Abstract
Vancomycin is a recommended therapy in multiple national guidelines. Despite the common use, there is a poor understanding of the mechanistic drivers and potential modifiers of vancomycin-mediated kidney injury. In this review, historic and contemporary rates of vancomycin-induced kidney injury (VIKI) are described, and toxicodynamic models and mechanisms of toxicity from preclinical studies are reviewed. Aside from known clinical covariates that worsen VIKI, preclinical models have demonstrated that various factors impact VIKI, including dose, route of administration, and thresholds for pharmacokinetic parameters. The degree of acute kidney injury (AKI) is greatest with the intravenous route and higher doses that produce larger maximal concentrations and areas under the concentration curve. Troughs (i.e., minimum concentrations) have less of an impact. Mechanistically, preclinical studies have identified that VIKI is a result of drug accumulation in proximal tubule cells, which triggers cellular oxidative stress and apoptosis. Yet, there are several gaps in the knowledge that may represent viable targets to make vancomycin therapy less toxic. Potential strategies include prolonging infusions and lowering maximal concentrations, administration of antioxidants, administering agents that decrease cellular accumulation, and reformulating vancomycin to alter the renal clearance mechanism. Based on preclinical models and mechanisms of toxicity, we propose potential strategies to lessen VIKI.
Keywords: vancomycin, acute kidney injury, biomarkers, nephrotoxicity, history, pharmacokinetics, pharmacology
Vancomycin is a recommended therapy in multiple national guidelines,1–6 with a dedicated guideline for dosing and monitoring strategies designed to maximize efficacy and minimize vancomycin-induced kidney injury (VIKI). Despite the common use, there is a poor understanding of the drivers and modifiers of VIKI. Several mechanisms underlying the nephrotoxicity of vancomycin have been identified; however, this has thus far not been translated into safer therapy for patients. Polypharmacy with other nephrotoxins is a major reason for compounded toxicity, and “avoiding nephrotoxic agents” is simply not an option for most patients. One variable well within the control of the clinician is the overall exposure of vancomycin that their patients receive. Vancomycin pharmacokinetic (PK) parameters, such as maximal concentrations (Cmax) and area under the concentration-time curve (AUC), allow for the exposures at the individual patient level to be compared to outcomes of efficacy and toxicity. Understanding the therapeutic window (i.e., minimum threshold exposures that define efficacy and ceiling concentrations that result in toxicity without meaningful gains in efficacy) is important for safe and effective care of patients. In this review, we chronicle rates of VIKI against the backdrop of dosing and monitoring guidelines that have been employed through the years. We explore preclinical models evaluating VIKI and describe how these models can provide insight into relationships that are difficult to discern clinically. We specifically review the pharmacokinetic/toxicodynamic (PK/TD) relationship for vancomycin derived from preclinical studies, mechanisms of toxicity derived from cellular and animal studies, and describe surrogates that have been used and are emerging to define VIKI. Finally, we propose future strategies for the early detection of VIKI and explore the potential for rescue/pro-phylactic therapy.
History of Vancomycin-Induced Kidney Injury (VIKI)
Vancomycin is a glycopeptide antibiotic originally isolated from the soil bacteria Amycolatopsis orientalis,7 previously known as Streptomyces orientalis, and was approved by the United States Food and Drug Administration (FDA) for patient use in 1958.8 Vancomycin use remained low after FDA approval because of a variety of toxicities related to purity that included pyrogen reactions, cochleotoxicity, vestibulotoxicity, and phlebitis.9 These adverse effects were initially ascribed to impurities in the formulation10 that was nicknamed “Mississippi mud,” because of its muddy brown look.11 However, the rise of methicillin-resistant Staphylococcus aureus infections (MRSA)12 led to increased vancomycin use. By the 1980s, vancomycin with a purity of 90% to 95% was available,10 and acute kidney injury (AKI) was relatively infrequent at approximately 5% of patients treated with vancomycin.13 Dosing guidelines from the 1980s until 2008 recommended vancomycin trough concentrations between 5 and 15 μg/ml.14 With AKI concerns very low and uncertainties about target concentrations being reached, multiple groups (including our own) recommended higher vancomycin exposures to maximize efficacy.14–16
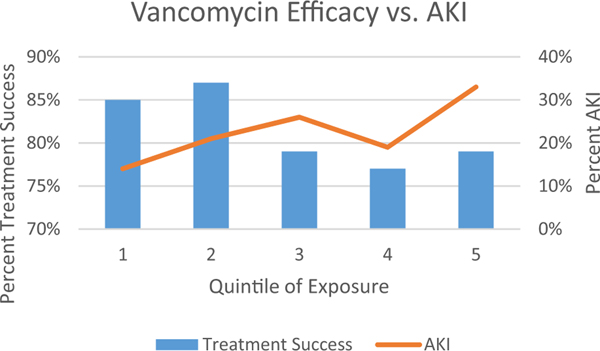
A standard for relating vancomycin use to AKI was created in the 2009 vancomycin therapeutic guidelines and was based on serial measurements of serum creatinine (an increase of 0.5 mg/dl or > 50% increase from baseline).14 Indeed after a definition was created in 2009 and the pneumonia17 and vancomycin guidelines14 recommended vancomycin trough concentrations of 15 to 20 μg/ml for serious infection, reports of kidney injury increased (Figure 1). In critically ill patients where higher vancomycin exposures were sought, rates of kidney injury approached 30%.18 From the clinical data, it also became clear that numerous factors amplified the risk for VIKI,19 including receipt of concomitant nephrotoxins, larger vancomycin doses (e.g., 4 g/day),20 total duration of therapy,21 elevated trough concentrations (e.g., above ~ 15 μg/ml), infusion type,22 patient severity of ill-ness, pre-existing renal disease, and obesity. The interested reader is referred to well-written reviews that have summarized the clinical data.19, 23, 24 Since those reviews were composed, the latest and most comprehensive prospective study identified overall AKI rates of 26% among patients infected with MRSA bacteremia who were treated with vancomycin.25 From this work, it became apparent that the exposure-efficacy curve was relatively flat with efficacy obtained with vancomycin AUCs as low as 94 mg/L × 24 hours. Maximal efficacy occurred at an upper threshold of 515 mg/L × 24 hours, whereas toxicity followed a linear trend across the ordinal categories (Figure 2). It should be noted that more work is needed to fully define the therapeutic window as prospective studies evaluating vancomycin efficacy and toxicity are limited. Contemporary strategies will focus on achieving enough vancomycin exposure to ensure efficacy, but limiting exposure to ensure that excess AKI does not occur. This review will focus on the mechanisms of VIKI and potential ways to further decrease the inherent risk of utilizing an antibiotic where exposure-toxicity and exposure-efficacy curves overlap.
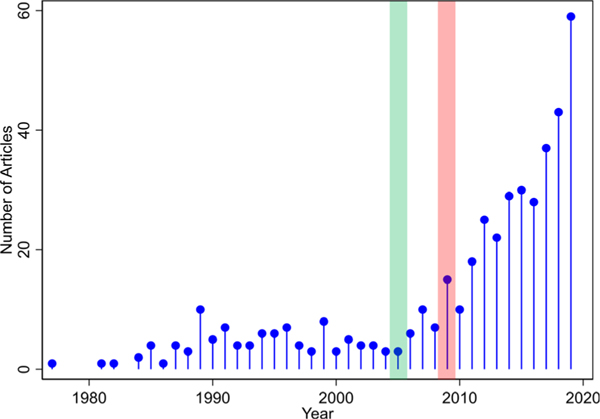
Figure 1.
Number of citations in PubMed mentioning vancomycin and nephrotoxicity between 1977 and 2019. The pneumonia guidelines released in 2005 (highlighted in green) and vancomycin guidelines released in 2009 (highlighted in pink) both recommended higher trough concentrations.
Figure 2.
Quintile of Exposure by AUC: (1) 94–387, (2) 392–515, (3) 516–621, (4) 621–757, (5) 758–1755.25 AUC, area under the curve; AKI, acute kidney injury.
Animal Models and Toxicodynamics
Despite more than 60 years of experience, the drivers of VIKI are not yet fully understood.26 Understanding VIKI in the clinical arena is challenged by the high cost of conducting clinical trials, which have been estimated to exceed a median of $40,000 per enrolled patient.27 For drugs like vancomycin that are already approved and in use, this financial barrier relegates most investigations to retrospective study. Retrospective analyses are subject to many sources of bias, and obtaining patients that are homogenous enough to detect differences in outcomes without confounding (or enrolling a large enough group to control and mitigate confounding) is challenging. Animal models, while surrogates of human physiology and subject to their own limitations, allow for the careful isolation of single variables and direct comparison of treatments. Additionally, animal models allow for the capture of copious amounts of data and samples that are only available clinically under the conduct of large-scale prospective trials. The FDA advises that acute toxicity studies are carried out to test the short-term adverse effects of a drug when administered either in single or multiple doses during a 24-hour period in two mammalian species (including one non-rodent) to identify species differences in toxicity.28 Chronic toxicity studies are carried out to test adverse effects occurring after the repeated or continuous administration of a drug for up to 6 months.29
Acute, chronic, and developmental toxicity studies of vancomycin have been conducted in experimental animals including mice, rats, dogs, cats, rhesus monkeys,30 and rabbits. 31 In this review, we focus primarily on toxicity studies in rats and dogs as they are the most common and are more relevant for human translation as described below. To allometrically scale doses for the rat and dog, rat doses should be divided by 6.2 and dog doses should be divided by 1.8.32 This normalizes the total exposures between humans and the animal model of toxicity by matching AUCs between the various species (i.e., accounting for more rapid clearance of smaller mammals).
Rats
Routes of Administration and Nephrotoxicity
Acute toxicity studies conducted in the 1950s demonstrated that rats died in clonic convulsions immediately following intravenous administration of vancomycin (LD50 319 mg/kg; lethal dose 50% is the amount of the substance required to kill 50% of the test population), likely as a function of central nervous system toxicity. However at this point in history, the product was not yet purified, and impurities may have driven this adverse event10, 33 Intraperitoneal administration of vancomycin was better tolerated and high doses were needed to cause lethal events (LD50 2218 mg/kg).33 Later in the 1980s when doses of 75, 150, and 350 mg/kg were given intraperitoneally twice daily for 4 days, the nephrotoxic dose of vancomycin was determined to lie between 150 to 350 mg/kg.30
Studies have also been performed where vancomycin was administered via the subcutaneous route. It has been previously demonstrated that the subcutaneous route results in low levels of absorption, even in chronic toxicity studies with daily subcutaneous doses of 400 mg/kg or less for 7 months.33 Large doses of 400 mg/kg/day given subcutaneously were not well tolerated and caused considerable necrosis of the skin at the site of injection34 due to the acidic pH of the vancomycin solution.35 Even with low absorption, a linear relationship was observed between subcutaneously administered vancomycin dose and vancomycin concentration in renal parenchymal tissue that ranged from 26 to 614 μg/g in rats receiving 10 to 400 mg/kg/day. Thus although studies employing the subcutaneous administration of vancomycin have occasionally induced histopathologic changes to the kidney tissue at high doses, low absorption from this route of administration and the lack of studies measuring blood concentrations preclude many conclusions.34
It was further identified that exposure-toxicity relationships with vancomycin may be dependent upon dosing route for rat studies.36 These authors demonstrated that vancomycin nephrotoxicity was highest with intravenous administration, followed by intraperitoneal, and lowest after intramuscular administration. A 500 mg/kg iv dose was lethal following the first, second, or third injection. This study suggested that absorption from the site of administration directly impacted exposure-toxicity relationships. Further, it showed that vancomycin has a high affinity for renal tissue. Following a single intravenous dose, vancomycin serum concentrations decreased with a half-life of 0.6 hours, but vancomycin kidney concentrations remained high for several days and accumulated in renal tissue after repeated dosing. A reduction of the nephrotoxic effect was observed when vancomycin was combined with D-glucaro-1.5-lactam, a β-glucuronidase inhibitor, which suggested that the nephrotoxic effect of vancomycin may be activated by lysosomal enzymes in the kidney cells.
Dose Effect and Nephrotoxicity
Dose-ranging studies revealed that an increase in the vancomycin dose (up to 450 mg/kg intraperitoneally) and duration of treatment (up to 28 days, subacute) in rats is associated with increased histopathologic damage and elevations in urinary biomarkers of AKI.37, 38 Damage is most prevalent at the proximal tubule, which is further supported by urinary biomarkers, such as kidney injury molecule-1 (KIM-1), clusterin, and osteopontin (OPN). Biomarkers KIM-1 and clusterin were the best predictors of histopathologic damage within 24 hours in the intraperitoneally dosed rat model of VIKI.39
Pharmacokinetic/Toxicodynamic (PK/TD) Relationships: VIKI
The rat model has also been used to explore PK/TD relationships. Since vancomycin efficacy correlates with exposure relative to the minimum inhibitory concentration (AUC:MIC),40 and since AUC remains constant with total daily dose, the authors asked whether the nephrotoxic potential of vancomycin (400 mg/kg/day intraperitoneally for 7 days) could be reduced if vancomycin was administered in divided doses (200 mg/kg/day twice daily intraperitoneally for 7 days).41 Although vancomycin concentrations were not measured, dose fractionation resulted in decreased kidney injury. Intravenous studies, where careful control of exposures is possible and exposures are not compromised by variable absorption rates, have been used to probe the PK parameters that cause toxicity and quantitative thresholds for injury. Rat studies employing intravenous dosing have demonstrated that vancomycin AUC0–24 or Cmax0–24, rather than minimum concentration (Cmin), predict urinary biomarker response.42–44 A threshold AUC0–24 of 482.2 mg h/L has been identified and correlates directly with similar human exposure-response data. Thus, moving to AUC monitoring to prevent toxicity (such as suggested in the published guidelines45) is supported by preclinical models. Future work is still needed to determine if minimizing Cmax while maintaining AUC is a beneficial strategy. Corroborating the work from this group,41 recent intravenous studies in rats have demonstrated lower urinary biomarkers in rats that received their daily vancomycin dose fractioned as opposed to consolidated (i.e., 3 to 4 times daily compared to once or twice daily). In this study, 24-hour AUCs were held functionally constant while Cmax was lowered. The Cmax better described the kidney injury as defined by KIM-1 urinary concentrations when compared to AUCs (Akaike information criterion of −5.28 vs −1.95)46; however, additional work is needed to further delineate this relationship and determine if altering administration schemes (e.g., continuous infusions) can lessen VIKI by decreasing Cmax even for similar AUCs.
Combination Therapy and Nephrotoxicity
Finally, it is well known in the preclinical literature that additional nephrotoxic agents increase vancomycin-mediated nephrotoxicity in an additive or synergistic fashion. Rat studies evaluating toxicity of combination therapy have demonstrated that concurrent use of vancomycin with aminoglycosides, including gentamicin, tobramycin, and arbekacin, potentiates nephrotoxicity30, 35, 47, 48 and should be avoided when clinically possible. Human studies have likewise confirmed synergistic toxicity between vancomycin and aminoglyco-sides.49, 50 We are unaware of preclinical data evaluating the combination of vancomycin with plazomicin, sisomicin, dibekacin, TS2037, and two novel arbekacin derivatives.
Fetal VIKI
The impact of vancomycin on the kidneys of the fetus has been studied. The effect of vancomycin on the developing rat fetus has been examined in developmental toxicology studies.31 No adverse effects were noted on fetal viability, weight, or morphology that included external anatomical and internal visceral anomalies and skeletal examination, at vancomycin doses up to 200 mg/kg; though, this dose was nephrotoxic to dams. A more recent study51 assessing KIM-1, a sensitive biomarker for VIKI,39 demonstrated that pups from dams dosed during the first trimester had higher concentration of KIM-1 in the fetal kidneys compared to the second and third trimesters. This suggests an inverse relationship between trimester of exposure and kidney damage, which warrants further examination.51
Dogs
Kidney toxicity in dogs is comparable to that observed in rats. In early dose-escalation acute toxicity studies, dogs died several days following vancomycin dosing (intravenous LD50 292 mg/kg) due to renal failure (blood urea nitrogen (BUN) 250–300 mg/dl) rather than to effects on the central nervous system. Similar to rat data, when vancomycin was administered to dogs as a rapid intravenous injection, it caused a drop in blood pressure that was mediated through a histaminergic response.30 In chronic toxicity studies with vancomycin in dogs, 19 of 22 dogs tolerated a 50 mg/kg dose given iv daily for 311 days; slight renal damage was observed in four of the 22 dogs. Emesis was frequent with loss of weight in some dogs.30, 33 More recently, a retrospective study of 29 dogs treated by a veterinary clinic concluded that vancomycin was reasonably tolerated with an all-cause AKI rate of 17% when given in clinical doses with therapeutic intent. The most common dose was 15 mg/kg intravenously every 6 hours, and all dogs received at least one additional antibiotic during the course of their therapy.52
Mechanisms of VIKI
There is some consensus that vancomycin-induced renal damage is caused by intracellular accumulation of the drug,36 though the exact method of vancomycin transport in proximal tubule cells has not been fully clarified. Vancomycin is excreted via glomerular filtration53, 54 and tubular secretion. Vancomycin is actively transported across the basolateral membrane into proximal tubule cells via the organic cation transporter system55, 56 (Figure 3). The visualization of large amounts of vancomycin at the brush border also points to either ongoing secretion or reabsorption of the drug at the apical side (i.e., from the urine).57, 58 In support, recent preclinical data have demonstrated vancomycin transport across the brush border membrane by apical endocytosis via dehydropeptidase59 and megalin60 and vancomycin-mediated inhibition of the expression and function of P-glycoprotein, an efflux transporter.61 Although mechanistically plausible, the involvement of the multidrug and toxin extrusion (MATE1 and MATE2-K) family of proteins in the secretion of vancomycin has yet to be demonstrated.
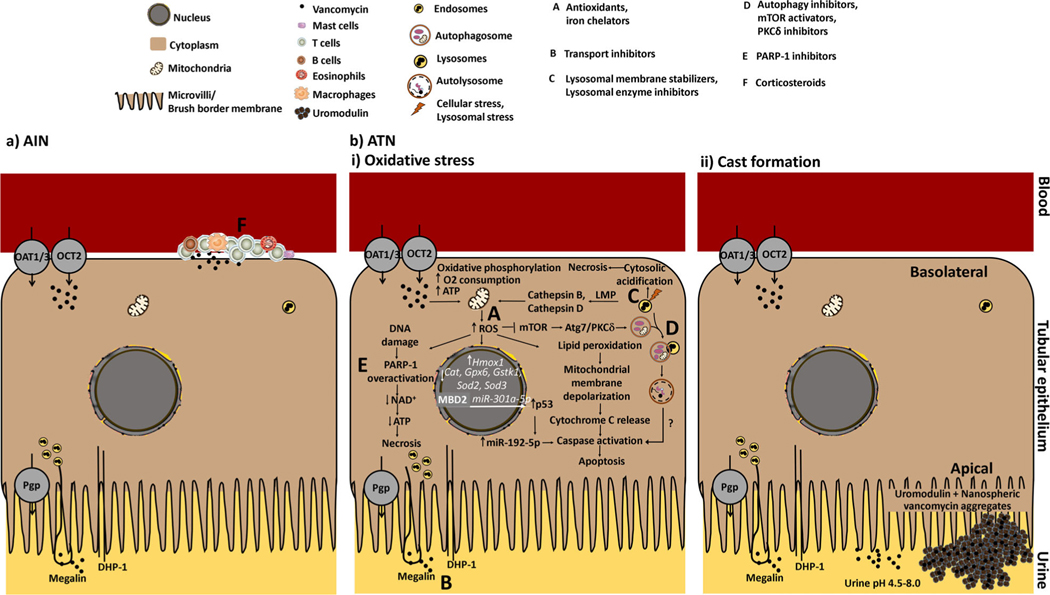
Figure 3.
Mechanisms of vancomycin-induced nephrotoxicity. (a) AIN: Vancomycin-induced AIN is caused by an immunologic response to vancomycin with interstitial infiltrate consisting of eosinophils, mast cells, plasma cells, lymphocytes, and macrophages. (b) ATN: (i) Oxidative stress: Vancomycin stimulates oxidative phosphorylation, as measured by an increase in oxygen consumption and an increase in ATP concentrations, which generate free radicals via formation of an iron complex. Free radicals induce lipid peroxidation leading to mitochondrial membrane depolarization and release of cytochrome C with activation of caspases leading to apoptosis. High levels of mitochondrial ROS can promote DNA damage, which in turn is obligatory for activation of PARP-1, an enzyme involved in DNA repair. Overactivation of PARP-1 activity leads to depletion of NAD+/ATP and cell death by necrosis. High doses of vancomycin upregulate the transcriptional expression of the Hmox1 gene, an indicator of cellular oxidative stress, and downregulate the expression of Cat, Gpx6, Gstk1, Sod2, and Sod3, which encode some of the main cellular antioxidants. Methyl-CPG-binding domain proteins are protein readers of methylation. In VIKI, MBD2 binds to the methylated CpG elements of miR-301a-5p promoter and activates the promoter, thus increasing transcription of miR-301a-5p. miR-301a-5p upregulates p53, which in turn induces caspase activity leading to apoptosis via miR-192–5p. Receptor-mediated endocytosis of vancomycin from the urine and transporter-mediated secretion of vancomycin from the blood into tubular epithelial cells causes entrapment of vancomycin in endosomes. Endosomes fuse with lysosomes, which leads to accumulation of vancomycin in lysosomes. This accumulation of vancomycin in lysosomes triggers a massive autophagy that is enhanced by suppressing mTOR activation and mediated by the activation of PKCδ through the direct interaction of Atg7 and PKCδ. In addition, the production of ROS may induce the increased permeability of lysosomes that are near mitochondria. Lysosomal membrane damage releases proteases including cathepsins into the cytosol that activate apoptotic effectors such as the mitochondria and caspases. Complete disruption of lysosomes may induce cytosolic acidification, which in turn provokes uncontrolled cell death by necrosis. (b) (ii) Cast formation: Nanospheric vancomycin aggregates interact with uromodulin and obstruct the tubular lumen. This leads to reduced renal blood flow via tubuloglomerular feedback mechanisms with a resultant decline in renal function. Various factors could lead to vancomycin supersaturation and precipitation within the tubular lumen including high concentrations and low urinary pH. Potential strategies for prevention. A: antioxidants (e.g., vitamins C and E); B: transport inhibitors (e.g., cilastatin, JBP485, fosfomycin); C: lysosomal membrane stabilizers (e.g., fosfomycin), lysosomal enzyme inhibitors (e.g., D-glucaro-1.5-lactam); D: autophagy inhibitors (e.g. chloroquine, bafilomycin A1), mTOR activators (e.g., MHY1485), PKCδ inhibitors (e.g., rottlerin); E: PARP-1 inhibitors (e.g., 3-aminobenzamide, olaparib, rucaparib, niraparib, talazoparib, veliparib, pamiparib, CEP 9722, E7016); F: corticosteroids. Abbreviations: AIN, acute interstitial nephritis; ATN, acute tubular necrosis; Atg, autophagy-related gene; ATP, adenosine triphosphate; Cat, catalase; DHP-1, dehydropeptidase-1; Gpx6, glutathione peroxidase 6; Gstk1, glutathione S-transferase kappa 1; Hmox1, heme oxygenase 1; LMP, lysosomal membrane permeabilization; MBD2, methyl-CpG-binding domain protein 2; miR, microRNA; NAD, nicotinamide adenine dinucleotide; mTOR, mammalian target of rapamycin; O2, oxygen; OAT1/3, organic anion transporter 1/3; OCT-2, organic cation transporter 2; PARP-1, poly (adenosine diphosphate ribose) polymerase 1; PEG, polyethylene glycol; Pgp, P-glycoprotein; PKCδ, protein kinase C delta; ROS, reactive oxygen species, SOD, superoxide dismutase; VIKI, vancomycin-induced kidney injury.
The mechanisms that underlie the pathogenesis of VIKI are multifactorial (Figure 3).
Acute Interstitial Nephritis
Vancomycin has been observed with rare cases of acute interstitial nephritis (AIN).62–68 Vancomycin-induced AIN has been suggested to be cellular-mediated hypersensitivity66 (Figure 3a). Interstitial edema has been reported on renal biopsy with infiltrate consisting of eosinophils, mast cells, plasma cells, lymphocytes, and macrophages.67, 68 The mechanism of vancomycin-induced AIN is not known.
It has been suggested that VIKI may be mediated by complement activation followed by development of an inflammatory response. In their toxicogenomic study, kidneys of mice receiving high-dose vancomycin showed changes in the expression of several transcripts from the complement pathway, including C3 and C4b, and Cxcl1, a biomarker of ischemic acute kidney failure that is produced in a complement-dependent manner.69 The role of the complement pathway in VIKI needs to be elucidated.
Acute Tubular Necrosis
Vancomycin causes dose-dependent ATN,70–75 which is attributed to oxidative stress in proximal tubule cells, autophagy, and obstructive cast formation76, 77 (Figure 3).
Oxidative Stress
Oxidative stress is an imbalance between free radicals and antioxidants within cells that leads to mitochondrial dysfunction and cellular apoptosis. Vancomycin stimulates oxygen consumption and causes a dose-dependent increase in ATP concentrations in cultured renal cells,77 which indicates that vancomycin can stimulate mitochondrial oxidative phosphorylation.78 Oxygen consumption can lead to free radical generation,79 and free radicals are involved in the pathogenesis of VIKI through the inhibition of superoxide dismutase.78 Since 2,3-dihydroxybenzoic acid, an iron chelator, could protect against VIKI, this was evidence that VIKI is mediated at least partly through oxidative stress via formation of an iron complex that catalyzes free radical formation, specifically the hydroxyl radical, since iron is crucial in the generation of this radical.80 Free radicals can induce lipid peroxidation, a chain reaction influencing unsaturated fatty acids in cell membranes, leading to their damage, and production of malondialdehyde.81 As such, an increase in malondialdehyde levels and a decrease in antioxidative enzyme activities – reduced glutathione peroxidase and superoxide dismutase – as indicators of oxidative stress have been reported in the kidney tissue of rats treated with vancomycin; antioxidants, including a superoxide dismutase conjugate,78 reversed or prevented these effects and downstream histopathologic damage. In addition, the superoxides produced cause mitochondrial membrane depolarization and subsequent release of cytochrome C with activation of downstream caspases59, 82, ultimately leading to cell apoptosis.
Of note, superoxide anions are endogenous inducers of DNA single-strand breaks which is obligatory for activation of poly (adenosine diphosphate ribose) polymerase 1 (PARP-1),83 an enzyme involved in DNA repair. The enzyme PARP-1 uses NAD+ as a substrate in the repair process, subsequently, cells utilize ATP to regenerate NAD+ stores. With high amounts of DNA damage, PARP-1 overactivation leads to depletion of NAD+/ATP and cell death by necrosis. Further support for the oxidative stress mechanism in VIKI is demonstrated by the overactivation of PARP-1 activity in rats administered vancomycin and attenuation of the renal injury on treatment with 1,5-isoquinolinediol, a PARP inhibitor.84
The occurrence of oxidative stress and mitochondrial damage in VIKI has been confirmed at the genomic level. In mice treated with high-dose vancomycin (i.e., seven daily intravenous doses of 200 and 400 mg/kg vancomycin and seven daily intraperitoneal doses of 400 mg/kg vancomycin), the transcriptional expression of Hmox1, an indicator of cellular oxidative stress, was upregulated and the expression of Cat, Gpx6, Gstk1, Sod2, and Sod3, which encode some of the main cellular antioxidants, were downregulated.69 The miRNA appears to be an important part of this VIKI mechanistic pathway. The miRNAs miR-301a-5p and miR-192–5p have been implicated in the development of VIKI through methyl-CpG-binding domain protein 2 (MBD2) and p53.85, 86 Methyl-CpG-binding domain proteins, are protein readers of methylation and are actively involved in DNA-methylation-mediated transcriptional regulation by binding to methylated CpG on the DNA and either blocking other protein factors from binding to DNA or by binding to promoters of actively transcribed genes.87 In VIKI, MBD2 binds to the methylated CpG elements of miR-301a-5p promoter and activates the promoter, thus increasing transcription of miR-301a-5p, which upregulates p53, caspase activity, and vancomycin-induced apoptosis in HK-2 cells. Additionally, p53 has been shown to induce apoptosis via miR-192–5p in wild-type mice, which was suppressed in p53-KO mice.86 Blocking miR-301a-5p85 or inhibiting miR-192–5p86 with respective antisense oligonucleotides ameliorated VIKI in C57BL/6 mice.
Receptor-mediated endocytosis of vancomycin from the urine and transporter-mediated secretion of vancomycin from the blood into tubular epithelial cells causes entrapment of vancomycin in endosomes. Endosomes fuse with lysosomes, which leads to accumulation of vancomycin in lysosomes. In 1992, vancomycin accumulation in the lysosomes of proximal tubule cells was first visualized using immunoelectron microscopy58; and later confirmed by another group.57 This accumulation of vancomycin in lysosomes may trigger a detrimental autophagy process. Autophagy is a lysosomal-dependent process of self-degradation of cellular components for the maintenance of cell homeostasis or in response to stress88 and may serve as an adaptive and protective mechanism for cell survival. As such, autophagy in proximal tubules has been shown to protect against AKI.89 However, massive autophagy and dysregulated autophagy may cause cell death.90, 91 Several autophagy-related genes (Atgs) or proteins have been identified that are required for the maturation of autophagosomes, deficiency of which can inhibit the autophagy process. Using a proximal tubule specific-Atg7-knockout mouse model, these authors succeeded in attenuating VIKI thus demonstrating a destructive role for autophagy in VIKI.92 Further, morphologic analysis of HK-2 cells showed that vancomycin-induced cell apoptosis was enhanced by rapamycin, an mTOR inhibitor, reduced by chloroquine, an autophagy inhibitor, and confirmed with a caspase activity assay. Using a coimmunoprecipitation and various Atg7 deletion constructs in renal cells treated with vancomycin, they concluded that Atg7 mediates renal tubule cell apoptosis through direct interaction and activation of PKCδ. Further research is necessary to confirm this molecular mechanism.
In addition, various stressors and the production of reactive oxygen species (ROS), may induce the permeabilization of lysosomes that are near mitochondria. Lysosomal membrane damage releases proteases including cathepsins into the cytosol that activate apoptotic effectors, such as the mitochondria and caspases. Complete disruption of lysosomes may induce cytosolic acidification, which in turn provokes uncontrolled cell death by necrosis.93
Cast Formation
Cast formation may also exacerbate vancomycin-induced ATN. Uromodulin, the most abundant urinary protein, is exclusively produced by renal epithelial cells where it regulates salt transport, protects against urinary tract infection and kidney stones, and has immunomodulatory functions. In the tubular lumen, uromodulin forms filaments that constitute the matrix of hyaline casts.94 Uromodulin has been shown to interact with vancomycin aggregates resulting in intratubular cast formation, inflammation, and ATN.76 These authors detected vancomycin within uromodulin casts in nine patients with ATN and replicated the pathophysiology in a mouse model. Various factors could lead to vancomycin supersaturation and precipitation within the tubular lumen including high concentrations and low urinary pH.95 In addition, vancomycin accumulation at the brush border has been observed. Using a monoclonal antibody to vancomycin with immunocytochemistry the presence of significant amounts of vancomycin was detected in the tubular lumen and in the microvilli, nuclei, and cytoplasm specifically in the S1 and S2 segments of the proximal tubules.57 Nevertheless, further studies are warranted to determine whether vancomycin accumulation in the tubular lumen is a result of supersaturation and precipitation at urinary pH.
Human Translation
The current practice for classifying VIKI is based on the 2009 vancomycin guidelines.14 These guidelines suggest that nephrotoxicity is considered when at least two instances of elevated serum creatinine concentrations are observed in the setting of several days of vancomycin therapy. Elevated serum creatinine is defined as an increase of 0.5 mg/dl or greater than 50% increase from baseline, whichever is greater. Other common methods for classifying AKI are the Risk of renal dysfunction, Injury to the kidney, Failure of kidney function, Loss of kidney function, End-stage kidney disease (RIFLE),96 Acute Kidney Injury Network (AKIN),97 and Kidney Disease Improving Global Outcomes (KDIGO)98 criteria (Table 1). These methods have similar discriminatory power for predicting mortality in critically ill patients.99 The newest vancomycin guidelines (in draft form45) report that most studies have assessed the relationship between vancomycin and nephrotoxicity using the aforementioned definition of a serum creatinine increase of 0.5 mg/dl or greater than 50% increase from baseline; however, some studies have begun to adapt a lower threshold (such as a serum creatinine absolute increase of ≥ 0.3 mg/dl), which qualifies patients as Stage 1 AKI by the AKIN and KDIGO criteria. The move to utilize absolute changes in creatinine as opposed to relative percentage changes is because the time to detection of renal insult is more heterogeneous across various stages of baseline kidney disease when using percentage changes as opposed to absolute changes.100, 101 That is, absolute changes, specifically when set to a lower value such as 0.3 mg/dl can identify an injury sooner and more consistently regardless of patient renal disease at baseline. The time to reach any set percentage rise in serum creatinine depends on the level of kidney dysfunction that a patient has prior to the AKI event. For instance in patients with an ultimate 50% reduction in creatinine clearance, patients with no chronic kidney disease (CKD) achieve a 100% increase in serum creatinine (SCr) within 12 hours, whereas those with stage 2 CKD approach the same doubling of SCr around 30 hours and those with stage 3 CKD do not double their SCr until after 60 hours.102
Table 1.
| Serum Creatinine | Urine Output | GFR | |
|---|---|---|---|
| RIFLEa criteria | |||
| Risk | 1.5 times baseline | < 0.5 ml/kg/hr for 6 hrs | ↓ by > 25% |
| Injury | 2 times baseline | < 0.5 ml/kg/hr for 12 hrs | ↓ by > 50% |
| Failure | 3 times baseline or ≥ 4 mg/dl with acute ↑ of ≥ 0.5 mg/dl | < 0.3 ml/kg/hr for 24 hrs or anuria for 12 hrs | ↓ by ≥ 75% |
| Loss | Persistent failure = complete loss of function for > 4 weeks (requiring dialysis) | ||
| ESKD | Complete loss of kidney function for > 3 months (requiring dialysis) | ||
| AKIN criteria | |||
| Stage 1 | ↑ of ≥ 0.3 mg/dl within 48 hrs or 1.5–2.0 times baseline within 48 hrs | ↓ to < 0.5 ml/kg/hr for > 6 hrs | |
| Stage 2 | 2–3 times baseline | ↓ to < 0.5 ml/kg/hr for > 12 hrs | |
| Stage 3 | > 3 times baseline or ≥ 4 mg/dl with acute ↑of ≥ 0.5 mg/dl | ↓ to < 0.3 ml/kg/hr for 24 hrs or anuria for 12 hrs | |
| KDIGO criteria | |||
| Stage 1 | ↑ of ≥ 0.3 mg/dl within 48 hrs or 1.5–1.9 times baseline within 7 days | < 0.5 ml/kg/hr for 6–12 hrs | |
| Stage 2 | 2.0–2.9 times baseline | < 0.5 ml/kg/hr for ≥ 12 hrs | < 18 yrs, ↓ to |
| Stage 3 | 3 times baseline or ≥ 4 mg/dl or initiation of RRT | < 0.3 ml/kg/hr for ≥ 24 hrs or anuria for ≥ 12 hrs | < 35 ml/min/1.73 m2 |
GFR = glomerular filtration rate; RIFLE = Risk of renal dysfunction, Injury to the kidney, Failure of kidney function, Loss of kidney function, End-stage kidney disease; ESKD = end-stage kidney disease; AKIN = Acute Kidney Injury Network; KDIGO = Kidney Disease Improving Global Outcomes; RRT = renal replacement therapy.
Classification is based on the highest score for any category.
More work will be needed to define the level of clinical kidney injury that should be utilized for adverse event attribution (i.e., VIKI). Within the criteria to define AKI, serum creatinine is the most commonly applied methodology because it is commonly measured in practice and thus available for assessment in retrospective studies. Creatinine is known to be an insensitive and slowly reactive marker of kidney function (vis-à-vis extrapolated glomerular filtration rate).103 Glomerular filtration rates can drop as much as 50% before changes in creatinine are detected.104–106 Patient hydration status also can have a big effect on serum creatinine values.107 Acute kidney injury-induced fluid retention and exogenous fluid administration can actually make serum creatinine values drop, delaying recognition of AKI. Therefore, there has been an invigorated interest in biomarkers that might detect injury closer to the actual event. Within 3 to 6 hours after an ischemic-reperfusion event, KIM-1 is elevated in experimental studies; whereas, creatinine elevations in this time period were less detectable and did not consistently elevate within the 120-hour study period.37 Hence, understanding when the injurious event occurs might be more beneficial to the clinical care of the patient than utilizing a surrogate marker (e.g., SCr) of a functional clearance metric (e.g., glomerular filtration rate (GFR)). Future studies will be required to understand how biomarkers predict VIKI in humans; these studies will be facilitated once biomarkers are approved for clinical use. Presently, urinary biomarkers (e.g., KIM-1 and clusterin) are qualified for rat108 by the FDA (i.e., for drug-induced AKI); a composite panel of six biomarkers that include KIM-1 and clusterin has been qualified in human drug trials.109 These biomarkers (i.e., KIM-1 and clusterin) will likely have direct relevance to identifying and quantifying VIKI. An understanding of time course and magnitude of change will be needed before updated vancomycin guidelines can be developed to prevent additional injurious exposures.
Potential Strategies for Prevention
Understanding the mechanisms underlying the pathogenesis of VIKI has aided the design of strategies to prevent it (Table 2; Figure 3; Table S1); yet few of the preclinical studies that have shown promise have been implemented in clinical trials to date for VIKI. We are only aware of a single trial, namely NCT03921099, where 40 patients are to be enrolled to evaluate the impact of ascorbic acid in the prevention of vancomycin-induced nephrotoxicity.110
Table 2.
Summary of Studies That Have Evaluated Potential Strategies for Prevention of VIKI
| Compounds to Inhibit or Reverse VIKI | Models | |
|---|---|---|
| Antioxidants | ||
| 1 | Hexamethylenediamine-conjugated superoxide dismutase (AH-SOD) 78 | Wistar rats |
| 2 | Erdosteine122 | Wistar albino rats |
| 3 | a-lipoic acid, ginkgo biloba extract, melatonin, or amrinone131 | Sprague Dawley rats |
| 4 | Erythropoietin121 | Wistar albino rats |
| 5 | Caffeic acid phenyl ester (CAPE), vitamin C, vitamin E, N-acetylcysteine 20 | Wistar albino rats |
| 6 | 2,3-dihydrobenzoic acid (DHB)80 | Wistar albino rats |
| 7 | Curcumin139 | Sprague Dawley rats |
| 8 | 1,5-isoquinolinediol (ISO)84 | Wistar rats |
| 9 | Thymoquinone140 | Wistar albino rats |
| 10 | Atorvastatin119 | Wistar albino rats |
| 11 | Spirulina, pycnogenol, spirulina + pycnogenol112 | Wistar albino rats |
| 12 | Vitamin E, mitoTEMPO (mitochondria-targeted antioxidant), vitamin C, N-acetylcysteine, glutathione141 | Porcine proximal tubular epithelial LLC-PK1 cell line |
| 13 | Naringenin113 | Wistar albino rats |
| 14 | Zingerone114 | Sprague Dawley rats |
| 15 | Docosahexaenoic acid-enriched phosphatidylcholine (DHA-PC)116 | Balb/c mice |
| 16 | Rutin117 | Wistar rats |
| 17 | Silymarin118 | Wistar albino rats |
| 18 | Zingerone115 | Sprague Dawley rats |
| Transport inhibitors | ||
| 1 | Imipenem-cilastatin, flomoxef sodium, fosfomycin sodium142 | Rabbits |
| 2 | Cilastatin123 | Rabbits |
| 3 | Cilastatin60 | C57BL/6J mice |
| 4 | Cilastatin61 | C57BL/6J mice |
| 5 | JBP485125 | Wistar rats |
| Formulations | ||
| 1 | Polyethylene glycol (PEG)-vancomycin conjugate143 | Bacterial efficacy studies |
| 2 | Vancomycin with 2 inactive ingredients, D-mannitol and Macrogol400 (PEG400), referred to as MEEK132,149 | Sprague Dawley rats |
| 3 | Nanoconjugated vancomycin138 | Swiss mice |
| 4 | Vancomycin PEGylated liposomes134 | 42 CF-1 mice |
| 5 | Dicetylphosphate (DCP) liposomal vancomycin144 | CD-1 mice |
| 6 | Liposomal vancomycin145 | Kunming mice |
| 7 | Vancomycin loaded pH-responsive chitosan nanoparticles containing anionic gemini surfactant (DL_CSSNPs)137 | Mice |
| 8 | Vancomycin analog compounds146 | Human renal proximal tubule epithelial cells (HK-2), human liver cell line (HL-7702) |
| 9 | Vancomycin loaded chitosan sponges136 | Bacterial inhibition assay |
| 10 | Vancomycin-dextran sulfate sodium nanoplexes147 | Adenocarcinoma human alveolar basal epithelial cells (A549), embryonic kidney cells (HEK-293), and liver hepatocellular carcinoma (Hep G2) cell lines |
| 11 | pH-responsive liposomal formulations DOAPA-VAN-Lipo, and DLAPA-VAN-Lipo135 | Balb/c mice |
| 12 | Vancomycin loaded silver nanoparticles148 | Bacterial efficacy studies |
| Others | ||
| 1 | Potassium salt of D-glucaro-1.5-lactam (a β-glucuronidase inhibitor)36 | Wistar albino rats |
| 2 | Fosfomycin126 | Wistar albino rats |
| 3 | Fosfomycin, imipenem/cilastatin127, 150 | Wistar albino rats |
| 4 | Fosfomycin128 | Wistar rats |
| 5 | Dexmedetomidine130 | Wistar albino rats |
| 6 | Chloroquine92 | Proximal tubule Atg7 wild type and deficient mice |
AH-SOD = hexamethylenediamine-conjugated superoxide dismutase; Atg = Autophagy related gene; AUC = area under the concentration time curve; BUN = blood urea nitrogen; CAPE = caffeic acid phenyl ester; CMC = carboxy methyl cellulose; DCP = Dicetylphosphate; DL_CSSNPs = vancomycin loaded pH-responsive chitosan nanoparticles containing anionic gemini surfactant; DMSO = dimethylsulfoxide; DHA-PC = Docosahexaenoic acid-enriched phosphatidylcholine; DHB = 2,3-dihydrobenzoic acid; ERK = extracellular signal related kinase; i.m. = intramuscular; i.p. = intraperitoneal; ISO = isoquinolinediol; mTOR = mammalian target of rapamycin; NAR = naringenin; PEG = polyethylene glycol; PKCδ = protein kinase C delta; s.c. = subcutaneously; VIKI = vancomycin-induced kidney injury; VRSA = van-comycin resistant staphylococcus aureus; VSSA = vancomycin susceptible staphylococcus aureus.
Hydration
The impact of hydration on VIKI has been tested. Water 40 ml/kg given orally 30 minutes before and 30 minutes following a 350 mg/kg twice daily intraperitoneal dose of vancomycin appeared to reduce the degree of renal injury as evaluated by the reduction in BUN and creatinine values for rats treated with vancomycin compared with controls who did not receive vancomycin, however, it was not clear if this effect was secondary to hemodilution. Supporting that the effect may have been driven by hemodilution, biochemical indicators of nephrotoxicity including transport and metabolism functions in renal cortical slices, and relative kidney weights, were lower than controls not given vancomycin.30 Thus blood dilution of the biomarkers may have been solely responsible for the differences. Additional work is needed to see if improving hydration status can help minimize vancomycin toxicity.
Antioxidants
As oxidative stress has been described as one of the mechanisms of VIKI (Figure 3), several antioxidants have been studied preclinically in an attempt to prevent VIKI. The effects of different antioxidants, such as α-lipoic acid, ginkgo biloba, melatonin, erdosteine, vitamin E, vitamin C, N-acetylcysteine, caffeic acid phenethyl ester, erythropoietin, 2,3-dihydroxybenzoic acid, tempol, thymoquinone, curcumin, atorvastatin, 1,5-isoquinolinediol and a superoxide dismutase conjugate, reversed or prevented oxidative stress and downstream histopathologic damage. These studies have been reviewed in detail.111 Since then, spirulina and pycnogenol,112 mitoTEMPO, naringenin,113 a free radical scavenger zingerone,114, 115 DHA-enriched phosphatidylcholine (DHA-PC),116 rutin,117 and silymarin118 have been evaluated in animal studies. In our opinion, the antioxidants that are most ready for clinical trials include preparations that are already clinically available, such as atorvastatin, vitamin C, vitamin E, N-acetylcysteine, erythropoietin, and erdosteine.
The data that underpin the potential role of the antioxidants in preventing VIKI follow. Atorvastatin provided a significant degree of protection in a rat model, but it depended on the dose of atorvastatin used: 5 mg/kg was insufficient, 10 mg/kg was most effective, and 20 mg/kg was toxic.119 The effects of vitamin C, vitamin E, and N-acetylcysteine on vancomycin-induced nephrotoxicity were investigated in a rat model.120 The three antioxidants decreased the histologic damage caused by vancomycin in the kidney, but they did not completely prevent it. The vancomycin plus vitamins C and E groups showed mild epithelial desquamation, interstitial edema, and disarrangement. Vitamin E was the most effective in preventing vancomycin-induced tubular damage, followed by vitamin C and N-acetylcysteine.120 Erythropoietin and erdosteine decreased histopathologic damage to rat kidneys.121, 122
Transport Inhibitors
One strategy to minimizing VIKI is limiting the amount of cellular accumulation. A number of studies in mice and in primary porcine proximal tubule cells have demonstrated that cilastatin can decrease cellular vancomycin concentrations in the kidney and attenuate VIKI in a dose-dependent manner123 by inhibiting the apical uptake of vancomycin through the megalin receptor,60 the enzyme dehydropeptidase-I,59 and by increasing P-glycoprotein expression and function.61 Although cilastatin might decrease the plasma concentration of vancomycin,124 which is essential for efficacy.
It has been confirmed that the renal expression of P-glycoprotein is decreased with vancomycin along with other proximal tubule transporters – organic anion transporter (Oat) 1, Oat3, organic cation transporter 2 (Oct2), and another efflux protein, multidrug resistance-associated protein 2 (Mrp2). This decrease in transporter expression with vancomycin could lead to accumulation of endogenous endotoxins thus contributing to VIKI. Compound JBP485, a dipeptide isolated from the human placenta, demonstrated antioxidant and antiapoptotic effects and regulated the expression of the proximal tubule transporters to enhance renal excretion of endogenous uremic toxins and attenuate VIKI in rats without affecting plasma concentrations of vancomycin.125
Miscellaneous Mechanisms
D-glucaro-1.5-lactam, β-glucuronidase inhibitor, has been reported to reduce the nephrotoxic effect of vancomycin in the rat as measured by impact of renal tubule cells,36 presumably due to inhibition of lysosomal enzymes. The protective effects of fosfomycin against VIKI in rats have been studied by different groups.126–128 Fosfomycin significantly decreased renal accumulation of vancomycin and reduced nephrotoxicity in a dose-dependent manner. The renal protective effects of fosfomycin on vancomycin-induced nephrotoxicity may be due to the inhibition of the tubular transport of vancomycin.127 Of note, fosfomycin has been shown to protect the kidneys against dibekacin-induced nephrotoxicity in rats by stabilizing the lysosomal membrane.129 Although this mechanism has not yet been demonstrated in VIKI, it is plausible. Another compound, an α−2 receptor agonist, dexmedetomidine, was shown to reduce the extent of vancomycin-induced renal damage in rats by preventing the elevation of vasoconstrictor agents.130 Amrinone, a phosphodiesterase type III inhibitor,131 was effective in reducing VIKI in rats but may not find clinical application because of its cardiovascular side effects. Recently, the autophagy inhibitor chloroquine has been shown to protect against vancomycin-induced nephrotoxicity in rats.92 Whether the above-mentioned modalities are protective against VIKI in human subjects remains to be determined.
Formulation Alterations
Multiple attempts have been made to alter the vancomycin formulation to lessen VIKI, and various formulations are under development to improve the safety and efficacy of vancomycin. Liposomes and chitosans are among the best examples, though one miscellaneous formulation bears mention. The inactive ingredients D-mannitol and Macrogol 400 (polyethylene glycol 400 (PEG 400), in MEEK, a generic vancomycin formulation, reduced the nephrotoxicity of vancomycin in rats.132 It is unclear if this has clinical implication.
Liposomes
Liposomal encapsulation has been employed for multiple nephrotoxic drugs and has decreased kidney injury. Liposomal encapsulation enhances reticuloendothelial system uptake, consequently killing bacteria trapped within macrophages, and resulting in increased levels of vancomycin in spleen and liver. Liposomes deliver higher concentration of vancomycin to lung tissue while reducing accumulation in kidney tissue133; surface PEGylation augments this effect.134 Novel fatty acid-based zwitterionic lipids have been used to develop pH-responsive liposomes for the targeted delivery of vancomycin to acidic environments.135 Bacteria thrive in these conditions whereas antibiotics often lose their activity. There is potential for liposomes to release vancomycin in response to change in pH at the site of infection. Reduction in kidney cellular concentrations may translate to safer therapies for patients, but these formulations need to be further developed.
Chitosans
A chitosan sponge as a carrier matrix for sustained vancomycin release was evaluated in vitro for its sustained-release effect.136 The high hydrophilic nature of vancomycin was cited as the reason it did not produce the desired sustained release, but sustained-release formulations such as these could potentially reduce vancomycin nephrotoxicity if site-concentrated therapy is desired. In another study, vancomycin loaded pH-responsive chitosan nanoparticles for controlled and targeted delivery of vancomycin showed an increase in vancomycin release at pH 6.5 compared to pH 7.4 and reduced MRSA burden 8-fold compared to vancomycin.137
Nanoconjugated vancomycin prepared from a carboxymethyl derivative of chitosan with folic acid showed beneficial effects against a lymphocyte model with S. aureus.138
Summary
In summary, preclinical studies have demonstrated that VIKI is a result of drug accumulation in proximal tubule cells, specifically in the lysosomes. Vancomycin accumulation at the proximal tubule triggers degradative autophagy, oxidative stress, and apoptosis. Altered PK exposure and a variety of compounds may help to circumvent these mechanisms of toxicity. Future studies are needed to bridge preclinical knowledge to the bedside and determine if these strategies will result in safer vancomycin therapy for patients.
Supplementary Material
Table S1. Summary of studies that have evaluated potential strategies for prevention of VIKI.
Acknowledgments
The authors would like to thank Alyse Rehberger, Anna Najem, Samar Shaheen and Alexis Vanderlee for their help with obtaining articles cited in this manuscript. The research reported in this publication was supported in part by the National Institute of Allergy and Infectious Diseases under award number R21AI149026. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Scheetz has ongoing research contracts with Nevakar. Dr. Scheetz and Rhodes have patent US 2019/0099500 A1 pending. Dr. Downes receives research support from Merck Inc., unrelated to the current project.
Footnotes
Conflict of interest: All other authors have no other related conflicts of interest to declare.
Supporting Information
The following supporting information is available in the online version of this paper:
References
- 1.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of America. Clin Infect Dis 2011;4:e56–93. [DOI] [PubMed] [Google Scholar]
- 2.Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;5:e61–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011;3:e18–55. [DOI] [PubMed] [Google Scholar]
- 4.Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019;7:e45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect (Larchmt) 2010;1:79–109. [DOI] [PubMed] [Google Scholar]
- 6.Taplitz RA, Kennedy EB, Bow EJ, et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America clinical practice guideline update. J Clin Oncol 2018;14:1443–53. [DOI] [PubMed] [Google Scholar]
- 7.Kahne D, Leimkuhler C, Lu W, Walsh C. Glycopeptide and lipoglycopeptide antibiotics. Chem Rev 2005;2:425–48. [DOI] [PubMed] [Google Scholar]
- 8.Levine DP. Vancomycin: a history. Clin Infect Dis 2006;42: S5–12. [DOI] [PubMed] [Google Scholar]
- 9.Geraci JE, Nichols DR, Wellman WE. Vancomycin in serious staphylococcal infections. Arch Intern Med 1962;109: 507–15. [DOI] [PubMed] [Google Scholar]
- 10.Bailie GR, Neal D. Vancomycin ototoxicity and nephrotoxicity. A review. Med Toxicol Adverse Drug Exp 1988;5: 376–86. [DOI] [PubMed] [Google Scholar]
- 11.Griffith RS. Introduction to vancomycin. Rev Infect Dis 1981;3:S200–4. [PubMed] [Google Scholar]
- 12.Rodvold KA, McConeghy KW. Methicillin-resistant Staphylococcus aureus therapy: past, present, and future. Clin Infect Dis 2014;58:S20–7. [DOI] [PubMed] [Google Scholar]
- 13.Farber BF, Moellering RC Jr. Retrospective study of the toxicity of preparations of vancomycin from 1974 to 1981. Antimicrob Agents Chemother 1983;1:138–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rybak MJ, Lomaestro BM, Rotschafer JC, et al. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis 2009;3:325–7. [DOI] [PubMed] [Google Scholar]
- 15.Scheetz MH, Wunderink RG, Postelnick MJ, Noskin GA. Potential impact of vancomycin pulmonary distribution on treatment outcomes in patients with methicillin-resistant Staphylococcus aureus pneumonia. Pharmacotherapy 2006;4:539–50. [DOI] [PubMed] [Google Scholar]
- 16.Martin JH, Norris R, Barras M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Clin Biochem Rev 2010;1:21–4. [PMC free article] [PubMed] [Google Scholar]
- 17.American Thoracic S, Infectious Diseases Society of A. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;4:388–416. [DOI] [PubMed] [Google Scholar]
- 18.Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis 2009;4:507–14. [DOI] [PubMed] [Google Scholar]
- 19.Filippone EJ, Kraft WK, Farber JL. The nephrotoxicity of vancomycin. Clin Pharmacol Ther 2017;3:459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lodise TP, Lomaestro B, Graves J, Drusano GL. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother 2008;4:1330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bosch K, McLaughlin MM, Esterly JS, Rhodes NJ, Postelnick MJ, Scheetz MH. Impact of vancomycin treatment duration and dose on kidney injury. Int J Antimicrob Agents 2014;3:297–8. [DOI] [PubMed] [Google Scholar]
- 22.Ingram PR, Lye DC, Fisher DA, Goh WP, Tam VH. Nephrotoxicity of continuous versus intermittent infusion of vancomycin in outpatient parenteral antimicrobial therapy. Int J Antimicrob Agents 2009;6:570–4. [DOI] [PubMed] [Google Scholar]
- 23.van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother 2013;2:734–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bamgbola O Review of vancomycin-induced renal toxicity: an update. Ther Adv Endocrinol Metab 2016;3:136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lodise TP, Rosenkranz SL, Finnemeyer M, et al. The emperor’s new clothes: prospective observational evaluation of the association between initial vancomycin exposure and failure rates among adult hospitalized patients with MRSA blood-stream infections (PROVIDE). Clin Infect Dis 2020;70 (8):1536–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodvold KA. 60 plus years later and we are still trying to learn how to dose vancomycin. Clin Infect Dis 2020;70 (8):1546–49. [DOI] [PubMed] [Google Scholar]
- 27.Moore TJ, Zhang H, Anderson G, Alexander GC. Estimated costs of pivotal trials for novel therapeutic agents approved by the US food and drug administration, 2015–2016. JAMA Intern Med 2018;11:1451–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for Industry. Single Dose Acute Toxicity Testing for Pharmaceuticals. PT1. 1996. “Government Document”.
- 29.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Center for Biologics Evaluation and Research (CBER). Guidance for Industry. M3(R2) Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals. 2010. “Government Document”.
- 30.Wold JS, Turnipseed SA. Toxicology of vancomycin in laboratory animals. Rev Infect Dis 1981;3:S224–9. [PubMed] [Google Scholar]
- 31.Byrd RA, Gries CL, Buening MK. Developmental toxicology studies of vancomycin hydrochloride administered intravenously to rats and rabbits. Fundam Appl Toxicol 1994;4:590–7. [DOI] [PubMed] [Google Scholar]
- 32.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluatio and Research (CDER). Guidance for Industry. Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. 2005. “Government Document”.
- 33.Anderson RC, Worth HM, Harris PN, Chen KK. Vancomycin, a new antibiotic. IV. Pharmacologic and toxicologic studies. Antibiot Annu 1956;75–81. [PubMed] [Google Scholar]
- 34.Aronoff GR, Sloan RS, Dinwiddie CB Jr, Glant MD, Fineberg NS, Luft FC. Effects of vancomycin on renal function in rats. Antimicrob Agents Chemother 1981;2:306–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wood CA, Kohlhepp SJ, Kohnen PW, Houghton DC, Gilbert DN. Vancomycin enhancement of experimental tobramycin nephrotoxicity. Antimicrob Agents Chemother 1986;1:20–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marre R, Schulz E, Anders T, Sack K. Renal tolerance and pharmacokinetics of vancomycin in rats. J Antimicrob Chemother 1984;3:253–60. [DOI] [PubMed] [Google Scholar]
- 37.Vaidya VS, Ozer JS, Dieterle F, et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol 2010;5:478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuchs TC, Frick K, Emde B, Czasch S, von Landenberg F, Hewitt P. Evaluation of novel acute urinary rat kidney toxicity biomarker for subacute toxicity studies in preclinical trials. Toxicol Pathol 2012;7:1031–48. [DOI] [PubMed] [Google Scholar]
- 39.Pais GM, Avedissian SN, O’Donnell JN, et al. Comparative performance of urinary biomarkers for vancomycin-induced kidney injury according to timeline of injury. Antimicrob Agents Chemother 2019;63(7):e00079–19. 10.1128/AAC.00079-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown J, Brown K, Forrest A. Vancomycin AUC24/MIC ratio in patients with complicated bacteremia and infective endocarditis due to methicillin-resistant Staphylococcus aureus and its association with attributable mortality during hospitalization. Antimicrob Agents Chemother 2012;2:634–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Konishi H, Morita Y, Mizumura M, Iga I, Nagai K. Difference in nephrotoxicity of vancomycin administered once daily and twice daily in rats. J Chemother 2013;5:273–8. [DOI] [PubMed] [Google Scholar]
- 42.Avedissian SN, Pais GM, O’Donnell JN, et al. Twenty-four hour pharmacokinetic relationships for intravenous vancomycin and novel urinary biomarkers of acute kidney injury in a rat model. J Antimicrob Chemother 2019;8:2326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rhodes NJ, Prozialeck WC, Lodise TP, et al. Evaluation of vancomycin exposures associated with elevations in novel urinary biomarkers of acute kidney injury in vancomycin-treated rats. Antimicrob Agents Chemother 2016;10:5742–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Donnell JN, Rhodes NJ, Lodise TP, et al. 24-hour pharmacokinetic relationships for vancomycin and novel urinary biomarkers of acute kidney injury. Antimicrob Agents Chemother 2017;61(11):e00416–17. 10.1128/AAC.00416-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. American Journal of Health-System Pharmacy 2020, zxaa036 10.1093/ajhp/zxaa036 [DOI] [PubMed] [Google Scholar]
- 46.Avedissian SN, Pais GM, Liu J, et al. The Pharmacodynamic-Toxicodynamic Relationship of AUC and CMAX in Vancomycin Induced Kidney Injury. Poster presented at: IDWeek 2019; 2019 Oct 2–6; Washington, DC: 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Appel GB, Given DB, Levine LR, Cooper GL. Vancomycin and the kidney. Am J Kidney Dis 1986;2:75–80. [DOI] [PubMed] [Google Scholar]
- 48.Fauconneau B, De Lemos E, Pariat C, Bouquet S, Courtois P, Piriou A. Chrononephrotoxicity in rat of a vancomycin and gentamicin combination. Pharmacol Toxicol 1992;1:31–6. [DOI] [PubMed] [Google Scholar]
- 49.Rybak MJ, Albrecht LM, Boike SC, Chandrasekar PH. Nephrotoxicity of vancomycin, alone and with an aminoglycoside. J Antimicrob Chemother 1990;4:679–87. [DOI] [PubMed] [Google Scholar]
- 50.Rybak MJ, Abate BJ, Kang SL, Ruffing MJ, Lerner SA, Drusano GL. Prospective evaluation of the effect of an aminoglycoside dosing regimen on rates of observed nephrotoxicity and ototoxicity. Antimicrob Agents Chemother 1999;7:1549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joshi MD, Pais GM, Chang J, et al. Evaluation of fetal and maternal vancomycin-induced kidney injury during pregnancy in a rat model. Antimicrob Agents Chemother 2019;63 (10):e00761–19. 10.1128/AAC.00761-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DeStefano IM, Wayne AS, Rozanski EA, Babyak JM. Parenterally administered vancomycin in 29 dogs and 7 cats (2003–2017). J Vet Intern Med 2019;1:200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nielsen HE, Hansen HE, Korsager B, Skov PE. Renal excretion of vancomycin in kidney disease. Acta Med Scand 1975;4:261–4. [DOI] [PubMed] [Google Scholar]
- 54.Golper TA, Noonan HM, Elzinga L, et al. Vancomycin pharmacokinetics, renal handling, and nonrenal clearances in normal human subjects. Clin Pharmacol Ther 1988;5:565–70. [DOI] [PubMed] [Google Scholar]
- 55.Sokol PP. Mechanism of vancomycin transport in the kidney: studies in rabbit renal brush border and basolateral membrane vesicles. J Pharmacol Exp Ther 1991;3:1283–7. [PubMed] [Google Scholar]
- 56.Nakamura T, Takano M, Yasuhara M, Inui K. In-vivo clearance study of vancomycin in rats. J Pharm Pharmacol 1996;11:1197–200. [DOI] [PubMed] [Google Scholar]
- 57.Fujiwara K, Yoshizaki Y, Shin M, Miyazaki T, Saita T, Nagata S. Immunocytochemistry for vancomycin using a monoclonal antibody that reveals accumulation of the drug in rat kidney and liver. Antimicrob Agents Chemother 2012;11:5883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beauchamp D, Gourde P, Simard M, Bergeron MG. Subcellular localization of tobramycin and vancomycin given alone and in combination in proximal tubular cells, determined by immunogold labeling. Antimicrob Agents Chemother 1992;10:2204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Humanes B, Jado JC, Camano S, et al. Protective effects of cilastatin against vancomycin-induced nephrotoxicity. Biomed Res Int 2015;2015:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hori Y, Aoki N, Kuwahara S, et al. Megalin blockade with cilastatin suppresses drug-induced nephrotoxicity. J Am Soc Nephrol 2017;6:1783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Im DS, Shin HJ, Yang KJ, et al. Cilastatin attenuates vancomycin-induced nephrotoxicity via P-glycoprotein. Toxicol Lett 2017;277:9–17. [DOI] [PubMed] [Google Scholar]
- 62.Blumenthal KG, Patil SU, Long AA. The importance of vancomycin in drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. Allergy Asthma Proc 2012;2:165–71. [DOI] [PubMed] [Google Scholar]
- 63.O’Meara P, Borici-Mazi R, Morton AR, Ellis AK. DRESS with delayed onset acute interstitial nephritis and profound refractory eosinophilia secondary to vancomycin. Allergy Asthma Clin Immunol 2011;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ratner SJ, Roberts DK. Vancomycin-induced interstitial nephritis. Am J Med 1988;3(Pt 1):561–2. [DOI] [PubMed] [Google Scholar]
- 65.Bergman MM, Glew RH, Ebert TH. Acute interstitial nephritis associated with vancomycin therapy. Arch Intern Med 1988;10:2139–40. [PubMed] [Google Scholar]
- 66.Azar R, Bakhache E, Boldron A. [Acute interstitial nephropathy induced by vancomycin]. Nephrologie 1996;6:327–8. [PubMed] [Google Scholar]
- 67.Htike NL, Santoro J, Gilbert B, Elfenbein IB, Teehan G. Biopsy-proven vancomycin-associated interstitial nephritis and acute tubular necrosis. Clin Exp Nephrol 2012;2:320–4. [DOI] [PubMed] [Google Scholar]
- 68.Wai AO, Lo AM, Abdo A, Marra F. Vancomycin-induced acute interstitial nephritis. Ann Pharmacother 1998;11:1160–4. [DOI] [PubMed] [Google Scholar]
- 69.Dieterich C, Puey A, Lin S, et al. Gene expression analysis reveals new possible mechanisms of vancomycin-induced nephrotoxicity and identifies gene markers candidates. Toxicol Sci 2009;1:258–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Belen C, Budhiraja P, Bracamonte E, Popovtzer M. Biopsy-proven acute tubular necrosis associated with vancomycin in an adult patient. Ren Fail 2012;4:502–5. [DOI] [PubMed] [Google Scholar]
- 71.Sawada A, Kawanishi K, Morikawa S, et al. Biopsy-proven vancomycin-induced acute kidney injury: a case report and literature review. BMC Nephrol 2018;1:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shah-Khan F, Scheetz MH, Ghossein C. Biopsy-proven acute tubular necrosis due to vancomycin toxicity. Int J Nephrol 2011;2011:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stidham T, Reiter PD, Ford DM, Lum GM, Albietz J. Successful utilization of high-flux hemodialysis for treatment of vancomycin toxicity in a child. Case Rep Pediatr 2011;2011:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wicklow BA, Ogborn MR, Gibson IW, Blydt-Hansen TD. Biopsy-proven acute tubular necrosis in a child attributed to vancomycin intoxication. Pediatr Nephrol 2006;8:1194–6. [DOI] [PubMed] [Google Scholar]
- 75.Wu CY, Wang JS, Chiou YH, Chen CY, Su YT. Biopsy proven acute tubular necrosis associated with vancomycin in a child: case report and literature review. Ren Fail 2007;8:1059–61. [DOI] [PubMed] [Google Scholar]
- 76.Luque Y, Louis K, Jouanneau C, et al. Vancomycin-associated cast nephropathy. J Am Soc Nephrol 2017;6:1723–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.King DW, Smith MA. Proliferative responses observed following vancomycin treatment in renal proximal tubule epithelial cells. Toxicol In Vitro 2004;6:797–803. [DOI] [PubMed] [Google Scholar]
- 78.Nishino Y, Takemura S, Minamiyama Y, et al. Targeting superoxide dismutase to renal proximal tubule cells attenuates vancomycin-induced nephrotoxicity in rats. Free Radic Res 2003;4:373–9. [DOI] [PubMed] [Google Scholar]
- 79.Young IS, Woodside JV. Antioxidants in health and disease. J Clin Pathol 2001;3:176–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Naghibi B, Ghafghazi T, Hajhashemi V, Talebi A, Taheri D. The effect of 2,3-dihydroxybenzoic acid and tempol in prevention of vancomycin-induced nephrotoxicity in rats. Toxicology 2007;3:192–9. [DOI] [PubMed] [Google Scholar]
- 81.Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin Chem 1997;7:1209–14. [PubMed] [Google Scholar]
- 82.Arimura Y, Yano T, Hirano M, Sakamoto Y, Egashira N, Oishi R. Mitochondrial superoxide production contributes to vancomycin-induced renal tubular cell apoptosis. Free Radic Biol Med 2012;9:1865–73. [DOI] [PubMed] [Google Scholar]
- 83.Heller B, Wang ZQ, Wagner EF, et al. Inactivation of the poly(ADP-ribose) polymerase gene affects oxygen radical and nitric oxide toxicity in islet cells. J Biol Chem 1995;19:11176–80. [DOI] [PubMed] [Google Scholar]
- 84.Dalaklioglu S, Tekcan M, Gungor NE, et al. Role of the poly (ADP-ribose) polymerase activity in vancomycin-induced renal injury. Toxicol Lett 2010;2:91–6. [DOI] [PubMed] [Google Scholar]
- 85.Wang J, Li H, Qiu S, Dong Z, Xiang X, Zhang D. MBD2 upregulates miR-301a-5p to induce kidney cell apoptosis during vancomycin-induced AKI. Cell Death Dis 2017;10: e3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen J, Wang J, Li H, Wang S, Xiang X, Zhang D. p53 activates miR-192–5p to mediate vancomycin induced AKI. Sci Rep 2016;6:38868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wood KH, Zhou Z. Emerging molecular and biological functions of MBD2, a reader of DNA methylation. Front Genet 2016;7:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 2009;43:67–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jiang M, Wei Q, Dong G, Komatsu M, Su Y, Dong Z. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int 2012;12:1271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baehrecke EH. Autophagy: dual roles in life and death? Nat Rev Mol Cell Biol 2005;6:505–10. [DOI] [PubMed] [Google Scholar]
- 91.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 2007;9:741–52. [DOI] [PubMed] [Google Scholar]
- 92.Xu X, Pan J, Li H, et al. Atg7 mediates renal tubular cell apoptosis in vancomycin nephrotoxicity through activation of PKC-delta. FASEB J 2019;3:4513–24. [DOI] [PubMed] [Google Scholar]
- 93.Boya P, Kroemer G. Lysosomal membrane permeabilization in cell death. Oncogene 2008;50:6434–51. [DOI] [PubMed] [Google Scholar]
- 94.Devuyst O, Olinger E, Rampoldi L. Uromodulin: from physiology to rare and complex kidney disorders. Nat Rev Nephrol 2017;9:525–44. [DOI] [PubMed] [Google Scholar]
- 95.Stokes MB. Vancomycin in the kidney-a novel cast nephropathy. J Am Soc Nephrol 2017;6:1669–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative w. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;4:R204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;2:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Khwaja A KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;4:c179–84. [DOI] [PubMed] [Google Scholar]
- 99.Levi TM, de Souza SP, de Magalhaes JG, et al. Comparison of the RIFLE, AKIN and KDIGO criteria to predict mortality in critically ill patients. Rev Bras Ter Intensiva 2013;4:290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Waikar SS, Betensky RA, Bonventre JV. Creatinine as the gold standard for kidney injury biomarker studies? Nephrol Dial Transplant 2009;11:3263–5. [DOI] [PubMed] [Google Scholar]
- 101.Lopes JA, Jorge S. The RIFLE and AKIN classifications for acute kidney injury: a critical and comprehensive review. Clin Kidney J 2013;1:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol 2009;3: 672–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Waikar SS, Betensky RA, Emerson SC, Bonventre JV. Imperfect gold standards for kidney injury biomarker evaluation. J Am Soc Nephrol 2012;1:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shemesh O, Golbetz H, Kriss JP, Myers BD. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int 1985;5:830–8. [DOI] [PubMed] [Google Scholar]
- 105.Swan SK. The search continues–an ideal marker of GFR. Clin Chem 1997;6(Pt 1):913–4. [PubMed] [Google Scholar]
- 106.Duarte CG, Preuss HG. Assessment of renal function–glomerular and tubular. Clin Lab Med 1993;1:33–52. [PubMed] [Google Scholar]
- 107.Macedo E, Bouchard J, Soroko SH, et al. Fluid accumulation, recognition and staging of acute kidney injury in critically-ill patients. Crit Care 2010;3:R82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.US Food and Drug Administration. Critical Path Institute’s Predictive Safety Testing Consortium Nephrotoxicity Working Group (CPATH PSTC-NWG), and Foundation for the National Institutes of Health’s Biomarker Consortium Kidney Safety Biomarker Project Team (FNIH BC-KSP). Urinary nephrotoxicity biomarker panel as assessed by immunoassays. Safety biomarker to be used with traditional indicators to indicate renal injury in rat. Available from https://www.fda.gov/media/87781/download
- 109.US Food and Drug Administration. Critical Path Institute’s Predictive Safety Testing Consortium Nephrotoxicity Working Group (CPATH PSTCNWG), and Foundation for the National Institutes of Health’s Biomarker Consortium Kidney Safety Biomarker Project Team (FNIH BC-KSP). Safety biomarker panel to aid in the detection of kidney tubular injury in phase 1 trials in healthy volunteers. Available from https://www.fda.gov/media/135310/download
- 110.National Institutes of Health. U.S. National Library of Medicine. ClinicalTrials.gov Available from https://clinicaltrials.gov/ct2/show/NCT03921099. Accessed March 14, 2020.
- 111.Elyasi S, Khalili H, Hatamkhani S, Dashti-Khavidaki S. Prevention of vancomycin induced nephrotoxicity: a review of preclinical data. Eur J Clin Pharmacol 2013;4:747–54. [DOI] [PubMed] [Google Scholar]
- 112.Bayomy NA, Abdelaziz EZ, Said MA, Badawi MS, El-Bakary RH. Effect of pycnogenol and spirulina on vancomycin-induced renal cortical oxidative stress, apoptosis, and autophagy in adult male albino rat. Can J Physiol Pharmacol 2016;8:838–48. [DOI] [PubMed] [Google Scholar]
- 113.Uckun Z, Guzel S, Canacankatan N, Yalaza C, Kibar D, Coskun Yilmaz B. Potential protective effects of naringenin against vancomycin-induced nephrotoxicity via reduction on apoptotic and oxidative stress markers in rats. Drug Chem Toxicol 2020;43(1):104–11. [DOI] [PubMed] [Google Scholar]
- 114.Kandemir FM, Yildirim S, Kucukler S, Caglayan C, Mahamadu A, Dortbudak MB. Therapeutic efficacy of zingerone against vancomycin-induced oxidative stress, inflammation, apoptosis and aquaporin 1 permeability in rat kidney. Biomed Pharmacother 2018;105:981–91. [DOI] [PubMed] [Google Scholar]
- 115.Caglayan C, Taslimi P, Demir Y, Kucukler S, Kandemir FM, Gulcin I. The effects of zingerone against vancomycin-induced lung, liver, kidney and testis toxicity in rats: the behavior of some metabolic enzymes. J Biochem Mol Toxicol 2019;10:e22381. [DOI] [PubMed] [Google Scholar]
- 116.Shi H, Zou J, Zhang T, et al. Protective effects of DHA-PC against vancomycin-induced nephrotoxicity through the inhibition of oxidative stress and apoptosis in BALB/c mice. J Agric Food Chem 2018;2:475–84. [DOI] [PubMed] [Google Scholar]
- 117.Qu S, Dai C, Lang F, et al. Rutin attenuates vancomycin-induced nephrotoxicity by ameliorating oxidative stress, apoptosis, and inflammation in rats. Antimicrob Agents Chemother 2019;63(1):e01545–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guzel S, Sahinogullari ZU, Canacankatan N, Antmen SE, Kibar D, Coskun Yilmaz B. Potential renoprotective effects of silymarin against vancomycin-induced nephrotoxicity in rats. Drug Chem Toxicol 2019. 10.1080/01480545.2019.1584208 [DOI] [PubMed] [Google Scholar]
- 119.Panonnummal R, Varkey J, Dinoop DR. Are statins nephroprotective?: a dose dependent study in albino rats. Int J Pharm Pharm Sci 2013;3:182–90. [Google Scholar]
- 120.Ocak S, Gorur S, Hakverdi S, Celik S, Erdogan S. Protective effects of caffeic acid phenethyl ester, vitamin C, vitamin E and N-acetylcysteine on vancomycin-induced nephrotoxicity in rats. Basic Clin Pharmacol Toxicol 2007;5:328–33. [DOI] [PubMed] [Google Scholar]
- 121.Cetin H, Olgar S, Oktem F, et al. Novel evidence suggesting an anti-oxidant property for erythropoietin on vancomycin-induced nephrotoxicity in a rat model. Clin Exp Pharmacol Physiol 2007;11:1181–5. [DOI] [PubMed] [Google Scholar]
- 122.Oktem F, Arslan MK, Ozguner F, et al. In vivo evidences suggesting the role of oxidative stress in pathogenesis of vancomycin-induced nephrotoxicity: protection by erdosteine. Toxicology 2005;3:227–33. [DOI] [PubMed] [Google Scholar]
- 123.Toyoguchi T, Takahashi S, Hosoya J, Nakagawa Y, Watanabe H. Nephrotoxicity of vancomycin and drug interaction study with cilastatin in rabbits. Antimicrob Agents Chemother 1997;9:1985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kusama M, Yamamoto K, Yamada H, Kotaki H, Sato H, Iga T. Effect of cilastatin on renal handling of vancomycin in rats. J Pharm Sci 1998;9:1173–6. [DOI] [PubMed] [Google Scholar]
- 125.Wen S, Wang C, Huo X, et al. JBP485 attenuates vancomycin-induced nephrotoxicity by regulating the expressions of organic anion transporter (Oat) 1, Oat3, organic cation transporter 2 (Oct2), multidrug resistance-associated protein 2 (Mrp2) and P-glycoprotein (P-gp) in rats. Toxicol Lett 2018;295:195–204. [DOI] [PubMed] [Google Scholar]
- 126.Marre R, Schulz E, Hedtke D, Sack K. Influence of fosfomycin and tobramycin on vancomycin-induced nephrotoxicity. Infection 1985;4:190–2. [DOI] [PubMed] [Google Scholar]
- 127.Nakamura T, Hashimoto Y, Kokuryo T, Inui KI. Effects of fosfomycin and imipenem/cilastatin on nephrotoxicity and renal excretion of vancomycin in rats. Pharm Res 1998;5:734–8. [DOI] [PubMed] [Google Scholar]
- 128.Yoshiyama Y, Yazaki T, Wong PC, Beauchamp D, Kanke M. The effect of fosfomycin on glycopeptide antibiotic-induced nephrotoxicity in rats. J Infect Chemother 2001;4:243–6. [DOI] [PubMed] [Google Scholar]
- 129.Inouye S, Niizato T, Komiya I, Yuda Y, Yamada Y. Mode of protective action of fosfomycin against dibekacin-induced nephrotoxicity in the dehydrated rats. J Pharmacobiodyn 1982;12:941–50. [DOI] [PubMed] [Google Scholar]
- 130.Bayram A, Erkan GN, Talih G, et al. The alpha-2 receptor agonist dexmedetomidine attenuates vancomycin induced acute kidney injury. Bratisl Lek Listy 2019;6:429–33. [DOI] [PubMed] [Google Scholar]
- 131.Celik I, Cihangiroglu M, Ilhan N, Akpolat N, Akbulut HH. Protective effects of different antioxidants and amrinone on vancomycin-induced nephrotoxicity. Basic Clin Pharmacol Toxicol 2005;5:325–32. [DOI] [PubMed] [Google Scholar]
- 132.Hodoshima N, Nakano Y, Izumi M, et al. Protective effect of inactive ingredients against nephrotoxicity of vancomycin hydrochloride in rats. Drug Metab Pharmacokinet 2004;1:68–75. [DOI] [PubMed] [Google Scholar]
- 133.Liu J, Wang Z, Li F, Gao J, Wang L, Huang G. Liposomes for systematic delivery of vancomycin hydrochloride to decrease nephrotoxicity: characterization and evaluation. Asian J Pharm Sci 2015;3:212–22. [Google Scholar]
- 134.Muppidi K, Wang J, Betageri G, Pumerantz AS. PEGylated liposome encapsulation increases the lung tissue concentration of vancomycin. Antimicrob Agents Chemother 2011;10:4537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Makhathini SS, Kalhapure RS, Jadhav M, et al. Novel two-chain fatty acid-based lipids for development of vancomycin pH-responsive liposomes against Staphylococcus aureus and methicillin-resistant Staphylococcus aureus (MRSA). J Drug Target 2019;10:1094–107. [DOI] [PubMed] [Google Scholar]
- 136.Pawar V, Bulbake U, Khan W, Srivastava R. Chitosan sponges as a sustained release carrier system for the prophylaxis of orthopedic implant-associated infections. Int J Biol Macromol 2019;134:100–12. [DOI] [PubMed] [Google Scholar]
- 137.Kalhapure RS, Jadhav M, Rambharose S, et al. pH-responsive chitosan nanoparticles from a novel twin-chain anionic amphiphile for controlled and targeted delivery of vancomycin. Colloids Surf B Biointerfaces 2017;158:650–57. [DOI] [PubMed] [Google Scholar]
- 138.Chakraborty SP, Kar Mahapatra S, Sahu SK, et al. Internal-ization of Staphylococcus aureus in lymphocytes induces oxidative stress and DNA fragmentation: possible ameliorative role of nanoconjugated vancomycin. Oxid Med Cell Longev 2011;2011:942123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ahmida MH. Protective role of curcumin in nephrotoxic oxidative damage induced by vancomycin in rats. Exp Toxicol Pathol 2012;3:149–53. [DOI] [PubMed] [Google Scholar]
- 140.Basarslan F, Yilmaz N, Ates S, et al. Protective effects of thymoquinone on vancomycin-induced nephrotoxicity in rats. Hum Exp Toxicol 2012;7:726–33. [DOI] [PubMed] [Google Scholar]
- 141.Sakamoto Y, Yano T, Hanada Y, et al. Vancomycin induces reactive oxygen species-dependent apoptosis via mitochondrial cardiolipin peroxidation in renal tubular epithelial cells. Eur J Pharmacol 2017;800:48–56. [DOI] [PubMed] [Google Scholar]
- 142.Toyoguchi T, Nakagawa Y. [Nephrotoxicity and drug interaction of vancomycin (2)]. Nihon Yakurigaku Zasshi 1996;5:225–35. [DOI] [PubMed] [Google Scholar]
- 143.Greenwald RB, Zhao H, Xia J, Martinez A. Poly(ethylene glycol) transport forms of vancomycin: a long-lived continuous release delivery system. J Med Chem 2003;23:5021–30. [DOI] [PubMed] [Google Scholar]
- 144.Sande L, Sanchez M, Montes J, et al. Liposomal encapsulation of vancomycin improves killing of methicillin-resistant Staphylococcus aureus in a murine infection model. J Antimicrob Chemother 2012;9:2191–4. [DOI] [PubMed] [Google Scholar]
- 145.Liu J, Wang Z, Li F, Gao J, Wang L, Huang G. Liposomes for systematic delivery of vancomycin hydrochloride to decrease nephrotoxicity: characterization and evaluation. Asian J Pharm Sci 2015;10:212–22. [Google Scholar]
- 146.Guan D, Chen F, Xiong L, et al. Extra sugar on vancomycin: new analogues for combating multidrug-resistant Staphylococcus aureus and vancomycin-resistant enterococci. J Med Chem 2018;1:286–304. [DOI] [PubMed] [Google Scholar]
- 147.Hassan D, Omolo CA, Gannimani R, et al. Delivery of novel vancomycin nanoplexes for combating methicillin resistant Staphylococcus aureus (MRSA) infections. Int J Pharm 2019;558:143–56. [DOI] [PubMed] [Google Scholar]
- 148.Kaur A, Preet S, Kumar V, Kumar R, Kumar R. Synergetic effect of vancomycin loaded silver nanoparticles for enhanced antibacterial activity. Colloids Surf B Biointerfaces 2019;176:62–69. [DOI] [PubMed] [Google Scholar]
- 149.Hodoshima N, Masuda S, Inui K. Decreased renal accumulation and toxicity of a new VCM formulation in rats with chronic renal failure. Drug Metab Pharmacokinet 2007;6:419–27. [DOI] [PubMed] [Google Scholar]
- 150.Nakamura T, Kokuryo T, Hashimoto Y, Inui KI. Effects of fosfomycin and imipenem-cilastatin on the nephrotoxicity of vancomycin and cisplatin in rats. J Pharm Pharmacol 1999;2:227–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of studies that have evaluated potential strategies for prevention of VIKI.