Abstract
Objective
to provide a preliminary estimate of the effectiveness of the prevention of delirium (POD) system of care in reducing incident delirium in acute hospital wards and gather data for a future definitive randomised controlled trial.
Design
cluster randomised and controlled feasibility trial.
Setting
sixteen acute care of older people and orthopaedic trauma wards in eight hospitals in England and Wales.
Participants
patients 65 years and over admitted to participating wards during the trial period.
Interventions
participating wards were randomly assigned to either the POD programme or usual care, determined by existing local policies and practices. The POD programme is a manualised multicomponent delirium prevention intervention that targets 10 risk factors for delirium. The intervention wards underwent a 6-month implementation period before trial recruitment commenced. Main outcome measure incidence of new-onset delirium measured using the Confusion Assessment Method (CAM) measured daily for up to 10 days post consent.
Results
out of 4449, 3274 patients admitted to the wards were eligible. In total, 714 patients consented (713 registered) to the trial, thirty-three participants (4.6%) withdrew. Adherence to the intervention was classified as at least medium for seven wards. Rates of new-onset delirium were lower than expected and did not differ between groups (24 (7.0%) of participants in the intervention group versus 33 (8.9%) in the control group; odds ratio (95% confidence interval) 0.68 (0.37–1.26); P = 0.2225).
Conclusions
based on these findings, a definitive trial is achievable and would need to recruit 5220 patients in 26 two-ward hospital clusters.
Trial registration: ISRCTN01187372. Registered 13 March 2014.
Keywords: delirium, hospitals, multicomponent interventions, older people, prevention
Key points
Multicomponent delirium prevention interventions can reduce incident delirium in hospitalised patients by about one third.
The National Health Service in England does not have a delirium prevention system of care suitable for national implementation.
We feasibility tested a multicomponent delirium prevention intervention capable of widespread NHS implementation.
The intervention was tested in a multicentre, pragmatic, cluster randomised, controlled feasibility study.
Based on the findings, a definitive trial is achievable and would need to recruit 5220 patients in 26 two-ward hospital clusters.
Introduction
Delirium is a common and serious condition in older people associated with distress for individuals, families and health care staff [1], increased mortality, protracted lengths of hospital stay, lasting functional and cognitive decline and increased requirement for long-term care placement [2]. Prevention of delirium (POD) is therefore highly desirable and multicomponent prevention interventions that aim to attenuate modifiable delirium risk factors have consistently been shown to reduce incident delirium in hospitalised patients by about one third in various inpatient specialties [3–5]. As a consequence of this evidence base, several national guidance documents have recommended that multicomponent delirium prevention interventions should be incorporated into routine care [6–8]. A major issue faced by the National Health Service (NHS) in England and Wales, and acknowledged by the National Institute for Health and Care Excellence (NICE) [6], is the lack of a delirium prevention system of care suitable for widespread national implementation. To address this, we developed [9] and preliminary tested [10] the POD programme based on the Hospital Elder Life Program [11] and NICE guideline [6].
Previous multicomponent delirium prevention trials have been predominantly single centre, explanatory (‘proof of concept’) studies. Ideally, future studies should be designed and conducted as multicentre, pragmatic evaluations in which the intervention is implemented and delivered by existing ward teams rather than by research staff [12]. The design of such trials requires critical information, such as prior estimates of effectiveness and recruitment rates. We therefore report here a multicentre, pragmatic, cluster randomised, controlled feasibility trial to obtain preliminary estimates of effectiveness of the modified version of the POD programme (Version 2), to assess recruitment and follow-up rates and to assess adherence to the intervention (reported in a companion paper [13]).
Methods
Trial design
A pragmatic, multicentre, cluster randomised, controlled feasibility trial to investigate the feasibility of a future definitive trial to assess effectiveness of the POD system of care compared to usual care. Trial methods have been fully described previously [14] and are summarised here. The study was approved by the UK National Research Ethics Service (reference 13/YH/0400). Data collection was undertaken by locally based research assistants (RAs) who were trained in study procedures and outcome measures.
Study setting
We aimed to recruit 16 care of older people and orthopaedic trauma wards in 8 NHS hospitals in England and Wales. Wards needed to be ‘site-ready’ defined as having the following: (i) involvement from a senior nurse, ward manager and voluntary services manager (if volunteers were to be part of the programme); (ii) a named person responsible for implementation of the POD programme (e.g. the senior nurse); (iii) dedicated time (equivalent to 1 day a week) of an experienced senior nurse to lead the implementation; and (iv) adequate ward staffing levels (Supplementary A1). Wards that had participated in previous studies within the research programme or were intending to implement delirium prevention initiatives during the duration of the study were not eligible.
Participants
Patients were eligible for trial recruitment if they were aged over 65 years and admitted to the study wards during the study period. Patients were excluded if delirium was present on admission to the ward, discharge was planned within 48 h of admission, delirium assessment had not been performed by an RA within 24 h of admission (older people’s care patients) or preoperatively (orthopaedic trauma patients), consent had not been obtained with 48 h of admission to the ward, end of life care was being provided or the patient was under the care of another ward.
Study intervention
The POD system of care [9] targets the 10 modifiable delirium risk factors highlighted by NICE [6]. It is directed at changing staff practices and engaging volunteers (where available) to facilitate enhancement of care in defined areas. It incorporates systems and mechanisms aimed at introducing, implementing, embedding and sustaining the intervention in routine care and as such has common content and mechanisms but with flexible implementation [9]. Further details are provided in Supplementary A1.
Screening assessment (prior to recruitment)
Patients admitted to participating wards were screened by RAs using the trial eligibility criteria. Screening included the following: the collection of demographic information (age, sex and ethnicity), date and time of admission to hospital and ward, assessment of capacity, assessment for prevalent delirium using the Confusion Assessment Method (CAM) [15] the scoring of which was informed by the Months of the Year Backwards (MOTYB) test for inattention [16] and the abbreviated mental test (AMT) for cognitive impairment [17].
Baseline assessment
Baseline demographic information included the following: living arrangements (alone, another person, nursing home, residential care home and other); reason for admission (hip fracture, other orthopaedic condition or medical condition); co-morbidities (Charlson index) [18]; presence of specific delirium risk factors (hearing/visual impairment, cognitive impairment and/or dementia); specific medications most closely associated with delirium (benzodiazepines, opiates and antihistamines) [19]; illness severity (early warning score) [20]; pre-admission independence [21].
Patient outcomes
Most new-onset delirium occurs in the first few days after admission [22]. We therefore assessed for differences in new-onset delirium within 10 days of providing consent between patients in the intervention group (POD programme) and control group (usual care) as this is the expected primary outcome for a definitive trial. Delirium was assessed using the four-item CAM [15]. A CAM training and monitoring process was developed that followed recommended practices [23].
We also investigated between-group differences in the number, severity [24] and duration of delirium episodes; length of stay in hospital; mortality; and discharge destination. Physical and social independence were measured by the RAs at baseline and at 3 months (postal questionnaire) using the Nottingham extended activities of daily living scale [21]. Anxiety and depression were measured using the clinical anxiety scale [25] and the geriatric depression scale short form [26], respectively, at 30 days at an RA visit.
Blinding
The RAs administering and collecting the outcome measures had no role in the intervention development or delivery. As RAs were visiting the wards daily to conduct delirium assessments it was unrealistic for them to remain blind to treatment allocation. Post-discharge outcomes were undertaken blind to allocation.
Sample size
As this was a feasibility study, a formal power calculation was not considered appropriate. Based on assumptions of (i) a 6 month recruitment period, (ii) an average length of stay of 14 days, (iii) 25-bed wards, (iv) 50% of patients fulfilling the entry criteria, (v) 30% of patients providing consent/consultee declaration [27,28], we expected 720 patients could be recruited in 6 months, with approximately 45 patients recruited per ward, across 16 wards.
Randomisation
The POD programme is a ward-based intervention that aims to affect staff skills, knowledge and clinical practice. Cluster randomisation was therefore chosen to reduce between-group contamination. Randomisation was stratified by ward type/speciality (older people’s care versus orthopaedic trauma) in a two-stage process. Hospitals were first randomised to either hospital or ward level delivery, following this, the wards in those hospitals randomised to ward-level implementation were then randomised (Supplementary A2). Wards randomised to deliver the intervention were given 6 months to implement the POD programme followed by 6 months of intervention delivery during which patient recruitment took place.
Statistical methods
All analyses and data summaries were conducted on the intention-to-treat population, defined as all participants registered, regardless of non-compliance with the protocol or withdrawal from the study. For this feasibility study, the analysis focused on descriptive statistics and confidence interval (CI) estimation rather than on formal hypothesis testing.
For estimation of effectiveness, we calculated the incidence of new-onset delirium within 10 days of providing consent by study arm and overall, together with corresponding 95% CIs. We used multilevel logistic regression that adjusted for age, gender, risk factors for delirium (medications associated with delirium, hearing impairment, visual impairment and early warning score category), cognitive impairment and/or dementia, Charlson comorbidity index, ward type. The intracluster correlation coefficient was calculated using the incidence of new-onset delirium expressed as a proportion of the recruited study population.
Results
Recruitment rate and follow-up
Twenty hospitals expressed interest of which 12 returned site survey forms and eight hospitals were recruited. Of the four hospitals not recruited, two withdrew, one did not respond to the request for a site visit and one was unable to supply timely regulatory approvals. Of the 16 recruited wards, nine were older people’s care medicine (five in the intervention group and four in the control group) and seven were orthopaedic trauma (three in the intervention group and four in the control group).
In 16 wards, 4449 patients were admitted and screened for eligibility of whom 3274 (73.6%) were considered initially eligible (Figure 1). The principal reasons for study exclusion were prevalent delirium (12.1%) and expected duration of stay less than 48 h (7.9%). Delirium assessment was performed by the RAs on 1537 (46.9%) of initially eligible patients; the main reasons for non-assessment of delirium included the research staff missing the patient (21.1% of initially eligible) and ward staff advised not to approach (11.4%). A further 113 of these patients had delirium and were excluded. Consent to participate in the study was obtained for 714 (50.4% of 1418 patients) with 343 and 370 (10.5% and 11.3% of those eligible) participants registered to the intervention and control groups, respectively (one person was discharged before registration). Only 33 (4.6%) of participants withdrew from the study (19 (57.6%) withdrawals occurred within 10 days of providing consent).
Figure 1.
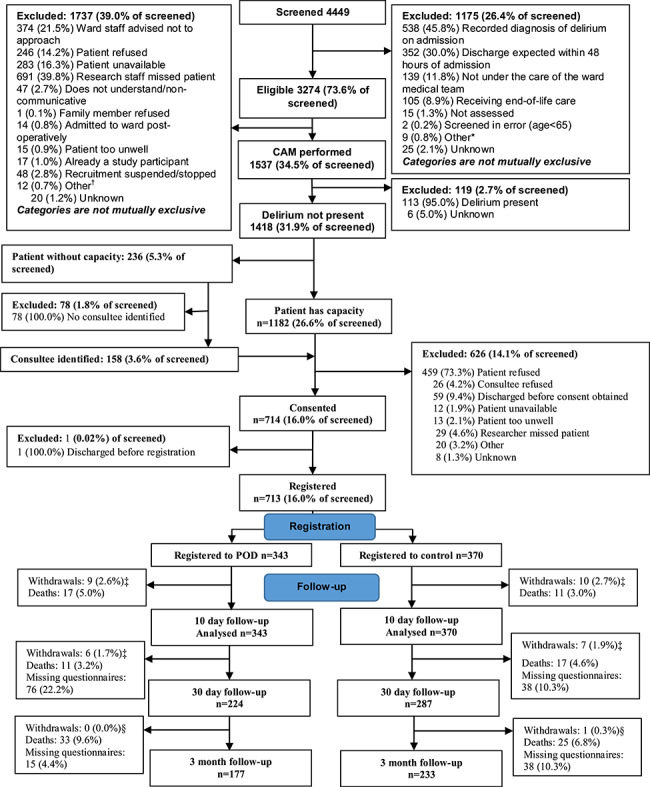
CONSORT diagram. Asterisk shows other reasons for ineligibility were listed as: previously screened (7); patient died (1); and non-UK resident (1). Dagger shows other reasons delirium assessment was not performed were listed as: staffing issues (2); unable to complete CAM (2); patient on another ward (1); previously screened (1); patient on holiday (1); and patient lives 1 h drive away (1). Double dagger shows withdrawals here are from researcher questionnaires. Section sign shows withdrawals here are from postal questionnaires, one patient in the POD arm withdrew from researcher visits at 30 days but not from postal questionnaires at 3 months.
Baseline characteristics of wards and randomised participants
There were more patients admitted with a medical condition in the intervention group compared to the control group (211 (61.5%) versus 169 (45.7%), respectively) due to an imbalance in ward type randomisation (Table 1). Participant demographic characteristics were similar between the arms. The intervention group had more participants with cognitive impairment/dementia than the control group (83 (24.2%) versus 67 (18.1%), respectively), and more participants who were severely ill (66 (19.2%) versus 47 (12.7%), respectively) as measured using the early warning score [20].
Table 1.
Characteristics of participants. Values are numbers (percentages) unless stated otherwise
| Characteristics | Intervention group (n = 343) | Control group (n = 370) |
|---|---|---|
| Ward type | ||
| Older people’s care | 212 (61.8) | 180 (48.6) |
| Orthopaedic trauma | 131 (38.2) | 190 (51.4) |
| Participant characteristics | ||
| Mean (SD) age (years) | 82.5 (7.9) | 83.0 (7.8) |
| Women | 231 (67.3) | 256 (69.2) |
| White | 326 (95.0) | 328 (88.6) |
| Living at home | 311 (90.7) | 339 (91.6) |
| Reason for hospital admission | ||
| Hip fracture | 71 (20.7) | 99 (26.8) |
| Other orthopaedic condition | 60 (17.5) | 102 (27.6) |
| Medical condition | 211 (61.5) | 169 (45.7) |
| Missing | 1 (0.3) | 0 (0.0) |
| Risk factors for delirium | ||
| Cognitive impairment and/or dementia | 83 (24.2) | 67 (18.1) |
| Severely illa | 66 (19.2) | 47 (12.7) |
| Hearing impairment | 120 (35.0) | 112 (30.3) |
| Hearing aid use | 78 (65.0) | 72 (64.3) |
| Visual impairment | 298 (86.9) | 336 (90.8) |
| Benzodiazepines prescribed | 17 (5.0) | 15 (4.1) |
| Opiates prescribed | 145 (42.3) | 172 (46.5) |
| H1 antihistamines prescribed | 43 (12.5) | 33 (8.9) |
| Comorbidities | 236 (68.8) | 244 (65.9) |
| Mean (SD) Charlson comorbidity index score | 1.7 (2.0) | 1.7 (1.9) |
aMedium or high national early warning score category within 48 h of admission.
Implementation of and adherence to the intervention
All wards allocated to the intervention implemented the POD programme (Supplementary A3). Overall adherence to the POD programme measured in four domains (ward set up, content and frequency of delivery, intervention coverage and duration of delivery) was classified as high in two wards, medium in five wards and low in the remaining ward (low in all domains except ward set up) (Supplementary A4).
Study outcomes
Delirium
Taking into account length of patient stay, deaths and withdrawals and discharges, there were a possible 5645 in-hospital CAM assessments of which 5065 (89.7%) were conducted by the RAs. The CAM scoring was informed by the AMT and the MOTYB test with completion rates of 100% and 99.5%, respectively. Out of 713, 57 (8.0%) participants developed new-onset delirium within 10 days of providing consent: 24 (7.0%) of 343 participants in the intervention group and 33 (8.9%) of 370 participants in the control group (Table 2). New-onset delirium was higher in the orthopaedic trauma wards compared to the older people’s care wards (10.0% versus 6.4%, respectively) (Supplementary A5). Delirium incidence in sites ranged between 4.6 and 12.9% (Supplementary A5). Delirium incidence rates in those sites randomised at the hospital level were similar between the arms (2.5% intervention versus 2.3% control); for those randomised at a ward level rates were 1.6% intervention versus 1.9% control.
Table 2.
Outcomes. Values are numbers (percentages) unless stated otherwise
| Outcome | Intervention group (n = 343) | Control group (n = 370) |
|---|---|---|
| New-onset delirium within 10 days of providing consent | 24 (7.0)a | 33 (8.9)a |
| No. of CAM assessments indicating delirium within 10 days of providing consent | 48/2382 (2.0) | 57/2683 (2.1) |
| Mean (SD) severity of delirium episodesb | 3.9 (1.0) | 3.8 (1.0) |
| Mean (SD) duration of delirium episodes (days) | 2.3 (2.0) | 2.2 (1.9) |
| Mean (SD) time to first delirium episode (days) | 4.0 (1.9) | 4.2 (2.3) |
| Delirium at 30 days | 6 (1.7) | 3 (0.8) |
| Mean (SD) severity of delirium at 30 daysb | 3.7 (0.8) | 3.3 (0.6) |
| Fallsc | ||
| Number of participants | 12 (3.5) | 17 (4.6) |
| Number of falls | 19 | 20 |
| Mean (SD) number of falls per participant | 1.6 (1.0) | 1.2 (0.4) |
| Deaths | ||
| Within 10 days of providing consent | 17 (5.0) | 11 (3.0) |
| Overall | 61 (17.8) | 53 (14.3) |
| Mean (SD) time to death (days) | 33.1 (26.9) | 36.8 (29.2) |
| Mean (SD) length of hospital stay (days) | 9.7 (7.1) | 9.8 (6.9) |
| Within-hospital ward moves | 58 (16.9) | 77 (20.8) |
| Discharge | (n = 248) | (n = 288) |
| New care home placement | 47 (19.0) | 71 (24.7) |
| Overnight stays in hospitald | (n = 144) | (n = 193) |
| Mean (SD) | 18.1 (22.3) | 15.9 (17.3) |
| Nottingham extended activities of daily living scale (Total score) | ||
| Baseline | (n = 334) | (n = 364) |
| Mean (SD) | 36.7 (18.4) | 39.7 (19.0) |
| Three months | (n = 173) | (n = 220) |
| Mean (SD) | 29.5 (20.3) | 33.1 (20.9) |
| Geriatric depression score at 30 days | (n = 199) | (n = 278) |
| Mean (SD) total score | 4.7 (3.5) | 4.2 (3.3) |
| Clinical anxiety scale at 30 days | (n = 180) | (n = 276) |
| Mean (SD) total score | 16.8 (15.4) | 16.9 (14.8) |
P = 0.2225, odds ratio 0.68 (95% CI 0.37, 1.26).
bScored with the CAM-S, score range 0–7.
cBetween consent and hospital discharge, death or withdrawal (whichever was sooner).
dBetween index ward admission and 3 months.
Multilevel logistic regression analysis (adjusting for baseline covariates: age, sex, prescription of benzodiazepines, opiates and antihistamines [19], hearing impairment, visual impairment, ward type, early warning score, cognitive impairment and Charlson comorbidity index) was used to explore the differences in incidence of delirium between randomised arms. Patients in the POD programme arm had lower odds of developing delirium but this result was not statistically significant (odds ratio 0.68; 95% CI 0.37 to 1.26; P-value 0.2225) (Supplementary A6). The intracluster correlation coefficient was calculated as 0.0002.
Other outcomes
Falls, mortality, length of hospital stay, social and physical activities and depression and anxiety were similar between the intervention and control groups (Table 2).
Discussion
The multicomponent (non-pharmacological) delirium literature is dominated by small to medium sized, single site, predominantly non-randomised evaluation studies [3–6] that are prone to several biases [29]. This is the first successfully completed multicentre multicomponent delirium prevention randomised controlled trial, albeit a preliminary feasibility study. We were able to recruit a vulnerable group of patients and achieved high outcome assessment and follow-up rates. The other similar multicentre trial involved only two hospitals but was unable to recruit sufficient patients and had large amounts of missing data [30]. We were able to consent 714 patients from eight hospitals/16 wards over 6 months against our target of 720. Our target was based on several assumptions. We assumed that approximately 50% of patients would be at risk of developing delirium. In fact nearly three quarters of the patients on these older people’s care and orthopaedic trauma wards were at risk based on the criteria published by NICE [6]. We further assumed that 30% of patients eligible for the study would be recruited; in this study it was 21.8%. In practice, we found that the major barrier to recruitment was inability to conduct a baseline CAM assessment to exclude prevalent delirium, largely because some patients were judged too sick by the ward staff or because some patients were not identified for assessment within 24 h of admission (older people’s care patients) or preoperatively (orthopaedic patients). The baseline CAM assessment was not achieved for 1737/3274 (53.1%) of the apparently eligible patients. The consent rate for eligible patients for whom delirium was not present was higher than anticipated at 50.4%. The overall recruitment rate was 16% of screened patients, which was consistent with our prior assumption of 15% but the recruitment rate might be readily enhanced if there was a greater focus on RA engagement with patients during the first 24 h of admission. Losses to follow-up were low: only 4.6% of patients withdrew from study follow-up and 16.0% of patients died within the study. We did not detect high levels of contamination in those sites that included both intervention and control wards suggesting that it is possible to randomise wards within a hospital to receive different interventions. These are important findings which can be used to plan future similar studies involving this mixed population of older people’s care and orthogeriatric patients.
The population recruited to this trial were older people (mean age 82.7 years, SD = 7.8); 21.0% had dementia/cognitive impairment; 67.3% had co-morbidities; 23.8% had been admitted with a hip fracture and 44.5% were taking an opiate. It might be reasonably expected, therefore, that this patient population would be at high risk for delirium [6]. Indeed, at least 14.6% of the 4449 patients screened (not all patients assessed due to time constraints) had prevalent delirium (delirium on admission). However, the rate of incident (new) delirium was lower than anticipated: 8% compared to 17.7% for a combined medical and orthogeriatric population reported in the randomised studies included in the Cochrane Review (N = 39 studies; n = 16,082 patients) [5]. The explanation for the low delirium incidence in the trial population is unclear. It is possible that greater awareness of delirium has resulted in improvements in NHS ward care and a subsequent reduction in delirium incidence. However, successive rounds of the National Audit of Dementia Care in General Hospitals have demonstrated continuing dementia and delirium care deficiencies in NHS hospitals in England and Wales [31]. Despite this, it is possible that staff and wards with a pre-existing interest in delirium prevention were more likely to participate with our study resulting in a study site selection bias and lower than anticipated rates of delirium.
The low rate of delirium was not related to missing delirium assessments as the RAs completed 89.7% of expected CAM assessments during the 10 days after patient randomisation. The delirium incidence rates showed some variation between sites (4.6–12.9%). This suggests some variation in either individual RA delirium assessment performance, or differences in local care environments that influenced the development of delirium. However, the between-site variation in delirium incidence was well within, and in no case exceeded, the pooled estimate value reported in the Cochrane review.
The adjusted odds ratio of 0.68 for delirium incidence for the patients randomised to the POD programme compared to usual care is entirely consistent with previous studies [3–6]. However, the 95% CIs were wide: 0.37–1.26. This finding is not surprising as the study was not powered to provide a definitive evaluation of the POD programme. Successful implementation and delivery are critical for multicomponent interventions. Intervention adherence was classified as high in two wards, medium in five wards and low in one ward. Obviously, greater levels of intervention adherence might have influenced the effectiveness estimate of POD. However, the trial was purposefully designed as a pragmatic study, that is, ward changes were led by existing ward staff rather than research staff. The findings are therefore likely to be generalisable to delirium prevention in routine care and form a more reliable basis to plan future studies. Based on the delirium incidence rates we observed, a definitive cluster randomised study would need to be far larger than any previous multicomponent delirium prevention study. Assuming a significance level of 5%, a study power of 90%, a delirium incidence reduction of 30% (consistent with previous studies and our own), incorporating the observed control group incidence rate of 8.9%, allowing for 15% loss to follow-up, and using the unadjusted intracluster correlation coefficient value obtained here (0.0002), the trial would need to recruit 5220 patients in 26 two-ward hospital clusters (200 patients per cluster). This clearly represents a substantial trial but is the only way to obtain robust evidence of effectiveness to support or refute a national roll-out of the POD programme, or a similar intervention. The findings from our feasibility trial suggest a larger study would be achievable and provides valuable underpinning methodological information to design the study.
Supplementary Material
Acknowledgements
We would like to thank the Principal Investigators from the participating centres: Dr Julie Brache, Ipswich Hospital, Ipswich Hospital NHS Trust; Dr Ruchi Chugh, Queen Elizabeth Hospital, University Hospitals Birmingham; Dr Premila Fade, Poole Hospital, Poole Hospital NHS Foundation Trust; Rev Jamie Hartwell, Whiston Hospital St Helens & Knowsley NHS Teaching Hospitals Trust; Dr David Heseltine, York Hospital, York Teaching Hospitals NHS Foundation Trust; Dr Sion Jones, Betsi Cadwaladr, Wales; Prof Tahir Masud, Queens Medical Centre, Nottingham University Hospitals NHS Trust; Dr Joyce Yeo, Wythenshawe Hospital, University Hospitals South Manchester and their colleagues and patients and their families who agreed to participate in the study.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
We acknowledge the contribution of the Hospital Elder Life Program, LLC. Dr. Inouye’s time was supported by Grant R24AG054259 (SKI) from the U.S. National Institute on Aging. This article presents independent research funded by the National Institute for Health Research (NIHR) under the Programme Grants for Applied Research programme RP-PG-0108-10037. The views expressed in this article are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
References
- 1. Belanger L, Ducharme F. Patients' and nurses' experiences of delirium: a review of qualitative studies. Nurs Crit Care 2011; 16: 303–15. [DOI] [PubMed] [Google Scholar]
- 2. Witlox J, Euelings LSM, Jonghe JFM et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. J Am Med Assoc 2010; 304: 443–51. [DOI] [PubMed] [Google Scholar]
- 3. Hshieh TT, Yue J, Oh E et al. Effectiveness of multicomponent nonpharmacologic delirium interventions: a meta-analysis. J Am Med Assoc Intern Med 2015; 175: 512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martinez F, Tobar C, Hill N. Preventing delirium: should non-pharmacological, multicomponent interventions be used? A systematic review and meta-analysis of the literature. Age Ageing 2015; 44: 196–204. [DOI] [PubMed] [Google Scholar]
- 5. Siddiqi N, Harrison JK, Clegg A et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev 2016; CD005563. doi: 10.1002/14651858.CD005563.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Institute for Health and Clinical Excellence Delirium: diagnosis, prevention and management, Clinical Guideline 103. London: National Clinical Guideline Centre, 2010. [Google Scholar]
- 7. AHMAC Health Care of Older Australians Standing Committee on behalf of the Australian health Ministers' advisory council. Delirium Care Pathwayshttps://www.health.vic.gov.au/acute-agedcare (19 February 2020, date last accessed).
- 8. American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc 2015; 63: 142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Godfrey M, Smith J, Green J et al. Developing and implementing an integrated delirium prevention system of care: a theory driven, participatory research study. BMC Health Serv Res 2013; 13: 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Godfrey M, Green J, Smith J et al. Process of implementing and delivering the prevention of delirium system of care: a mixed method preliminary study. BMC Geriatr 2020; 20: 1. doi: 10.1186/s12877-019-1374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Inouye SK, Bogardus ST Jr, Charpentier PA et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999; 340: 669–76. [DOI] [PubMed] [Google Scholar]
- 12. Rowland M, Torgerson D. Understanding controlled trials: what are pragmatic trials? BMJ 1998; 316: 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith J, Green J, Siddiqi N et al. Investigation of ward fidelity to a multicomponent delirium prevention intervention during a multicentre, pragmatic, cluster randomised, controlled feasibility trial. Age and Ageing (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Young J, Cheater F, Collinson M et al. Prevention of delirium (POD) for older people in hospital: study protocol for a randomised controlled feasibility trial. Trials 2015; 16: 340. doi: 10.1186/s13063-015-0847-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Inouye SK, Dyck CH, Alessi CA et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990; 113: 941–8. [DOI] [PubMed] [Google Scholar]
- 16. O’Regan NA, Ryan DJ, Boland E et al. Attention! A good bedside test for delirium? J Neurol Neurosurg Psychiatry 2014; 85: 1122–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hodkinson HM. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing 1972; 1: 233–8. [DOI] [PubMed] [Google Scholar]
- 18. Charlson M, Szatrowski TP, Peterson J et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994; 47: 1245–51. [DOI] [PubMed] [Google Scholar]
- 19. Clegg A, Young JB. Which medications to avoid in people at risk of delirium: a systematic review. Age Ageing 2011; 40: 23–9. [DOI] [PubMed] [Google Scholar]
- 20. Royal College of Physicians National Early Warning Score (NEWS): Standardising the assessment of acute illness severity in the NHS In: Report of a Working Party. London: Royal College of Physicians, 2012. [Google Scholar]
- 21. Nouri FM, Lincoln N. An extended activities of daily living scale for stroke patients. Clin Rehabil 1987; 1: 301–5. [Google Scholar]
- 22. Kalisvaart KJ, Vreeswijk R, Jonghe JF et al. Risk factors and prediction of postoperative delirium in elderly hip-surgery patients: implementation and validation of a medical risk factor model. J Am Geriatr Soc 2006; 54: 817–22. [DOI] [PubMed] [Google Scholar]
- 23. Inouye SK. The Confusion Assessment Method (CAM): Short CAM Training Manual and Coding Guide. 2014; Boston, MA: Hospital Elder Life Program, LLC; http://www.hospitalelderlifeprogram.org/uploads/disclaimers/Long_CAM_Training_Manual_10-9-14.pdf (l5 March 2018, date last accessed). [Google Scholar]
- 24. Inouye SK, Kosar CM, Tommet D et al. The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med 2014; 160: 526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Westhuis D, Thyer BA. Development and validation of the clinical anxiety scale: a rapid assessment instrument for empirical practice. Educ Psychol Meas 1989; 49: 153–63. [Google Scholar]
- 26. Sheikh JI, Yesavage JA. Geriatric depression scale (GDS): recent evidence and development of a shorter version. Clin Gerontol 1986; 5: 165–73. [Google Scholar]
- 27. Holt R, Young J, Heseltine D. Effectiveness of a multi-component intervention to reduce delirium incidence in elderly care wards. Age Ageing 2013; 42: 721–7. [DOI] [PubMed] [Google Scholar]
- 28. Nixon J, Nelson EA, Cranny G et al. Pressure relieving support surfaces: a randomised evaluation. Health Technol Assess 2006; 10, 1–163. [DOI] [PubMed] [Google Scholar]
- 29. Teale E, Young J. Multicomponent delirium prevention: not as effective as NICE suggest? Age Ageing 2015; 44: 915–7. [DOI] [PubMed] [Google Scholar]
- 30. Heim N, Stel HF, Ettema RG et al. HELP! Problems in executing a pragmatic, randomized, stepped wedge trial on the hospital elder life program to prevent delirium in older patients. Trials 2017; 18: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Royal College of Psychiatrists National Audit of Dementia Care in General Hospitals 2016–17. Third round of audit report London: Royal College of Psychiatrists, 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


