Abstract
Background:
Systemic administrations of conventional antithrombotics reduce neointima formation after angioplasty in experimental animals. However, clinical translation of these results has not been successful due to high risk for bleeding.
Objectives:
We sought to determine whether novel annexin-V (ANV)-Kunitz protease inhibitor fusion proteins, TAP-ANV and ANV-6L15, can specifically target to vascular injury site and limit neointima formation without inducing systemic hypo-coagulation in a rat carotid artery balloon angioplasty injury model.
Methods:
Near infrared imaging was carried out after balloon-injury and injection of fluorescent ANV or ANV-6L15 to examine their bio-distributions. For peri-procedure treatment, TAP-ANV or ANV-6L15 was administered as i.v. boluses 3 times: 30-minutes before balloon-injury, immediate after procedure, and 120-minutes postballoon-injury. For extended treatment, additional i.v. bolus injection was given on day-2, day-3 and every other day thereafter. Carotid arteries were collected on day-7 and day-14 for analysis. Blood was collected for measurement of clotting parameters.
Results:
Near infrared imaging and immunochemistry showed that fluorescent ANV and ANV-6L15 specifically localized to injured carotid artery and significant amount of ANV-6L15 remained bound to the injured artery after 24-h. Peri-procedure injections of TAP-ANV or ANV-6L15 resulted in decrease of intima/media ratio by 56%. Extended injections of both yielded similar results. Both decreased the expression of PCNA on day-7 and increased the expression calponin on day-14 in the intima post-balloon-injury.
Conclusions:
TAP-ANV and ANV-6L15 can specifically localize to balloon injured carotid arteries after i.v. bolus injections, resulting in substantial attenuation of intimal hyperplasia without inducing a state of systemic hypo-coagulation.
Keywords: Anticoagulants, Thrombosis, Balloon angioplasty, Intimal hyperplasia, Recombinant Kunitz protease inhibitor
1. Introduction
Arteries [1–3] and veins [4–5] respond to injury by a sequential healing process that includes an acute thrombotic phase (minutes to days), a sub-acute neointima formation phase (weeks to months), and a late vascular remodeling phase (months to years). Such healing process suggests that the mural thrombus formed at the site of vascular injury assumes an important role in subsequent neointima formation by providing a biodegradable matrix with abundant chemokines/cytokines/mitogens into which smooth muscle cell proliferate and elaborate matrix. These concepts led to extensive search for antithrombotic agents to limit intima hyperplasia and restenosis, among of which, however, were mostly ineffective [6–19]. Subsequent studies using genetic [20–23] or pharmacological [24–33] manipulation showed that inhibition of TF/FVIIa by recombinant tissue factor pathway inhibitor (rTFPI) or inactivated FVIIa (FVIIai) were more effective in reducing intimal hyperplasia and restenosis than inhibition of thrombin or FXa by r-hirudin and r-tick anticoagulant protein (r-TAP). However, systemic pharmacological inhibition of TF/FVIIa required continuous infusion of very high doses of rTFPI (25 μg/kg per min for 3 or 14 days) to reduce intimal hyperplasia and restenosis [24,25,28]. Such treatment regimens were impractical for clinical application and would render patients at high risk for bleeding.
A series of novel anticoagulant fusion proteins each comprising an annexin-V (ANV) domain linking to a Kunitz Protease Inhibitor (KPI) domain were developed recently [34]. The fusion proteins possess the following distinctive properties: 1) Upon i.v. bolus injection, the fusion proteins can specifically localize to sites of vascular injury due to Ca++-dependent high-affinity binding to phosphatidylserine (PS) on the membrane surfaces of damaged, activated, stressed and apoptotic/necrotic cells [34,35]; 2) the fusion proteins bind to the PS-exposed membranes via the ANV domains and greatly enhance the inhibition of coagulation enzyme/cofactor complexes on the membrane surfaces by the KPI domains. Previous in vitro studies have suggested the fusion proteins may promote endothelial cell membrane repair and reduce inflammation in addition to anticoagulation [36–39,40,41]. In this study, we hypothesize that fusion proteins with such unique multi-functional properties may potently inhibit thrombo-inflammation at sites of vascular injury, resulting in subsequent attenuation of neointimal hyperplasia without inducing a state of systemic hypo-coagulation.
2. Materials and methods
2.1. Recombinant TAP-ANV and ANV-6L15
TAP-ANV and ANV-6L15 were cloned into the expression vector pET20b(+) (Novagen, Madison, WI, USA) and expressed in Escherichia coli BL21(DE3)pLysS as described before [34]. The recombinant proteins were purified by chromatography on Q-Sepharose™ fast flow (GE Healthcare, Piscataway, NJ).
2.2. Rat carotid artery balloon injury model
Animal care and surgical procedures complied with Guide for the Care and Use of Laboratory Animals (NIH publication no. 86-23) and were approved by the Institutional Animal Care and Use Committee of Chang Gung Memorial Hospital. Adult male Wistar rats weighing 350–400 g were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (5 mg/kg). Angioplasty of the left external carotid artery was performed using an inflated 2F Fogarty embolectomy catheter. The balloon was inflated with saline (~0.05 ml) with inflation pressure ~2.0 atm. For histology and immunofluorescence study (Figs. 3A, B, 4B and D), the rats were administered with (1) vehicle (PBS), n = 15, (2) TAP-ANV at 25, 50 or 100 μg/kg respectively (n = 15 for each group) and (3) ANV-6L15 at 25, 50 or 100 μg/kg respectively (n = 15 for each group). For PCNA immunofluorescence (Fig. 4A and C), additional rats were treated with PBS (vehicle), TAP-ANV (50 μg/kg), or ANV-6L15 (50 μg/kg) respectively (n = 10 for each group). The regimens were given three times via tail-vein bolus injections at 30 min before balloon procedure, immediate after procedure, and 120 min post-angioplasty. After surgery, the treated rats were sacrificed on day 1 for Near infrared (near-IR) fluorescence imaging and immunofluorescence (Fig. 1), on day 7 for immunofluorescence (Fig. 4A and C) and on day 14 for histology and immunofluorescence (Figs. 3A, B, 4B and D) respectively.
Fig. 3.
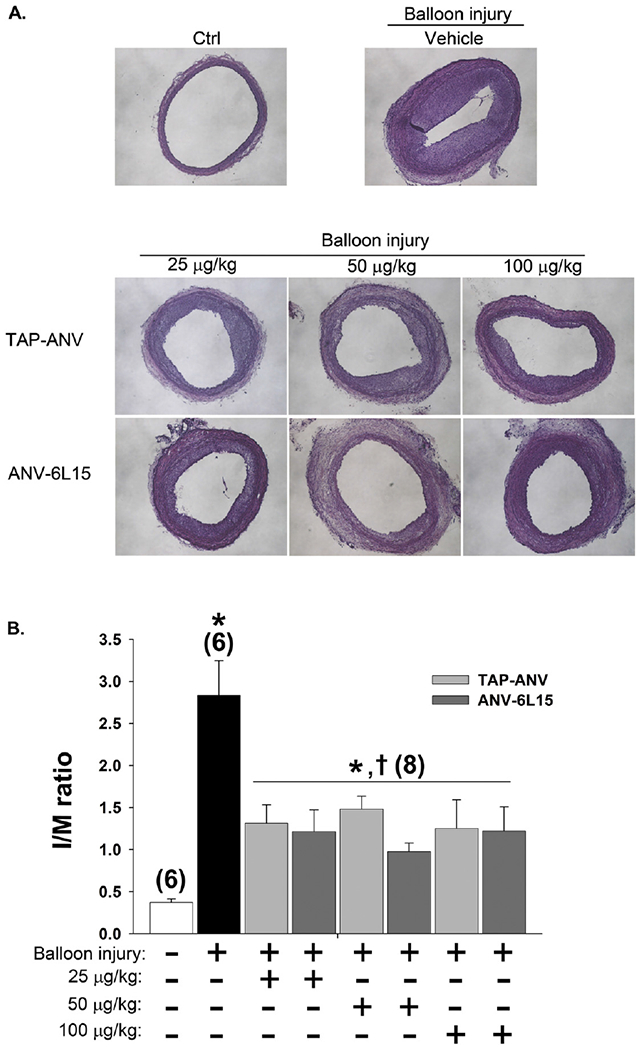
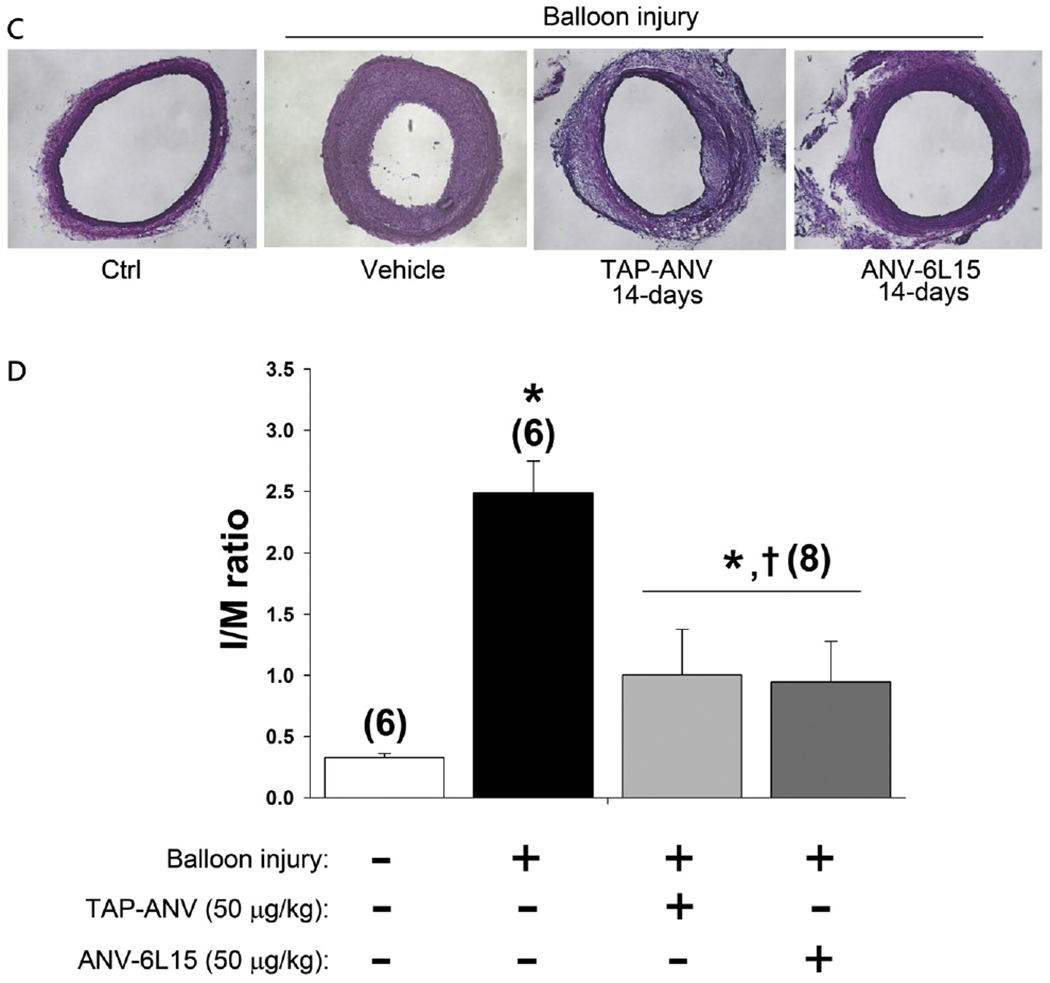
Effects of TAP-ANV and ANV-6L15 on neointima formation after balloon angioplasty of carotid artery. A. Representative histological cross-section of uninjured carotid artery (Ctrl), balloon-injured carotid arteries treated with 3 injections of PBS (vehicle), TAP-ANV (25, 50, 100 μg/kg), and ANV-6L15 (25, 50, 100 μg/kg) via tail vein at 30 min before balloon procedure, immediate after procedure and 120 min post-angioplasty. The carotid arteries were harvested 14 days after balloon injury for histological analysis and measurements of areas of the intimal and the media. B. The intima/media ratios (I/M ratio) were calculated for the uninjured carotid arteries (Ctrl) and balloon-injured carotid arteries treated with 3 bolus injections of PBS (vehicle), TAP-ANV (25, 50, 100 μg/kg), and ANV-6L15 (25, 50, 100 μg/kg). The I/M ratios represent mean ± SD (n = 6–8). The symbols * and † indicated significant difference compared with uninjured control and vehicle-treated group respectively. C and D. Effects of extended treatments: Rats received 3 i.v. bolus injections of PBS, (vehicle),TAP-ANV (50 μg/kg), or ANV-6L15 (50 μg/kg) during balloon procedure on day 1, followed by one additional bolus injection (50 μg/kg) on day 2, day 3 and every 2 days thereafter until day 14. C. Representative histological cross-sections of uninjured carotid artery (Control), balloon-injured carotid arteries treated with PBS (Vehicle), TAP-ANV, and ANV-6L15. D. The I/M ratio of each treatment group represents mean ± SEM (n = 6–8). The symbols * and † indicated significant difference compared with uninjured control and vehicle-treated group respectively.
Fig. 4.
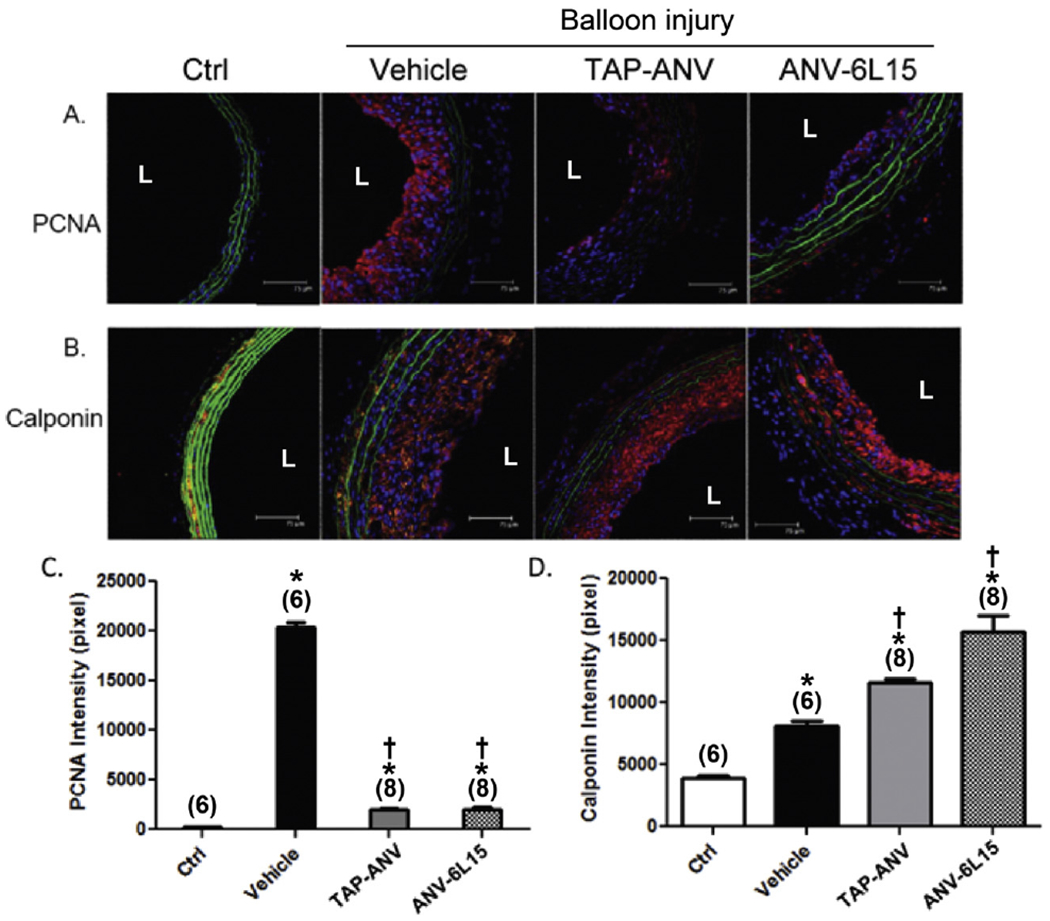
Effects of TAP-ANV and ANV-6L15 treatments on smooth muscle cell proliferation and differentiation after balloon angioplasty of carotid artery. Rats received 3 i.v. bolus injections of PBS (vehicle), TAP-ANV (50 μg/kg), or ANV-6L15 (50 μg/kg) during balloon procedure on day 1. Carotid arteries were harvested on day 7 and day 14 post-surgery for histochemical staining with (A) PCNA and (B) calponin, respectively. Sections were stained with anti-PCNA or anti-calponin antibodies followed by Cy3-conjugated second antibody (red). The mean ± SD intensities of PCNA and calponin were plotted for each group in C and D, respectively (n = 5–8). The symbols * and † indicated significant difference compared with uninjured control and vehicle-treated group respectively.
Fig. 1.
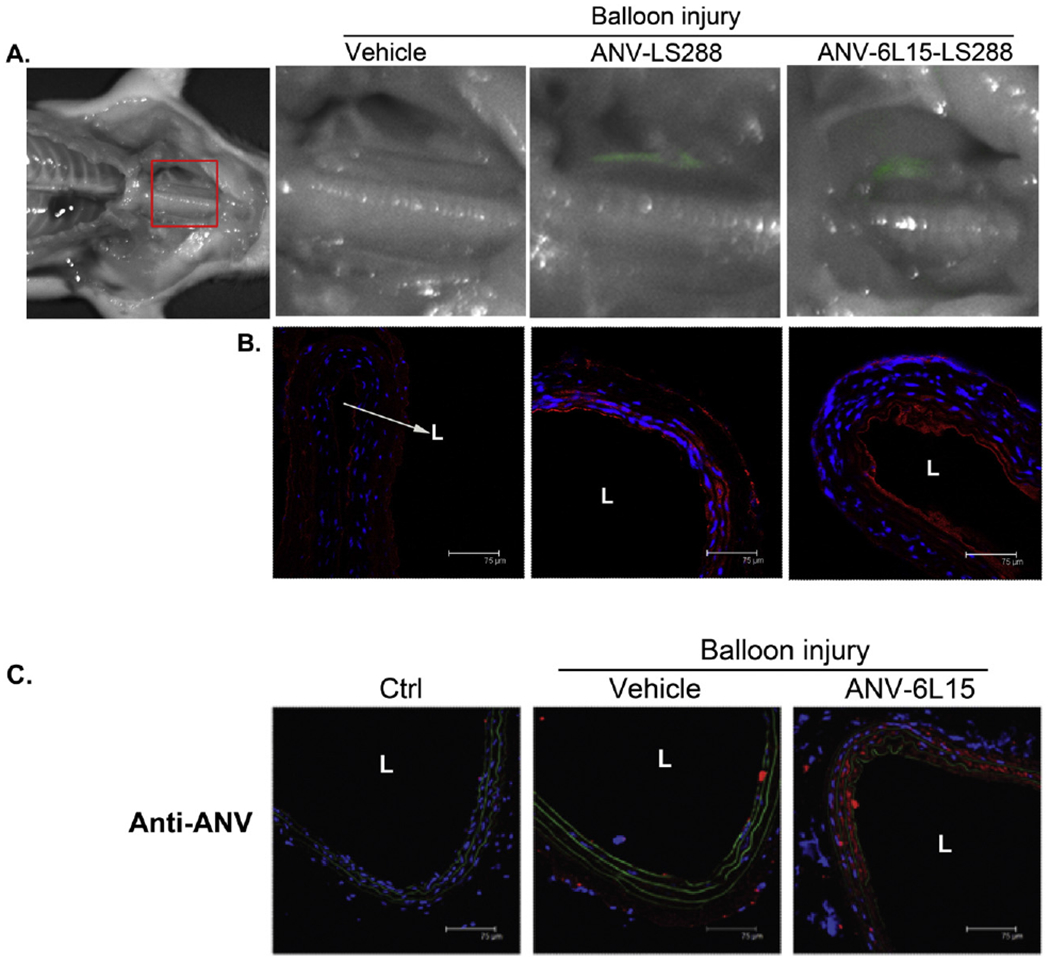
Binding of ANV-LS288 and ANV-6L15-LS288 to the balloon-injured carotid artery. A. Near infrared (near-IR) fluorescence imaging was carried out 8 h after balloon injury of the left external carotid artery and i.v. bolus injection of vehicle (PBS), ANV-LS288 (50 μg/kg), or ANV-6L15-LS288 (50 μg/kg). The balloon injured, but not the uninjured, carotid arteries showed near-IR (pseudo-color: green) fluorescence due to the binding of ANV-LS288 or ANV-6L15-LS288. B. Immunohistological staining of the injured carotid artery sections using anti-ANV antibody, and Cy3-conjugated secondary antibodies (red), and DAPI nucleus stain (blue). The anti-ANV antibody detected the bound ANV-LS288 or ANV-6L16-LS288 on the injured arterial wall. L: lumen. C. Immunohistological detection of ANV-6L15 bound to the balloon-injured carotid artery 24 h after i.v. bolus injection. The left external carotid artery of rats was injured by balloon angioplasty and treated with PBS vehicle or a bolus of ANV-6L15 (50 μg/kg). After 24 h, the rats were sacrificed and the uninjured and balloon-injured carotid artery sections were immuno-stained with anti-ANV antibody and Cy3-conjugated second antibody (red). The uninjured carotid artery (Ctrl) barely showed red color stain; the PBS-treated balloon-injured carotid artery (Vehicle) lightly stained red; and the ANV-6L15-treated balloon-injured carotid artery (ANV-6L15) stained red intensely. L: lumen.
For the study of extended treatment (Fig. 3C and D), the rats were administered with (1) vehicle (PBS), n = 10,(2) TAP-ANV at 50 μg/kg (n = 10) and (3) ANV-6L15 at 50 μg/kg (n = 10) respectively by bolus injections via tail-vein for a total of 10 times, including 3 peri-procedure bolus injections plus one bolus injection each on day 2, 3, 5, 7, 9, 11 and 13. The rats were sacrificed on day 14 after surgery.
2.3. Morphometric measurement
The injured segments of the artery were collected and fixed in 4% paraformaldehyde. Then the middle one-third portion of the samples was embedded in paraffin and transverse histological sections (5 μm) were made from each segment and stained with hematoxylin-eosin. Three discontinuous sections (2 mm apart) from each vessel were measured in a rat. Morphometry was performed using a video microscope. The cross-sectional area of the neointima, media and the ratio of the areas of neointima to media (I/M) were measured using IMAGE-PRO PLUS (Media Cybernetics Inc.).
2.4. Immunohistochemistry
The method and quantification of immunohistochemical staining were performed as previously described [48,49]. Immunohistochemical staining was performed using anti-annexin V-Ig (Abcam, USA), anti-calponin-Ig (Dako, Carpinteria, CA), or anti-PCNA-Ig (Abcam, USA) as primary antibodies and Cy3- (red) conjugated secondary antibodies (Chemicon, Temecula, CA), and examined by confocal immunofluorescent microscopy. For quantification, a basic Cy3 intensity was set as threshold to disrupt background noise. The Cys-positive area was calculated and compared to the mean value of control group as relative folds of inductions. All images were processed and analyzed using MetaMorph software (Universal Imaging Corp., West Chester, PA, USA). The results presented from 3 random sections of injured or non-injured arteries of individuals of every experiment group.
2.5. Blood coagulation test
The rats (N = 6) received a bolus injection of PBS, heparin (100 U/kg, i.v.), TAP-ANV (50 μg/kg, i.v.) or ANV-6L15 (50 μg/kg, i.v.); then 9 volume of blood was collected into 1 volume of acid-citrate-dextrose at 5, 30, 60, 120 min after injection for measurement of Activated Clotting Time (ACT). Plasma samples were separated by centrifugation at 2000g for 15 min for measurement of Prothrombin Time (PT) and Activated Partial Thromboplastin Time (aPTT) using GEM PCL® Plus coagulation system (Instrumentation Laboratory, Milano).
2.6. Near infrared (near-IR) fluorescence imaging
A cypate fluorescence probe, LS288 [42], was conjugated to ANV or ANV-6L15 by a two-step EDC/sulfo-NHS coupling process recommended by the manufacturer (Thermo Fisher Scientific, Waltham, MA). ANV-LS288 and ANV-6L15-LS288 conjugates had probe:protein molar ratio of 0.59 and 0.81, respectively. Rats received bolus injections of ANV-LS288 (50 μg/kg) or ANV-6L15-LS288 (50 μg/kg) after balloon injury of the carotid arteries. Fluorescence imaging was performed after 8 h using Pearl® Impulse system (Li-COR Biosciences, Lincoln, NE).
3. Statistical analysis
All values were expressed as the mean ± SEM. Unpaired Student’s t-test for two groups and one-way ANOVA with post hoc Tukey’s tests for multiple comparisons were applied. A value of P < 0.05 was considered to be significant.
4. Results
4.1. Binding of ANV and ANV-6L15 to the balloon-injured carotid artery
Previous studies showed that both ANV and ANV-6L15 bound specifically to anionic phospholipids exposed on the membrane surfaces of damaged, activated or apoptotic cells [35,43]. In this study, we conjugated a near-IR fluorescent probe, LS288, to ANV and ANV-6L15 and investigated the binding of these conjugates to the balloon-injured carotid artery in vivo. Fig. 1A showed near-IR fluorescence imaging of the carotid arteries 8 h after balloon angioplasty and injection of ANV-LS288 or ANV-6L16-LS288. ANV-LS288 specifically bound to the injured, but not uninjured, carotid artery 8 h after balloon injury. ANV-6L15-LS288 yielded higher fluorescence intensity than ANV-LS288, partly due to higher avidity of ANV-6L15 for anionic phospholipids than ANV [43]. ANV-6L15 also appeared to have stronger avidity for heparan sulfate proteoglycans on endothelial glycocalyx than ANV (unpublished results), which might account for the higher background fluorescence. The balloon-injured carotid arteries were sectioned and immuno-stained with anti-ANV antibody and Cy3-second antibody (red color). Fig. 1B showed greater extent of ANV-6L15-LS288 binding to the balloon-injured carotid artery than ANV-LS288.
The carotid arteries of rats were subject to balloon angioplasty and treated with vehicle, or a bolus of ANV-6L15 (50 μg/kg) via tail-vein bolus injection. After 24 h, the rats were sacrificed and the uninjured and balloon-injured carotid artery sections were immuno-stained with anti-ANV antibody and Cy3-conjugated second antibody (red). Fig. 1C showed that the uninjured carotid artery (Ctrl) barely stained red color, the PBS-treated/balloon-injured carotid artery (vehicle) lightly stained red, and the ANV-6L15-treated/balloon-injured carotid artery (ANV-6L15) stained red more intensely. These results suggested that a significant amount of ANV-6L15 remained bound to the balloon-injured carotid artery 24 h after bolus injection. Thus, despite relatively short initial circulating half-life (t1/2α < 2 min) [35], ANV-6L15 quickly bound to sites of vascular damage and significant amounts remained bound to the sites for >24 h.
4.2. Effects of ANV-6L15 and TAP-ANV on whole blood ACT and plasma aPTT and PT after i.v. bolus injections into rats
Blood samples were collected from the tail veins of rats at 5, 30, 60, and 120 min after bolus injection of PBS (Control), heparin (100 U/kg), ANV-6L15 (50 μg/kg), or TAP-ANV (50 μg/kg). Whole blood ACT and plasma aPTT and PT were measured. Fig. 2A, B, C showed that bolus injection of heparin (100 U/kg) led to rapid rise of ACT, aPTT and PT > 2 fold within 5 min followed by gradual decline over 2 h. In contrast, little or no changes in ACT, aPTT and PT were observed following bolus i.v. injection of ANV-6L15 (50 μg/kg), or TAP-ANV (50 μg/kg). These results suggested that i.v. bolus injections of TAP-ANV or ANV-6L15 led to targeted delivery and accumulation of these antithrombotic proteins to vascular damage sites without significant impact on the coagulation parameters in the systemic circulating blood.
Fig. 2.
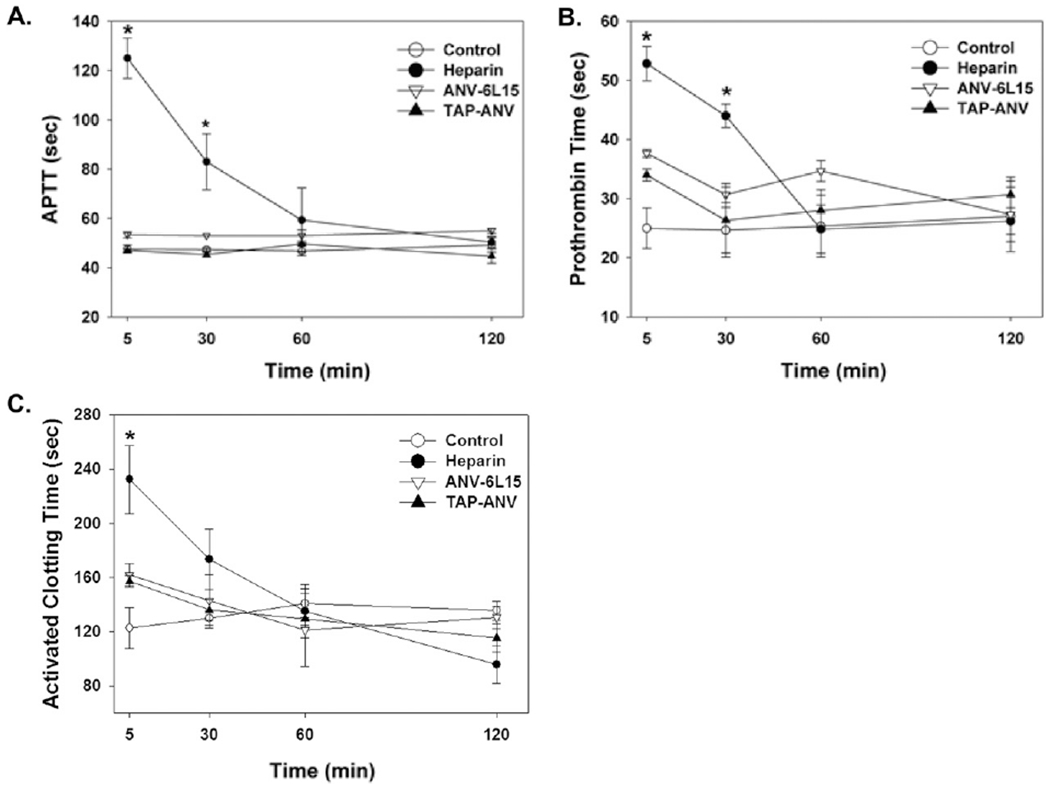
Activated Clotting Time (ACT), Activated Partial Thromboplastin Time (aPTT) and Prothrombin Time (PT). Blood samples were collected from the tail veins of rats at 5,30,60, and 120 min after bolus injection of PBS (control), heparin (100 U/kg), ANV-6L15 (50 μg/kg), or TAP-ANV (50 μg/kg). Plasma (A) APTT, (B) Prothrombin Time and whole blood (C) ACT were measured and expressed as mean ± SEM (n = 6). The symbol, * indicated statistically significant difference compared with control group (P < 0.05).
4.3. Intravenous bolus injections of TAP-ANV and ANV-6L15: effects on neointima formation after balloon injury of carotid arteries
The pharmacological effects of TAP-ANV and ANV-6L15 were previously studied in a mouse photochemical-induced carotid artery thrombosis model [34] and a rat myocardial ischemia-reperfusion injury model [44]. Both molecules inhibited thrombosis and myocardial ischemia-reperfusion injury effectively in 10–100 μg/kg bolus dose range. Based on these results, we chose 25–100 μg/kg bolus-doses for this rat carotid artery balloon angioplasty study. Rats received peri-procedure i.v. bolus injections of PBS (vehicle), TAP-ANV (25, 50, 100 μg/kg) or ANV-6L15 (25, 50, 100 μg/kg) three times at 30 min before procedure, immediately after procedure and 120 min post-angioplasty. Neointima formation after 14 days was assessed by measuring I/M ratio. Fig. 3A showed representative histological sections of uninjured carotid artery (Ctrl), and balloon-injured carotid arteries treated with vehicle, TAP-ANV, and ANV-6L15. Fig. 3B compared the I/M ratios for these groups. TAP-ANV and ANV-6L15 treatments both inhibited intima hyperplasia as reflected in decreases of I/M ratio. The I/M ratios for all the groups treated with different doses of TAP-ANV and ANV-6L15 did not differ significantly. The results suggested that the dosing schemes for both proteins possibly all achieved maximal inhibition of local thrombogenesis at the balloon injury site. The averaged I/M ratio for all the TAP-ANV and ANV-6L15 treatment groups was 1.24 ± 0.17 (mean ± SD), which represented a 56.2% decrease in I/M ratio compared with that of vehicle-treated group.
It had been reported that balloon injury was associated with upregulation of tissue factor (TF) on the arterial wall [45] and that the injured artery retained FXa/FVa on the luminal surface for 3–7 days [46,47]. Therefore, we tested whether extended treatment with TAP-ANV or ANV-6L15 afforded greater inhibition of intimal hyperplasia after balloon angioplasty. In addition to 3 i.v. bolus injections with PBS (vehicle), TAP-ANV (50 μg/kg) or ANV-6L15 (50 μg/kg) at the time of balloon procedure, the rats received one additional bolus injection each on 2nd and 3rd days and every 2 days thereafter until day 14. Fig. 3C and D showed that extended treatment with either TAP-ANV or ANV-6L15 during the 14-day period decreased the I/M ratio about 60% compared with that of PBS treated control. Thus, extended treatment with TAP-ANV or ANV-6L15 resulted in marginal further decrease in intimal hyperplasia compared with those of peri-procedure treatments.
4.4. Effects of TAP-ANV and ANV-6L15 on smooth muscle cell proliferation and differentiation after balloon angioplasty
To investigate the molecular mechanism underlying the effects of TAP-ANV and ANV-6L15 in reducing neointima hyperplasia, we evaluated the expression of PCNA (proliferating cell nuclear antigen) and calponin in the layer of intima after balloon angioplasty. PCNA and calponin were considered as vascular smooth muscle cell proliferation and differentiation markers respectively [49].
Rats received 3 i.v. bolus injections of PBS (vehicle), TAP-ANV (50 μg/kg), or ANV-6L15 (50 μg/kg) at 30 min before balloon procedure, immediately after procedure and 120 min post-angioplasty. Fig. 4A and C showed intense staining of PCNA in hyperplastic intima in vehicle-treated/balloon-injured carotid artery harvested on day 7 post-angioplasty. TAP-ANV or ANV-6L15 treatment greatly diminish the expression of PCNA in the intima. Fig. 4B and D showed that the neointima of vehicle-treat/balloon-injured carotid artery harvested on day 14 post-angioplasty had relatively lower expression of calponin. TAP-ANV and ANV-6L15 treatments increased the expression of calponin in the neointima. Thus, the results implicated these site-targeted fusion proteins may reduce the angioplasty-related neointima formation by inhibiting vascular smooth muscle cell proliferation.
5. Discussion
In this study we presented that, distinct from all these systemically active antithrombotic agents which induce systemic hypo-coagulation [24,25,28], i.v. bolus injection of TAP-ANV or ANV-6L15 apparently resulted in targeted delivery to vascular injury sites (Fig. 1) with long-lasting localized inhibitory effects (Fig. 3) without inducing systemic hypo-coagulation in circulating blood (Fig. 2). These results suggested that TAP-ANV and ANV-6L15 might be safer and more effective than previously available antithrombotic agents for prevention of acute thrombosis, intimal hyperplasia and restenosis after angioplasty.
The initial and final steps of blood clotting cascade are driven by TF/FVIIa complex and FVa/FXa prothrombinase complex, respectively, assembled on anionic phospholipid membrane surfaces. Both TAP-ANV and ANV-6L15 contain ANV domains which enable high-affinity docking of the fusion proteins onto anionic phospholipids and facilitate the inhibition of TF/FVIIa and FVa/FXa complexes on 2-dimensional membrane surfaces by 6L15 and TAP, respectively. In TF-induced plasma clotting assay, TAP-ANV and ANV-6L15 are 86-fold and 6000-fold more potent than TAP and 6L15, respectively [34], suggesting that phospholipid-docking is an important mechanism for the efficient inhibition of thrombogenesis by TAP-ANV and ANV-6L15. In this study, we showed that peri-procedure bolus injections of TAP-ANV or ANV-6L15 at 25–100 μg/kg per bolus range resulted in approximately 56.2 ± 5.8% reduction in intima hyperplasia 2-weeks post-angioplasty with minimal alterations in coagulation parameters in circulating blood. In this study we did not present the pathways how annexin-V and Kunitz protease inhibitors attenuated intimal hyperplasia since there were already numerous studies showing the molecular mechanisms of anti-coagulants and anti-thrombotics in preventing the intimal hyperplasia [16–18, 20–30]. We highlighted that targeting anionic phospholipids for efficient inhibition of TF/VIIa and FVa/FXa provides a much simpler and safer means for effective control of acute thrombogenesis and subsequent intimal hyperplasia after arterial injury.
There are several limitations in regard to the relevance of the present results to clinical percutaneous interventions. First, the rat arterial injury model involves balloon injury to normal carotid artery which only has rare intimal cells. Thus, the rat carotid model differs substantially to angioplasty to diseased human vessels. The results cannot be directly extrapolated to clinical situations. Second, thrombus formation and leukocyte infiltration in injured rat arteries are much less pronounced compared to vascular injury in rabbits, swine, and non-human primates. How these differences impact on progression to intimal hyperplasia is not known. Third, vascular smooth muscle cell proliferation and dedifferentiation contribute to the formation of intimal hyperplasia [49]. We found that TAP-ANV and ANV-6L15 may inhibit vascular smooth muscle cell proliferation and promote its differentiation. Because vascular smooth muscle cell proliferation and dedifferentiation are regulated by distinct mechanism [49], the mechanism underling the role of TAP-ANV and ANV-6L15 in vascular smooth muscle cell differentiation merits further study. Finally, the 14-day follow-up was rather shortterm, which cannot be extrapolated to the long-term results. Studies with extended follow-up should be conducted to explore its clinical relevance.
In conclusion, peri-procedure i.v. bolus injections with TAP-ANV or ANV-6L15 resulted in substantial reduction of intima hyperplasia after balloon angioplasty of carotid arteries in rats without inducing a state of systemic hypo-coagulation or bleeding. Intimal hyperplasia also occurs pathologically in pulmonary hypertension, atherosclerosis, transplanted organs, vein grafts and hemodialysis vascular access. It would be of interest to investigate whether TAP-ANV and ANV-6L15 are useful for controlling intimal hyperplasia under these clinical settings.
Acknowledgements
The authors thank the Microscope Core Laboratory, Chang-Gung Memorial Hospital, Linkou about the assistance for confocal immunofluorescence.
Funding sources
Funding for this project was provided by Ministry of Science and Technology, Taiwan Grant (101-2314-B-182-092-MY2), Chang Gung Memorial Hospital, Taiwan (CMRPG3F0991-3, 3D1631-3), and National Heart, Lung and Blood Institute, NIH, USA (1R43HL77061, 1R43HL093848, 2R44HL093848 to TCW).
Footnotes
Disclosures
Tze-Chein Wun is a proprietor of EVAS therapeutics, currently developing ANV-KPIs fusion proteins for therapeutic applications. The other authors have no conflicts of interest to declare.
References
- [1].Ip JH, Fuster V, Israel D, Badimon L, Badimon J, Chesebro JH, The role of platelets, thrombin and hyperplasia in restenosis after coronary angioplasty, J. Am. Coll. Cardiol 17 (6 Suppl. B) (1991) 77B–88B. [DOI] [PubMed] [Google Scholar]
- [2].Schwartz RS, Holmes DR Jr., Topol EJ, The restenosis paradigm revisited: an alternative proposal for cellular mechanisms, J. Am. Coll. Cardiol 20 (1992) 1284–1293. [DOI] [PubMed] [Google Scholar]
- [3].Schwartz RS, Pathophysiology of restenosis: interaction of thrombosis, hyperplasia, and/or remodeling, Am. J. Cardiol 81 (7A) (1998) 14E–17E. [DOI] [PubMed] [Google Scholar]
- [4].Davies MG, Hagen PO, Pathophysiology of vein graft failure: a review, Eur. J. Vasc. Endovasc. Surg 9 (1995) 7–18. [DOI] [PubMed] [Google Scholar]
- [5].Motwani JG, Topol EJ, Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention, Circulation 97 (1998) 916–931. [DOI] [PubMed] [Google Scholar]
- [6].Mitra AK, Gangahar DM, Agrawal DK, Cellular, molecular and immunological mechanisms in the pathophysiology of vein graft intimal hyperplasia, Immunol. Cell Biol 84(2006)115–124. [DOI] [PubMed] [Google Scholar]
- [7].Lefkovits J, Topol EJ, Pharmacological approaches for the prevention of restenosis after percutaneous coronary intervention, Prog. Cardiovasc. Dis 40 (1997) 141–158. [DOI] [PubMed] [Google Scholar]
- [8].Bittl JA, Strony J, Brinker JA, et al. , Treatment with bivalirudin (Hirulog) as compared with heparin during coronary angioplasty for unstable or postinfarction angina. Hirulog Angioplasty Study Investigators, N. Engl. J. Med 333 (12) (September 21 1995) 764–769. [DOI] [PubMed] [Google Scholar]
- [9].Schussheim AE, Fuster V, Antithrombotic therapy and venous graft disease, Curr. Opin. Cardiol 13 (1998) 459–464. [DOI] [PubMed] [Google Scholar]
- [10].Herbert JM, Tissinier A, Defreyn G, Maffrand JP, Inhibitory effect of clopidogrel on platelet adhesion and intimal proliferation after arterial injury in rabbits, Arterioscler. Thromb 13 (8) (1993) 1171–1179. [DOI] [PubMed] [Google Scholar]
- [11].Waksman R, Pakala R, Roy P, et al. , Effect of clopidogrel on neointimal formation and inflammation in balloon-denuded and radiated hypercholesterolemic rabbit iliac arteries, J. Interv. Cardiol 21 (2) (2008) 122–128. [DOI] [PubMed] [Google Scholar]
- [12].Göncü T, Tiryakioğlu O, Ozcan A, et al. , Inhibitory effects of ticlopidine and clopidogrel on the intimal hyperplastic response after arterial injury, Anadolu Kardiyol. Derg 10 (1) (February 2010) 11–16. [DOI] [PubMed] [Google Scholar]
- [13].Wallitt EJ, Jevon M, Hornick PI, Therapeutics of vein graft intimal hyperplasia: 100 years on, Ann. Thorac. Surg 84 (2007) 317–323. [DOI] [PubMed] [Google Scholar]
- [14].Patel JH, Stoner JA, Owora A, Mathew ST, Thadani U, Evidence for using clopidogrel alone or in addition to aspirin in post coronary artery bypass surgery patients, Am. J. Cardiol 103 (2009) 1687–1693. [DOI] [PubMed] [Google Scholar]
- [15].Kulik A, Le May MR, Voisine P, et al. , Aspirin plus clopidogrel versus aspirin alone after coronary artery bypass grafting: the clopidogrel after surgery for coronary artery disease (CASCADE) trial, Circulation 122 (2010) 2680–2687. [DOI] [PubMed] [Google Scholar]
- [16].Sarembock IJ, Gertz SD, Gimple LW, et al. , Effectiveness of recombinant desulphatohirudin in reducing restenosis after balloon angioplasty of atherosclerotic femoral arteries in rabbits, Circulation 84 (1) (1991) 232–243. [DOI] [PubMed] [Google Scholar]
- [17].Unterberg C, Sandrock D, Nebendahl K, et al. , Reduced acute thrombus formation results in decreased neointimal proliferation after coronary angioplasty, J. Am. Coll. Cardiol 26 (7) (1995) 1747–1754. [DOI] [PubMed] [Google Scholar]
- [18].Abendschein DR, Recchia D, Meng YY, Oltrona L, Wickline SA, Eisenberg PR, Inhibition of thrombin attenuates stenosis after arterial injury in minipigs, J. Am. Coll. Cardiol 28 (7) (1996) 1849–1855. [DOI] [PubMed] [Google Scholar]
- [19].Serruys PW, Herrman JP, Simon R, et al. , A comparison of hirudin with heparin in the prevention of restenosis after coronary angioplasty. Helvetica Investigators, N. Engl. J. Med 333 (12) (September 21 1995) 757–763. [DOI] [PubMed] [Google Scholar]
- [20].Zoldhelyi P, Chen ZQ, Shelat HS, McNatt JM, Willerson JT, Local gene transfer of tissue factor pathway inhibitor regulates intimal hyperplasia in atherosclerotic arteries, Proc. Natl. Acad. Sci. U. S. A 98 (7) (March 27 2001) 4078–4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yin X, Yutani C, Ikeda Y, et al. , Tissue factor pathway inhibitor gene delivery using HVJ-AVE liposomes markedly reduces restenosis in atherosclerotic arteries, Cardiovasc. Res 56 (3) (December 2002) 454–463. [DOI] [PubMed] [Google Scholar]
- [22].Kopp CW, Holzenbein T, Steiner S, et al. , Inhibition of restenosis by tissue factor pathway inhibitor: in vivo and in vitro evidence for suppressed monocyte chemoattraction and reduced gelatinolytic activity, Blood 103 (5) (March 1 2004) 1653–1661. [DOI] [PubMed] [Google Scholar]
- [23].Pyo RT, Sato Y, Mackman N, Taubman MB, Mice deficient in tissue factor demonstrate attenuated intimal hyperplasia in response to vascular injury and decreased smooth muscle cell migration, Thromb. Haemost 92 (3) (September 2004) 451–458. [DOI] [PubMed] [Google Scholar]
- [24].Jang Y, Guzman LA, Lincoff AM, et al. , Influence of blockade at specific levels of the coagulation cascade on restenosis in a rabbit atherosclerotic femoral artery injury model, Circulation 92 (10) (November 15 1995) 3041–3050. [DOI] [PubMed] [Google Scholar]
- [25].Oltrona L, Speidel CM, Recchia D, Wickline SA, Eisenberg PR, Abendschein DR, Inhibition of tissue factor-mediated coagulation markedly attenuates stenosis after balloon-induced arterial injury in minipigs, Circulation 96 (1997) 646–652. [DOI] [PubMed] [Google Scholar]
- [26].St Pierre J, Yang LY, Tamirisa K, et al. , Tissue factor pathway inhibitor attenuates procoagulant activity and upregulation of tissue factor at the site of balloon-induced arterial injury in pigs, Arterioscler. Thromb. Vasc. Biol 19 (9) (September 1999) 2263–2268. [DOI] [PubMed] [Google Scholar]
- [27].Asada Y, Hara S, Tsuneyoshi A, et al. , Fibrin-rich and platelet-rich thrombus formation on neointima: recombinant tissue factor pathway inhibitor prevents fibrin formation and neointimal development following repeated balloon injury of rabbit aorta, Thromb. Haemost 80 (3) (September 1998) 506–511. [PubMed] [Google Scholar]
- [28].Roqué M, Reis ED, Fuster V, et al. , Inhibition of tissue factor reduces thrombus formation and intimal hyperplasia after porcine coronary angioplasty, J. Am. Coll. Cardiol 36 (7) (December 2000) 2303–2310. [DOI] [PubMed] [Google Scholar]
- [29].Ragosta M, Gimple LW, Gertz SD, et al. , Specific factor Xa inhibition reduces restenosis after balloon angioplasty of atherosclerotic femoral arteries in rabbits, Circulation 89 (3) (March 1994) 1262–1271. [DOI] [PubMed] [Google Scholar]
- [30].Schwartz RS, Holder DJ, Holmes DR, et al. , Neointimal thickening after severe coronary artery injury is limited by a short-term administration of a factor Xa inhibitor. Results in a porcine model, Circulation 93 (8) (April 15 1996) 1542–1548. [DOI] [PubMed] [Google Scholar]
- [31].Brown DM, Kania NM, Choi ET, et al. , Local irrigation with tissue factor pathway inhibitor inhibits intimal hyperplasia induced by arterial interventions, Arch. Surg 131 (1996) 1086–1090. [DOI] [PubMed] [Google Scholar]
- [32].Rapp JH, Pan XM, Ghermay A, et al. , A blinded trial of local recombinant tissue factor pathway inhibitor versus either local or systemic heparin in a vein bypass model, J. Vasc. Surg 25 (1997) 726–729. [DOI] [PubMed] [Google Scholar]
- [33].Huynh TT, Davies MG, Thompson MA, Ezekowitz MD, Hagen P, Annex BH, Local treatment with recombinant tissue factor pathway inhibitor reduces the development of intimal hyperplasia in experimental vein grafts, J. Vasc. Surg 33 (2001) 400–407. [DOI] [PubMed] [Google Scholar]
- [34].Chen HH, Vicente CP, He L, Tollefsen DM, Wun TC, Fusion proteins comprising annexin V and Kunitz protease inhibitors are highly potent thrombogenic site-directed anticoagulants, Blood 105 (2005) 3902–3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liao MH, Jan TR, Chiang CC, et al. , Investigation of a potential scintigraphic tracer for imaging apoptosis: radioiodinated annexin V-Kunitz protease inhibitor fusion protein, J. Biomed. Biotechnol 2011 (2011) 675701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ida M, Satoh A, Matsumoto I, Kojima-Aikawa K, Human annexin V binds to sulfatide: contribution to regulation of blood coagulation, J. Biochem 135 (2004) 583–588. [DOI] [PubMed] [Google Scholar]
- [37].Merten M, Beythien C, Gutensohn K, Kuhnl P, Meinertz T, Thiagarajan P, Sulfatides activate platelets through P-selectin and enhance platelet and platelet-leukocyte aggregation, Arterioscler. Thromb. Vasc. Biol 25 (2005) 258–263. [DOI] [PubMed] [Google Scholar]
- [38].Capila I, Hernaiz MJ, Mo YD, et al. , Annexin V-heparin oligosaccharide complex suggests heparan sulfate-mediated assembly on cell surfaces, Structure 9 (2001) 57–64. [DOI] [PubMed] [Google Scholar]
- [39].Kumar AV, Katakam SK, Urbanowitz AK, Gotte M, Heparan sulphate as a regulator of leukocyte recruitment in inflammation, Curr. Protein Pept. Sci 16 (2015) 77–86. [DOI] [PubMed] [Google Scholar]
- [40].Draeger A, Monastyrskaya K, Babiychuk EB, Plasma membrane repair and cellular damage control: the annexin survival kit, Biochem. Pharmacol 81 (2011) 703–712. [DOI] [PubMed] [Google Scholar]
- [41].Bouter A, Gounou C, Berat R, et al. , Annexin-A5 assembled into two-dimensional arrays promotes cell membrane repair, Nat. Commun 2 (2011) 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lee H, Mason C, Achilefu S, Heptamethine cyanine dyes with a robust C-C bond at the center position of the chromophore, J. Org. Chem 71 (2008) 7862–7865. [DOI] [PubMed] [Google Scholar]
- [43].Yen TC, Wey SP, Liao CH, et al. , Measurement of the binding parameters of annexin derivatives-erythrocyte membrane interactions, Anal. Biochem 406 (2010) 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Yeh CH, Chen TP, Wang YC, Fang SW, Wun TC, Potent cardioprotection from ischemia-reperfusion injury by a 2-domain fusion protein comprising Annexin V and Kunitz protease inhibitor, J. Thromb. Haemost 11 (2013) 1454–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Marmur JD, Rossikhina M, Guha A, et al. , Tissue factor is rapidly induced in arterial smooth muscle after balloon injury, J. Clin. Invest 91 (1993) 2253–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Abendschein DR, Yang LY, Chun J, Cho D, Scherrer D, St Pierre J, Prolonged procoagulant activity on overstretch-injured coronary arteries in pigs, J. Thromb. Haemost 1 (2003) 836–842. [DOI] [PubMed] [Google Scholar]
- [47].Ghigliotti G, Waissbluth AR, Speidel C, Abendschein DR, Eisenberg PR, Prolonged activation of prothrombin on the vascular wall after arterial injury, Arterioscler. Thromb. Vasc. Biol 18 (1998) 250–257. [DOI] [PubMed] [Google Scholar]
- [48].Chen WJ, Yeh YH, Lin KH, Chang GJ, Kuo CT, Molecular characterization of thyroid hormone-inhibited atrial L-type calcium channel expression: implication for atrial fibrillation in hyperthyroidism, Basic Res. Cardiol 106 (2) (2011) 163–174. [DOI] [PubMed] [Google Scholar]
- [49].Chen WJ, Chen YH, Lin KH, Ting CH, Yeh YH, Cilostazol promotes vascular smooth muscles cell differentiation through the cAMP response element-binding protein-dependent pathway, Arterioscler. Thromb. Vasc. Biol 31 (9) (2011. September) 2106–2113. [DOI] [PubMed] [Google Scholar]


