Abstract
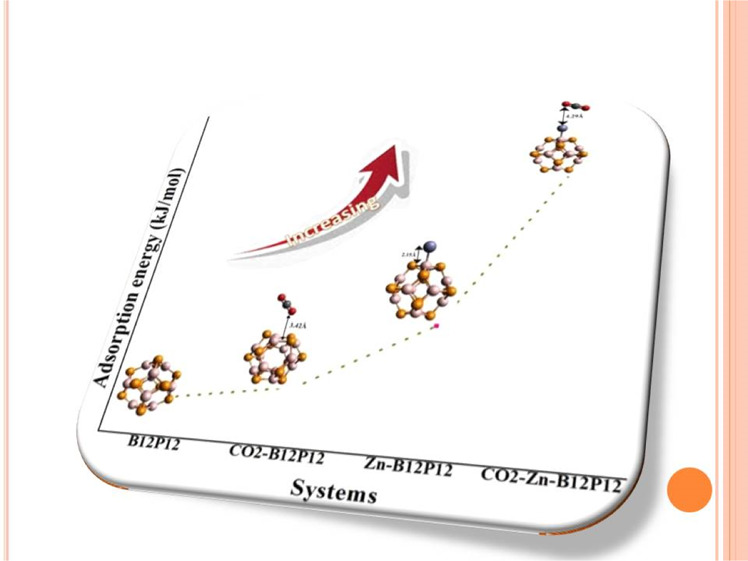
Gas sensing materials have been widely explored recently owing to their versatile environmental and agriculture monitoring applications. The present study advocates the electronic response of Zn-decorated inorganic B12P12 nanoclusters to CO2 gas. Herein, a series of systems CO2–Zn–B12P12 (E1–E4) are designed by adsorption of CO2 on Zn-decorated B12P12 nanoclusters, and their electronic properties are explored by density functional theory. Initially, placement of Zn on B12P12 delivers four geometries named as D1–D4, with adsorption energy values of −57.12, −22.94, −21.03, and −14.07 kJ/mol, respectively, and CO2 adsorption on a pure B12P12 nanocage delivers one geometry with an adsorption energy of −4.88 kJ/mol. However, the interaction of CO2 with D1–D4 systems confers four geometries named as E1 (Ead = −75.12 kJ/mol), E2 (Ead = −25.89 kJ/mol), E3 (Ead = −42.43 kJ/mol), and E4 (Ead = −28.73 kJ/mol). Various electronic parameters such as dipole moment, molecular electrostatic potential analysis, frontier molecular orbital analysis, QNBO, global descriptor of reactivity, and density of states are also estimated in order to understand the unique interaction mechanism. The results of these analyses suggested that Zn decoration on B12P12 significantly favors CO2 gas adsorption, and a maximum charge separation is also noted when CO2 is adsorbed on the Zn–B12P12 nanocages. Therefore, the Zn-decorated B12P12 nanocages are considered as potential candidates for application in CO2 sensors.
1. Introduction
Nanoscience has developed a revolutionary trend in various fields of science. Functional nanomaterials are now attracting the modern research community because of their distinct structural and electronic properties.1,2 Recently, nanostructure semiconductors have gained significant interest from the scientific community because of their distinct physical and chemical properties.2−4 Nanostructures such as fullerenes, nanotubes, and nanoclusters have also received great interest for many applications such as catalysis, biotechnology, gas sensors, and cluster protection.5−7
Nanomaterials also find applications in transistors and adsorption because of their high surface/volume ratio. Metals adsorbed on the surface of nanocages enhance catalytic and adsorption properties.8−10 Group III–V semiconductors have gained importance because of their extensive use in light-emitting diodes, nonlinear optics,11−13 and in microelectronic devices.14−16 These are also used as adsorbents/sensors for various analytes. The small-sized solid-state adsorbents can easily be synthesized at low cost. These are also reproducible, which makes them excellent candidates for sensor applications.17
Adsorption of gases on nanostructures, nanocages, and nanotubes are part of valuable literature. For example, Ahmadi et al. studied the adsorption energies of nitrogen oxide and carbon monoxide on MgO nanotubes.18 In addition, different reports specifically on the adsorption properties of different molecules on the surface of (AlP)x, (AlN)x, (BP)x, and (BN)x nanocages are also part of valuable literature. For example, Ayub19 studied the binding affinity of helium and neon atoms with X12Y12 (X = B, Al, and Y = N, P) in exohedral and endohedral modes. Similarly, Rad et al.20 demonstrated the ability of Al12N12 nanoclusters for adsorption of BCl3 by using density functional theory calculations. In another report, Rad and Ayub21 studied the geometries and electronic properties of Ni-doped Al12N12 nanocages. Baei et al. showed B12N12 nanoclusters as an outstanding adsorbent for aniline from groundwater to tackle the environmental issues.22 Soltani et al. studied phenol interactions with various nanocages through density functional theory (DFT) calculations.10 Significant literature is available for adsorption of various molecules such as pyridine,23 methylamine,24 hydrogen cyanide,25 and fluorine26 on B12N12 nanocages. Adsorption of biological molecules such as nitrogenous basis (uracil, cytosine, and adenine)27 and guanine28 on these nanocages are also part of valuable literature. In valuable literature, report on adsorption of pyrrole on Al12N12, Al12P12, B12P12, and B12N12 nanocages is also present.9 Rad et al. studied the effective adsorption of O3 on B12P12, Al12N12, and B12N12 nanocages previously.29 Similarly, B12P12 nanocages were also utilized for hydrogen gas adsorption.30
Recently, metal decorations (dopants) have been launched to improve the adsorption, electronic,31−35 and nonlinear optical properties of nanostructures.36,37 Zhang et al.38 carried out DFT calculations to examine the hydrogen adsorption on pure and nickel metal-decorated aluminum nitride nanocages. They studied that in a pure AlN nanocage, a single Al atom avails only one H2, whereas the nickel atom in Ni–AlN has a tendency to adsorb three hydrogen molecules. Ayub et al. studied that nickel decoration on B12N1239 and B12P1230 significantly enhanced the adsorption of hydrogen gas. Nickel metal decoration on (XY)12 was also proved useful for adsorption of SO240 and acetylene.41 Similarly, Shakerzadeh et al. investigated adsorption of phosgene gas on Al- and Ga-doped B12N12 and B16N16 nanoclusters.42 Decoration of metal is useful in order to enhance the effective role of these nanocages in NLO materials.43,44 In addition, these nanocages exhibit a variety of applications such as field-effective transistors,45 storage devices,46 and magnetic nanoparticles.47
Because of the rapid increase in industrialization, population, and traffic, the percentage of CO2 also increases in the atmosphere as a result of fossil fuel burning. Ultimately, the greenhouse effect is increasing and making the earth warmer. Oceans act as a sink of CO2 and the dissolution of CO2 affecting the system by lowering the pH. Therefore, it is important to monitor and control this pollutant to make the environment more safe and friendly. Recently, the research is devoted to develop some gas sensors for monitoring hazardous gas for its optimum level.48−50
Carbon sequestration is a process through which atmospheric freely available carbon dioxide (CO2) is captured and stored through a natural process, so it has become a most important feature in environment protection. For better results, the sequestration material should be of large surface area for effective absorption and easy accessibility to atmospheric carbon dioxide.51,52 Therefore, it is important to capture this dangerous gas from the environment in order to make the environment green and clean. Recently, Cu-decorated B12N12 has been used to detect the harmful phosgene gas.53 Similarly, Hussain et al. explored the remarkable response of Zn-doped B12P12 to SO2 gas.54
In the literature, there is no detailed report on the adsorption of carbon dioxide on Zn-decorated nanocages. Analysis is performed for all promising relaxed structures of CO2-adsorbed nanocages on the above-mentioned surfaces. We discuss the result on adsorption through the net charge transfer, values of binding energy, molecular electrostatic potential (MEP) analysis, dipole moment, density of states (DOS), global descriptor of energy, and the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) distribution on all possible forms of B12P12 nanocages. Finally, we recommend a kind of novel systems with promising electronic properties for CO2 sensing materials.
2. Results and Discussion
The relaxed geometry of a B12P12 nanocage at the B3LYP method with the 6-31G(d,p) level of DFT is shown in Figure 1. Two types of rings are present in the B12P12 nanocage, one is tetragonal and the other is hexagonal. Both rings are interconnected in order to gain a three-dimensional nanocage. Similarly, two types of bonds are present in B12P12, one is b64 (bond shared between tetragonal and hexagonal ring) and the other is b66 (bond shared between two hexagonal rings). In an optimized geometry of B12P12 nanocage, the b64 and b66 bond lengths are 1.93 and 1.91 Å, respectively.
Figure 1.
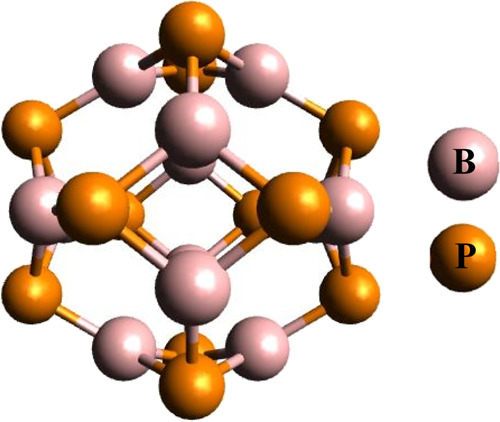
B12P12 DFT-based relaxed structure with the aid of the B3LYP method along with the 6-31G(d,p) level of DFT.
2.1. Interaction Energies along with Bond Lengths
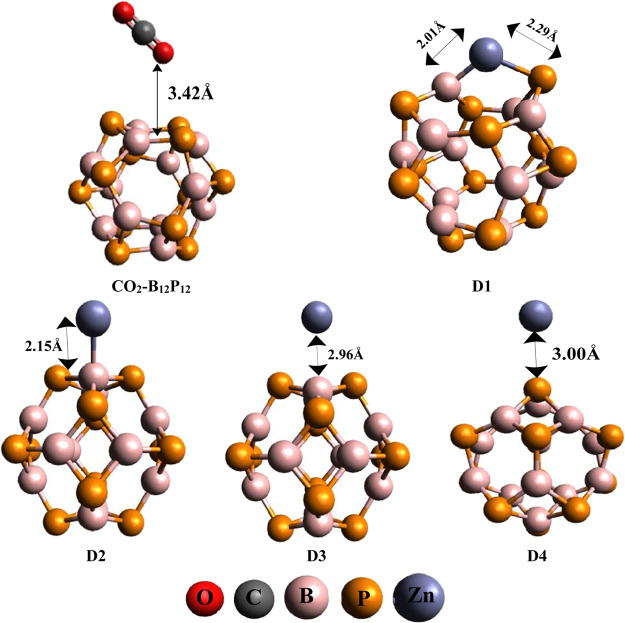
Pure B12P12 has equal number of electropositive and electronegative atoms. Six different sites are present for the decoration of a late transition metal (Zn). These sites are named as: (i) b66 [decoration of Zn on a common bond present between two six-member (hexagonal) rings], (ii) b64 [placement of Zn metal specifically on a bond shared between one four-member (tetragonal) and one six-member (hexagonal) ring], (iii) Btop (Zn metal installed on boron top), (iv) Ptop (positing of Zn metal on phosphorus top), (v) r6 (bringing Zn metal on the top center of the hexagonal ring), and (vi) r4 (putting Zn metal on top of the tetragonal ring). We tried all the above-mentioned positions, but only four geometries could be optimized because some initial input geometries switched to other geometries. The obtained geometries are named as D1 (Zn@b64), D2 (Zn@Btop), D3 (Zn@r4), and D4 (Zn@Ptop). In the D1 geometry, the positioning of the late transition metal (Zn) elongates the B–P bond length to 3.66 Å (as compared to 1.93 Å in pure BP) with an interaction energy value of −57.12 kJ/mol. In the D2 geometry, the decoration of Zn metal on B12P12 does not bring much change in the B–P bond length (bond length(B–P) = 1.98 Å), and the interaction energy value in this case is −22.94 kJ/mol. In the D3 and D4 geometries, the interaction of Zn metal with the B12P12 nanocage executes a negligible alteration in the B–P bond length with interaction energy values of −21.03 and −14.07 kJ/mol, respectively. Similarly, the distances of Zn metal from the B12P12 nanocage in D1, D2, D3, and D4 geometries are 2.15, 2.15, 2.96, and 3.00 Å, respectively, as shown in Figure 2. From the preceding discussion, it is cleared that D1 is the most stable and D4 is the least stable geometry among D1–D4. This might be due to the shape of the geometries; that is, in D1, adsorption of Zn metal changes the normal shape of B12P12 to an open envelop shape, which ultimately reduces the strain of the tetragonal ring and becomes stable, whereas in D4, B12P12 does not provide enough room for adsorption of Zn metal, which decreases the stability of the Zn-doped system. Apart from this, the distance of Zn metal from the BP nanocage is very crucial, which suggests that large distance of Zn from BP in D4 causes weak adsorption and vice versa.
Figure 2.
Optimized geometries of CO2–B12P12 and D1–D4 systems.
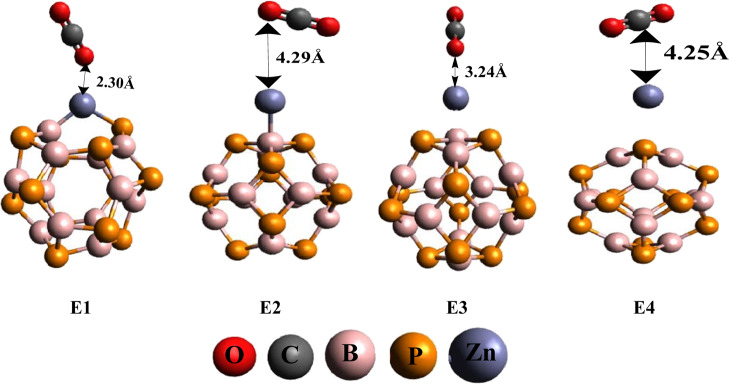
Further, adsorption of CO2 gas on pure and Zn-doped B12P12 nanocages is being analyzed. CO2 absorption on bare B12P12 exhibits the physisorption phenomenon. The large distance of gas from the nanocage (dCO2–BP = 3.42 Å) and the low adsorption value (Ed = −4.88 kJ/mol) indicated that CO2 is not favorably adsorbed on bare B12P12. Besides this, CO2 is also adsorbed on Zn-doped B12P12 nanocages (D1–D4), which deliver four geometries named as E1, E2, E3, and E4. We gained E1–E4 after CO2 adsorption on D1–D4, respectively, as shown in Figure 3. The adsorption energy values in E1 (Ead = −75.12 kJ/mol), E2 (Ead = −25.89 kJ/mol), E3 (Ead = −42.43 kJ/mol), and E4 (Ead = −28.73 kJ/mol) suggest that CO2 gas is favorably adsorbed on Zn-doped systems, which suggested potential utilization of these materials for CO2 gas sensing as compared to pure B12P12. In all geometries (E1–E4), orientation of CO2 on Zn-decorated BP is different (Figure 3). Different orientations display different distances from the Zn–BP system. Among E1–E4, E1 and E3 disclosed high adsorption energy values, and these are due to the small distance of CO2 from the Zn–B12P12 nanocage [dCO2–Zn–BP (E1 = 2.30 Å and E3 = 3.24 Å)] as shown in Table 1. The closest distance of CO2 from Zn–B12P12 with high adsorption energy is found in the case of E1, where Zn metal effectively offers better adsorption because of the small distance of Zn from the B12P12 nanocage. Therefore, small distance allows favorable adsorption of CO2 on Zn-doped B12P12 nanocages. From the above discussion, it is concluded that adsorption of late transition metal (Zn) brings some structural changes in the B12P12 nanocage, which significantly enhance the adsorption rate of CO2 gas.
Figure 3.
Optimized geometries of CO2-adsorbed Zn–B12P12 (E1–E4) systems.
Table 1. Distance of Zn Metal from the B12P12 Nanocage (Å), Distance between the Closest Atom of CO2 and Zn Metal (Å), Natural Bonding Orbital Charge (QNBO) on Metal and Gas (eV), Dipole Moment (Debye), and Adsorption Energy of All Systems (kJ/mol).
| systems | dZn–BP (Å) | dCO2–Zn(Å)a | QNBO on CO2 (eV) | QNBO on Zn (eV) | μD (D) | Eadb (kJ/mol) |
|---|---|---|---|---|---|---|
| B12P12 | 0.00 | |||||
| B12P12–CO2 | –0.624 | 0.33 | –4.88 | |||
| Zn–BP (D1) | 2.15 | 1.937 | 2.25 | –57.12 | ||
| Zn–BP–CO2 (E1) | 2.30 | –0.692 | 5.36 | –75.12 | ||
| Zn–BP (D2) | 2.15 | 0.524 | 3.24 | –22.94 | ||
| Zn–BP–CO2 (E2) | 4.25 | –0.677 | 3.07 | –25.89 | ||
| Zn–BP (D3) | 2.96 | 0.076 | 0.94 | –21.03 | ||
| Zn–BP–CO2 (E3) | 3.24 | –0.685 | 3.00 | –42.43 | ||
| Zn–BP (D4) | 3.00 | –0.422 | 0.61 | –14.07 | ||
| Zn–BP–CO2 (E4) | 4.25 | –0.681 | 3.30 | –28.73 |
Distance between the nearest CO2 atom from the Zn metal.
Adsorption energies of different systems.
The literature is quite extensive on the utilization of different surfaces for CO2 detection and adsorption. We have now given comparison of our results with those available in the literature on different nanoclusters. Moreover, in valuable literature, reports related to CO2 adsorption on different nanocages are present. Baei et al.5 studied different orientations of CO2 on B12N12 nanocages with adsorption energies ranging from −14.99 to −15.45 kcal/mol. Similarly, Kauffman et al.55 studied the interaction of CO2 with the Au25 cluster with an adsorption energy value of 0.13 eV. Liang et al.56 studied the physisorption of CO2 on B12N12, Li@B12N12, Na@B12N12, and K@B12N12 with adsorption energies of −1.86, −2.96, −3.18, and −2.66 kcal/mol, respectively. Based on DFT study, Jiang et al.57 reported that Al11Mg3– nanoclusters had excellent capturing capacity of CO2 (Ead = 0.114 eV). Guo et al.58 reported the adsorption of CO2 on a hexagonal BN sheet, where the adsorption energies are as high as 0.42 eV (parallel orientation of CO2 on the h-BN sheet) and 0.44 eV (vertical orientation of CO2 on the h-BN sheet). The adsorption energies of Zn-doped boron phosphide nanocages (in our case) are much higher (−6.18 to −17.96 kcal/mol) than the reported values of adsorption energies of CO2 on different surfaces. These results illustrate the efficiency and potential of the Zn–B12P12 nanocluster for CO2 adsorption.
2.2. Dipole Moment
Dipole moment is another tool to explore the electronic change in the B12P12 nanocluster upon Zn decoration and CO2 adsorption. As we know, the electropositive and electronegative atoms are equal in number; therefore, a pure B12P12 nanocage is a symmetrical structure with zero dipole moment. However, decoration with late transition metal such as Zn brings significant change in the dipole moment as shown in Table 1. The dipole moments in D1–D4 geometries are noted as 2.25, 3.24, 0.94, and 0.61 D, respectively. All values of dipole moments revealed that the placement of Zn significantly disturbs the charge separation in the B12P12 nanocage. The disturbance in charge separation is high in the case of D1 and D2 systems, which might be due to the small distance of Zn from BP. In addition, the disturbance in charge separation of B12P12 is also analyzed in CO2–B12P12 and E1–E4 geometries. Initially, when carbon dioxide is placed on top of B12P12, the dipole moment value is quite small (0.33 D), which indicates that adsorption of CO2 does not bring significant change in charge separation. However, when CO2 is positioned on Zn-decorated B12P12 nanocages (E1–E4), significant changes in the dipole moment value are noted. The dipole moment values for E1–E4 are 5.36, 3.07, 3.00, and 3.30 D, respectively. These values suggest that CO2 adsorption on D1–D4 geometries significantly affects the charge separation with dipole moment values as compared to the rest of the geometries. Large adsorption energies cause high charge separation (large dipole moment value) in E1–E4. The decreasing order of the dipole moment for all systems is E1 > E4 > D2 > E2 > E3 > D1 > D3 > D4 > CO2–B12P12. From the preceding discussion, it is cleared that CO2 adsorption on Zn-decorated B12P12 nanocages enhanced charge separation of B12P12 with large values of dipole moment.
2.3. QNBO
In support of dipole moment, QNBO analysis is performed in order to understand the strong interaction of Zn and CO2 with the B12P12 nanocage. In the case of D1–D4 geometries, the increase in QNBO is consistent with the increase in the dipole moment value. A highest QNBO value is noted in the D1 system with large dipole moment and Ead. The decreasing order of QNBO for D1–D4 is D1 > D2 > D3 > D4. This trend is consistent with the dipole moment trend of D1–D4 geometries. However, the QNBO analysis in CO2-adsorbed Zn-doped B12P12 systems (E1–E4) exhibited negative values. This might be due to shifting of charge from Zn–BP, which makes CO2 slightly negative in nature (Table 1). Therefore, from QNBO discussion, it is illustrated that Zn decoration on B12P12 nanocages shows a consistent trend with dipole moment, and CO2 adsorption on these metal-decorated B12P12 nanocages makes carbon dioxide slightly negative in nature.
2.4. MEP Analysis
MEP analysis is another useful parameter to explore the extent of charge separation within a molecule. MEP analysis also correlates the geometry of a system with physiochemical properties such as dipole moment, chemical reactivity, and partial charges. MEP analysis is estimated at the B3LYP/6-31G(d,p) level of DFT. Figure 4 discloses the charge separation. Generally, blue area represents the electropositive end (boron in the present case), yellow area specifies the electronegative end (phosphorus in the present case), while green area represents the mean potential (the area between the two extremes) in web version. Pure B12P12 being symmetrical shows equal charges. Fixing of CO2 on pure B12P12 does not bring significant charge separation as shown in Figure 4. However, placement of Zn on pure nanocage causes positive charge shifting on top of the Zn metal (blue color) because of the electropositive nature of late transition metal and the yellow area in the nanocage becomes less intense. However, in the case of E1–E4, major charge separation occurs as the blue area is shifted to Zn metal and the carbon center of CO2 while both oxygen atoms of CO2 exhibit extreme negative charge. All this charge shifting is attributed to an increase in dipole moment (D). For instance, pure B12P12 has zero dipole moment, whereas Zn-decorated B12P12 has some value of dipole moment.
Figure 4.
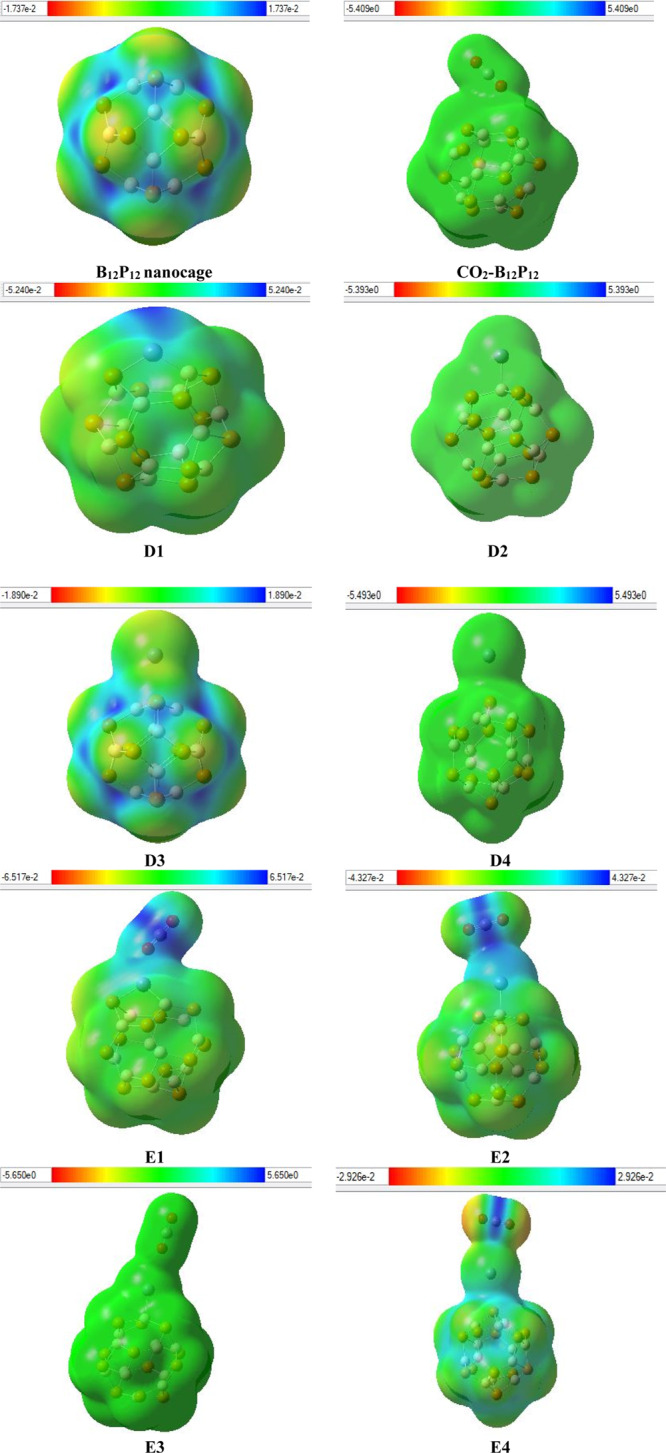
MEP of all systems (for understanding the colors in these figures, the reader must read the web version of this article). The isosurface value is 0.02e/Å3.
Next, the correlation of the distribution of charges (in MEP) with the dipole moment is explored. In D1–D4 systems, an irregular trend of dipole moment is observed. A maximum dipole moment is noted in the D2 geometry; however, this value does not bring much change in the electrostatic potential plot, probably because of the large distance of metal and B-top adsorption of metal on BP. The second largest dipole moment value is obtained in the D1 geometry. In D1, some charge density is shifted toward the metal end upon Zn adsorption on BP, probably because of the incorporation of metal with the bond present between the tetragonal and hexagonal ring. The electrostatic potential plots of geometries D3 and D4 also exhibit a similar potential. Again, this is probably due to large distances and low values of dipole moment along with low adsorption energies values, which do not bring any change in charge distribution. Overall, the electrostatic potential (ESP) of D1–D4 is different from each other because of the difference in dipole moment values and orientation along with the distance of metal from the cage.
2.5. Electronic Properties
The frontier molecular orbitals (FMOs) (energies of HOMOs and LUMOs), Fermi level, and the HOMO–LUMO gap of systems are observed at the B3LYP/6-31G(d,p) level of density functional theory. The results of these key parameters are tabulated in Table 2. Pure B12P12 is viewed as semiconductor, which holds a HOMO–LUMO gap of 3.70 eV. The HOMO and LUMO orbitals of B12P12 are located at −6.83 and −3.13 eV, respectively. Generally, the Fermi level (EFL) of a molecule is observed at the midpoint of the HOMO–LUMO gap.59 The Fermi level is found at −4.98 eV for the B12P12 nanocage. When Zn is installed on the B12P12 nanocage (D1–D4), a remarkable change in the energies of HOMO and LUMO is observed. The energies of HOMO for D1–D4 are −5.92, −6.25, −5.63, and −5.57 eV, whereas the energies of LUMO are −2.97, −3.16, −3.19, and −3.17 eV, respectively. In all Zn-doped BP systems (D1–D4), the energies of HOMO are increased, whereas the LUMO energies are decreased, which ultimately causes narrowing of the HOMO–LUMO gap. The HOMO–LUMO gap (Eg) is related to the conductivity of a material,60,61 and this direct relation is commonly measured with the aid of the following equation
| 1 |
Table 2. Energies of HOMO, LUMO, and Fermi Level (EFL) along with the HOMO–LUMO Energy Gap (Eg) in eV of All Systems.
| system | EHOMO (eV) | EFL (eV) | ELUMO (eV) | Eg (eV) |
|---|---|---|---|---|
| B12P12 | –6.83 | –4.98 | –3.13 | 3.70 |
| B12P12–CO2 | –6.81 | –4.96 | –3.11 | 3.69 |
| Zn–BP (D1) | –5.92 | –4.45 | –2.97 | 2.95 |
| Zn–BP–CO2 (E1) | –5.64 | –4.21 | –2.77 | 2.88 |
| Zn–BP (D2) | –6.25 | –4.71 | –3.16 | 3.09 |
| Zn–BP–CO2 (E2) | –6.27 | –4.73 | –3.19 | 3.08 |
| Zn–BP (D3) | –5.63 | –4.41 | –3.19 | 2.43 |
| Zn–BP–CO2 (E3) | –6.16 | –4.59 | –3.02 | 3.41 |
| Zn–BP (D4) | –5.57 | –4.37 | –3.17 | 2.40 |
| Zn–BP–CO2 (E4) | –5.61 | –4.40 | –3.19 | 2.42 |
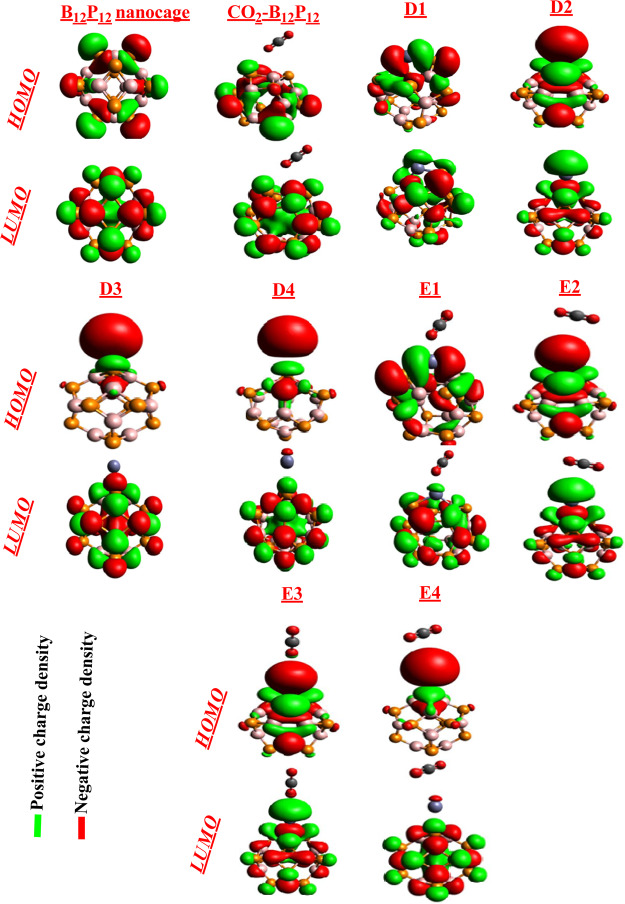
The widths of H–L gaps for D1–D4 are 2.95, 3.09, 2.43, and 2.40 eV, respectively. These values disclosed the great conductivity of Zn-decorated B12P12 nanocages. When CO2 is adsorbed on pure B12P12, no such sifting of HOMO and LUMO orbital is observed. The difference in the energy gap of pure and CO2–B12P12 is very minute as shown in Table 2. However, adsorption of CO2 on Zn-decorated B12P12 nanocages (E1–E4) appreciably destabilizes the HOMO orbitals and stabilizes the LUMO orbitals. The HOMO energies of E1–E4 geometries are −4.21, −4.73, −4.59, and −4.40 eV, whereas the LUMO energies are −2.77, −3.19, −3.02, and −3.19 eV, respectively. All these values indicated destabilization of HOMO orbital and stabilization of LUMO orbital (after CO2 adsorption on D1–D4), which results in narrowing of the HOMO–LUMO energy gap. The HOMO–LUMO energy gap upon Zn decoration on B12P12 decreases from 3.70 to 2.95 (D1), 3.09 (D2), 2.43 (D3), and 2.40 (D4). Therefore, Zn decoration significantly causes narrowing of the HOMO–LUMO energy gap with high conductivity as compared to pure and CO2–B12P12 nanocages. The HOMO–LUMO energy gaps of E1–E4 are 2.88, 3.08, 3.41, and 2.42 eV, respectively. The values of HOMO–LUMO energy gap show that CO2 adsorption on D1–D4 significantly reduces the HOMO–LUMO energy gap as compared to pure and CO2–B12P12, which suggest that Zn metal decoration favors CO2 adsorption. However, the Fermi levels for E1–E4 are noted at −4.21, −4.73, −4.59, and −4.40 eV, respectively. The decreasing order of HOMO–LUMO energy gap for all systems including the pure B12P12 nanocage is B12P12 > CO2–B12P12 > E3 > D2 > E2 > D1 > E1 > D3 > E4 > D4. From this trend and the above-mentioned discussion, it is noted that Zn decoration on B12P12 significantly enhances the conductivity of the B12P12 nanocage, and Zn decoration also makes the BP nanocage an efficient material for CO2 adsorption (Figure 5).
Figure 5.
Side views of HOMO and LUMO of different systems. The isosurface value is 0.02e/Å3.
The distribution of HOMO and LUMO densities on all systems at B3LYP in conjunction with the 6-31G(d,p) level of DFT is shown in Figure 6.
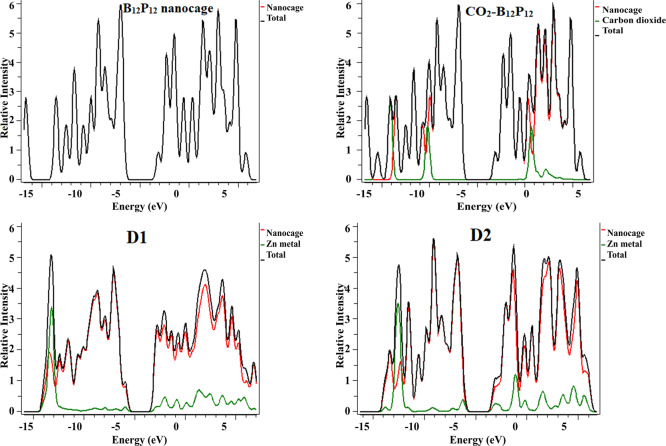
Figure 6.
DOS for all systems at the B3LYP/6-31G(d,p) level of DFT.
Generally, DOS is performed in support of FMOs. The DOS graphs are shown in Figure 6, which reveal that the HOMO and LUMO densities are equally shared within the whole B12P12 nanocage. However, placement of Zn on B12P12 shifted the HOMO and LUMO toward the Zn center in the D1 and D2 geometries. However, in the case of D3 and D4 geometries, the position of Zn metal on BP causes shifting of a major part of HOMO on the metal center and LUMO on the cage end. This happens because of the presence of many electronegative atoms that make the metal atom electron-rich. A metal being electropositive cannot retain these electrons, and therefore, they are spread out as excess electrons causing high energy level of the newly formed HOMO. Moreover, in the E1–E4 geometries, a similar distribution pattern of HOMO and LUMO densities is observed as shown in Figure 6.
2.6. Global Descriptor of Reactivity
The global descriptor of reactivity of investigated systems is inspected at the B3LYP/6-31G(d,p) level of DFT. Various properties such as global hardness (η), global softness (S), global ionization potential (IP), global electron affinity (EA), electrophilic index (ω), global chemical potential (μ), and electronegativity (χ) are carried out in order to illustrate the effect of Zn doping (Zn–BP) on the capturing of CO2. The conventional energy of LUMO expresses the electron accepting property, whereas the energy of HOMO represents the IP (according to the Koopmann theorem62). The results in Table 3 reveal that all Zn-doped and CO2-adsorbed Zn-decorated systems exhibit good affinity values with fine IP. The values of EA and IP suggested that our strategy of Zn decoration on pure B12P12 is useful for CO2 adsorption. Similarly, the electrophilic index expresses the great chemical reactivity of a compound. The pure BP nanocage disclosed an electrophilic index of 6.703 eV. Fixing of Zn on B12P12 (D1–D4) boosted the electrophilic index value to 7.951 from 6.703 eV for bare nanocage. Likewise, adsorption of CO2 on D1–D4 geometries improved the electrophilic index values in E1–E4 geometries, which recommended that Zn decoration is an effective way for CO2 adsorption. Global hardness and global softness parameters also point out that Zn-decorated and CO2-adsorbed Zn–BP nanocages are hard in nature with low values of global softness. The great chemical stability of a system is directly related to the chemical potential of that system. A compound with large value of chemical potential is supposed to be least reactive and most stable in nature. The Pure B12P12 nanocage exhibited a chemical potential value of −4.980 eV, whereas the positioning of Zn metal on the B12P12 nanocage increased the chemical potential in D1 (μ(=–4.445 eV)), D2 (μ(=–4.705 eV)), D3 (μ(=–4.410 eV)), and D4 (μ(=–4.370 eV)). Nonetheless, the adsorption of CO2 on Zn-doped B12P12 nanocages (E1–E4) significantly raised the chemical potential. The decreasing order of chemical potential among E1–E4 is [E1 (μ(=–4.205 eV))] > [E4 (μ(=–4.400 eV))] > [E3 (μ(=–4.590 eV))] > [E2 (μ(=–4.70 eV))]. Therefore, designed systems show good values of chemical potential and electronegativity. From the preceding discussion, it is concluded that all designed systems (Zn-decorated and CO2-adsorbed B12P12 nanocages) exhibited ordinary reactivity with remarkable stability and thus proved to be the best candidates for CO2 sensing materials.
Table 3. IP, EA, X (Electronegativity), μ (Chemical Potential), η (Global Hardness), S (Global Softness), and ω (Global Electrophilicity) of All Systems.
| system | IP (eV) | EA (eV) | X (eV) | μ (eV) | Η (eV) | S (eV–1) | Ω (eV) |
|---|---|---|---|---|---|---|---|
| BP | 6.830 | 3.130 | 4.980 | –4.980 | 1.850 | 0.270 | 6.703 |
| BP–CO2 | 6.810 | 3.110 | 4.960 | –4.960 | 1.850 | 0.270 | 6.649 |
| Zn–BP (D1) | 5.920 | 2.970 | 4.445 | –4.445 | 1.475 | 0.339 | 6.698 |
| Zn–BP–CO2 (E1) | 5.640 | 2.770 | 4.205 | –4.205 | 1.435 | 0.348 | 6.161 |
| Zn–BP (D2) | 6.250 | 3.160 | 4.705 | –4.705 | 1.545 | 0.324 | 7.164 |
| Zn–BP–CO2 (E2) | 6.270 | 3.190 | 4.730 | –4.730 | 1.540 | 0.325 | 7.264 |
| Zn–BP (D3) | 5.630 | 3.190 | 4.410 | –4.410 | 1.220 | 0.410 | 7.971 |
| Zn–BP–CO2 (E3) | 6.160 | 3.020 | 4.590 | –4.590 | 1.570 | 0.318 | 6.710 |
| Zn–BP (D4) | 5.570 | 3.170 | 4.370 | –4.370 | 1.200 | 0.417 | 7.957 |
| Zn–BP–CO2 (E4) | 5.610 | 3.190 | 4.400 | –4.400 | 1.210 | 0.413 | 8.000 |
3. Conclusions
In summary, the changes in the electronic behavior of B12P12 nanocage on Zn decoration and CO2 adsorption are studied. Zn decoration on B12P12 followed by CO2 adsorption on bare and Zn-decorated B12P12 causes narrowing of the HOMO–LUMO energy gap. The binding energy of CO2-adsorbed Zn–B12P12 nanocages [E1 (Ead = −75.12 kJ/mol), E2 (Ead = −25.89 kJ/mol), E3 (Ead = −42.43 kJ/mol), and E4 (Ead = −28.73 kJ/mol)] is remarkably higher than those of Zn–B12P12 [D1 (Ead = −57.12 kJ/mol), D2 (Ead = −22.94 kJ/mol), D3 (Ead = −21.03 kJ/mol), and D4 (Ead = −14.07 kJ/mol)] systems, which suggested the strong adsorption of CO2 on Zn-decorated BP systems. The dipole moment and QNBO analysis showed that maximum charge separation is observed for CO2–Zn–B12P12 geometries. MEP analysis confirmed the different charge zones on all systems. DOS analysis is also performed in support of FMO analysis, which explored the different distribution patterns of HOMO and LUMO orbitals on CO2-adsorbed and Zn-decorated B12P12 geometries. Finally, the global descriptors of reactivity are investigated, which demonstrated the great stability and least reactivity of our designed (Zn–B12P12 and CO2–Zn–B12P12) systems. The results of all analyses recommended our Zn-decorated B12P12 nanocages as potential candidates for application in CO2 sensors.
4. Computational Methods
The B3LYP method of DFT along with the 6-31G(d,p) basis set is utilized for performing all calculations through Gaussian 09.63 The same level of DFT is used in order to gain optimized geometries of all systems of interest. B3LYP64,65/6-31G(d,p)66,67 is a reliable level of DFT for computing the geometric and electronic properties of nanocages23,53,54, and other systems.68,69 Different parameters such as adsorption energy, QNBO, dipole moment, analysis of MEP, FMO distribution, and DOS are estimated at the B3LYP/6-31G(d,p) level of DFT. The HOMO–LUMO gap is directly related to the conductivity of a material and also plays a key role in evaluating the global descriptor of reactivity. Global hardness, global softness, electronegativity, chemical potential, IP, EA, and electrophilic index of all systems are calculated by using HOMO–LUMO gap values. Different equations are used to calculate the interaction energies of CO2 gas and Zn metal with B12P12 nanocages.
Equation 2 is useful for calculating the adsorption energy of the Zn–B12P12 system
| 2 |
In this equation, EZn–BP stands for the zinc metal-decorated B12P12 nanocage. Similarly, EBP expresses the energy of bare B12P12 nanocage and EZn points out the energy of Zn (metal). The adsorption energies in kJ/mol for CO2-adsorbed pure and Zn-decorated B12P12 are calculated with the aid of eqs 3 and 4.
| 3 |
| 4 |
The Eint(BP) in eq 2 and the Eint(Zn–BP) in eq 4 define the interaction energies of CO2 with pristine B12P12 and Zn-decorated B12P12, respectively. The ECO2–BP and ECO2–Zn–BP fragments highlight the total interaction energies of the CO2-adsorbed BP nanocage and CO2-adsorbed Zn–BP nanocages, respectively. Similarly, ECO2 indicates the single CO2 molecule energy in kJ/mol.
Valuable literature suggests that different equations were utilized for calculating different global indices of reactivity [electrophilicity index (ω), global softness (S), electronegativity (X), global hardness (η), and chemical potential (μ)], which are given below70,71
| 5 |
| 6 |
| 7 |
| 8 |
| 9 |
The visualization software utilized to study the different properties of investigated systems are GaussView 5.0 (used to manage the input files), Avogadro (for interpreting the HOMO and LUMO distribution), and Pymolyz (for DOS graphs).
Acknowledgments
The authors acknowledge the financial and technical support from Shakarganj Limited, Jhang, Punjab, Pakistan.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01686.
Cartesian coordinates of the optimized geometries (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Murray C. B.; Kagan C. R.; Bawendi M. G. Synthesis and Characterization of Monodisperse Nanocrystals and Close-Packed Nanocrystal Assemblies. Annu. Rev. Mater. Sci. 2000, 30, 545–610. 10.1146/annurev.matsci.30.1.545. [DOI] [Google Scholar]
- Wu H.-S.; Cui X.-Y.; Qin X.-F.; Strout D. L.; Jiao H. Boron Nitride Cages from B12N12 to B36N36: Square–hexagon Alternants vs Boron Nitride Tubes. J. Mol. Model. 2006, 12, 537–542. 10.1007/s00894-005-0042-6. [DOI] [PubMed] [Google Scholar]
- Li J. L.; Hu Z. S.; Yang G. W. High-Capacity Hydrogen Storage of Magnesium-Decorated Boron Fullerene. Chem. Phys. 2012, 392, 16–20. 10.1016/j.chemphys.2011.08.017. [DOI] [Google Scholar]
- Yin B.; Wang G.; Sa N.; Huang Y. Bonding Analysis and Stability on Alternant B16N16 Cage and Its Dimers. J. Mol. Model. 2008, 14, 789–795. 10.1007/s00894-008-0303-2. [DOI] [PubMed] [Google Scholar]
- Baei M. T.; Peyghan A. A.; Bagheri Z. A DFT Study on CO2 Interaction with a BN Nano-Cage. Bull. Korean Chem. Soc. 2012, 33, 3338–3342. 10.5012/bkcs.2012.33.10.3338. [DOI] [Google Scholar]
- Lee S.-S.; Yee K.-A.; Yi H.-S.; Kang S.-K.; Seong S.-Y. The Electronic Structure and Stability of the Heterofullerene:C (60-2x) (BN) X. Bull. Korean Chem. Soc. 2003, 24, 494–498. 10.5012/bkcs.2003.24.4.494. [DOI] [Google Scholar]
- Hadipour N. L.; Ahmadi Peyghan A.; Soleymanabadi H. Theoretical Study on the Al-Doped ZnO Nanoclusters for CO Chemical Sensors. J. Phys. Chem. C 2015, 119, 6398–6404. 10.1021/jp513019z. [DOI] [Google Scholar]
- Bachtold A. Logic Circuits with Carbon Nanotube Transistors. Science 2001, 294, 1317–1320. 10.1126/science.1065824. [DOI] [PubMed] [Google Scholar]
- Rad A. S.; Ayub K. Adsorption of Pyrrole on Al12N12, Al12P12, B12N12, and B12P12 Fullerene-like Nano-Cages; a First Principles Study. Vacuum 2016, 131, 135–141. 10.1016/j.vacuum.2016.06.012. [DOI] [Google Scholar]
- Soltani A.; Baei M. T.; Taghartapeh M. R.; Lemeski E. T.; Shojaee S. Phenol Interaction with Different Nano-Cages with and without an Electric Field: A DFT Study. Struct. Chem. 2015, 26, 685–693. 10.1007/s11224-014-0504-5. [DOI] [Google Scholar]
- Ullah F.; Kosar N.; Ali A.; Maria; Mahmood T.; Ayub K. Alkaline Earth Metal Decorated Phosphide Nanoclusters for Potential Applications as High Performance NLO Materials; A First Principle Study. Phys. E 2020, 118, 113906. 10.1016/j.physe.2019.113906. [DOI] [Google Scholar]
- Ullah F.; Kosar N.; Ali A.; Maria; Mahmood T.; Ayub K. Design of Novel Inorganic Alkaline Earth Metal Doped Aluminum Nitride Complexes (AEM@Al12N12) with High Chemical Stability, Improved Electronic Properties and Large Nonlinear Optical Response. Optik 2020, 207, 163792. 10.1016/j.ijleo.2019.163792. [DOI] [Google Scholar]
- Munsif S.; Maria; Khan S.; Ali A.; Gilani M. A.; Iqbal J.; Ludwig R.; Ayub K. Remarkable Nonlinear Optical Response of Alkali Metal Doped Aluminum Phosphide and Boron Phosphide Nanoclusters. J. Mol. Liq. 2018, 271, 51–64. 10.1016/j.molliq.2018.08.121. [DOI] [Google Scholar]
- Kandalam A. K.; Blanco M. A.; Pandey R. Theoretical Study of AlnNn , GanNn , and Inn Nn (n = 4, 5, 6) Clusters. J. Phys. Chem. B 2002, 106, 1945–1953. 10.1021/jp0140062. [DOI] [Google Scholar]
- Costales A.; Kandalam A. K.; Franco R.; Pandey R. Theoretical Study of Structural and Vibrational Properties of (AlP)n, (AlAs)n, (GaP)n, (GaAs)n, (InP)n, and (InAs)n Clusters with n = 1, 2, 3. J. Phys. Chem. B 2002, 106, 1940–1944. 10.1021/jp013906f. [DOI] [Google Scholar]
- Yong Y.; Liu K.; Song B.; He P.; Wang P.; Li H. Coalescence of BnNn Fullerenes: A New Pathway to Produce Boron Nitride Nanotubes with Small Diameter. Phys. Lett. A 2012, 376, 1465–1467. 10.1016/j.physleta.2012.03.011. [DOI] [Google Scholar]
- Guldi D. M.; Illescas B. M.; Atienza C. M.; Wielopolski M.; Martín N. Fullerene for Organic Electronics. Chem. Soc. Rev. 2009, 38, 1587. 10.1039/b900402p. [DOI] [PubMed] [Google Scholar]
- Beheshtian J.; Kamfiroozi M.; Bagheri Z.; Ahmadi A. Computational Study of CO and NO Adsorption on Magnesium Oxide Nanotubes. Phys. E 2011, 44, 546–549. 10.1016/j.physe.2011.09.016. [DOI] [Google Scholar]
- Ayub K. Binding Affinity and Permeation of X12Y12 Nanoclusters for Helium and Neon. J. Mol. Liq. 2017, 244, 124–134. 10.1016/j.molliq.2017.08.118. [DOI] [Google Scholar]
- Rad A. S.; Ayub K. DFT Study of Boron Trichloride Adsorption on the Surface of Al12N12 Nanocluster. Mol. Phys. 2017, 115, 879–884. 10.1080/00268976.2017.1290843. [DOI] [Google Scholar]
- Rad A. S.; Ayub K. Detailed Surface Study of Adsorbed Nickel on Al12N12 Nano-Cage. Thin Solid Films 2016, 612, 179–185. 10.1016/j.tsf.2016.05.055. [DOI] [Google Scholar]
- Baei M. T.; Mohammadian H.; Hashemian S. B12N12 Nanocage as a Potential Adsorbent for the Removal of Aniline from Environmental Systems. Bulg. Chem. Commun. 2014, 46, 735–742. [Google Scholar]
- Baei M. T. Remove of Toxic Pyridine from Environmental Systems by Using B12N12 Nano-Cage. Superlattices Microstruct. 2013, 58, 31–37. 10.1016/j.spmi.2013.02.009. [DOI] [Google Scholar]
- Esrafili M. D.; Nurazar R. Methylamine Adsorption and Decomposition on B12N12 Nanocage: A Density Functional Theory Study. Surf. Sci. 2014, 626, 44–48. 10.1016/j.susc.2014.03.028. [DOI] [Google Scholar]
- Baei M. T. B12N12 Sodalite like Cage as Potential Sensor for Hydrogen Cyanide. Comput. Theor. Chem. 2013, 1024, 28–33. 10.1016/j.comptc.2013.09.018. [DOI] [Google Scholar]
- Wang H. Density Functional Investigation of Fluorinated B12N12 Clusters. Chin. J. Chem. 2010, 28, 1897–1901. 10.1002/cjoc.201090316. [DOI] [Google Scholar]
- Baei M. T.; Taghartapeh M. R.; Lemeski E. T.; Soltani A. A Computational Study of Adenine, Uracil, and Cytosine Adsorption upon AlN and BN Nano-Cages. Phys. Rev. B: Condens. Matter Mater. Phys. 2014, 444, 6–13. 10.1016/j.physb.2014.03.013. [DOI] [Google Scholar]
- Rad A. S.; Ayub K. A Comparative Density Functional Theory Study of Guanine Chemisorption on Al12N12, Al12P12, B12N12, and B12P12 Nano-Cages. J. Alloys Compd. 2016, 672, 161–169. 10.1016/j.jallcom.2016.02.139. [DOI] [Google Scholar]
- Rad A. S. High Ozone Chemisorption by Using Metal–cluster Complexes: A DFT Study on the Nickel-Decorated B12P12 Nanoclusters. Can. J. Chem. 2017, 95, 845–850. 10.1139/cjp-2017-0119. [DOI] [Google Scholar]
- Rad A. S.; Ayub K. How Can Nickel Decoration Affect H2 Adsorption on B12P12 Nano-Heterostructures?. J. Mol. Liq. 2018, 255, 168–175. 10.1016/j.molliq.2018.01.149. [DOI] [Google Scholar]
- Rad A. S.; Abedini E. Chemisorption of NO on Pt-Decorated Graphene as Modified Nanostructure Media: A First Principles Study. Appl. Surf. Sci. 2016, 360, 1041–1046. 10.1016/j.apsusc.2015.11.126. [DOI] [Google Scholar]
- Rad A. S. Adsorption of C2H2 and C2H4 on Pt-Decorated Graphene Nanostructure: Ab-Initio Study. Synth. Met. 2016, 211, 115–120. 10.1016/j.synthmet.2015.11.031. [DOI] [Google Scholar]
- Rad A. S.; Jouibary Y. M.; Foukolaei V. P.; Binaeian E. Study on the Structure and Electronic Property of Adsorbed Guanine on Aluminum Doped Graphene: First Principles Calculations. Curr. Appl. Phys. 2016, 16, 527–533. 10.1016/j.cap.2016.02.004. [DOI] [Google Scholar]
- Rad A. S.; Foukolaei V. P. Density Functional Study of Al-Doped Graphene Nanostructure towards Adsorption of CO, CO2 and H2O. Synth. Met. 2015, 210, 171–178. 10.1016/j.synthmet.2015.09.026. [DOI] [Google Scholar]
- Rad A. S. Al-Doped Graphene as a New Nanostructure Adsorbent for Some Halomethane Compounds: DFT Calculations. Surf. Sci. 2016, 645, 6–12. 10.1016/j.susc.2015.10.036. [DOI] [Google Scholar]
- Arshad Y.; Khan S.; Hashmi M. A.; Ayub K. Transition Metal Doping: A New and Effective Approach for Remarkably High Nonlinear Optical Response in Aluminum Nitride Nanocages. New J. Chem. 2018, 42, 6976–6989. 10.1039/c7nj04971d. [DOI] [Google Scholar]
- Gilani M. A.; Tabassum S.; Gul U.; Mahmood T.; Alharthi A. I.; Alotaibi M. A.; Geesi M.; Sheikh R.; Ayub K. Copper-Doped Al12N12 Nano-Cages: Potential Candidates for Nonlinear Optical Materials. Appl. Phys. A 2018, 124, 14. 10.1007/s00339-017-1425-0. [DOI] [Google Scholar]
- Zhang Y.; Zheng X.; Zhang S.; Huang S.; Wang P.; Tian H. Bare and Ni Decorated Al12N12 Cage for Hydrogen Storage: A First-Principles Study. Int. J. Hydrogen Energy 2012, 37, 12411–12419. 10.1016/j.ijhydene.2012.06.056. [DOI] [Google Scholar]
- Rad A. S.; Ayub K. Enhancement in Hydrogen Molecule Adsorption on B12N12 Nano-Cluster by Decoration of Nickel. Int. J. Hydrogen Energy 2016, 41, 22182–22191. 10.1016/j.ijhydene.2016.08.158. [DOI] [Google Scholar]
- Rad A. S.; Mirabi A.; Peyravi M.; Mirzaei M. Nickel-Decorated B12P12 Nanoclusters as a Strong Adsorbent for SO2 Adsorption: Quantum Chemical Calculations. Can. J. Phys. 2017, 95, 958–962. 10.1139/cjp-2017-0119. [DOI] [Google Scholar]
- Rad A. S.; Ayub K. Adsorption Properties of Acetylene and Ethylene Molecules onto Pristine and Nickel-Decorated Al12N12 Nanoclusters. Mater. Chem. Phys. 2017, 194, 337–344. 10.1016/j.matchemphys.2017.04.002. [DOI] [Google Scholar]
- Shakerzadeh E.; Khodayar E.; Noorizadeh S. Theoretical Assessment of Phosgene Adsorption Behavior onto Pristine, Al- and Ga-Doped B12N12 and B16N16 Nanoclusters. Comput. Mater. Sci. 2016, 118, 155–171. 10.1016/j.commatsci.2016.03.016. [DOI] [Google Scholar]
- Ayub K. Are Phosphide Nano-Cages Better than Nitride Nano-Cages? A Kinetic, Thermodynamic and Non-Linear Optical Properties Study of Alkali Metal Encapsulated X12Y12 Nano-Cages. J. Mater. Chem. C 2016, 4, 10919–10934. 10.1039/c6tc04456e. [DOI] [Google Scholar]
- Maria; Iqbal J.; Ayub K. Enhanced Electronic and Non-Linear Optical Properties of Alkali Metal (Li, Na, K) Doped Boron Nitride Nano-Cages. J. Alloys Compd. 2016, 687, 976–983. 10.1016/j.jallcom.2016.06.121. [DOI] [Google Scholar]
- Radosavljević M.; Appenzeller J.; Derycke V.; Martel R.; Avouris P.; Loiseau A.; Cochon J.-L.; Pigache D. Electrical Properties and Transport in Boron Nitride Nanotubes. Appl. Phys. Lett. 2003, 82, 4131–4133. 10.1063/1.1581370. [DOI] [Google Scholar]
- Ayub K. Transportation of Hydrogen Atom and Molecule through X12Y12 Nano-Cages. Int. J. Hydrogen Energy 2017, 42, 11439–11451. 10.1016/j.ijhydene.2017.02.202. [DOI] [Google Scholar]
- Tokoro H.; Fujii S.; Oku T. Iron Nanoparticles Coated with Boron Nitride Nanolayers Synthesized by a Solid Phase Reaction. IEEE Trans. Magn. 2003, 39, 2761–2763. 10.1109/tmag.2003.815591. [DOI] [Google Scholar]
- Zhuiykov S.; Wlodarski W.; Li Y. Nanocrystalline V2O5–TiO2 Thin-Films for Oxygen Sensing Prepared by Sol–gel Process. Sens. Actuators, B 2001, 77, 484–490. 10.1016/s0925-4005(01)00739-0. [DOI] [Google Scholar]
- Chang H.; Lee J. D.; Lee S. M.; Lee Y. H. Adsorption of NH3 and NO2 Molecules on Carbon Nanotubes. Appl. Phys. Lett. 2001, 79, 3863–3865. 10.1063/1.1424069. [DOI] [Google Scholar]
- Lu J.; Nagase S.; Maeda Y.; Wakahara T.; Nakahodo T.; Akasaka T.; Yu D.; Gao Z.; Han R.; Ye H. Adsorption Configuration of NH3 on Single-Wall Carbon Nanotubes. Chem. Phys. Lett. 2005, 405, 90–92. 10.1016/j.cplett.2005.01.122. [DOI] [Google Scholar]
- Sedjo R.; Sohngen B. Carbon Sequestration in Forests and Soils. Annu. Rev. Resour. Econ. 2012, 4, 127–144. 10.1146/annurev-resource-083110-115941. [DOI] [Google Scholar]
- Holloway S.; Pearce J.; Hards V.; Ohsumi T.; Gale J. Natural Emissions of CO2 from the Geosphere and Their Bearing on the Geological Storage of Carbon Dioxide. Energy 2007, 32, 1194–1201. 10.1016/j.energy.2006.09.001. [DOI] [Google Scholar]
- Hussain S.; Hussain R.; Mehboob M. Y.; Chatha S. A. S.; Hussain A. I.; Umar A.; Khan M. U.; Ahmed M.; Adnan M.; Ayub K. Adsorption of Phosgene Gas on Pristine and Copper-Decorated B12N12 Nanocages: A Comparative DFT Study. ACS Omega 2020, 5, 7641–7650. 10.1021/acsomega.0c00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S.; Chatha S. A. S.; Hussain A. I.; Hussain R.; Mehboob M. Y.; Muhammad S.; Ahmad Z.; Ayub K. Zinc-Doped Boron Phosphide Nanocluster as Efficient Sensor for SO2. Acad. J. Chem. 2020, 2020, 1–12. 10.1155/2020/2629596. [DOI] [Google Scholar]
- Kauffman D. R.; Alfonso D.; Matranga C.; Qian H.; Jin R. Experimental and Computational Investigation of Au25 Clusters and CO2: A Unique Interaction and Enhanced Electrocatalytic Activity. J. Am. Chem. Soc. 2012, 134, 10237–10243. 10.1021/ja303259q. [DOI] [PubMed] [Google Scholar]
- Liang X.; Zhang Q.; Zhao Q.; Zhao H.; Feng Y.; Suo B.; Han H.; Song Q.; Li Y.; Zou W.; et al. CO2 Adsorption on the B12N12 Nanocage Encapsulated with Alkali Metals: A Density Functional Study. Nano 2019, 14, 1950034. 10.1142/s1793292019500346. [DOI] [Google Scholar]
- Jiang Y.; Xie X.; Hamid I.; Chen C.; Duan H. Theoretical Simulation of CO2 Capture by Al11Mg3- Cluster. Mater. Res. Express 2017, 4, 046302. 10.1088/2053-1591/aa5fbf. [DOI] [Google Scholar]
- Guo H.; Zhang W.; Lu N.; Zhuo Z.; Zeng X. C.; Wu X.; Yang J. CO2 Capture on h -BN Sheet with High Selectivity Controlled by External Electric Field. J. Phys. Chem. C 2015, 119, 6912–6917. 10.1021/acs.jpcc.5b00681. [DOI] [Google Scholar]
- Li S. S.Scattering Mechanisms and Carrier Mobilities in Semiconductors. In Semiconductor Physical Electronics; Springer: New York, NY, 2006; pp 211–245 [Google Scholar]
- Hussain R.; Khan M. U.; Mehboob M. Y.; Khalid M.; Iqbal J.; Ayub K.; Adnan M.; Ahmed M.; Atiq K.; Mahmood K. Enhancement in Photovoltaic Properties of N , N -diethylaniline Based Donor Materials by Bridging Core Modifications for Efficient Solar Cells. ChemistrySelect 2020, 5, 5022–5034. 10.1002/slct.202000096. [DOI] [Google Scholar]
- Afzal Z.; Hussain R.; Khan M. U.; Khalid M.; Iqbal J.; Alvi M. U.; Adnan M.; Ahmed M.; Mehboob M. Y.; Hussain M.; et al. Designing Indenothiophene-Based Acceptor Materials with Efficient Photovoltaic Parameters for Fullerene-Free Organic Solar Cells. J. Mol. Model. 2020, 26, 137. 10.1007/s00894-020-04386-5. [DOI] [PubMed] [Google Scholar]
- Koopmans T. Ordering of Wave Functions and Eigenenergies to the Individual Electrons of an Atom. Phys 1933, 1, 104–113. [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.; Nakatsuji H.; Caricato M.; Li X.; Hratchian H. P.; Izmaylov A. F.; Bloino J.; Zheng G.; Sonnenberg J. L.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Montgomery J. A. Jr.; Peralta J. E.; Ogliaro F.; Bearpark M.; Heyd J. J.; Brothers E.; Kudin K. N.; Staroverov V. N.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Rega N.; Millam J. M.; Klene M.; Knox J. E.; Cross J. B.; Bakken V.; Adamo C.; Jaramillo J.; Gomperts R.; Stratmann R. E.; Yazyev O.; Austin A. J.; Cammi R.; Pomelli C.; Ochterski J. W.; Martin R. L.; Morokuma K.; Zakrzewski V. G.; Voth G. A.; Salvador P.; Dannenberg J. J.; Dapprich S.; Daniels A. D.; Farkas Ö.; Foresman J. B.; Ortiz J. V.; Cioslowski J.; Fox D. J.. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford CT, 2013.
- Becke A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 492, 5648–5652. 10.1063/1.464913. [DOI] [Google Scholar]
- Lee C.; Yang W.; Parr R. G. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behaviour. Phys. Rev. B: Condens. Matter Mater. Phys. 1988, 37, 785–789. 10.1103/physrevb.37.785. [DOI] [Google Scholar]
- Parr R.; Hehre W. J.; Pople J. A. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 1971, 54, 724–728. 10.1063/1.1674902. [DOI] [Google Scholar]
- Hehre W. J.; Ditchfield R.; Pople J. A. Self—Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian—Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. 10.1063/1.1677527. [DOI] [Google Scholar]
- Ashraf A.; Carter-Fenk K.; Herbert J. M.; Farooqi B. A.; Farooq U.; Ayub K. Interaction of Graphene Quantum Dots with Oligothiophene: A Comprehensive Theoretical Study. J. Phys. Chem. C 2019, 123, 29556–29570. 10.1021/acs.jpcc.9b08090. [DOI] [Google Scholar]
- Fazl-i-Sattar; Ahmed A.; Ullah H.; Ullah Z.; Tariq M.; Ayub K. External Stimulus Controlled Recombination of Hydrogen in Photochromic Dithienylethene Frustrated Lewis Pairs. Int. J. Hydrogen Energy 2019, 44, 31141–31152. 10.1016/j.ijhydene.2019.10.051. [DOI] [Google Scholar]
- Parr R. G.; Szentpály L. v.; Liu S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. 10.1021/ja983494x. [DOI] [Google Scholar]
- Pearson R. G. The Transition Metal-Carbon Monoxide Bond. Inorg. Chem. 1984, 23, 4675–4679. 10.1021/ic00194a051. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.