBs were put into the basket chamber. The collection chamber was screwed onto the device, and the device was left on a heating plate until all of the water had passed through the GCB and reached the collection chamber. Five milliliters of the BC was pipetted into a PP vial and kept for further analysis.
Filter Machine (FIM)
A commercially available and pre-used filter machine was used for this brewing method (Figure 5). A commercially available paper coffee filter was placed into the machine, and the GCB was filled in. The cold water from a reservoir was heated in an internal tubing system and poured over the GCB. This led to a slow extraction with the BC collected in a glass pot. Five milliliters of the BC was pipetted into a PP vial and kept for further analysis.
Capsules (CAPs)
The capsules were placed in the machine, which has a piercing mechanism that allows the automatically heated water to pass through the GCB. The BC was collected in a mug, and 5 mL was pipetted into a PP vial and kept for further analysis.
Sample Preparation for Al Determination in Brewed Coffee
In preparation for elemental analyses, BC samples were digested using 5 mL of the liquid coffee, 2 mL of 69% HNO3, and 1 mL of hydrogen peroxide using microwave digestion as well, and the digested samples were brought to 10 mL in PP volumetric flasks.
Aluminum in Brewing Water
To determine the contribution of the extracted Al originating from the brewing devices to the total Al content in brewed coffee, all methods were carried out without adding the coffee powder to the brewing cycles. The same mineral water as for the brewing of coffee was used for the determination. For the analyses of water samples, 10 mL of the brewing water and mineral water, respectively, were acidified with 0.2 mL of 69% HNO3 and directly used for further analysis.
Analytical Device and Method for Al Analysis
The Al content of all samples was determined with graphite furnace atomic absorption spectroscopy (GF-AAS) using a “PinAAcle 900Z” (Perkin Elmer, Waltham). The GF-AAS method for the measurement was taken from House et al.62 Calibration was done by preparing five different concentrations of Al by diluting a 1000 mg L–1 aluminum standard solution (Merck, Darmstadt, Germany). Quality control was ensured by digesting the certified reference material “BCR-482” (lichen, n = 5) from the Institute for Reference Materials and Measurements (European Commission) in the same manner as the GCB samples. The recovery rates of Al for the certified reference material were at 94 ± 8%, showing that the sample preparation and method were appropriate.
Statistical Analysis
Statistical analysis was carried out using SigmaPlot 14.0. Normal distribution was tested with the Shapiro–Wilk test (p < 0.05). The normality test failed; therefore, the nonparametric Mann–Whitney Rank-Sum test was used to determine significant differences. Sample size (n) is given in the Materials and Methods section, as well as in the Results and Discussion section, where the respective level of significance is also provided.
Acknowledgments
The authors would like to thank Michael Stachowitsch for proofreading this article. Open access funding provided by the University of Vienna.
The authors declare no competing financial interest.
References
- Holleman A. F.Lehrbuch der Anorganischen Chemie; Gruyter D., Ed.; Walter: Berlin, 1976; pp 81–90. [Google Scholar]
- Ginsberg H.Aluminium; Enke: Stuttgart, 1962; p 218. [Google Scholar]
- Exley C. Darwin, natural selection and the biological essentiality of aluminium and silicon. Trends Biochem. Sci. 2009, 34, 589–593. 10.1016/j.tibs.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Markert B.; Fraenzle S.; Wuenschmann S.. Chemical Evolution; Springer International Publishing: Switzerland, 2015. [Google Scholar]
- Exley C. Human exposure to aluminium. Environ. Sci.: Processes Impacts 2013, 15, 1807–1816. 10.1039/C3EM00374D. [DOI] [PubMed] [Google Scholar]
- Verstraeten S. V.; Aimo L.; Oteiza P. I. Aluminium and lead: molecular mechanisms of brain toxicity. Arch. Toxicol. 2008, 82, 789–802. 10.1007/s00204-008-0345-3. [DOI] [PubMed] [Google Scholar]
- Exley C. Aluminum in antiperspirants: More than just skin deep. Am. J. Med. 2004, 117, 969–970. 10.1016/j.amjmed.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Darbre P. D. Aluminium, antiperspirants and breast cancer. J. Inorg. Biochem. 2005, 99, 1912–1919. 10.1016/j.jinorgbio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Morris G.; Puri B. K.; Frye R. E. The putative role of environmental aluminium in the development of chronic neuropathology in adults and children. How strong is the evidence and what could be the mechanisms involved?. Metab. Brain Dis. 2017, 32, 1335–1355. 10.1007/s11011-017-0077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberg J. P.; McDonald B.; Watt F. Absence of aluminium in neuritic plaque cores in Alzheimer’s disease. Nature 1992, 360, 65–68. 10.1038/360065a0. [DOI] [PubMed] [Google Scholar]
- Mirick D. K.; Davis S.; Thomas D. B. Antiperspirant Use and the Risk of Breast Cancer. J. Natl. Cancer Inst. 2002, 94, 1578–1580. 10.1093/jnci/94.20.1578. [DOI] [PubMed] [Google Scholar]
- Aguilar F.; Autrup H.; Barlow S.; Castle L.; Crebelli R.; Dekant W.; Engel K. H.; Gontard N.; Gott D.; Grilli S.; Gurtler R.; Larsen J. C.; Leclercq C.; Leblanc J. C.; Malcata F. X.; Mennes W.; Milana M. R.; Pratt I.; Rietjens I.; Tobback P.; Toldra F.; Members P. Annex of the opinion on Safety of aluminium from dietary intake. EFSA J. 2008, 6, 754. [Google Scholar]
- Trapp G. A. Plasma aluminum is bound to transferrin. Life Sci. 1983, 33, 311–316. 10.1016/S0024-3205(83)80002-2. [DOI] [PubMed] [Google Scholar]
- Yokel R. A. Brain uptake, retention, and efflux of aluminum and manganese. Environ. Health Perspect. 2002, 110, 699–704. 10.1289/ehp.02110s5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sole P.; Rossi C.; Chiarpotto M.; Ciasca G.; Bocca B.; Alimonti A.; Bizzarro A.; Rossi C.; Masullo C. Possible relationship between Al/ferritin complex and Alzheimer’s disease. Clin. Biochem. 2013, 46, 89–93. 10.1016/j.clinbiochem.2012.10.023. [DOI] [PubMed] [Google Scholar]
- Darbre P. D.; Mannello F.; Exley C. Aluminium and breast cancer: Sources of exposure, tissue measurements and mechanisms of toxicological actions on breast biology. J. Inorg. Biochem. 2013, 128, 257–261. 10.1016/j.jinorgbio.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Darbre P. D.; Pugazhendhi D.; Mannello F. Aluminium and human breast diseases. J. Inorg. Biochem. 2011, 105, 1484–1488. 10.1016/j.jinorgbio.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Mannello F.; Ligi D.; Canale M. Aluminium, carbonyls and cytokines in human nipple aspirate fluids: Possible relationship between inflammation, oxidative stress and breast cancer microenvironment. J. Inorg. Biochem. 2013, 128, 250–256. 10.1016/j.jinorgbio.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Ruipérez F.; Mujika J. I.; Ugalde J. M.; Exley C.; Lopez X. Pro-oxidant activity of aluminum: Promoting the Fenton reaction by reducing Fe(III) to Fe(II). J. Inorg. Biochem. 2012, 117, 118–123. 10.1016/j.jinorgbio.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Polizzi S.; Ferrara M.; Bugiani M.; Barbero D.; Baccolo T. Aluminium and iron air pollution near an iron casting and aluminium foundry in Turin district (Italy). J. Inorg. Biochem. 2007, 101, 1339–1343. 10.1016/j.jinorgbio.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Exley C.; Begum A.; Woolley M. P.; Bloor R. N. Aluminum in tobacco and cannabis and smoking-related disease. Am. J. Med. 2006, 119, 276.e9. 10.1016/j.amjmed.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Exley C. Does antiperspirant use increase the risk of aluminium-related disease, including Alzheimer’s disease?. Mol. Med. Today 1998, 4, 107–109. 10.1016/S1357-4310(98)01209-X. [DOI] [PubMed] [Google Scholar]
- Stahl T.; Falk S.; Rohrbeck A.; Georgii S.; Herzog C.; Wiegand A.; Hotz S.; Boschek B.; Zorn H.; Brunn H. Migration of aluminum from food contact materials to food—a health risk for consumers? Part III of III: migration of aluminum to food from camping dishes and utensils made of aluminum. Environ. Sci. Eur. 2017, 29, 17. 10.1186/s12302-017-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiyed S. M.; Yokel R. A. Aluminium content of some foods and food products in the USA, with aluminium food additives. Food Addit. Contam. 2005, 22, 234–244. 10.1080/02652030500073584. [DOI] [PubMed] [Google Scholar]
- Reinke C. M.; Breitkreutz J.; Leuenberger H. Aluminium in Over-the-Counter Drugs. Drug Saf. 2003, 26, 1011–1025. 10.2165/00002018-200326140-00003. [DOI] [PubMed] [Google Scholar]
- Flaten T. P. Aluminium in tea—concentrations, speciation and bioavailability. Coord. Chem. Rev. 2002, 228, 385–395. 10.1016/S0010-8545(02)00036-X. [DOI] [Google Scholar]
- Chenery E. M. A preliminary study of aluminium and the tea bush. Plant Soil 1955, 6, 174–200. 10.1007/BF01343446. [DOI] [Google Scholar]
- Dong D.; Xie Z.; Du Y. The bioavailability of Al in soils to tea plants. J. Appl. Geochem. 2001, 16, 1413–1418. 10.1016/S0883-2927(01)00052-X. [DOI] [Google Scholar]
- Haarer A. E.Modern Coffee Production; Leonard Hill Books Limited: London, 1956. [Google Scholar]
- Poschenrieder C.; Gunse B.; Corrales I.; Barcelo J. A glance into aluminum toxicity and resistance in plants. Sci. Total Environ. 2008, 400, 356–68. 10.1016/j.scitotenv.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Cagliani L. R.; Pellegrino G.; Giugno G.; Consonni R. Quantification of Coffea arabica and Coffea canephora var. robusta in roasted and ground coffee blends. Talanta 2013, 106, 169–173. 10.1016/j.talanta.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Grembecka M.; Malinowska E.; Szefer P. Differentiation of market coffee and its infusions in view of their mineral composition. Sci. Total Environ. 2007, 383, 59–69. 10.1016/j.scitotenv.2007.04.031. [DOI] [PubMed] [Google Scholar]
- Ky C. L.; Louarn J.; Dussert S.; Guyot B.; Hamon S.; Noirot M. Caffeine, trigonelline, chlorogenic acids and sucrose diversity in wild Coffea arabica L. and C. canephora P. accessions. Food Chem. 2001, 75, 223–230. 10.1016/S0308-8146(01)00204-7. [DOI] [Google Scholar]
- Cruz R.; Morais S.; Casal S.. Mineral Composition Variability of Coffees: A Result of Processing and Production. In Processing and Impact on Active Components in Food; Preedy V., Ed.; Academic Press: San Diego, 2015; pp 549–558. [Google Scholar]
- Martín M. J.; Pablos F.; González A. G. Characterization of green coffee varieties according to their metal content. Anal. Chim. Acta 1998, 358, 177–183. 10.1016/S0003-2670(97)00610-7. [DOI] [Google Scholar]
- Karbouj R.; Desloges I.; Nortier P. A simple pre-treatment of aluminium cookware to minimize aluminium transfer to food. Food Chem. Toxicol. 2009, 47, 571–577. 10.1016/j.fct.2008.12.028. [DOI] [PubMed] [Google Scholar]
- Wellinger M.; Smrke S.; Yeretzian C.. Water for Extraction—Composition, Recommendations, and Treatment. In The Craft and Science of Coffee; Folmer B., Eds.; Academic Press, 2017; pp 381–398. [Google Scholar]
- Fibrianto K.; Ardianti A. D.; Pradipta K.; Sunarharum W. B. The influence of brewing water characteristic on sensory perception of pour-over local coffee. IOP Conf. Ser.: Earth Environ. Sci. 2018, 102, 012095 10.1088/1755-1315/102/1/012095. [DOI] [Google Scholar]
- Hendon C. H.; Colonna-Dashwood L.; Colonna-Dashwood M. The Role of Dissolved Cations in Coffee Extraction. J. Agric. Food Chem. 2014, 62, 4947–4950. 10.1021/jf501687c. [DOI] [PubMed] [Google Scholar]
- Anderson K. A.; Smith B. W. Chemical Profiling To Differentiate Geographic Growing Origins of Coffee. J. Agric. Food Chem. 2002, 50, 2068–2075. 10.1021/jf011056v. [DOI] [PubMed] [Google Scholar]
- Martín M. J.; Pablos F.; González A. G. Characterization of arabica and robusta roasted coffee varieties and mixture resolution according to their metal content. Food Chem. 1999, 66, 365–370. 10.1016/S0308-8146(99)00092-8. [DOI] [Google Scholar]
- Krivan V.; Barth P.; Morales A. F. Multielement analysis of green coffee and its possible use for the determination of origin. Microchim. Acta 1993, 110, 217–236. 10.1007/BF01245106. [DOI] [Google Scholar]
- Fraňková A.; Drábek O.; Havlík J.; Száková J.; Vaněk A. The effect of beverage preparation method on aluminium content in coffee infusions. J. Inorg. Biochem. 2009, 103, 1480–1485. 10.1016/j.jinorgbio.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Lamberti M.; Escher F. Aluminium Foil as a Food Packaging Material in Comparison with Other Materials. Food Rev. Int. 2007, 23, 407–433. 10.1080/87559120701593830. [DOI] [Google Scholar]
- Müller J. P.; Steinegger A.; Schlatter C. Contribution of aluminium from packaging materials and cooking utensils to the daily aluminium intake. Z. Lebensm.-Unters. Forsch. 1993, 197, 332–341. 10.1007/BF01242057. [DOI] [PubMed] [Google Scholar]
- Malik J.; Szakova J.; Drabek O.; Balik J.; Kokoska L. Determination of certain micro and macroelements in plant stimulants and their infusions. Food Chem. 2008, 111, 520–525. 10.1016/j.foodchem.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Wei F.; Furihata K.; Koda M.; Hu F.; Miyakawa T.; Tanokura M. Roasting Process of Coffee Beans as Studied by Nuclear Magnetic Resonance: Time Course of Changes in Composition. J. Agric. Food Chem. 2012, 60, 1005–1012. 10.1021/jf205315r. [DOI] [PubMed] [Google Scholar]
- Redgwell R. J.; Trovato V.; Curti D.; Fischer M. Effect of roasting on degradation and structural features of polysaccharides in Arabica coffee beans. Carbohydr. Res. 2002, 337, 421–431. 10.1016/S0008-6215(02)00010-1. [DOI] [PubMed] [Google Scholar]
- Kaikake K.; Hoaki K.; Sunada H.; Dhakal R. P.; Baba Y. Removal characteristics of metal ions using degreased coffee beans: Adsorption equilibrium of cadmium(II). Bioresour. Technol. 2007, 98, 2787–2791. 10.1016/j.biortech.2006.02.040. [DOI] [PubMed] [Google Scholar]
- Tokimoto T.; Kawasaki N.; Nakamura T.; Akutagawa J.; Tanada S. Removal of lead ions in drinking water by coffee grounds as vegetable biomass. J. Colloid Interface Sci. 2005, 281, 56–61. 10.1016/j.jcis.2004.08.083. [DOI] [PubMed] [Google Scholar]
- Krachler R.; von der Kammer F.; Jirsa F.; Süphandag A.; Krachler R. F.; Plessl C.; Vogt M.; Keppler B. K.; Hofmann T. Nanoscale lignin particles as sources of dissolved iron to the ocean. Global Biogeochem. Cycles 2012, 26, 400 10.1029/2012GB004294. [DOI] [Google Scholar]
- Klöcking R.; Hofmann R.; Mücke D. Stoffe vom Huminsäuretyp in Röstkaffee-Extrakten. Z. Lebensm.-Unters. Forsch. 1971, 146, 79–83. 10.1007/BF01784145. [DOI] [Google Scholar]
- Klöcking R.; Hofmann R.; Mücke D. Stoffe vom Huminsäuretyp in Röstkaffee-Extrakten. Z. Lebensm.-Unters. Forsch. 1967, 135, 1. 10.1007/BF02438853. [DOI] [Google Scholar]
- Van den Bergh J.Vort-Ort-Charakterisierung von aquatischen Huminstoffen und ihren Metallspezies. Dissertation; Universität Dortmund, 2001. [Google Scholar]
- Tipping E.Cation Binding by Humic Substances, 1st ed.; Cambridge University Press: New York, 2002. [Google Scholar]
- Kaim W.; Schwederski B.. Bioanorganische Chemie: zur Funktion chemischer Elemente in Lebensprozessen, 4th ed.; Teubner: Wiesbaden, 2005. [Google Scholar]
- Pennington J. A. T.; Schoen S. A. Estimates of dietary exposure to aluminium. Food Addit. Contam. 1995, 12, 119–128. 10.1080/02652039509374286. [DOI] [PubMed] [Google Scholar]
- Ysart G.; Miller P.; Crews H.; Robb P.; Baxter M.; De L’Argy C.; Lofthouse S.; Sargent C.; Harrison N. Dietary exposure estimates of 30 elements from the UK Total Diet Study. Food Addit. Contam. 1999, 16, 391–403. 10.1080/026520399283876. [DOI] [PubMed] [Google Scholar]
- Ensminger A. H.; Ensminger M. E.; Konlande J. E.; Robson J. R. K.. Foods & Nutrition Encyclopedia, 2nd ed.; CRC Press, 1994; Vol. 1. [Google Scholar]
- Tran L.; Greenwood-Van Meerveld B. Age-associated remodeling of the intestinal epithelial barrier. J. Gerontol., Ser. A 2013, 68, 1045–1056. 10.1093/gerona/glt106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easto J.; Willhoff A.. Craft Coffee: A Manual: Brewing a Better Cup at Home, 1st ed.; Agate Publishing: Chicago, 2017. [Google Scholar]
- House E.; Esiri M.; Forster G.; Ince P. G.; Exley C. Aluminium, iron and copper in human brain tissues donated to the medical research council’s cognitive function and ageing study. Metallomics 2012, 4, 56–65. 10.1039/C1MT00139F. [DOI] [PubMed] [Google Scholar]