Abstract
Objective
Previous research has shown that women have an advantage on verbal episodic memory and processing speed tasks, while men show an advantage on spatial ability measures. Previous work has also found differences in cognition across age. The current study examines gender differences in neurocognitive functioning across adulthood, whether age moderates this effect, and whether these differences remain consistent with practice across multiple testing sessions.
Method
Data from the Virginia Cognitive Aging Project were used, which included participants between the ages of 18 and 99 years (N = 5125). Participants completed measures assessing five cognitive domains: episodic memory, processing speed, reasoning, spatial visualization, and vocabulary.
Results
Results showed that gender was significantly related to memory, speed, and spatial visualization, but not to vocabulary or reasoning. Results of invariance analyses across men and women provided evidence of configural and metric invariance, along with partial scalar invariance. Additionally, there was little evidence that age or practice influenced the gender effect on neurocognition.
Conclusions
Consistent with the previous research, these results suggest that there is a female advantage in episodic memory and processing speed, and a male advantage in spatial visualization. Gender was shown to influence cognition similarly across adulthood. Furthermore, the influence of gender remained the same across three sessions, which is consistent with the previous work that has shown that training does not differentially impact performance on spatial ability measures for females compared to males.
Keywords: Aging, Cognition, Cross-sectional, Memory, Processing speed, Sex differences, Lifespan, Spatial ability
Neurocognitive differences between men and women have been a topic of research for decades, and recent debate surrounding the lack of proportionate representation of women in science-related fields has included the examination of cognitive differences between genders (Berenbaum & Resnick, 2007). Research has consistently demonstrated a female advantage in verbal episodic memory tasks (e.g., Herlitz, Airaksinen, & Nordström, 1999; Herlitz, Nilsson, & Backman, 1997; Schaie & Willis, 1993; Zelinski, Gilewski, & Schaie, 1993) and a male advantage in spatial tasks (e.g., Maeda & Yoon, 2013). There is also evidence of a female advantage in processing speed measures (e.g., Daseking, Petermann, & Waldmann, 2017; Irwing, 2012). Although some researchers have found a male advantage in general intelligence (referred to as g) (Irwing, 2012), many others have found minimal or null effects of gender on g (e.g., Saggino et al., 2014; Salthouse, 2004a; Salthouse & Ferrer-Caja, 2003).
Age-associated differences in cognition are also well documented. Cognition can be partitioned into crystallized (or product) and fluid (or process) domains. Cross-sectionally, age is generally associated with increases in crystallized intelligence (assessed with measures such as vocabulary) until about age 60 (e.g., Salthouse, 2014a), and decreases in fluid intelligence (e.g., Salthouse, 2019). Fluid domains that have demonstrated age-associated declines include reasoning (e.g., Salthouse, 2004b) and spatial ability (e.g., Borella, Meneghetti, Ronconi, & De Beni, 2014; Salthouse, Babcock, Skovronek, Mitchell, & Palmon, 1990). To illustrate, a recent meta-analysis by Techentin, Voyer, and Voyer (2014) reported a large (mean d = 1.01) age-related decrease in performance on tests of spatial ability between young and older adults. In addition, verbal episodic memory (e.g., Lundervold Wollshlaeager, & Wehling, 2014; Whitley et al., 2016) and processing speed (e.g., Salthouse, 2004b) also show age-associated declines.
Gender Differences in Cognition Across the Adult Lifespan
Gender differences in intelligence, often referred to as general cognitive ability (or g), has been a controversial topic in the field of psychology. Savage-McGlynn (2012) states that “No other concept in psychology has generated more debate (Johnson, 2004), and may arguably be the longest-running and most impassioned controversy in psychology’s history (Halpern, [2011])” (p. 137). However, the number of studies that have comprehensively examined gender differences in broad domains of cognition across the adult lifespan is limited (but see Daseking et al., 2017; Irwing, 2012; Salthouse, 2004a; Salthouse & Ferrer-Caja, 2003). Daseking et al. (2017) recently assessed gender differences in cognitive abilities in the German standardization sample for the Wechsler Adult Intelligence Scale-IV (WAIS-IV); participants ranged from 16 to 89 years of age. Daseking et al. found that men scored higher on the visual processing, fluid reasoning, and verbal comprehension indices, and women scored higher on the processing speed index. The gender effect was consistently small across the subtests (i.e., the maximum eta squared was .05). Education level had a greater effect on cognitive performance than did gender. Similarly, Irwing (2012) examined gender differences in the WAIS-III using the US standardization sample spanning across ages 16–89 years using both hierarchical and bi-factor multigroup confirmatory factor analysis. Results indicated a male advantage in g, and in the information, arithmetic, and symbol search tasks, and, consistent with Daseking et al. (2017), there was a female advantage in processing speed.
McCarrey, An, Kitner-Triolo, Ferrucci, and Resnick (2016) recently examined gender differences in cognitive trajectories for a large battery of cognitive tasks in a large sample of individuals over the age of 50. They found that after controlling for age, education, and race, women performed better than men on most tests of cognition, except for two visuospatial tasks, in which men performed better than women. Results also showed that men demonstrated steeper rates of decline over time for a global measure of cognition, a processing speed task, and two visuospatial tasks, whereas there was no evidence of women having steeper declines in cognition over time, compared to men.
Age Moderation on Gender Differences in Cognition
There is evidence to suggest that age moderates gender differences in childhood and adolescence. For example, Lynn (1994, 1999) suggested there are negligible gender differences in g among children and adolescents until age 16, at which time a male advantage emerges and increases into adulthood (but see Savage-McGlynn, 2012). However, few studies have examined whether age moderates gender differences across adulthood. This is a compelling question because some researchers have suggested that the magnitude of gender differences may decline over time due to changes in societal expectations (e.g., Priess & Hyde, 2010). As summarized by Perales, Lersch, and Baxter (2019), “The second half of the 20th century brought about unprecedented historical changes in the socio-economic standing of women in developed nations, collectively labelled as the ‘gender revolution’ (England, 2010)” (p. 8). Commensurate with these changes, the endorsement of traditional gender attitudes and ideologies decreased. A great deal of research that has examined changes regarding society’s endorsement of traditional gender roles has used the General Social Survey (GSS). Research examining trends between 1977 and 1998 showed substantial and fairly monotonic increases in attitudes towards gender equality and less restrictive gender roles in the United States (e.g., Brooks & Bolzendahl, 2004). Recent work examining the GSS suggests that this trend may have reversed slightly in the 1990s, and then rebounded in the early 2000s (Cotter, Hermsen, & Vanneman, 2011). In addition, recent work by Reilly, Neumann, and Andrews (2016) found that sex-role identity (e.g., masculine, feminine) accounted for more of the variance in spatial ability and language ability than did sex itself in sample of college-aged participants. Furthermore, masculine sex-roles partially mediated the relationship between sex and a spatial ability composite, and feminine sex-roles fully mediated the relationship between sex and a language ability composite. Thus, one may expect larger gender effects in older samples and smaller gender effects in younger samples due to shifts in societal beliefs as a result of changes in traditional roles for both men and women.
Practice Effects
There is evidence that gender differences in some cognitive domains may be attributed to differential practice and experience (e.g., Reilly, Neumann, & Andrews, 2017). This has been studied extensively within the field of spatial ability. As noted by Reilly et al. (2017), “A large number of studies have examined the effects of brief training interventions to improve spatial ability. While there is wide variation in effectiveness, almost all such interventions show some improvement in spatial ability” (p. 18). It has been suggested that women may show increased improvements with training in spatial ability tasks because they have less spatial experience (Fennema & Sherman, 1977; Sherman, 1967). However, two separate meta-analyses of spatial training interventions have failed to detect differential improvement in women (Baenninger & Newcombe, 1989; Uttal et al., 2013), indicating that the gender gap in spatial ability was not reduced through training. Taking the same cognitive test more than once may be considered a type of intervention in which an individual is provided with experience practicing items from a test. One unique aspect of the current study is that participants completed the same 16 measures of cognition on three occasions within a 2-week period. One session used the original versions of the tasks, and the other two sessions used alternate versions with the same instructions but different items. Although practice or training does not appear to impact spatial ability differentially across women and men (Baenninger & Newcombe, 1989; Uttal et al., 2013), there has been little assessment of the influence of practice on the gender effect within other cognitive domains. Thus, an interesting empirical question is whether the effect of gender on cognitive abilities, such as memory and processing speed, diminishes with increased practice. That is, does repeated exposure to the task result in a reduction in the influence of gender on performance?
The Current Study
The goals of the current study are to (1) examine gender differences in cognition across adulthood, (2) examine whether age moderates the gender effect on cognition, and (3) examine whether the gender effect is consistent across multiple sessions.
METHODS
Participants
Data from the Virginia Cognitive Aging Project (VCAP; Salthouse, 2014b), a prospective study of cognition in community dwelling adults between the ages of 18 and 99 years, are used. Participants were recruited from the community through newspaper advertisements, flyers, and referrals from other participants. To participate, individuals needed to be fluent in English, have the equivalent of a high school level of education, and have sufficient hearing and vision to perform the tasks. Participant characteristics are presented in Table 1, by gender and across age. Gender was assessed with the question, “Are you male or female?” The current study uses data from the first measurement occasion of 5125 individuals who participated in VCAP. Participants visited the lab three different times within a period of 2 weeks to complete a comprehensive cognitive assessment. In each session, participants completed the same cognitive tasks but different versions of each task. All data were collected with the approval of the local Institutional Review Board, and in compliance with the Helsinki Declaration.
Table 1.
Participant characteristics
| Total sample |
18–39 years |
40–64 years |
65–99 years |
|||||
|---|---|---|---|---|---|---|---|---|
|
N = 5125 |
n = 1425 |
n = 2482 |
n = 1218 |
|||||
| Men | Women | Men | Women | Men | Women | Men | Women | |
| n | 1795 | 3330 | 543 | 882 | 747 | 1735 | 505 | 713 |
| Age | 51.09 (19.92) | 50.68 (17.17) | 25.85 (5.84) | 27.89 (6.30) | 53.37 (6.83) | 52.88 (6.70) | 74.86 (6.88) | 73.51 (6.56) |
| Race | ||||||||
| % American Indian/Alaskan Native | 1.9 | 2.0 | 0.7 | 1.0 | 2.0 | 1.8 | 3.2 | 3.5 |
| % Asian | 1.7 | 1.2 | 3.1 | 2.7 | 1.2 | 0.7 | 1.0 | 0.0 |
| % Native Hawaiian or Other Pacific Islander | 0.4 | 0.1 | 0.0 | 0.3 | 0.7 | 0.1 | 0.4 | 0.6) |
| % Black or African American | 9.5 | 13.4 | 13.3 | 21.8 | 12.6 | 13.3 | 1.0 | 3.2 |
| % White | 81.0 | 77.7 | 75.5 | 67.8 | 79.8 | 78.9 | 88.7 | 87.0 |
| % More than one | 4.4 | 4.9 | 6.8 | 5.9 | 2.5 | 4.4 | 4.6 | 4.9 |
| % Not reported | 0.2 | 0.1 | 0.2 | 0.2 | 0.3 | 0.1 | 0.0 | 0.1 |
| % Hispanic/Latino | 1.8 | 1.5 | 3.5 | 2.7 | 1.3 | 1.4 | 0.8 | 0.3 |
| Self-rated health | 2.20 (.91) | 2.17 (.89) | 2.03 (.88) | 2.06 (.83) | 2.22 (.92) | 2.15 (.89) | 2.36 (.89) | 2.36 (.90) |
| Education | 15.78 (2.82) | 15.38 (4.34) | 14.99 (2.52) | 14.89 (4.52) | 15.75 (2.88) | 15.64 (3.82) | 16.68 (2.79) | 15.38 (5.19) |
| Vocab | 49.25 (11.94) | 49.26 (11.87) | 49.46 (11.44) | 46.74 (12.27) | 48.44 (13.13) | 49.98 (12.06) | 50.22 (10.46) | 50.63 (10.36) |
| Picture vocab | 18.28 (5.41) | 17.15 (5.84) | 16.01 (5.43) | 14.04 (5.56) | 19.04 (5.40) | 18.21 (5.72) | 19.61 (4.62) | 18.38 (5.05) |
| Synonym vocab | 6.91 (2.82) | 6.66 (2.95) | 5.94 (2.85) | 5.30 (2.88) | 7.00 (2.79) | 6.90 (2.92) | 7.84 (2.50) | 7.73 (2.48) |
| Antonym vocab | 6.37 (2.91) | 6.22 (2.98) | 5.62 (2.80) | 5.20 (2.73) | 6.47 (2.97) | 6.51 (2.99) | 7.05 (2.76) | 6.76 (2.93) |
| Matrix reasoning | 7.66 (3.62) | 7.38 (3.29) | 10.20 (3.32) | 9.18 (3.18) | 7.33 (3.08) | 7.41 (2.96) | 5.32 (2.85) | 5.02 (2.68) |
| Shipley | 12.74 (3.94) | 13.04 (3.60) | 14.86 (3.19) | 14.52 (2.97) | 12.49 (3.75) | 13.06 (3.53) | 10.82 (3.83) | 11.26 (3.64) |
| Letter sets | 10.66 (2.99) | 11.03 (2.77) | 11.63 (2.72) | 11.65 (2.53) | 10.62 (2.99) | 11.20 (2.69) | 9.57 (2.92) | 9.80 (2.91) |
| Spatial relations | 9.51 (5.20) | 7.77 (4.73) | 12.09 (5.36) | 9.42 (5.26) | 9.16 (4.95) | 7.82 (4.57) | 7.15 (3.97) | 5.59 (3.34) |
| Paper folding | 6.39 (2.94) | 5.76 (2.67) | 8.16 (2.79) | 6.96 (2.70) | 6.08 (2.74) | 5.72 (2.56) | 4.91 (2.35) | 4.37 (2.14) |
| Form boards | 7.82 (4.63) | 6.44 (4.01) | 10.71 (4.84) | 8.54 (4.46) | 7.18 (4.09) | 6.25 (3.60) | 5.50 (3.28) | 4.21 (2.87) |
| Recall | 32.44 (7.25) | 34.78 (6.37) | 36.77 (5.84) | 37.34 (5.27) | 32.38 (6.54) | 35.01 (5.91) | 27.86 (6.77) | 31.04 (6.93) |
| Paired associates | 2.62 (1.77) | 2.98 (1.77) | 3.70 (1.71) | 3.67 (1.74) | 2.51 (1.63) | 3.00 (1.72) | 1.61 (1.35) | 2.11 (1.54) |
| Logical memory | 41.63 (10.62) | 44.44 (10.17) | 45.34 (10.45) | 47.05 (10.31) | 41.40 (10.25) | 44.39 (9.81) | 37.90 (9.98) | 41.34 (9.98) |
| Digit symbol | 67.00 (18.38) | 73.19 (17.52) | 80.01 (16.71) | 84.83 (15.52) | 66.51 (14.73) | 73.48 (14.84) | 53.69 (14.90) | 58.05 (14.27) |
| Pattern comparison | 15.92 (4.01) | 16.06 (3.64) | 18.83 (3.83) | 18.50 (3.36) | 15.69 (3.18) | 15.96 (3.16) | 13.08 (3.04) | 13.24 (2.89) |
| Letter comparison | 10.15 (2.71) | 10.45 (2.40) | 11.82 (2.50) | 11.74 (2.21) | 10.07 (2.35) | 10.51 (2.16) | 8.45 (2.30) | 8.71 (2.08) |
Measures
Five cognitive domains were examined: episodic memory, processing speed, reasoning, spatial visualization, and vocabulary. Episodic memory was assessed with tests of word recall (Wechsler, 1997b), paired associate learning (Salthouse, Fristoe, & Rhee, 1996), and logical memory (Wechsler, 1997b). Processing speed was assessed with the digit symbol substitution test (Wechsler, 1997a) and pattern comparison and letter comparison tests (Salthouse & Babcock, 1991). Reasoning was assessed with tests of matrix reasoning (Raven, 1962), series completion (Zachary, 1986), and letter sets (Ekstrom, French, Harman, & Dermen, 1976). Spatial visualization was assessed with tests of spatial relations (Bennett et al., 1997), paper folding (Ekstrom et al., 1976), and form boards (Ekstrom et al., 1976). Vocabulary was assessed with tests of vocabulary (Wechsler, 1997a), picture vocabulary (Woodcock & Johnson, 1990), synonym vocabulary, and antonym vocabulary (Salthouse, 1993). See the Supplemental Table [dummy]for a brief description of each task.
Statistical Analyses
Structural equation modeling is used to conduct the analyses using Amos 24.0 (Arbuckle, 2015). To evaluate model fit, several fit indices are examined, such as the chi-square test statistic, including the chi-square ratio ( χ2/df), the root mean square error of approximation (RMSEA; <.06 as good fit, <.08 as acceptable fit; Browne & Cudeck, 1993; Hu & Bentler, 1999), and the comparative fit index (CFI) in which values ≥.95 are indicative of a good fit (Hu & Bentler, 1999). To assess invariance across gender, increasingly stringent levels of invariance are assessed: (1) configural invariance analyses assess whether the factor structure (i.e., the relations among the variables) is the same across groups, (2) metric invariance analyses assess whether the magnitude of the factor loadings from the observed variables to the latent constructs are invariant across groups, and (3) scalar invariance analyses assess whether the magnitude of the observed variable intercepts are invariant across groups. The fit of each model is compared to the preceding model. In evaluating model fit, Cheung and Rensvold (2002) suggest that a change in the CFI value of ≤ −.010 is indicative of no substantial change in model fit. When an invariance model fits worse than the preceding model that it is nested within, partial invariance is investigated and is demonstrated when allowing one or more non-invariant items to differ between groups (Millsap & Kwok, 2004).
A p value of .01 is used for all analyses. Full-information maximum likelihood estimation is used to deal with missing data. Unless otherwise specified, analyses were performed on data from session one.
RESULTS
Zero Order Correlations
Table 2 presents the zero-order correlations between gender (coded 0 for male and 1 for female) and each of the cognitive variables. Gender was significantly negatively associated with picture vocabulary, synonym vocabulary, matrix reasoning, spatial relations, paper folding, and form boards such that men performed better on those tests. Gender was significantly positively associated with letter sets, word recall, paired associates, logical memory, and letter comparison indicating that women performed better on those tests.
Table 2.
Correlation matrix
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. | 14. | 15. | 16. | 17. | 18. | 19. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Gender | 1 | ||||||||||||||||||
| 2. Age | −.01 | 1 | |||||||||||||||||
| 3. Self-rated health | −.02 | .13* | 1 | ||||||||||||||||
| 4. Education | −.05* | .12* | −.10* | 1 | |||||||||||||||
| 5. Vocab | .00 | .09* | −.15* | .37* | 1 | ||||||||||||||
| 6. Picture vocab | −.10* | .28* | −.13* | .30* | .71* | 1 | |||||||||||||
| 7. Synonym vocab | −.04* | .30* | −.13* | .36* | .74* | .71* | 1 | ||||||||||||
| 8. Antonym vocab | −.03 | .21* | −.13* | .35* | .69* | .66* | .78* | 1 | |||||||||||
| 9. Matrix reasoning | −.04* | −.49* | −.18* | .17* | .41* | .28* | .27* | .30* | 1 | ||||||||||
| 10. Shipley | .04 | −.37* | −.21* | .21* | .51* | .39* | .39* | .40* | .69* | 1 | |||||||||
| 11. Letter sets | .06* | −.25* | −.19* | .21* | .47* | .36* | .37* | .39* | .60* | .69* | 1 | ||||||||
| 12. Spatial relations | −.17* | −.32* | −.17* | .16* | .41* | .38* | .32* | .33* | .67* | .57* | .52* | 1 | |||||||
| 13. Paper folding | −.11* | −.40* | −.18* | .15* | .38* | .32* | .26* | .28* | .66* | .59* | .52* | .70* | 1 | ||||||
| 14. Form boards | −.15* | −.43* | −.13* | .08* | .28* | .23* | .16* | .19* | .61* | .53* | .43* | .65* | .59* | 1 | |||||
| 15. Word recall | .16* | −.42* | −.16* | .12* | .34* | .22* | .20* | .23* | .50* | .52* | .44* | .37* | .42* | .34* | 1 | ||||
| 16. Paired associates | .10* | −.38* | −.13* | .14* | .39* | .28* | .27* | .28* | .51* | .52* | .44* | .45* | .45* | .40* | .61* | 1 | |||
| 17. Logical memory | .13* | −.25* | −.13* | .18* | .47* | .38* | .36* | .36* | .46* | .54* | .44* | .39* | .41* | .32* | .58* | .54* | 1 | ||
| 18. Digit symbol | .16* | −.55* | −.24* | .11* | .24* | .10* | .09* | .14* | .54* | .57* | .49* | .40* | .44* | .41* | .51* | .43* | .38* | 1 | |
| 19. Pattern comparison | .02 | −.56* | −.19* | .05* | .17* | .07* | .03 | .08* | .48* | .45* | .34* | .40* | .41* | .45* | .43* | .38* | .31* | .64* | 1 |
| 20. Letter comparison | .06* | −.48* | −.21* | .10* | .22* | .10* | .11* | .16* | .47* | .52* | .41* | .33* | .35* | .36* | .43* | .34* | .34* | .66* | .66* |
Note. For gender, 0 = male and 1 = female.
p < .01.
Invariance Analyses
Cross-sectional gender differences in cognition were first examined via invariance analyses across the total sample of participants between the ages of 18 and 99 years (N = 5125). There was strong evidence of configural and metric invariance (see Table 3), which are important prerequisites for making comparisons across groups. However, the scalar invariance model in which intercepts are constrained to be equal across men and women fit worse relative to the metric invariance model (see Model 3 in Table 3), as indicated by a change in CFI > −.01 (Cheung & Rensvold, 2002). To examine which intercepts were contributing the most to the reduction in fit, and to establish partial scalar invariance, the intercept for each of the 16 variables was constrained to be equal between the two groups one at a time. Constraining the intercepts of the spatial relations, form board, word recall, and digit symbol variables yielded the largest reductions in overall fit. Thus, when those four intercepts were allowed to vary between men and women, partial scalar invariance was obtained. There was also evidence of invariance at the structural level since the change in CFI was < −.01, and the RMSEA indicated a slight improvement in Model 5 as compared to Model 4.
Table 3.
Model fit for the multigroup invariance analyses across men (n = 1795) and women (n = 3330)
| Model | χ2 | df | χ2/df | CFI | RMSEA | ΔCFI |
|---|---|---|---|---|---|---|
| Model 1: Configural invariance | 2661.52 | 188 | 14.16 | 0.951 | 0.051 | |
| Model 2: Invariant factor loadings | 2742.50 | 199 | 13.78 | 0.950 | 0.050 | −0.001 |
| Model 3: Model 2 and invariant intercepts | 3758.80 | 215 | 17.48 | 0.930 | 0.057 | −0.021 |
| Model 4: Model 3 and partially invariant intercepts | 3199.50 | 211 | 15.16 | 0.941 | 0.053 | −0.010 |
| Model 5: Model 4 and invariant latent variable variances and covariances | 3309.29 | 226 | 14.64 | 0.939 | 0.052 | −0.002 |
Gender Differences in Cognitive Factors, Covarying for Age, Education, and Health
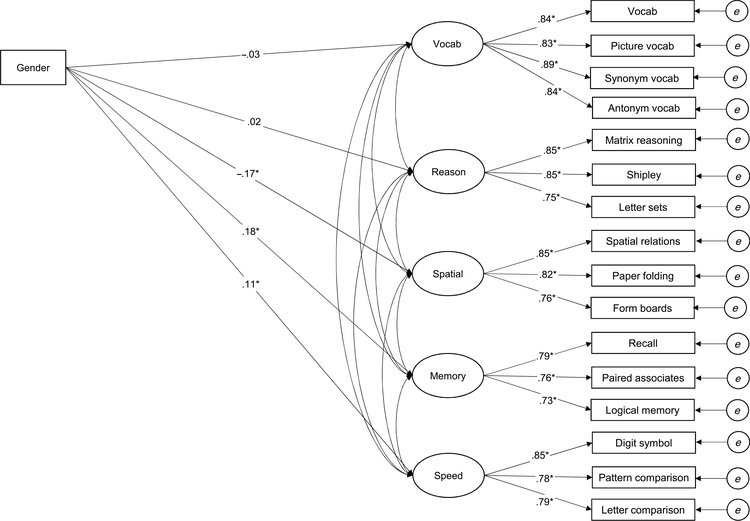
In order to statistically control for several variables that may influence cognitive performance, a five-factor model comprising the five latent cognitive constructs (memory, speed, vocabulary, reasoning, and spatial visualization) was examined in which performance on each factor was predicted by gender (male = 0, female = 1) with age, self-rated health, and education included as covariates (see Figure 1). The resulting model fit the data fairly well, χ2 = 3564.46, df = 138, χ2/df = 25.83, CFI = .941, RMSEA = .070. Gender was significantly related to memory (.18*), speed (.11*), and spatial visualization (−.17*) such that women performed better on measures of memory and speed, and men performed better on measures of spatial visualization. Gender was not significantly related to vocabulary (−.03) or reasoning (.02).
Fig. 1.
Structural equation model depicting the relationship of gender to cognitive factors. Note. For gender, 0 = male and 1 = female. Age, self-rated health, and education are included as covariates but are not depicted in the figure for presentation purposes. Observed variables are depicted as rectangles, and latent variables are depicted as ovals or circles. The latent variables labeled “e” represent the error and unique variance associated with each observed variable. *p < .01.
Age Moderation of the Gender Effect on Cognition
To examine whether age moderated the gender effect on cognition, the sample was divided into three age groups corresponding to 18–39 years (n = 1425), 40–64 years (n = 2482), and 65–99 years (n = 1218). The model depicted in Figure 1 was examined for each age group. Standardized coefficients and 99% confidence intervals are presented in Table 4. Gender was associated with performance on spatial visualization ability, memory, and speed consistently across all three age groups. Gender was significantly associated with reasoning in the middle-aged group only, with women performing better than men (β = .05*), but the confidence intervals of the coefficients overlapped suggesting the differences in coefficients were not significant across the age groups. There was evidence that age moderated the gender effect on vocabulary. Specifically, in the younger group the gender effect was significant ( β = −.14*) on vocabulary, with men performing better than women. The confidence intervals in the middle and older adult age groups do not overlap with the confidence intervals from the younger group, suggesting that the magnitude of the coefficient in the younger group is significantly greater than in the other two age groups.
Table 4.
Standardized coefficients (99% confidence intervals) of gender to each cognitive construct across age groups
| 18–39 years |
40–64 years |
65–99 years |
|
|---|---|---|---|
| n = 1425 | n = 2482 | n = 1218 | |
| gender -> vocab | −.14* (−.20, −.07) | .00 (−.04, .04) | −.01 (−.07, .06) |
| gender -> reasoning | −.06 (−.13, .01) | .05* (.003, .11) | .01 (−.06, .08) |
| gender -> spatial visualization | −.23* (−.29, −.16) | −.14* (−.19, −.09) | −.27* (−.35, −.19) |
| gender -> memory | .10* (.02, .17) | .20* (.15, .26) | .24* (.16, .31) |
| gender -> speed | .11* (.03, .18) | .16* (.11, .21) | .07* (.002, .15) |
Note. For gender, 0 = male and 1 = female.
p < .01.
Effect of Practice on the Gender Effect
To examine whether increased practice is associated with an attenuation of the gender effect, the standardized coefficients from gender to each cognitive construct was examined for sessions 1, 2, and 3, which took place across a 2-week period. Inspection of Table 5 shows that the gender effect was consistent across the three sessions. There was a female advantage in memory with standardized loadings of .18*, .21*, and .22* across the three sessions respectively, and in speed, the standardized loadings were .11*, .11*, and .11* across the three sessions respectively. Likewise, there was a male advantage in spatial ability with standardized loadings of −.17*, −.13*, and −.10* across the three sessions, which showed an attenuation in magnitude across the sessions, but overlapping 99% confidence intervals indicate that the differences in magnitude were not significant.
Table 5.
Standardized coefficients (99% confidence intervals) of gender to each cognitive construct across each session
| Session 1 | Session 2 | Session 3 | |
|---|---|---|---|
| gender-> vocab | −.03 (−.06, .00) | −.02 (−.07, .03) | −.03 (−.09, .03) |
| gender -> reasoning | .02 (−.02, .06) | −.01 (−.04, .02) | .05* (.01, .09) |
| gender -> spatial visualization | −.17* (−.20, −.14) | −.13* (−.18, −.08) | −.10* (−.15, −.05) |
| gender -> memory | .18* (.15, .21) | .21* (.17, .25) | .22* (.17, .27) |
| gender-> speed | .11* (.08, .14) | .11* (.07, .15) | .11* (.07, .15) |
Note. For gender, 0 = male and 1 = female.
N = 5195.
p < .01.
DISCUSSION
The results of the current study provide additional evidence that the meaning of cognitive constructs of episodic memory, processing speed, reasoning, spatial visualization, and vocabulary are invariant across gender. Specifically, we found strong evidence of both configural (invariant factor structure) and metric (invariant factor loadings) invariance. This is important because configural and metric invariance are considered to be a necessary prerequisite for making unambiguous comparisons across groups (e.g., Horn & McArdle, 1992). A demonstration of configural and metric invariance means that the relations among the variables, as well as the magnitude of the loadings from the observed variables to the latent constructs are not substantially different across men and women. Furthermore, partial scalar invariance (invariant intercepts) was obtained by allowing the intercepts of two spatial visualization variables (spatial relations and form boards), an episodic memory variable (word recall), and processing speed variable (digit symbol) to vary across the two groups. These variables have some of the strongest associations with age, as indicated by the correlations reported in Table 2.
Consistent with the previous research, we found a female advantage in verbal episodic memory (Herlitz et al., 1999, 1997; Salthouse, 2004a; Salthouse & Ferrer-Caja, 2003; Schaie & Willis, 1993; Zelinski et al., 1993) and in processing speed (Camarata & Woodstock, 2006; Daseking et al., 2017; Irwing, 2012), and a male advantage in spatial visualization ability (e.g., Maeda & Yoon, 2013; Salthouse, 2004a; Salthouse & Ferrer-Caja, 2003). These advantages were evident after statistically controlling for age, self-rated health, and education. Demographic norms that adjust for age are common in neuropsychological batteries. Some batteries (e.g., WAIS, WMS) also include norms adjusted for gender (e.g., Lange, Chelune, Taylor, Woodward, & Heaton, 2006). Although gender effects are modest, they are consistently found which suggests that it may be worthwhile to include norms adjusted for gender on most batteries that assess episodic verbal memory, processing speed, and/or spatial visualization.
Despite speculation that changing societal expectations regarding gender norms may influence the effect of gender on cognition, there was little evidence of age moderation. Rather, gender influenced cognition similarly across the adult lifespan, with the exception of vocabulary. There was a male advantage on vocabulary, but only in the young adult group. The finding of a male advantage in the young adult group is consistent with the work by Camarata and Woodcock (2006), who reported that males performed better on a crystallized ability (Gc) construct in three different samples spanning preschool through adulthood. The lack of consistent age moderation is interesting because it suggests that whatever contributes to gender differences, whether biological or experiential in nature, persists throughout adulthood.
A novel contribution of the current study was our ability to examine the influence of practice on the effect of gender on cognitive performance. Participants completed the tasks three different times across a 2-week period, using alternate versions of the task each time. The influence of gender on cognition was remarkably consistent across sessions, suggesting that repeated exposure to a task does not attenuate the effect of gender on cognition. This is consistent with the work (examining more intensive practice interventions) in the spatial ability domain which has shown that training or practice does not differentially impact performance in spatial ability tasks for women as compared to men (e.g., Baenninger & Newcombe, 1989; Uttal et al., 2013). However, some researchers have found that trajectories of improvement in spatial ability tasks may be moderated by initial level of performance (e.g., Terlecki, Newcombe, & Little, 2008), and Uttal et al. (2013) state that, “This difference in learning trajectory is important because it suggests that if training periods are not sufficiently long, female participants will appear to benefit less from training and show smaller training-related gains than male participants” (p. 367). Therefore, the brevity of the current study’s practice intervention makes it difficult to draw strong conclusions about the absence of a gender effect. It is possible that additional practice may have minimized gender differences.
Explanations for the Gender Effect on Cognition
There is currently no clear-cut explanation for gender differences in neurocognition. Some researchers speculate that biological factors, such as prenatal and postnatal hormones (Halpern et al., 2007) and biological predispositions (e.g., genes associated with the X chromosome; Johnson, Carothers, & Deary, 2009), are primarily responsible, whereas others postulate environmental factors, such as parental care (e.g., parents monitoring boys less closely and being more restrictive of girls and parental assumptions of their child’s interests; Halpern et al., 2007), education (Halpern et al., 2007; Jones & Wheatley, 1990), and stereotypes (e.g., Cadinu, Maas Rosablanca, & Kiesner, 2005; Halpern et al., 2007), are primarily responsible. In terms of education, it has been reported that males and females receive differential treatment according to gender, such that teachers attend to and call out male students more often in class for disruptive behavior and encourage male students to ask more questions in math and science classes, creating different learning environments for males and females (Jones & Wheatley, 1990). Alternatively, the concept of stereotype threat is well documented in the literature. In the context of race, Steele (1997) initially proposed that a stereotype pertaining to one’s own group can negatively impact performance when the stereotype becomes “activated” without conscious awareness. This finding has been demonstrated when examining gender differences. For example, Cadinu et al. (2005) found that women who were informed that “recent research has shown that there are clear differences in the scores obtained by men and women in logical-mathematical tasks” (p. 574) showed a decrease in performance on a difficult math test compared to control participants in a no-threat condition. This finding was mediated by an increase in negative thoughts related to mathematics. The use of stereotype threat and education as potential explanations for gender differences in cognition indicates the need to consider a more comprehensive framework, beyond solely biological factors, when discussing gender differences in cognition. Halpern and LaMay (2000) propose a psychobiosocial model as a rationale as to why no explanations have emerged to adequately interpret gender differences in cognitive performance. The model describes an integrative framework in which the influences of biological and environmental (e.g., psychosocial) factors operate interdependently to impact outcomes.
In the current study, gender effects showed little age moderation and persisted throughout adulthood. This suggests that mechanisms to explain gender differences in cognition are likely present prior to adulthood. Most of the proposed explanations described above precede adulthood. Potential mechanisms therefore include differential exposure to prenatal hormones, biological predispositions, and/or environmental influences such gender socialization in childhood. Despite increased endorsement of gender equality across the past 50–70 years, research shows that gender stereotypes still exist and are evident even in young children (e.g., Bian, Leslie, & Cimpian, 2017; Cvencek et al., 2011).
Limitations and Future Directions
The current study is limited by the fact that our assessment of gender was restricted; we asked whether participants identified as male or female (i.e., “Are you male or female?”) but there is a continuum of potential responses related to gender identity that may be informative when investigating gender differences (e.g., Smiler & Epstein, 2010). In addition, our sample comprised healthy community-dwelling adults who are generally high functioning and thus our results may not generalize to other samples. We found little evidence that age moderated the gender effect on cognition; future research should examine whether other variables (e.g., self-reported masculinity and femininity) moderate the gender effect on cognition across adulthood.
CONCLUSION
Consistent with previous work, we found a female advantage in episodic memory and processing speed and a male advantage in spatial visualization. Notably, we found little evidence for age moderation of the gender effect on cognition, suggesting that the influence of gender persists throughout adulthood. This indicates that the mechanism to explain gender differences is long-lasting, whether it is biological or experiential in nature. Our results also indicate that there is little influence of practice on the gender effect.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by National Institute on Aging Grant RO1AG024270 to TAS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health. There are no conflicts of interest.
Footnotes
CONFLICTS OF INTEREST
The authors have nothing to disclose.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/S1355617719000821
REFERENCES
- Arbuckle JL (2015). Amos (Version 24.0) [Computer Program]. Chicago, IL: IBM SPSS. [Google Scholar]
- Baenninger M & Newcombe N (1989). The role of experience in spatial test performance: A meta-analysis. Sex Roles, 20(5–6), 327–344. doi: 10.1007/BF00287729. [DOI] [Google Scholar]
- Bennett GK, Seashore HG, & Wesman AG (1997). Differential aptitude test. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Berenbaum SA & Resnick SM (2007). The seeds of career choices: Prenatal sex hormone effects on psychological sex differences, In Ceci SJ & Williams WM (Eds.), Why aren’t more women in science? (pp. 147–157). Washington DC: APA Books. [Google Scholar]
- Bian L, Leslie S-J, & Cimpian A (2017). Gender stereotypes about intellectual ability emerge early and influence children’s interests. Science, 355, 389–391. [DOI] [PubMed] [Google Scholar]
- Borella E, Meneghetti C, Ronconi L, & De Beni R (2014). Spatial abilities across the adult life span. Developmental Psychology, 50, 384–392. doi: 10.1037/a0033818. [DOI] [PubMed] [Google Scholar]
- Brooks C & Bolzendahl C (2004). The transformation of U.S. gender role attitudes: cohort replacement, social-structural change, and ideological learning. Social Science Research, 33, 106–133. [Google Scholar]
- Browne MW & Cudeck R (1993). Alternative ways of assessing model fit In Bollen KA & Long JS (Eds.), Testing structural equation models (pp. 445–455). Newbury Park, CA: Sage. [Google Scholar]
- Cadinu M, Maas A, Rosablanca A, & Kiesner J (2005). Why do women underperform under stereotype threat? Evidence for the role of negative thinking. Psychological Science, 16, 572–578. doi: 10.1111/j.0956-7976.2005.01577.x. [DOI] [PubMed] [Google Scholar]
- Camarata S & Woodcock R (2006). Sex differences in processing speed: Developmental effects in males and females. Intelligence, 34, 231–252. doi: 10.1016/j.intell.2005.12.001. [DOI] [Google Scholar]
- Cheung GW & Rensvold RB (2002). Evaluating goodness-of-fit indexes for testing measurement invariance. Structural Equation Modeling: A Multidisciplinary Journal, 9, 233–255. doi: 10.1207/s15328007sem0902_5. [DOI] [Google Scholar]
- Cotter D, Hermsen JM, & Vanneman R (2011). The end of the gender revolution? Gender role attitudes from 1977 to 2008. American Journal of Sociology, 117, 259–289. [DOI] [PubMed] [Google Scholar]
- Cvencek D, Meltzoff AN, & Greenwald AG (2011). Math–gender stereotypes in elementary school children. Child Development, 82, 766–779. doi: 10.1111/j.1467-8624.2010.01529.x. [DOI] [PubMed] [Google Scholar]
- Daseking M, Petermann F, & Waldmann H-C. (2017). Sex differences in cognitive abilities: Analyses for the German WAIS-IV. Personality and Individual Differences, 114, 145–150. doi: 10.1016/j.paid.2017.04.003. [DOI] [Google Scholar]
- England P (2010). The gender revolution uneven and stalled. Gender & Society, 24, 149–166. doi: 10.1177/0891243210361475. [DOI] [Google Scholar]
- Ekstrom RB, French JW, Harman HH, & Dermen D (1976). Manual for Kit of Factor-Referenced Cognitive Tests. Princeton, NJ: Educational Testing Service. [Google Scholar]
- Fennema E & Sherman J (1977). Sex-related differences in mathematics achievement, spatial visualization and affective factors. American Educational Research Journal, 14(1), 51–71. doi: 10.2307/1162519. [DOI] [Google Scholar]
- Halpern DF (2011). Sex Differences in Cognitive Abilities (4th ed.). Mahwah, NJ: Lawrence Erlbaum Associates Publishers. [Google Scholar]
- Halpern DF, Benbow CP, Geary DC, Gur RC, Hyde JS, & Gernsbacher MA (2007). The science of sex differences in science and mathematics. Psychological Science in the Public Interest, 8(1), 1–51. doi: 10.1111/j.1529-1006.2007.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern DF & LaMay ML (2000). The smarter sex: A critical review of sex differences in intelligence. Educational Psychology Review, 12(2), 229–246. [Google Scholar]
- Herlitz A, Airaksinen E, & Nordström E (1999). Sex differences in episodic memory: The impact of verbal and visuospatial ability. Neuropsychology, 13, 590–597. doi: 10.1037/0894-4105.13.4.590. [DOI] [PubMed] [Google Scholar]
- Herlitz A, Nilsson L-G, & Backman L (1997). Gender differences in episodic memory. Memory and Cognition, 25, 801–811. doi: 10.3758/BF03211324. [DOI] [PubMed] [Google Scholar]
- Horn JL & McArdle JJ (1992). A practical and theoretical guide to measurement invariance in aging research. Experimental Aging Research, 18, 117–144. [DOI] [PubMed] [Google Scholar]
- Hu L-T & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6, 1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- Irwing P (2012). Sex differences in g: An analysis of the US standardization sample of the WAIS-III. Personality and Individual Differences, 53, 126–131. doi: 10.1016/j.paid.2011.05.001. [DOI] [Google Scholar]
- Johnson W (2004). Just one g: Consistent results from three test batteries. Intelligence, 32(1), 95–107. doi: 10.1016/S0160-2896(03)00062-X. [DOI] [Google Scholar]
- Johnson W, Carothers A, & Deary IJ (2009). A role for the X chromosome in sex differences in variability in general intelligence? Perspectives on Psychological Science, 4(6), 598–611. doi: 10.1111/j.1745-6924.2009.01168.x. [DOI] [PubMed] [Google Scholar]
- Jones MG & Wheatley J (1990). Gender differences in teacher-student interactions in science classrooms. Journal of Research in Science Teaching, 27(9), 861–874. doi: 10.1002/tea.3660270906. [DOI] [Google Scholar]
- Lange RL, Chelune GJ, Taylor MJ, Woodward TS, & Heaton RK (2006). Development of demographic norms for four new WAIS-III/WMS-III indexes. Psychological Assessment, 18, 174–181. doi: 10.1037/1040-3590.18.2.174. [DOI] [PubMed] [Google Scholar]
- Lundervold AJ, Wollshlaeager D, & Wehling E (2014). Age and sex related changes in episodic memory function in middle aged and older adults. Scandinavian Journal of Psychology, 55, 225–232. doi: 10.1111/sjop.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn R (1994). Sex differences in intelligence and brain size: A paradox resolved. Personality and Individual Differences, 17, 257–271. doi: 10.1016/0191-8869(94)90030-2. [DOI] [Google Scholar]
- Lynn R (1999). Sex differences in intelligence and brain size: a developmental theory. Intelligence, 27, 1–12. doi: 10.1016/S0160-2896(99)00009-4. [DOI] [Google Scholar]
- Maeda Y & Yoon SY (2013). A meta-analysis on gender differences in mental rotation ability measured by the Purdue spatial visualization tests: Visualization of rotations (PSVT:R). Educational Psychology Review, 25, 69–94. doi: 10.1007/s10648-012-9215-x. [DOI] [Google Scholar]
- McCarrey AC, An Y, Kitner-Triolo MH, Ferrucci L, & Resnick SM (2016). Sex differences in cognitive trajectories in clinically normal older adults. Psychology and Aging, 31, 166–175. doi: 10.1037/pag0000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millsap RE & Kwok O-M (2004). Evaluating the impact of partial factorial invariance on selection in two populations. Psychological Methods, 9, 93–115. doi: 10.1037/1082-989X.9.1.9. [DOI] [PubMed] [Google Scholar]
- Perales F, Lersch PM, & Baxter J (2019). Birth cohort, ageing and gender ideology: Lessons from British panel data. Social Science Research, 79, 85–100. doi: 10.1019/j.ssresearch.2018.11.003. [DOI] [PubMed] [Google Scholar]
- Priess HA & Hyde JS (2010). Gender and academic abilities and preferences In Chrisler JC & McCreary DR (Eds.), Handbook of gender research in psychology. New York, NY: Springer Science+Business Media, LLC. doi: 10.1007/978-1-4419-1465-1_15. [DOI] [Google Scholar]
- Raven J (1962). Advanced progressive matrices, Set II. London: Lewis. [Google Scholar]
- Reilly D, Neumann DL, & Andrews G (2016). Sex and sex-role differences in specific cognitive abilities. Intelligence, 54, 147–158. doi: 10.1016/j.intell.2015.12.004. [DOI] [Google Scholar]
- Reilly D, Neumann DL, & Andrews G (2017). Gender differences in spatial ability: Implications for STEM education and approaches to reducing the gender gap for parents and educators In Khine MS (Ed.), Visual-spatial ability: Transforming research into practice (pp. 195–224). Switzerland: Springer International. doi: 10.1007/978-3-319-44385-0_10. [DOI] [Google Scholar]
- Saggino A, Pezzuti L, Tommasi M, Cianci L, Colom R, & Orsini A (2014). Null sex differences in general intelligence among elderly. Personality and Individual Differences, 63, 53–57. doi: 10.1016/j.paid.2014.01.047. [DOI] [Google Scholar]
- Salthouse TA (1993). Speed and knowledge as determinants of adult age differences in verbal tasks. Journal of Gerontology: Psychological Sciences, 48, P29–P36. doi: 10.1037/0278-7393.20.6.1486. [DOI] [PubMed] [Google Scholar]
- Salthouse TA (2004a). Localizing age-related individual differences in a hierarchical structure. Intelligence, 32, 541–561. doi: 10.1016/j.intell.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2004b). What and when of cognitive aging. Current Directions in Psychological Science, 13, 140–144. doi: 10.1111/j.0963-7214.2004.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2014a). Quantity and structure of word knowledge across adulthood. Intelligence, 46, 122–130. doi: 10.1016/j.intell.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2014b). Correlates of cognitive change. Journal of Experimental Psychology: General, 143, 1026–1048. doi: 10.1037/a0034847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2019). Trajectories of normal cognitive aging. Psychology and Aging, 34, 17–24. doi: 10.1037/pag0000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA & Babcock RL (1991). Decomposing adult age differences in working memory. Developmental Psychology, 27, 763–776. doi: 10.1037/0012-1649.27.5.763. [DOI] [Google Scholar]
- Salthouse TA, Babcock RL, Skovronek E, Mitchell DRD, & Palmon R (1990). Age and experience effects in spatial visualization. Developmental Psychology, 26, 128–136. doi: 10.1037/0012-1649.26.1.128. [DOI] [Google Scholar]
- Salthouse TA & Ferrer-Caja E (2003). What needs to be explained to account for age-related effects on multiple cognitive variables? Psychology and Aging, 18, 91–110. doi: 10.1037/0882-7974.18.1.91. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Fristoe N, & Rhee SH (1996). How localized are age-related effects on neuropsychological measures? Neuropsychology, 10, 272–285. doi: 10.1037/0894-4105.10.2.272. [DOI] [Google Scholar]
- Savage-McGlynn E (2012). Sex differences in intelligence in younger and older participants of the Raven’s Standard Progressive Matrices Plus. Personality and Individual Differences, 53, 137–141. doi: 10.1016/j.paid.2011.06.013. [DOI] [Google Scholar]
- Schaie KW & Willis SL (1993). Age difference patterns of psychometric intelligence in adulthood: Generalizability within and across ability domains. Psychology and Aging, 8, 44–55. doi: 10.1037/0882-7974.8.1.44. [DOI] [PubMed] [Google Scholar]
- Sherman JA (1967). Problem of sex differences in space perception and aspects of intellectual functioning. Psychological Review, 74(4), 290–299. doi: 10.1037/h0024723. [DOI] [PubMed] [Google Scholar]
- Smiler AP & Epstein M (2010). Measuring gender: Options and issues In Chrisler JC & McCreary DR (Eds.), Handbook of gender research in psychology, vol 1: Gender research in general and experimental psychology (pp. 133–157). New York, NY: Springer Science + Business Media. [Google Scholar]
- Steele CM (1997). A threat in the air: How stereotypes shape intellectual identity and performance. American Psychologist, 52(6), 613–629. doi: 10.1037/0003-066X.52.6.613. [DOI] [PubMed] [Google Scholar]
- Techentin C, Voyer D, & Voyer SD (2014). Spatial abilities and aging: A meta analysis. Experimental Aging Research, 40, 395–425. doi: 10.1080/0361073X.2014.926773. [DOI] [PubMed] [Google Scholar]
- Terlecki MS, Newcombe NS, & Little M (2008). Durable and generalized effects of spatial experience on mental rotation: Gender differences in growth patterns. Applied Cognitive Psychology, 22, 996–1013. doi: 10.1002/acp.1420. [DOI] [Google Scholar]
- Uttal DH, Meadow NG, Tipton E, Hand LL, Alden AR, Warren C, & Newcombe NS (2013). The malleability of spatial skills: A meta-analysis of training studies. Psychological Bulletin, 139(2), 352–402. doi: 10.1037/a0028446. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1997a). Wechsler Adult Intelligence Scale (3rd ed.). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wechsler D (1997b). Wechsler Memory Scale (3rd ed). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Whitley E, Deary I, Ritchie SJ, Batty D, Kumari M, & Benzeval M (2016) Variations in cognitive abilities across the life course: Cross-sectional evidence from Understanding Society: The UK Household Longitudinal Study. Intelligence, 59, 39–50. doi: 10.1016/j.intell.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock RW & Johnson MB (1990). Woodcock–Johnson Psycho-educational Battery—Revised. Allen, TX: DLM. [Google Scholar]
- Zachary RA (1986). Shipley Institute of Living Scale—Revised. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Zelinski EM, Gilewski MJ, & Schaie KW (1993). Individual differences in cross-sectional and 3-year longitudinal memory performance across the adult life span. Psychology and Aging, 8, 176–186. doi: 10.1037/0882-7974.8.2.176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.